Abstract
Triggering of the TCR/CD3 complex with specific antigen or anti-CD3 monoclonal antibody initiates activation-induced cell death (AICD) in mature T cells, an effect also mediated by the Fas/FasL system. We have previously shown that CD2 stimulation rescues T cells from TCR/CD3-induced apoptosis by decreasing the expression of Fas and FasL. In the present study, we examined whether the endogenous production of IL-2 plays a role in the effects mediated by CD2 triggering. The results indicated that transcription of Fas/FasL is controlled by interleukin-2 (IL-2) production and that CD2 triggering rescues a T-cell hybridoma from AICD via decreased production of IL-2. To ascertain whether modulation of IL-2 may be a general mechanism of AICD control, we examined other stimuli, capable of modulating the expression of the Fas/FasL system and the ensuing AICD, for ability to affect production of IL-2. We found that IL-6 reduced the level of TCR/CD3-induced apoptosis and the expression of Fas/FasL, yet failed to inhibit IL-2 production. Because IL-2 is involved in both apoptosis and activation events, these results indicate that, in contrast to CD2, which inhibits apoptosis and T cell activation, IL-6 inhibits apoptosis but not IL-2–induced activation. These observations may provide the basis for differential control of T-cell activation and apoptosis.
LIGATION OF THE TCR/CD3 complex of primary T cells by antigen peptides or monoclonal antibody (MoAb) to TCR/CD3 triggers a series of activation events, eventually leading to cell proliferation and cytokine production.1 In contrast, TCR/CD3 stimulation of previously activated T cells or T-cell hybridomas will induce apoptosis. This event has been termed activation-induced cell death (AICD).2 AICD is also mediated by the Fas/FasL system.3-5 Fas/APO-1 (CD95) is a type I membrane protein of the tumor necrosis factor (TNF)/nerve growth factor (NGF) receptor family,6,7 and FasL is a type II membrane protein belonging to the tumor necrosis factor (TNF) family.8,9 Binding of FasL to its receptor or agonist anti-Fas MoAb induces apoptosis.3-7 Anti-CD3 MoAb cross-linking induces the expression of FasL and upregulates Fas. The engagement of Fas by FasL activates the cell death program.3-5 10
Clonal activation and/or expansion are controlled by a balance between apoptotic and activating signals. It has been proposed that AICD serves a role in limiting the expansion of an immune response by eliminating lymphocytes that are no longer needed.10However, previously activated T cells are susceptible to Fas-mediated apoptosis, but in vitro stimulation induces AICD only in a portion of these cells, resulting in clonal expansion.2-5,10 These results suggest that the process must be controlled by additional stimuli such as cytokines, adhesion molecules, or glucocorticoid hormones (GCH).11-14 In particular, the role of interleukin-2 (IL-2) is not completely understood. In fact, IL-2 rescues activated T cells from apoptosis by upregulating the expression of apoptosis inhibitory proteins such as Bcl-2,15-19 but recent studies have shown that IL-2 cannot prevent AICD; in fact, the cytokine will prime T cells for TCR-activated apoptosis.10,20-22 This pro-apoptosis mechanism, occurring at high doses of antigen and IL-2, has been termed propriocidal regulation and may limit clonal expansion.21,23-25 This notion is supported by the observation that IL-2–deficient animals display overproduction of lymphocytes with uncontrolled T-cell activation and autoimmunity.26-28
We previously showed that several stimuli, including adhesion molecules such as CD2 and CD44, IL-4, or GCH, can modulate AICD.11,12,14 We also established an in vitro model in which a murine CD4+ hybridoma T-cell line, 3DO, undergoes apoptosis after stimulation of TCR/CD3. In this model, the Fas-FasL interaction plays a dominant role in mediating AICD.12 29In the present study, we have tested the effects of IL-6 on apoptosis and Fas/FasL expression. We also investigated whether changes in IL-2 production could account for the rescue of T cells from apoptosis operated by CD2 triggering and IL-6. We found that IL-6, like CD2, decreases anti-CD3–induced apoptosis by downregulating the expression of Fas and FasL. However, while the effect of CD2 triggering appeared to depend on inhibition of IL-2 production, IL-6 rescued T cells from apoptosis via a mechanism apparently independent of the presence of IL-2. These observations suggest that Fas/FasL expression and T-cell apoptosis may not always depend on IL-2 production.
MATERIALS AND METHODS
Animals.
C3H/HeN mice purchased from Charles River (Chalco, Milan, Italy) were used at the age of 8 weeks as donors of T cells.
Cell suspensions.
A CD3+, CD4+, CD2+, CD44+ subline of the OVA-specific hybridoma T-cell line 3DO30 developed in our laboratory was used in this study. Cells, maintained in suspension in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 10 μmol/L HEPES buffer, were centrifuged at preestablished times at 200g for 10 minutes, washed, and adjusted to the desired concentration (see below).
Lymph nodes were ground between frosted glass slides to produce a cell suspension and then passed twice through nylon columns, to obtain a 95% pure T-cell population, as assessed by CD4 and CD8 expression.
Antibody cross-linking and cell treatment.
Hamster anti-mouse CD3ε (clone 145-2C11; Pharmingen, San Diego, CA) MoAb at 1 μg/mL and/or rat anti-mouse CD2 (clone RM2-5; Pharmingen) MoAb, at a concentration of 5 μg/mL, were allowed to adhere in flat-bottom, high-binding 96-well plates (Costar, Cambridge, MA) at 4°C in 100 μL phosphate-buffered saline (PBS). After 20 hours, plates coated with MoAbs were washed, incubated at 37°C for 2 hours with PBS supplemented with 10% FCS, washed again, and the hybridoma T cells were then plated at 1 × 105cells/well with or without recombinant murine (rm) IL-6, rm interferon-γ (IFN-γ; Genzyme, Cambridge, MA), rmIL-13 (R&D System, Ltd, Abingdon, UK), and then incubated at 37°C for 18 hours. Isotype-matched rat anti-mouse IgG2b MoAb (clone R35-38; Pharmingen) was used as a control. Lymph node T cells, at the concentration of 2 × 105 cells/well, were cultured in 96-well plates coated with anti-CD3 MoAb (1 μg/mL) for 5 days in the presence of different concentrations of rmIL-6.
To evaluate Fas-mediated killing, 3DO cells (1 × 106) were incubated at room temperature for 30 minutes with 10 μg/mL of the antibody to Fas (hamster anti-mouse, clone Jo2; Pharmingen), then washed and plated in wells coated with an antibody to hamster IgG (5 μg/mL; Pharmingen) for the cross-linking of the anti-Fas MoAb. Some groups were treated with IL-6 (20 μg/mL).
To block anti-CD3–induced apoptosis, in selected experiments anti-Fas MoAb (clone Jo2) or isotype-matched hamster anti-mouse IgG MoAb (clone UC8-4B3; Pharmingen) were used in a soluble non–cross-linked form. For this purpose, the MoAbs were added after incubation of anti-CD3 pretreated plates with 100 μL/well FCS.
To neutralize IL-2 endogenous activity, anti–IL-2 MoAb, at 10 μg/mL (S4B6; Pharmingen), or isotype-matched control MoAb were added to cell cultures.
To test the effects of exogenous IL-2, 3DO cells were incubated for 18 hours at 37°C in anti-CD3–coated plates, in the presence or absence of human recombinant IL-2 (500 IU/mL; provided by Hoffmann La Roche, Nutley, NJ). After extensive washing, an aliquot of anti-CD3–primed cells was recultured for an additional 18 hours, without antigen, in the presence or absence of IL-2 (500 IU/mL). Apoptosis and Fas/FasL expression were evaluated as described above.
IL-2 assays.
Supernatants from cells stimulated for 18 hours with anti-CD3 and/or anti-CD2, and/or IFN-γ, and/or IL-13, and/or IL-6 were tested for their concentrations of IL-2 by two-site enzyme-linked immunosorbent assay (ELISA) using MoAb JES6-1A12 as the primary reagent and biotinylated monoclonal S4B6 as the secondary reagent. Both antibodies were purchased from Pharmingen. The IL-2 titers (means ± SD of replicate samples) were expressed as picograms per milliliter, calculated by reference to standard curves constructed with known amounts of IL-2. The sensitivity limit of the assay was approximately 20 pg/mL.
Flow cytometry analysis.
A single-cell suspension (1 × 106 cells/sample) was incubated for 30 minutes on ice in 50 μL staining buffer (PBS plus 5% FCS) containing 10 μg/mL hamster anti-mouse Fas MoAb directly conjugated to R-phycoerytrin (PE) or PE-hamster IgG (isotype control). Both MoAbs were purchased from Pharmingen. Cells were also stained with rabbit polyclonal antibody raised against a peptide, corresponding to amino acids 260-279 mapping at the carboxy terminus of human Fas-L (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), which has been shown to cross-react with mouse FasL12 or with isotype-matched antibody. Anti-rabbit IgG fluorescein isothiocyanate (FITC) conjugate, F(ab′)2 fragment (Sigma, St Louis, MO), was used as the second-step reagent. An aliquot of cells was stained with the secondary antibody alone (background). The blocking peptide used for rabbit immunization (Santa Cruz Biotechnology) was also used in competition studies, according to the manufacturer’s instructions. After 30 minutes the samples were washed and analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). In selected experiments, a portion of T cells was stained with anti–IL-2 receptor MoAb conjugated to R-phycoerythrin (IL-2R, P55; Pharmingen). The median or percentage values of Fas, FasL, and IL-2R histograms were calculated using Lysis II research software (Becton Dickinson).
Apoptosis evaluation by propidium iodide solution (PI).
Apoptosis was measured by flow cytometry as described elsewhere.31 After culturing, cells were centrifuged and the pellets gently resuspended in 1.5 mL hypotonic PI (50 μg/mL in 0.1% sodium citrate plus 0.1% Triton X-100; Sigma). The tubes were kept overnight at 4°C in the dark. The PI-fluorescence of individual nuclei was measured by flow cytometry using standard FACScan equipment (Becton Dickinson). The nuclei traversed a 488-nm Argon laser light beam. A 560-nm dichroid mirror (DM 570) and a 600-nm band pass filter (band width 35 nm) were used to collect the red fluorescence caused by PI DNA staining. The data were recorded in a logarithmic scale in a Hewlett Packard (HP 9000, model 310; Palo Alto, CA) computer. The percentage of apoptotic cell nuclei (sub-diploid DNA peak in the DNA fluorescence histogram) was calculated with specific FACScan research software (Lysis II).
Cytotoxicity assay.
The lysis of P815 Fas+ tumor cell line within 16 hours was used as an indicator of FasL expression. This tumor cell line was grown in RPMI 1640 and 10% FCS and subcultured two to three times per week. Different concentrations of 3DO cells were cultured for 18 hours on plates coated with anti-CD3 (1 μg/mL), or control medium with or without IL-6 (20 ng/mL). The 51Cr labeling and assay were as previously described.3 Spontaneous release or release in the presence of anti-CD3 and/or IL-6 with no effector cells was ≤15% of the total release. The percentage of specific lysis at various effector:target (E:T) ratios was calculated as follows:
where Test cpm is the mean cpm released in the presence of effector cells, Spontaneous Release is the mean of cpm released from targets cultured in medium alone, and Total Release is the mean cpm after lysing target cells with 0.5% Triton X-100.
Northern blot analysis.
Specific amounts (indicated in individual figure legends) of total cytoplasm RNA were separated in 1.2% agarose gel and transferred to nitrocellulose filters (Scheicher and Schuell, Dassel, Germany). DNA probes were 32P-labeled using the nick translation kit from Boehringer Mannheim (Mannheim, Germany). Hybridization was performed overnight. Filters were washed three times in 0.2× sodium citrate sodium chloride (SSC) with 0.5% sodium dodecyl sulfate (SDS) at 37°C followed by two washes at 65°C.
RNase protection analysis (RPA).
A probe for RPA was constructed by reverse transcriptase-polymerase chain reaction (RT-PCR) using the forward primer CACATATGGAACCGCTCTGATC and the reverse primer CATTAGCACCAGATCCT0CAGGA (located on FasL cDNA 610-631 bp and 772-793, respectively). PCR products (184 bp) cloned into pCRII vector using the TA cloning kit (Invitrogen BV, San Diego, CA) were sequenced to exclude the occurrence of point mutations. Plasmid DNA was linearized with BamHI (New England Biolabs, Beverly, MA) and transcribed with T7 RNA polymerase (GIBCO-BRL Life Technologies, Paisley, UK) in the presence of 50 μmol/L [α-32 P] UTP. After gel purification, a 2 × 105 cpm probe was hybridized to total RNA (20 μg) overnight at 60°C. The linearized β-actin probe was purchased from Ambion (Ambion Inc, Austin, TX).
RNase digestion was performed by using a RNase A (40 μg/mL; Boehringer Mannheim) and RNase T1 (1.5 U/μL; GIBCO-BRL) solution at 37°C for 15 minutes. The undigested products were treated with phenol-chloroform, precipitated with ethanol, and loaded on a denaturing polyacrylamide sequencing gel. Autoradiographic exposure was performed for 2 days.
Statistical analysis.
Each experiment was performed at least three times. Representative experiments are shown, unless otherwise indicated, in figure legends. The means ± SD of three different experiments are reported in the text. Due to non-normal distribution of the data, nonparametric tests (Kruskall-Wallis’ analysis of variance) were adopted for statistical evaluation.
RESULTS
AICD in 3DO cells: Role of IL-2.
It has previously been shown that treatment with anti-CD3 MoAb mimics the effect of Ag-TCR/CD3 interaction.32 We have established an in vitro model of AICD consisting of the mouse T-cell hybridoma 3DO that undergoes apoptosis after stimulation of the TCR/CD3 complex. In this model, the Fas/FasL interaction plays a dominant role in mediating AICD.12,29 33 We thus performed experiments to evaluate the possible role of IL-2 in this experimental system. 3DO cells were cultured for different times in 96-well plates coated with cross-linked (activating) anti-CD3 MoAb. Apoptosis was assessed by measuring DNA contents with the PI assay in a time-course experiment. Table 1 shows the results of a representative experiment. Before culturing and at 4 hours, less than 3% of the cells were dead. However, 8 and 18 hours later, 7.5% and 40% of the cells had died, respectively. Fas/FasL and IL-2R expression by fluorescence-activated cell sorting (FACS) analysis and IL-2 production by ELISA were determined in the same experiments. Results in Table 1 indicate increased expression of Fas/FasL, which correlated with a parallel increase in IL-2 and IL2R. The levels of IL-2 and Fas/FasL are consistent with apoptosis levels. To ascertain the specificity of FasL antibody, experiments of binding competition for FasL were performed by means of the peptide used for immunization. The antigen peptide completely blocked FasL staining of anti-CD3–stimulated 3DO cells, as stated in Table 1.
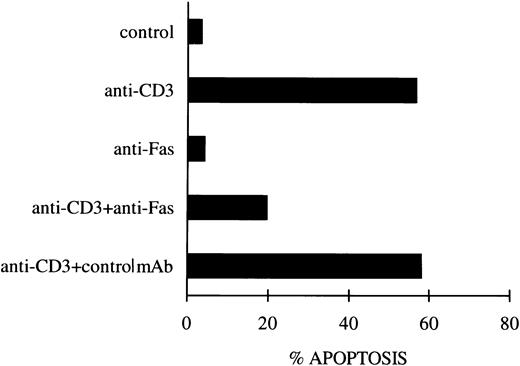
As an additional control, experiments were performed to block the Fas/FasL system using the soluble non–cross-linked anti-Fas MoAb, as previously described.12 Figure1 shows that blocking the Fas receptor induced a substantial inhibition of anti-CD3–induced apoptosis, whereas no effect was observed with the control antibody.
Inhibition of apoptosis in anti-CD3–stimulated 3DO cells by soluble anti-Fas MoAb. In one experiment representative of three, anti-CD3–stimulated cells (10 μg/mL) were cultured in the presence of soluble anti-Fas (1 μg/mL) or control MoAbs. Apoptosis was evaluated by flow cytometric analysis of PI-stained nuclei after 18 hours of culture.
Inhibition of apoptosis in anti-CD3–stimulated 3DO cells by soluble anti-Fas MoAb. In one experiment representative of three, anti-CD3–stimulated cells (10 μg/mL) were cultured in the presence of soluble anti-Fas (1 μg/mL) or control MoAbs. Apoptosis was evaluated by flow cytometric analysis of PI-stained nuclei after 18 hours of culture.
Role of IL-2 in the regulation of Fas/FasL expression.
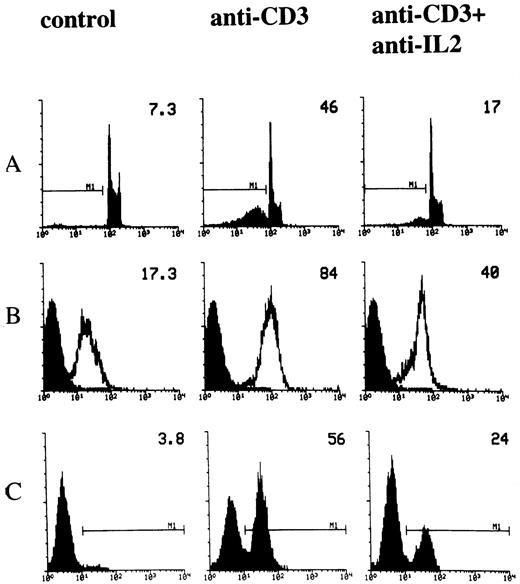
IL-2 is involved in T-cell activation.34 It has also been reported that IL-2 is important in the regulation of TCR-activated apoptosis.10,20,21,23,25 In fact, it has been shown that the blockade of IL-2 utilization inhibits both T-cell proliferation and death.24 We performed experiments to study the role of IL-2 in the modulation of anti-CD3–induced apoptosis and, in particular, we analyzed the role of IL-2 in regulating Fas and FasL expression. Cells were cultured for 18 hours in 96-well plates, coated with activating anti-CD3 MoAb in the presence or absence of neutralizing anti–IL-2 MoAb. Figure 2A shows the result of a representative experiment in which apoptosis was evaluated by the PI assay. Consistent with previous results,24 treatment with anti–IL-2 antibodies inhibited anti-CD3–induced apoptosis (Fig 2A). We also tested the effect of reduced IL-2 utilization on Fas/FasL expression. Figure 2B and C show the results of flow cytometry analysis, indicating that treatment with anti–IL-2 MoAb countered the anti-CD3–induced upregulation of Fas and FasL. Similar results were obtained when Northern blot and RNAse protection experiments were performed. Antibodies against IL-2 downregulated the mRNA for Fas and FasL, suggesting that IL-2 modulates the transcription of the Fas and FasL genes (Fig 3).
Effect of anti–IL-2 neutralizing MoAb on anti-CD3-induced apoptosis and Fas/FasL expression. 3DO cells were cultured in 96-well plates coated with anti-CD3 MoAb (1 μg/mL), in the presence or absence of anti–IL-2 neutralizing MoAb (10 μg/mL). Apoptosis (A), Fas (B), and FasL (C) expression were evaluated by flow cytometric analysis after 18 hours of culture, as described in Materials and Methods. The percentage of apoptotic nuclei, the Fas histogram median, the percentage of FasL+ cells, of a representative experiment, are indicated in each histogram.
Effect of anti–IL-2 neutralizing MoAb on anti-CD3-induced apoptosis and Fas/FasL expression. 3DO cells were cultured in 96-well plates coated with anti-CD3 MoAb (1 μg/mL), in the presence or absence of anti–IL-2 neutralizing MoAb (10 μg/mL). Apoptosis (A), Fas (B), and FasL (C) expression were evaluated by flow cytometric analysis after 18 hours of culture, as described in Materials and Methods. The percentage of apoptotic nuclei, the Fas histogram median, the percentage of FasL+ cells, of a representative experiment, are indicated in each histogram.
Expression of Fas and FasL mRNA in anti-CD3–activated 3DO cells in the presence of anti–IL-2 neutralizing MoAb. (B) Northern blot. Total RNA was extracted, separated on agarose gel (20 μg/line), and transferred to nitrocellulose filter. The filter was hybridized with a nick-translation–labeled Fas cDNA probe, washed, and exposed for autoradiography. (A) RNase protection analysis of FasL mRNA expression. The protected antisense mRNA FasL fragment is 184 bp. Each line was loaded with 20 μg of total RNA. 1, Control; 2, anti-CD3–treated cells; 3, anti-CD3 + anti–IL-2 control isotype-treated cells; 4, anti-CD3 + anti–IL-2 treated cells.
Expression of Fas and FasL mRNA in anti-CD3–activated 3DO cells in the presence of anti–IL-2 neutralizing MoAb. (B) Northern blot. Total RNA was extracted, separated on agarose gel (20 μg/line), and transferred to nitrocellulose filter. The filter was hybridized with a nick-translation–labeled Fas cDNA probe, washed, and exposed for autoradiography. (A) RNase protection analysis of FasL mRNA expression. The protected antisense mRNA FasL fragment is 184 bp. Each line was loaded with 20 μg of total RNA. 1, Control; 2, anti-CD3–treated cells; 3, anti-CD3 + anti–IL-2 control isotype-treated cells; 4, anti-CD3 + anti–IL-2 treated cells.
Moreover, IL-2R had the same expression pattern as IL-2 production (Table 1), further suggesting that the IL-2/IL-2R system plays a role in T-cell death. These data are in agreement with previous evidence showing that IL-2 activity is dependent on antigen-induced increase in IL-2R density.35,36 IL-2 alone, in fact, induces neither apoptosis nor Fas/FasL regulation. Because of the striking correlation between IL-2R expression, IL-2 production, and dose of antigen,21,24,35 36 only a modest increase of Fas/FasL expression and apoptosis were observed when exogenous IL-2 was added to anti-CD3–stimulated 3DO cells (data not shown). This modest increase could be explained by the fact that endogenous IL-2 has already engaged IL-2R. To verify this hypothesis, a series of experiments was performed in which the IL-2 containing conditioned medium was removed from anti-CD3–activated (18 hours) 3DO cell cultures, and cells were then recultured for 18 hours in the presence of exogenous IL-2. The results (Table 2) showed that exogenous IL-2 determined a significant increase in apoptosis and Fas/FasL expression.
CD2 triggering but not IL-6 modulates AICD via regulation of IL-2 levels.
We have previously shown that CD2 triggering rescues T cells from AICD by downmodulating the Fas/FasL system.12 In the present study, we investigated whether this effect could be mediated through modulation of endogenous IL-2 production and whether this mechanism is shared by other stimuli that modulate T-cell apoptosis. We tested the effect of various cytokines on apoptosis. 3DO cells were cultured for 18 hours in 96-well plates coated with cross-linked anti-CD3 MoAb in the presence or absence of cytokines, used at concentrations above those known to be active in vitro.37-39 As shown in Fig 4A, IL-13 had no effect, IFN-γ induced a significant increase, and IL-6 inhibited anti-CD3–induced apoptosis. The means ± SD of three experiments with 3DO are as follows: untreated control, 5 ± 1; anti-CD3–treated (1 μg/mL), 48 ± 5; anti-CD3–treated plus IFN-γ–treated (200 U/mL), 63 ± 3; anti-CD3–treated plus IL-13 (10 ng/mL), 47 ± 3; anti-CD3–treated plus IL-6 (20 ng/mL), 22 ± 2. The difference between anti-CD3–treated plus IFN-γ, or anti-CD3–treated plus IL-6 versus anti-CD3–treated groups was statistically significant (P < .01).
Effect of IL-6 or IFN-γ on anti-CD3–treated cells. Cells were cultured for 18 hours on anti-CD3 coated plates in the presence or absence of IL-6 (20 ng/mL), IFN-γ (200 U/mL), or IL-13 (10 ng/mL). Apoptosis (A) of PI-stained nuclei, Fas (B), and FasL (C) expression were evaluated by flow cytometric analysis after 18 hours of culture. Numbers on histograms represent the percentage of apoptotic nuclei, the value of Fas histogram median, and the percentage of FasL+ cells of a representative experiment calculated by Lysis II.
Effect of IL-6 or IFN-γ on anti-CD3–treated cells. Cells were cultured for 18 hours on anti-CD3 coated plates in the presence or absence of IL-6 (20 ng/mL), IFN-γ (200 U/mL), or IL-13 (10 ng/mL). Apoptosis (A) of PI-stained nuclei, Fas (B), and FasL (C) expression were evaluated by flow cytometric analysis after 18 hours of culture. Numbers on histograms represent the percentage of apoptotic nuclei, the value of Fas histogram median, and the percentage of FasL+ cells of a representative experiment calculated by Lysis II.
We next examined the expression of Fas/FasL in anti-CD3–treated 3DO cells, in the presence or absence of cytokines. As shown in Fig 4B and C, in which the expression of Fas and FasL was assessed by FACS analysis, IL-13 had no effect, IFN-γ induced a significant increase, and IL-6 significantly reduced the anti-CD3–induced expression of Fas/FasL.
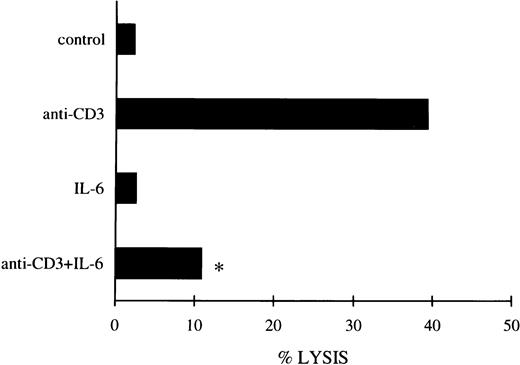
IL-6 titration was also performed to establish whether the concentrations effective in inhibiting apoptosis would be comparable to those able to downmodulate the Fas/FasL system. The results in Table 3 show that IL-6 countered apoptosis at the same concentrations as those capable of downmodulating the expression of Fas/FasL. Similar results were obtained when the IL-6 effect on FasL expression was assessed by functional analysis using an Fas+ P815 cell target in a cytotoxicity assay (Fig 5).
FasL expression evaluated by the cytotoxicity assay (see Materials and Methods) with 3DO cells untreated or treated with anti-CD3 (1 μg/mL) and/or IL-6 (20 ng/mL) for 18 hours. The results are the average of three experiments (each in triplicate culture). The standard errors (<10%) are omitted for clarity. E:T ratio, 25:1. *P < .01 comparing anti-CD3– + IL-6–treated versus anti-CD3–treated group.
FasL expression evaluated by the cytotoxicity assay (see Materials and Methods) with 3DO cells untreated or treated with anti-CD3 (1 μg/mL) and/or IL-6 (20 ng/mL) for 18 hours. The results are the average of three experiments (each in triplicate culture). The standard errors (<10%) are omitted for clarity. E:T ratio, 25:1. *P < .01 comparing anti-CD3– + IL-6–treated versus anti-CD3–treated group.
We also analyzed the effects of IL-6 on apoptosis induced by anti-CD3 MoAb on normal lymphocytes. As previously reported, chronic stimulation is required for FasL expression and induction of apoptosis in primary lymphocytes.9 40 Table 4 shows that lymphocytes, cultured for 5 days in the presence of anti-CD3 MoAb, upregulated Fas/FasL and underwent apoptosis. The effects on apoptosis and FasL expression were partially reverted by IL-6. In contrast, IL-6 did not affect anti-CD3–induced Fas expression (Table 4), suggesting that AICD is controlled by FasL expression.
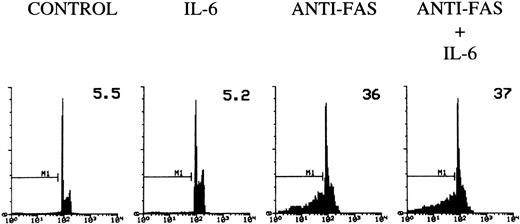
To determine whether IL-6 would inhibit the apoptosis induced by direct triggering of Fas, 3DO cells were treated with agonist anti-Fas MoAb and then plated on wells coated with an antibody to IgG in the presence or absence of IL-6. According to previous data, cross-linked anti-Fas MoAb induces apoptosis in 3DO cells.12 IL-6 failed to inhibit this Fas-induced death (Fig 6), suggesting that IL-6 regulates TCR-mediated expression of Fas/FasL, but does not interfere directly with Fas-activated cell death.
Fas-induced apoptosis of 3DO cells. Ninety-six–well plates were coated with an antibody to hamster IgG (5 μg/mL) with or without IL-6 (20 ng/mL). Cells were incubated for 30 minutes with medium alone or medium containing anti-Fas MoAb (10 μg/mL). Apoptosis was evaluated by flow cytometric analysis of PI-stained nuclei after 18 hours of culture. The percentage of apoptotic nuclei is indicated in each histogram. PI fluorescence versus number of nuclei is shown.
Fas-induced apoptosis of 3DO cells. Ninety-six–well plates were coated with an antibody to hamster IgG (5 μg/mL) with or without IL-6 (20 ng/mL). Cells were incubated for 30 minutes with medium alone or medium containing anti-Fas MoAb (10 μg/mL). Apoptosis was evaluated by flow cytometric analysis of PI-stained nuclei after 18 hours of culture. The percentage of apoptotic nuclei is indicated in each histogram. PI fluorescence versus number of nuclei is shown.
We also explored the possibility that IFN-γ, IL-6, or CD2 triggering could indirectly affect the expression of Fas/FasL, via the modulation of IL-2 levels. We measured the levels of IL-2 in supernatants from cells activated with anti-CD3 MoAb in the presence or absence of various stimuli. As shown in Table 5, significant inhibition of IL-2 production was induced by costimulation with cross-linked anti-CD2 antibody, whereas an increase was induced by IFN-γ. In contrast to CD2, IL-6 did not affect IL-2 production induced by stimulation with anti-CD3. Again, IL-2R expression displayed a pattern similar to that of IL-2 production (Table 5).
These data indicate that some signals, such as those activated by CD2 or IFN-γ, regulate the expression of Fas/FasL and the ensuing cell death by controlling the level of IL-2, whereas other signals, such as IL-6, fail to do so, suggesting the occurrence of different mechanisms responsible for the modulation of Fas/FasL expression and AICD.
DISCUSSION
This study shows that IL-6 rescues T cells from anti-CD3–induced apoptosis. This protective effect correlates with downmodulation of Fas/FasL expression in 3DO cells (Figs 4, 5, and Table 3) and FasL in primary lymphocytes (Table 4). Furthermore, IL-6 does not inhibit IL-2 production (Table 5), suggesting that IL-6–induced modulation of Fas/FasL expression and cell death may not be regulated by IL-2 production. On the contrary, CD2, which also inhibits Fas/FasL expression and T-cell apoptosis,12 and IFN-γ, which increases Fas/FasL expression (Fig 4) and activates apoptosis,37 will decrease and increase IL-2 levels, respectively (Table 5). Taken together, these results indicate that Fas/FasL expression may or may not be regulated by IL-2 and suggest that different mechanisms control AICD.
It is generally believed that IL-2 has potent activating effects on human lymphocytes and monocytes.34 However, recent data in IL-2–deficient mice indicate that IL-2 also exerts negative modulatory effects on the immune system.26-28 It has been shown that IL-2 prevents apoptosis of activated T cells by upregulating the expression of apoptosis inhibitory proteins such as Bcl-2.17,19 Moreover, removal of IL-2 from activated T cells in vitro leads to reduced Bcl-2 expression and cell death.15 On the other hand, it has been shown that IL-2 is involved in the propriocidal regulation of AICD.21 23-25This study shows that IL-2 neutralization by anti–IL-2 MoAb inhibits the anti-CD3–induced increases of Fas/FasL expression and T-cell apoptosis (Figs 2 and 3), suggesting that modulation of Fas/FasL expression may contribute to the propriocidal activity of IL-2.
Because it has been proposed that AICD plays an important role in the regulation of peripheral tolerance, it would be interesting to ascertain whether other factors could directly modulate AICD. Very few studies have been designed to address this question. We demonstrated previously that CD2 engagement decreases TCR/CD3-induced apoptosis, by decreasing Fas/FasL expression and level of activation.12This study investigated whether signals able to affect Fas/Fas-L may act via modulation of IL-2.
The results showed that CD2 triggering decreases the production of IL-2 and diminishes the expression of Fas/FasL12 (Table 5). Because anti–IL-2 MoAb reduced the expression of Fas/FasL when added to anti-CD3–stimulated 3DO cells (Figs 2 and 3), it is reasonable to suggest that CD2 could act via modulation of IL-2 production. Furthermore, IFN-γ increased IL-2 production, Fas/FasL expression, and apoptosis (Table 5, Fig 4). These results suggest that modulation of AICD, Fas, and FasL expression is a consequence of modulation of IL-2 production.
To further analyze whether the modulation of IL-2 production is a general mechanism involved in the control of apoptosis, the protective effect of IL-6 was also studied. IL-6 rescued T cells from TCR/CD3-induced apoptosis by downmodulating Fas/FasL expression (Figs4, 5, and Table 3). However, IL-6 did not affect the levels of IL-2, suggesting that an IL-2–independent mechanism may also modulate the expression of the Fas/FasL system (Table 5). IL-6 did not rescue 3DO cells from Fas-induced cell death (Fig 6), suggesting that IL-6 can interfere with TCR/CD3-, but not Fas-activated signals. This result, in apparent contrast with the data showing that IL-6 inhibits anti–Fas-induced apoptosis in multiple myeloma cells,41indicates that IL-6 may deliver different signals and/or induce different biological effects depending on the cell type and/or activation states of the cell. On the other hand, IL-6 can rescue resting T cells from apoptosis by inducing Bcl-2 expression42 or protect murine myeloma cells against dexamethasone-induced apoptosis, an Fas/FasL independent death mechanism.43
It has been reported that IL-6 has different effects, either pro-or anti-inflammatory, depending on the experimental system.44-50 However, there is increasing evidence to suggest a predominant anti-inflammatory and immunosuppressive role for IL-6.46-50 It has been shown that IL-6 is a potent inducer of IL-1rα, and also of soluble TNFR p55.51
The observation that IL-6 can downmodulate the Fas/FasL system and lymphocyte cell death without affecting IL-2 and T-cell activation suggests that it might be possible to control lymphocyte apoptosis without interfering with the process of T-cell activation. This approach would be of therapeutic interest in those syndromes in which disregulation of Fas/FasL plays an important role and a differential control of activation and apoptosis would be desirable.
Supported by the Italian Association for Cancer Research (AIRC), by Progetto Strategico “Ciclo Cellulare e Apoptosi” CNR Italy, and by Progetto finalizzato “Biotecnologie.”
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Emira Ayroldi, MD, Department of Clinical and Experimental Medicine, Pharmacology Section, University of Perugia, Via del Giochetto, 0600 Perugia, Italy.