Abstract
In type 2N von Willebrand disease (vWD), von Willebrand factor (vWF) is characterized by normal multimeric pattern, normal platelet-dependent function, but a markedly decreased affinity for factor VIII (FVIII). In this report, we describe the case of a vWD patient who has an abnormal vWF multimers distribution associated with a markedly decreased vWF ability to bind FVIII. Sequencing analysis of patient’s vWF gene showed, at heterozygous state, a G→A transition resulting in the substitution of Asn for Asp at position 116 of the mature vWF subunit and a C→T transition, changing the codon for Arg 896 into a stop codon. His sister who has a subnormal vWF level, but a normal FVIII/vWF interaction, was found to be heterozygous for the Arg896ter mutation only. Recombinant vWF (rvWF) containing the candidate (Asn116) missense mutation was expressed in COS-7 cells. The expression level of Asn116rvWF was significantly decreased compared with wild-type rvWF. The multimeric pattern of Asn116rvWF was greatly impaired as shown by the decrease in high molecular weight forms. The FVIII binding ability of Asn116rvWF was dramatically decreased. These data show that the Asp116Asn substitution is the cause of both the defective FVIII/vWF interaction and the impaired multimeric pattern observed in the patient’s vWF. The monoclonal antibody 31H3 against D’ domain of vWF (epitope aa 66-76) that partially inhibits the FVIII binding and recognizes only nonreduced vWF, showed a decreased ability to bind Asn116rvWF when used as capture-antibody in enzyme-linked immunosorbent assay (ELISA). This result suggests that a potential conformation change in the D’ domain is induced by the Asp116Asn substitution, which is localized in the D3 domain.
VON WILLEBRAND FACTOR (vWF) is a large multimeric glycoprotein, synthesized in endothelial cells and megakaryocytes, which plays two main hemostatic roles: it mediates platelet adhesion and aggregation to the damaged vessel wall under conditions of high shear rate, and it is the carrier of procoagulant factor VIII (FVIII), an essential cofactor in the generation of activated factor X (FXa) (for review, see Meyer and Girma1). The vWF gene, located on chromosome 12, contains 52 exons and is transcribed into an 8.7-kb mRNA, which encodes a 2,813 amino acids (aa) precursor, named pre-pro–vWF, consisting of a 22-aa signal peptide, a 741-aa propeptide, and a 2,050-aa mature vWF subunit.2,3 The analysis of the aa sequence for vWF precursor showed that over 90% of the sequence is contained within repeats of four types of homologous domains designated A to D and arranged as follows: D1-D2-D’-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2.4 In plasma, vWF is present as a series of multimers composed of a 250-kD subunit that ranges in size from 500 kD (protomer) to over 15,000 kD. The multimeric structure of vWF is crucial in platelet adhesion and aggregation, the larger multimers being the most functional.5 The different functions of vWF reside on well-characterized domains of the subunit. The FVIII binding site has been localized on a 31-kD tryptic fragment that contains the first 272 aa residues of the mature vWF subunit.6,7 This fragment is encoded by the exons 18 to 23 and is composed of the D’ (aa 6 to 102) and a part of the D3 (aa 103 to 479) homologous domains. The interaction between FVIII and vWF is necessary for normal survival of FVIII in blood circulation8,9 and stabilization of recombinant FVIII in cell culture media.10 Furthermore, vWF protects FVIII from inactivation by activated protein C11,12 and prevents FVIII activation and inactivation by FXa.13,14 vWF also acts as a cofactor for thrombin-catalyzed cleavage of the FVIII light chain.15
Von Willebrand disease (vWD), the most common cause for inherited bleeding disorder, with an estimated prevalence of up to 1% of the general population, is heterogeneous and results from quantitative and/or qualitative defects of vWF.16 Type 1 vWD is characterized by a dominant inheritance and a more or less pronounced quantitative defect of vWF, while type 3 vWD is recessive and associated with extremely low levels or undetectable vWF. Among type 2 vWD, defined by a qualitative vWF abnormality, the type 2N refers to patients with a markedly decreased binding to FVIII, but a normal multimeric pattern.17 This variant form of vWD is characterized by a disproportionately low level of FVIII as compared with normal or subnormal vWF level and is recessively inherited.18 Until now, type 2N vWD patients have been found homozygous or compound heterozygous for seven missense mutations (Thr28Met, Arg91Gln, Arg53Trp, Arg19Trp, His54Gln, Glu24Lys, and Gly22Glu) localized in the D’ domain.19 For six of these mutations, the expression of mutated recombinant vWF (rvWF) has confirmed that the corresponding single aa substitution results in decreased FVIII binding capacity.20-25 Furthermore, a subgroup of type 2N vWD patients was characterized by coinheritance of one allele containing a type 2N mutation with a vWD type 1 or type 3 mutation on the other allele.21,22 24-28
We report here the case of a vWD patient who has an abnormal vWF multimer distribution and a marked decrease in vWF ability to bind FVIII. The nucleotide (nt) sequencing of polymerase chain reaction (PCR)-amplified fragments of exons 18 to 28 from the patient’s vWF gene showed, on one allele, an nt transition in exon 20, which changes the aspartic acid residue 116 of mature subunit into asparagine (Asp116Asn). Furthermore, an nt substitution was also detected at heterozygous state, in exon 28, changing the codon for arginine residue 896 into a stop codon. The expression in mammalian cells of the full-length vWF cDNA showed that the Asp116Asn substitution is responsible for both FVIII binding defect and abnormal multimerization of vWF. The use of monoclonal antibodies (MoAbs) directed to the N-terminal part of mature vWF provided indirect evidence of a potential conformation change in the D’ domain induced by this Asp116Asn substitution localized in the D3 domain.
MATERIALS AND METHODS
Case report.
The patient, a man born in 1924, was referred to one of us in August 1984 before osteoarthritis surgery, because of a prolonged activated partial thromboplastin time (APTT) (49 seconds v39 seconds for normal plasma) and borderline bleeding time (10 minutes). In the past, the patient experienced bleeding symptoms after tooth extraction and puncture of femoral artery, which was followed by the development of an important hematoma. After he was diagnosed as a type 2 vWD patient, other surgeries (total hip replacement, tooth extraction, pacemaker implantation) were performed under treatment with vWF concentrates (LFB, Lille, France) without any bleeding problem. The biological investigations performed on four occasions are reported in Table 1. Plasma FVIII activity (FVIII:C) has ranged from 0.16 to 0.42 U/mL (normal, 0.5 to 1.5 U/mL). The vWF antigen (vWF:Ag) and ristocetin cofactor (vWF:RCo) activity levels have ranged from 0.22 to 0.55 U/mL (normal, 0.5 to 1.5) and from 0.10 to 0.25 U/mL (normal, 0.5 to 1.5), respectively. In addition, the patient suffers from noninsulin-dependent diabetes, hypertension, unstable angina, and obesity.
His sister, born in 1932, also had bleeding complications after a hysterectomy and described frequent ecchymosis. A recent blood sample showed that she has a normal level of FVIII:C (0.54 U/mL) and slightly reduced levels of vWF:Ag (0.36 U/mL) and vWF:RCo (0.48 U/mL).
DNA sequence analysis.
Genomic DNA was extracted from peripheral blood leukocytes and the exons 18 to 27 of vWF gene were amplified by PCR using, as primers, oligonucleotides localized in adjacent introns of which the sequences were derived from the gene sequence data already published.2 Exon 28 was amplified as previously described in detail.29 The use of 5′-biotinylated PCR primers and the purification of single-stranded DNA on streptavidin-coated magnetic beads (Dynal, Oslo, Norway) according to the manufacturer’s instructions, allowed subsequent direct solid-phase sequencing of each amplified exon using the T7 sequencing kit (Pharmacia, Uppsala, Sweden).
Site-directed mutagenesis.
The construction of expression vector pSVvWF, containing the full-length cDNA for human vWF has been previously described.29 The vWF cDNA nucleotides (nt) are numbered beginning at the A of the initiating methionine codon.30Plasmid pSV116N was derived from pSVvWF by introducing a G→A transition at nt 2635, using the Transformer site-directed mutagenesis kit (Clontech, Palo Alto, CA). The oligonucleotides used for mutagenesis were as follows: 5′-CCTCACCTTCAACGGGCTCAAA-3′ (vWF nt 2625-2646, substitution is underlined) and 5′-AGTTTCCCAGCTTCTTATTTTGATG-3′ (vWF nt 5101-5125). The first primer was used to introduce the desired mutation into pSVvWF used as template and the second primer to destroy the unique previously created NheI restriction site of the plasmid for the purpose of selection. Clones containing the desired mutation were identified by allele-specific PCR, and the nt substitution was confirmed by nt sequence analysis.
Transient expression of vWF.
COS-7 cells were transfected with plasmids pSVvWF and pSV116N by the diethyl aminoethyl (DEAE)-dextran method, as previously described.29 Forty hours after transfection, cells were cultured for 72 hours in serum-free Dulbecco’s modified Eagle’s medium. The concentration of vWF:Ag in conditioned media was measured by enzyme-linked immunosorbent assay (ELISA) using rabbit anti-vWF polyclonal antibodies.31
FVIII binding assay of vWF.
FVIII binding to vWF was assayed as described previously.25Briefly, increasing amounts of vWF were immobilized by binding to anti-vWF polyclonal antibody-coated microplates. Recombinant FVIII (Cutter, Biological Miles Inc, Berkeley, CA) was incubated with the captured vWF, and the bound FVIII was then measured by adding the reagents for a chromogenic assay of FVIII-dependent Factor X activation (Diagnostica Stago, Asnières, France). The amounts of vWF captured by immobilized antibodies were then quantified by ELISA.31
Comparative recognition of vWF by various MoAbs.
MoAbs 32B12 and 31H3 have been selected among a panel of MoAbs directed towards the N-terminal part of the vWF mature subunit. MoAb 32B12 is a potent inhibitor of the FVIII/vWF interaction and recognizes reduced vWF. MoAb 31H3, which is a moderate inhibitor of the FVIII binding to vWF, is sensitive to the reduction of vWF. The epitopes of MoAbs 32B12 and 31H3 have been precisely mapped to aa residues 51 to 60 and 66 to 76, respectively.32 Moreover, both MoAbs recognize all multimeric forms, whatever the degree of multimerization of vWF (unpublished observation). The ability of these MoAbs to capture plasma or recombinant vWF (rvWF) was tested as described previously,25 except that MoAbs were used at 5 μg/mL for coating. The results are expressed as percent of the absorbance, the values obtained with normal plasma and wild-type (WT) rvWF being considered 100%.
Plasmin digestion of vWF.
Plasmin digestion of vWF was performed as described previously33 with minor modifications. vWF was immunoisolated with anti-vWF MoAb 21A11 covalently linked to Sepharose-4B beads and the digestion was performed for 4 hours at 37°C after adding 0.045 U of bovine plasmin (Sigma, Chemical Co, St Louis, MO).
Protein characterization.
Ristocetin-induced binding of vWF to formalin-fixed platelets was assayed as previously described.34 The multimeric structure of plasma, platelet, or recombinant vWF was determined by 0.1% sodium dodecyl sulfate (SDS)-1.5% agarose gel electrophoresis as previously described,35 except that vWF was detected by anti-vWF polyclonal antibodies conjugated to alkaline-phosphatase. The percentages of the protomer and multimers were deduced from computerized scanning of the areas of the peaks obtained with a Helena pC24 densitometer.35 The subunit composition of rvWF was analyzed on vWF immunoisolated with rabbit anti-vWF polyclonal antibodies. The bound proteins were recovered by boiling for 5 minutes in electrophoresis sample buffer (10 mmol/L Tris, pH 8.0, 1 mmol/L EDTA, 2.5% SDS) containing 5% 2-mercaptoethanol and analyzed by electrophoresis on a 5% to 9% gradient polyacrylamide gel. After electrotransfer onto nitrocellulose as previously described,36 vWF was visualized with anti-vWF polyclonal antibodies (Dako, Glostrup, Denmark), followed by peroxidase-conjugated antirabbit IgG, which was subsequently revealed by the enhanced chemiluminescence method (Amersham, Buckinghamshire, UK).
RESULTS
Abnormal features of the patient’s vWF.
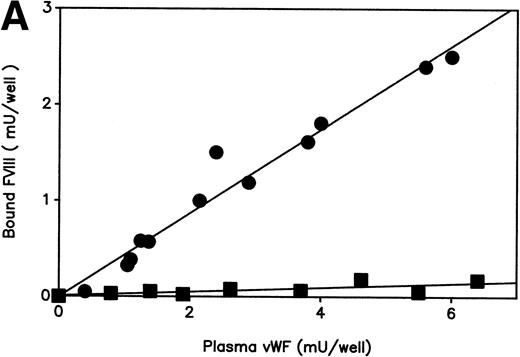
The patient’s plasma was characterized by several biological abnormalities. The vWF:Ag level was decreased or borderline and always higher than the vWF:RCo level (Table 1). Plasma vWF binding to ristocetin activated platelets was decreased as compared with a pool of normal plasmas (data not shown). In addition, electrophoretic pattern obtained in 2.5% agarose gel displayed a moderate decrease in high molecular weight (HMW) multimers and a loss of satellite bands (data not shown). At first, these data brought us to classify this patient as a type IIE vWD. At the time of the last examination, the patient’s FVIII:C level (0.16 U/mL) was decreased out of proportion of vWF:Ag level (0.48 U/mL) leading to a suspicion of type 2N vWD. In agreement with this assumption, the patient’s vWF showed a markedly decreased ability to bind FVIII (Fig 1A). Furthermore, the patient’s plasma and platelet vWF analyzed by 1.5% agarose gel electrophoresis, exhibited a loss of the largest multimeric forms as compared with a pool of normal plasmas and a normal platelet lysate, respectively (Fig1B). To accurately define the multimeric defect observed in the patient’s plasma vWF, the percentage of protomer and HMW forms were calculated from the densitometric scanning of multimeric patterns (Table 2). As compared with normal individual plasma, the patient’s plasma vWF was consistently characterized by a significant decrease in HMW ≥ 5-mer and ≥ 10-mer by 25.8% and 40%, respectively, associated with a relative increase in protomer.
Features of the patient’s vWF. (A) FVIII binding ability of plasma vWF. Serial dilutions of plasma samples were incubated into a microtiter plate coated with anti-vWF polyclonal antibodies. A constant amount of recombinant FVIII (0.1 U/mL) was then added and the activity of FVIII bound to immobilized vWF was determined using a chromogenic assay. The amounts of vWF captured by immobilized antibodies were measured by ELISA using a peroxidase-conjugated MoAb as described.25 Plasma samples : (•), pool of normal plasmas; (▪), patient’s plasma. Each dilution sample was analyzed in duplicate and the results were averaged. (B) Multimeric pattern of normal and patient’s vWF. Plasma and platelet lysate samples were electrophoresed in SDS-1.5% agarose gel and vWF was visualized with alkaline-phosphatase conjugated anti-vWF polyclonal antibodies. Lane 1, pool of normal plasmas; lane 2, patient’s plasma; lane 3, patient’s platelet lysate; and lane 4, normal platelet lysate.
Features of the patient’s vWF. (A) FVIII binding ability of plasma vWF. Serial dilutions of plasma samples were incubated into a microtiter plate coated with anti-vWF polyclonal antibodies. A constant amount of recombinant FVIII (0.1 U/mL) was then added and the activity of FVIII bound to immobilized vWF was determined using a chromogenic assay. The amounts of vWF captured by immobilized antibodies were measured by ELISA using a peroxidase-conjugated MoAb as described.25 Plasma samples : (•), pool of normal plasmas; (▪), patient’s plasma. Each dilution sample was analyzed in duplicate and the results were averaged. (B) Multimeric pattern of normal and patient’s vWF. Plasma and platelet lysate samples were electrophoresed in SDS-1.5% agarose gel and vWF was visualized with alkaline-phosphatase conjugated anti-vWF polyclonal antibodies. Lane 1, pool of normal plasmas; lane 2, patient’s plasma; lane 3, patient’s platelet lysate; and lane 4, normal platelet lysate.
Interestingly, his sister who has proportionately low vWF:Ag and vWF:RCo levels, was found to have normal binding of vWF to FVIII, as well as normal multimeric distribution of plasma vWF.
Identification of candidate mutations in the vWF gene.
To identify the abnormalities in vWF gene responsible for the FVIII binding defect and the abnormal multimeric pattern, PCR-amplified fragments of exons 18 to 28 were sequenced (exons 29 through 52 of the patient’s vWF gene were not sequenced). A single nt change was identified in exon 20, consisting of a G→A transition at position 2635, which modifies the encoded aa residue from aspartic acid to asparagine at position 116 of the mature vWF subunit (Fig 2A). Because both nucleotides G and A were identified on the sequencing gel, the patient is heterozygous for the Asp116Asn candidate mutation. In contrast, this mutation was not detected in the patient’s sister.
Identification of two mutations in patient’s vWF gene. Part of the nucleotide sequence gels of amplified vWF exon 20 (A) and exon 28 (B) for both normal control (c) and patient (p) are shown. The asterisks localize the point mutations: (A) the indicated G2635→A transition alters the encoded sequence from Asp 116 to Asn; (B) the indicated C4975→T transition alters the encoded sequence from Arg896 to stop codon.
Identification of two mutations in patient’s vWF gene. Part of the nucleotide sequence gels of amplified vWF exon 20 (A) and exon 28 (B) for both normal control (c) and patient (p) are shown. The asterisks localize the point mutations: (A) the indicated G2635→A transition alters the encoded sequence from Asp 116 to Asn; (B) the indicated C4975→T transition alters the encoded sequence from Arg896 to stop codon.
As shown on Fig 2B, a C→T substitution was detected in exon 28 at position 4975, changing the codon for arginine in position 896 of mature vWF into a stop codon. The patient is also heterozygous for this nonsense mutation. The C4975T substitution, which creates a DdeI restriction site, was detected by digestion of PCR-amplified exon 28 fragment at heterozygous state in the patient’s sister (data not shown).
Binding of FVIII to recombinant vWF.
To determine whether the Asp116Asn substitution alone could account for the defect in FVIII/vWF interaction, the corresponding mutated rvWF was expressed. Plasmids pSVvWF and pSV116N were transiently expressed in COS-7 cells in seven separate experiments and quantitative analysis of secreted rvWF, using ELISA, were performed on the conditioned media. The vWF:Ag concentration of WT rvWF present in the culture media was 80 ± 22 mU/mL (mean ± standard deviation [SD], n = 7), while the concentration of Asn116rvWF was significantly decreased to 37 ± 10 mU/mL. The hybrid rvWF, obtained by cotransfection of a 1:1 ratio of WT and mutated plasmids, was secreted in the conditioned media at the intermediate concentration of 59.4 ± 18 mU/mL.
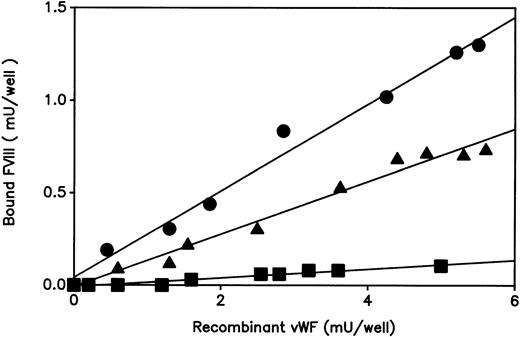
The ability of the expressed rvWF to bind FVIII was next examined using a solid-phase binding assay as described in experimental procedures (Fig 3). As normal plasma vWF, the WT rvWF bound FVIII in a dose-dependent manner. The mutated Asn116rvWF showed a markedly decreased FVIII binding affinity, whereas comparable amounts of rvWF were captured by the coated anti-vWF polyclonal antibodies. The hybrid Asp/Asn116rvWF gave an intermediate FVIII binding curve between that of WT and Asn116 rvWFs.
Binding of FVIII to recombinant vWF. The ability of WT or mutated rvWF to bind FVIII was determined as described in Fig 1A. Conditioned media samples: (•), WT rvWF; (▪) Asn116rvWF; (▴) hybrid Asp/Asn116rvWF. Each dilution sample was analyzed in duplicate and the results were averaged. Similar results were consistently observed when three independent tansfection media were tested in three separate experiments.
Binding of FVIII to recombinant vWF. The ability of WT or mutated rvWF to bind FVIII was determined as described in Fig 1A. Conditioned media samples: (•), WT rvWF; (▪) Asn116rvWF; (▴) hybrid Asp/Asn116rvWF. Each dilution sample was analyzed in duplicate and the results were averaged. Similar results were consistently observed when three independent tansfection media were tested in three separate experiments.
Structural characterization of mutated vWF.
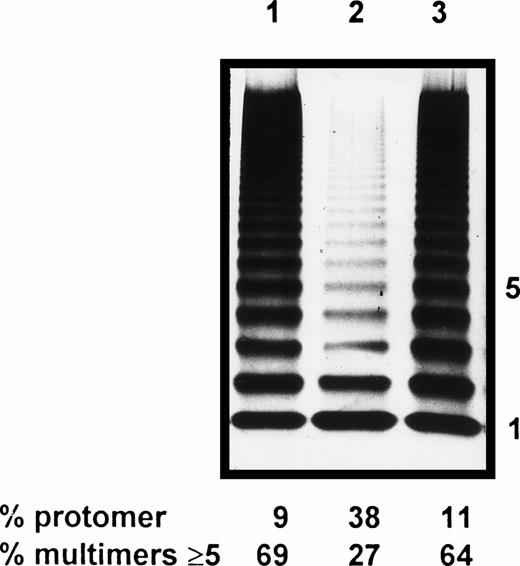
As the multimeric pattern of the patient’s plasma vWF was significantly altered, electrophoretic study of Asn116rvWF was performed to check the potential impact of Asp116Asn substitution on the multimerization. In comparison to WT rvWF, Asn116rvWF showed a marked decrease in HMW ≥ 5-mer (27% v 69% for WT rvWF), which is correlated to a relative increase in the amount of protomer (38% for mutated rvWF, as compared with 9% for WT rvWF) (Fig 4). These data give evidence that the multimerization abnormalities previously characterized in the patient’s plasma were reproduced in mutated rvWF, but to a more pronounced extent. However, the pattern of hybrid Asn116/WT rvWF appeared normal (Fig 4).
Multimeric analysis of rvWF by SDS-1.5% agarose gel electrophoresis. Lane 1, WT rvWF; lane 2, Asn116rvWF; lane 3, hybrid Asp/Asn116rvWF.
Multimeric analysis of rvWF by SDS-1.5% agarose gel electrophoresis. Lane 1, WT rvWF; lane 2, Asn116rvWF; lane 3, hybrid Asp/Asn116rvWF.
On SDS-polyacrylamide gel electrophoresis under reducing conditions, immunoisolated WT, mutated, and hybrid rvWFs showed a subunit band at 225 kD similar to that obtained with both normal and patient’s plasma vWF. In all of the rvWF proteins, no pro-vWF subunit was detected that is consistent with full processing in COS-7 cells. Moreover, minor bands corresponding to proteolytic fragments of vWF were detected at the same level in normal and patient’s plasmas (data not shown).
Conformation of the N-terminal part of mutated vWF.
To explain the FVIII binding capacity defect of plasma and rvWF harboring the mutation Asp116Asn, we sought a potential conformation change of the N-terminal part of the mature subunit. Immunoisolated plasma and recombinant vWFs (normal and mutated) were hydrolyzed by plasmin and analyzed by SDS-polyacrylamide gel electrophoresis under nonreducing conditions. The electrophoretic mobility of the N-terminal plasmin fragment, which has an apparent molecular weight of 31 kD, was similar for normal and patient’s plasma vWF. Moreover, the amino-terminal plasmin fragments obtained with WT rvWF, Asn116rvWF, and hybrid Asp/Asn116rvWF displayed the same electrophoretic mobility as compared with plasma fragments (data not shown). We also performed antigen-capture ELISA with anti-vWF MoAbs that inhibit the binding of FVIII. MoAb 32B12, which recognizes reduced vWF, bound in a similar way normal and patient’s plasma vWF, as well as WT and mutated rvWFs (Fig 5). In contrast, the recognition of the MoAb 31H3, which fails to bind reduced vWF, was decreased for the patient’s plasma, as well as for mutated and hybrid rvWFs to 41.2% ± 2.8%, 30.5% ± 4.7%, and 68.4% ± 11.3%, respectively, as compared with normal plasma and WT proteins (Fig 5).
Recognition of plasma and recombinant vWF by MoAbs. Plasma samples and conditioned media containing 0.01 U/mL vWF:Ag were incubated for 1 hour at 37°C into microtiter plate coated with (▪) MoAb 32B12 or (▧) MoAb 31H3. After washing, the bound vWF was detected with peroxidase-conjugated anti-vWF polyclonal antibodies. Values are expressed as a percent of the absorbance obtained with normal plasma or WT rvWF. Each value is the mean (± SD) of three experiments.
Recognition of plasma and recombinant vWF by MoAbs. Plasma samples and conditioned media containing 0.01 U/mL vWF:Ag were incubated for 1 hour at 37°C into microtiter plate coated with (▪) MoAb 32B12 or (▧) MoAb 31H3. After washing, the bound vWF was detected with peroxidase-conjugated anti-vWF polyclonal antibodies. Values are expressed as a percent of the absorbance obtained with normal plasma or WT rvWF. Each value is the mean (± SD) of three experiments.
DISCUSSION
Since the identification and confirmation of the first type 2N vWD mutation, ie, Thr28Met,20 36 six other missense mutations (Arg19Trp, Gly22Glu, Gly24Lys, Arg53Trp, His54Gln, and Arg91Gln) have been reported in patients characterized by a markedly decreased capacity of vWF to bind FVIII (database through the Internet at hppt : //mmg2.im.med.umich.edu/vWF). All of these mutations are localized in the first 102 aa residues of mature vWF subunit, which correspond to the D’ domain (aa 764-865 of pre-pro-vWF).
We report here the case of a patient who has been first classified as having type IIE vWD based on a discrepancy between vWF:Ag and vWF:RCo levels and a slightly disturbed vWF multimers distribution. In the context of a complete routine biologic analysis, a reduced FVIII level disproportionate to the vWF level underscored by a marked decrease in vWF ability to bind FVIII led us to consider a type 2N vWD. Despite the defective FVIII/vWF interaction, the discrepancy between plasma FVIII and vWF levels is not constant in our patient. The variations in the FVIII and vWF may be explained by the different diseases from which our patient suffers that influence plasma vWF and FVIII levels. The nt sequence analysis of exons 18 to 28 of vWF gene allowed us to identify in this patient both a nt transition in exon 20, changing Asp 116 into Asn and a nt transition in exon 28, changing the encoded Arg896 into a stop codon. The patient was heterozygous for these two mutations. To show that the Asp116Asn substitution is responsible for the observed FVIII binding defect, the corresponding rvWF was expressed by COS-7 cells. The Asn116rvWF exhibited a dramatic decrease in the ability to bind FVIII, while hybrid rvWF, resulting from the cotransfection with WT and mutated vWF cDNA, showed a moderately decreased binding. These data suggest that the Asp116Asn substitution, like the other type 2N vWD mutations already described, is recessive. Although the patient is heterozygous for the Asp116Asn mutation, the plasma vWF behavior was more consistent with Asn116rvWF than with hybrid Asp/Asn116rvWF, suggesting that the second allele not mutated in exon 20 is underexpressed. The nonsense mutation detected in exon 28 has been previously identified by Zhang et al,37 at homozygous state, in a type 3 vWD patient and, at heterozygous state, associated with mild type 1 vWD phenotype. In agreement with these data, the patient’s sister who was found to be heterozygous for the Arg896ter mutation has a moderate plasma vWF deficiency (0.36 U/mL) and a normal FVIII/vWF interaction. Thus, the propositus described here is likely a compound heterozygous type 3/2N vWD patient with the Asp116Asn mutation on one allele and the Arg896ter mutation inducing the lack of normal vWF expression on the second allele. When transiently expressed by COS-7 cells, the accumulation of Asn116rvWF in conditioned media was half that of WT rvWF. Charged to alanine scanning mutagenesis has previously shown that the substitution of Ala for Asp 116 also induces a secretion defect of mutated vWF protein expressed by 293/Tag cells (David Ginsburg, personal communication, October 1996). These data confirm the importance of aa residue at position 116 in the secretion of rvWF protein. Therefore, in addition to the FVIII binding defect, the Asp116Asn substitution appeared to induce a secretion impairment, which might also explain the low level of plasma vWF:Ag initially found in our patient.
Besides the FVIII binding defect, the patient’s plasma vWF displayed platelet-dependent function impairment, characterized by a lower vWF:RCo than vWF:Ag level and a moderate decrease in vWF binding to ristocetin-activated platelets, which seems to be related to the abnormal multimeric pattern.
In an attempt to gain more information on the origin of the patient’s vWF multimeric impairment, structural studies were performed on mutated rvWF. Analysis by SDS-polyacrylamide gel electrophoresis under reducing conditions indicated that WT and mutated rvWF proteins had similar subunit composition and proteolytic patterns and were both secreted as a processed form corresponding to the mature subunit. The possibility that rvWF was less stable outside of the cell was rendered unlikely by immunoblot analysis showing no detectable degradation product in agreement with the normal patient’s plasma vWF pattern. However, the multimeric structure of Asn116rvWF analyzed by SDS-agarose gel electrophoresis was strikingly different from that of WT rvWF. The amount of HMW multimers was significantly decreased, while the relative amount of protomer was increased. The multimeric profile of hybrid Asp/Asn116rvWF being similar to the one of WT rvWF, it appears that the Asp116Asn mutation does not exert a dominant effect on WT vWF in COS-7 cells. Therefore, the Asn116rvWF expressed by COS-7 cells reproduced the multimerization impairment found in the patient’s plasma vWF, but to a larger extent. However the pattern of patient’s platelet vWF, which displayed a lack of HMW multimers, was closer to that of Asn116rvWF. The difference in the extent of the loss of HMW multimers between plasma and platelet vWF is difficult to explain.
To define the mechanism by which the Asp116Asn substitution acts on FVIII binding and multimerization of vWF, we investigated the hypothesis of a conformation change. For this purpose, MoAbs directed to the N-terminal part of the mature vWF subunit were used. MoAb 32B12, a potent inhibitor of the FVIII/vWF interaction, which is not sensitive to the reduction of vWF, recognized equally well WT and mutated vWF. In contrast, MoAb 31H3, a moderate inhibitor of the FVIII binding to vWF, which only recognizes nonreduced vWF, showed a similar decreased capacity to capture the patient’s plasma vWF and Asn116rvWF in correlation with the absence of expression of the allele not mutated in exon 20. Moreover, as the epitope of MoAb 31H3 has been previously mapped with synthetic peptides to aa residues 66 to 76 in D’ domain,32 these data suggest that Asp116, localized in the D3 domain of the vWF, either belongs to or is required for the native conformation of FVIII binding site and epitope of MoAb 31H3.
The potential conformation change induced by the Asp116Asn mutation may explain the loss of FVIII binding capacity of vWF. Indeed, two other mutations (Gly22Glu, Thr28Met) localized in D’ domain, have been previously shown to induce a conformation change leading to a FVIII binding defect.25,36 The FVIII binding domain is constituted of at least three aa sequences (aa 19-28, 53-54, 91-95), as supported by naturally-occuring mutations and epitope mapping of anti-vWF MoAbs inhibiting the FVIII/vWF interaction.32,38,39 The stabilization of this domain by disulfide bridges is required,6 but we suggest that aa residues other than cysteine are also necessary to maintain the functional conformation of this site.
The multimerization of vWF is a complex process, which involves different parts of the precursor (pro-vWF) subunit. Previous studies have shown that the propeptide, as well as D’ and D3 domains of the mature subunit, are required for dimer assembly.40 The prosequence composed of the two homologous D1 and D2 domains contains two consensus sequences that are similar to those of the active site of disulfide isomerases that catalyzes thiol protein disulfide interchange.41 The D3 domain contains sulfhydryl groups, which are involved both in intra- and intermolecular disulfides bridges.42,43 In contrast, all of the cysteine residues of D’ domain are involved in intrachain disulfide bonds, and the possible role of this domain in multimerization is so far unknown. The potential conformation change induced by the Asp116Asn substitution may affect the accessibility necessary to promote the interchain disulfide bridges involved in the assembly of pro-vWF dimers into multimers. Recent studies by Eikenboom et al44 have shown that the Cys386Arg mutation, detected in a patient classified as type 1 vWD, induces a secretion defect linked to a decrease in the concentration of the largest vWF multimers. Our data confirm the key role of the D3 domain in the multimerization and secretion of vWF, but further studies are required to elucidate the mechanisms involved.
In conclusion, this report describes the first naturally-occurring mutation localized in the D3 domain, which affects both the FVIII binding and the structure, as well as the level of vWF. Thus, this peculiar mutation causes an unusual phenotype. As type 2N refers to variants with decreased affinity of vWF for FVIII, but normal vWF multimeric pattern, and type 2A refers to variants with decreased platelet-dependent function associated with the absence of HMW vWF multimers,45 we propose to classify Asp116Asn mutation as type 2N (A) vWD. The patient described here, who is a compound heterozygote with type 3 mutation (Arg896ter) on one allele and the Asp116Asn mutation on the other one, may be classified as type 3/2N (A) vWD.
ACKNOWLEDGMENT
We thank Cutter Biological Miles Inc for the generous gift of recombinant FVIII. We are grateful to S. Belmont and V. Barylo for their excellent technical assistance and to V. Tancré for typing the manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to S. Jorieux, Unité de Recherche, LFB, 59 rue de Trévise, BP 2006, 59011 Lille cédex, France.