Abstract
In this study a previously undescribed 3 bp deletion, AAT1030-1032, in the factor XIII A subunit gene, has been detected in a Thai patient. The inframe deletion results in the translation of a factor XIII A subunit that lacks Asn344. This is the first inframe deletion to be identified in the factor XIII A subunit gene because six previously reported deletions have all caused frameshifts. The deletion has been introduced into a factor XIII A subunit cDNA and the deleted polypeptide expressed in yeast. The mRNA encoding the mutant enzyme appears to have normal stability but the translated protein is subject to premature degradation. In addition, the mutated enzyme exhibited very little transglutaminase activity compared with the wild-type enzyme. Structural modeling of the deleted enzyme suggests that the absence of Asn344 would have a potent impact on the catalytic activity by reorienting the residues associated with the catalytic center. Thus, the Asn344 deletion strongly confirms the significance of the residues surrounding the catalytic center of the factor XIII A subunit.
BLOOD COAGULATION factor XIII is a proenzyme for a plasma transglutaminase previously known as fibrin stabilizing factor. It is involved in the modification of fibrin clots by the formation of tight covalent cross-links between fibrin monomers or between fibrin and α-2 plasmin inhibitor.1 This cross-linking enhances the mechanical strength of the clot and increases its resistance to proteolysis. Factor XIII has also been found in many cells and tissues (platelets, megakaryocytes, placenta, uterus, and monocytes/macrophages).2-7 Factor XIII circulating in plasma is composed of two A and two B subunits forming a noncovalent heterotetrameric complex (A2B2), whereas the intracellular form is a dimer of A subunits (A2).8,9 The A subunits of factor XIII are responsible for its catalytic activity,10,11 whereas the B subunits are thought to play a significant role as a protective carrier of the circulating A subunits.12-14 In addition, there is some evidence that the B subunit might regulate the activation of the proenzyme A subunits.10 11
Inherited factor XIII deficiency can result from mutations in either the A- or B-subunit genes, but the frequency of A-subunit mutations is far higher than that of B-subunit mutations. This difference is probably due to the fact that B-subunit deficiency is less severe and possibly goes undiagnosed. Congenital factor XIII A-subunit deficiency results in a severe life-long bleeding diathesis and frequently presents as umbilical bleeding a few days after birth.15Other clinical features of this disease including abnormal wound healing and spontaneous abortion have been reviewed by Board et al.16 Inherited factor XIII deficiency is an autosomal recessive disorder as the genes coding for the A and B subunits have been precisely mapped to chromosome band 6p24-2517 and chromosome band 1q31-32.1,18 respectively.
Inherited factor XIII deficiency has been reported in many racial groups,16 and although many mutations have been identified in the A-subunit gene, only 3 mutations have been detected in the B-subunit gene.19,20 Since the first two mutations causing factor XIII A-subunit deficiency were reported in 1992,21,22 around 30 mutations have been identified.23-37 It is notable that the mutations observed in the factor XIII A-subunit gene are heterogeneous, and only four mutations have been reported on multiple occasions (C → T transition at codon 661,25,35 G → A transition at codon 681,21 38 AATT deletion at codons 462-46331,34). All the nucleotide deletions reported so far have been found to disrupt the reading frame.
In this study, we identified a novel 3-bp deletion in a homozygous patient with Thai origins. This deletion causes the translation of a polypeptide missing Asn344. The effect of the deletion on the enzyme's expression and activity has been investigated by site-directed mutagenesis, and its effect on enzyme structure has been predicted by computer modeling.
MATERIALS AND METHODS
Patient.
The patient is a Thai girl born in 1992 and the third child in the family. According to the interview at the time of admission, her parents are not relatives and there is no history of bleeding disorders in either side of the family. The first child in this family died of intracranial hemorrhage before this disorder was diagnosed in the family. The patient's severe factor XIII deficiency first became evident when bleeding from the umbilical stump was noted 7 days after birth. Nineteen months later, she suffered from posttraumatic hematoma of the scalp, and at 2 years of age she had a posttraumatic subgaleal hematoma. Another year later, she became ill with posttraumatic intraventricular hemorrhage. A comprehensive hematological investigation when the patient was 3 years and 8 months showed that she had a complete blood count within the normal range and had normal values for basic blood coagulation and platelet function tests. However, her fibrin clots solubilized very rapidly in 8 mol/L urea (10 minutes), confirming a clinical diagnosis of factor XIII deficiency. The patient is not on regular substitution treatment but receives fresh-frozen plasma when she suffers bleeding episodes after accidents. The samples used in this study were collected at varying times after replacement therapy and could be potentially contaminated with residual donor factor XIII.
DNA amplification, heteroduplex analysis, and nucleotide sequencing.
Each of the 15 exons of the factor XIII A-subunit gene was amplified from genomic DNA from the patient and her immediate family members as described by Board et al.21 The polymerase chain reaction (PCR) products were then subjected to heteroduplex analysis.39 Exons showing heteroduplex formation were further purified by a Wizard PCR Preps DNA purification system (Promega, Madison, WI) and directly sequenced using a Thermo Sequenase cycle sequencing kit (Amersham, Arlington Heights, IL).
Genetic analysis.
Genetic transmission of the 3-bp deletion in this particular family has been linked to the length polymorphism of the tetranucleotide repeat element (AAAG)n occurring upstream of the coding sequence of the factor XIII A-subunit gene as described previously.40
Determination of factor XIII activity in plasma.
Recombinant factor XIII A subunit.
The plasmid pGal181 was used for the expression of wild-type and Asn344 deleted A-subunit cDNAs. These constructs have a similar configuration to that constructed by Jagadeeswaran and Haas.42 The expression of the factor XIII A subunit is under the control of theGAL1 promoter and can be induced by galactose.
In vitro mutagenesis.
The deletion of AAT at codon 344 was introduced into the factor XIII A-subunit cDNA by the primer (5′-CAAATTGGCATCATCATGGGC-3′) and the oligonucleotide-directed in vitro mutagenesis system version 2.1 (Amersham). A plasmid containing the desired mutant was transformed into Saccharomyces cerevisiae AH22 by the lithium acetate method43 and grown on selective media without leucine (SD/glucose-Leu).44
Characterization of the mutant enzyme in yeast lysate.
Yeast strains were grown overnight in YPD broth,44 washed with sterile water, and grown for another 30 hours in SD/galactose-Leu broth.44 The yeast pellet was resuspended in lysis buffer (50 mmol/L HEPES pH 7.5, 150 mmol/L NaCl, 5 mmol/L EDTA, 1% Triton X-100, 1 mmol/L phenylmethylsulphonyl fluoride) and lysed by the method described by Bartel et al.45Immunoblotting of the mutant protein after separation on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with rabbit anticoagulation factor XIII A-subunit serum (Calbiochem, San Diego, CA) as previously described.46 The transglutaminase activity of the mutant enzyme was determined by the incorporation of [1,4-14C] putrescine dihydrochloride (Amersham) into α casein (Sigma, St Louis, MO).41 A pulse-chase experiment was performed47 using 35S-Met (Amersham) to evaluate the possible degradation of mutant A subunits expressed in yeast.
RNA hybridization analysis.
Because no suitable samples from the patient and her family were available for mRNA analysis, mRNA was evaluated in yeast expressing the mutant enzyme. Yeast strains were grown overnight in SD/glucose-Leu, washed with sterile water, and grown for 8 hours in either YPD broth or in YPGal broth.44 Total RNA was prepared by the glass bead/phenol/chloroform extraction procedure,48and aliquots (approximately 10 μg) were electrophoresed in 1% (wt/vol) agarose gel in MOPS buffer/1% (vol/vol) formaldehyde as described by Finley et al.49 After electrophoresis, samples were transferred to a nylon membrane (Amersham Hybond N+) as described by the manufacturer. The probe was a 2.3-kb Pst I fragment that contained the entire factor XIII A-subunit cDNA coding region,50 which was labeled by a random primer fluorescein labeling kit (DuPont NEN, Wilmington, DE) and hybridized according to the manufacturer's instructions. Hybridization was detected using CDP-Star chemiluminescence (DuPont NEN).
Computer modeling.
Computer modeling was used to examine the likely effect of the deletion on the enzyme structure. A model for the mutant enzyme was created by deleting Asn344 from the wild-type structure and then performing energy minimization to resolve the excessive bond length between residues 343 and 345. The three-dimensional structure of factor XIII A subunit determined by Yee et al51 was used as the template to model the Asn344 deletion mutant. Modeling was performed with the Insight package from MSI/Biosym (San Diego, CA). Initially, all atoms of residue 344 were deleted and a pseudo peptide bond created between residues 343 and 345. Hydrogens were introduced into the model with the Builder module and residues 185-515, the “core” domain, were minimized with the Discover module for 100 steps of steepest descent minimization. In keeping with other simulations done in vacuum, a noncovalent interaction cutoff 12 Å was used with no cross or Morse terms. Subsequently, a short dynamics trajectory was calculated tethering all residues but those between 340 and 349 to their original positions with a moderate force constant of 100 kcal/mol. The system was equilibrated for 300 iterations before calculating a single trajectory for 500 iterations at 301°K. The resulting structure was then further minimized for 100 steps of steepest descent minimization followed by 200 steps of conjugate gradient minimization. The same procedure was also applied to the wild-type core domain as a control. The atomic coordinates of the protein structure were obtained from the Brookhaven Protein Data Bank under the identifier “1fie” (Http://www.pdb.bnl.gov).
RESULTS
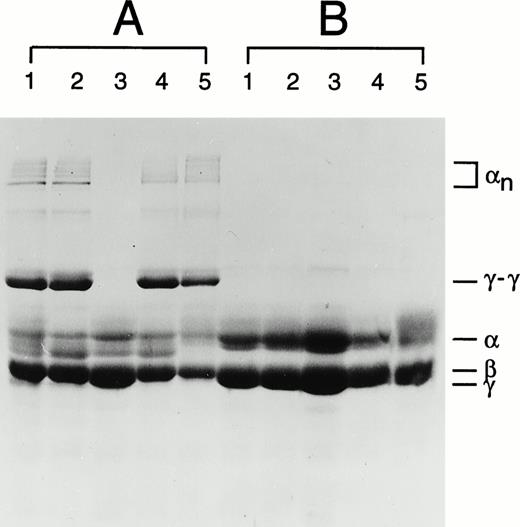
Plasma factor XIII activity was determined in the patient and other members of her family. The value obtained for the patient's sample was very low when compared with normal controls (Table1). The values obtained from the patient's heterozygous relatives are also low, but not lower than might be expected given the wide normal range. An analysis of the cross-linking in the fibrin clots of the patient and her immediate family members has also been performed. No γ-dimer or α polymer formation was observed in the patient's fibrin clot (Fig 1). A Western blot of the patient's plasma revealed only a trace band of A-subunit antigen (data not shown). These data confirm the diagnosis of A-subunit deficiency originally made after a positive urea solubility test in the patient.
Fibrin cross-linking in plasma clots of family members where the Asn344 deletion was identified. (A) Fibrin clots in the reaction containing CaCl2 and thrombin (test). (B) Fibrin clots in the reaction containing EDTA and thrombin (control). In each panel: lane 1, maternal sample; lane 2, paternal sample; lane 3, patient's sample; lane 4, brother's sample; and lane 5, a normal control sample. The symbols α, β, γ represent uncross-linked fibrin chains, αn represents α chain polymers, and γ-γ represents γ chain dimers.
Fibrin cross-linking in plasma clots of family members where the Asn344 deletion was identified. (A) Fibrin clots in the reaction containing CaCl2 and thrombin (test). (B) Fibrin clots in the reaction containing EDTA and thrombin (control). In each panel: lane 1, maternal sample; lane 2, paternal sample; lane 3, patient's sample; lane 4, brother's sample; and lane 5, a normal control sample. The symbols α, β, γ represent uncross-linked fibrin chains, αn represents α chain polymers, and γ-γ represents γ chain dimers.
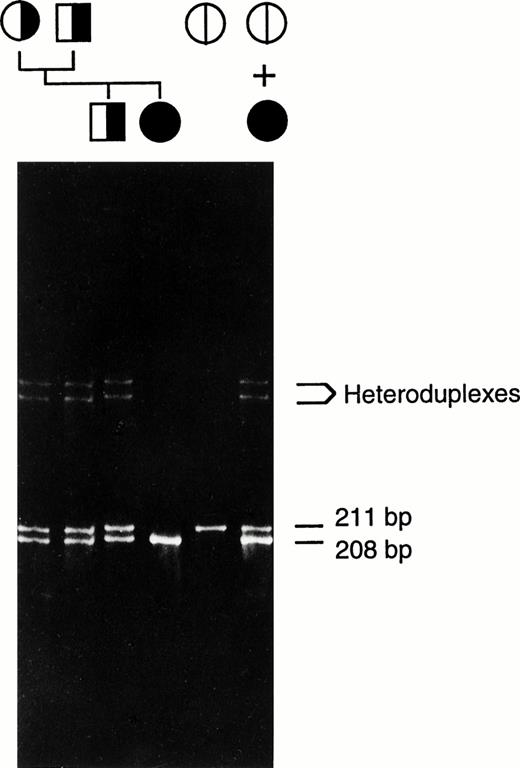
Heteroduplex analysis of each factor XIII A-subunit exon from the patient indicated the presence of a deletion in exon 8 (Fig2). Direct sequencing of amplified exon 8 DNA from the patient and her immediate family members revealed a 3-bp (AAT) deletion of nucleotides 1030-1032 (nucleotides and amino acids are numbered from the first serine of the mature factor XIII A-subunit protein). This deletion does not alter the reading frame and results in a protein without Asn344 as shown in Fig 3. There is no alteration in a restriction-cleavage site available for the PCR-restriction fragment length polymorphism (RFLP) diagnosis of this particular mutation; however, the mutation can be detected directly by separation of the amplified exon 8 DNA on 6% polyacrylamide gel since the deletion allele (208 bp) runs faster than the normal allele (211 bp; Fig 2). The data suggest that the patient is a homozygote having inherited the same deletion allele from both parents. In addition, a family study using the polymorphic short tandem repeat (AAAG)n in the 5′ flanking sequence of the factor XIII A-subunit gene (Fig 4) shows that the patient inherited a common repeat allele from each parent, thus supporting the conclusion that the patient is a homozygote. This study also revealed that a deletion allele in the patient's brother, who is heterozygous for this deletion, was inherited through the paternal line.
Heteroduplex analysis of amplified exon 8 DNA. The mixture of the patient's and a normal subject's amplified DNA was run on the first lane on the right. Amplified normal exon 8 DNA was run as a control (second from right). The unmixed amplified DNA from the patient, her immediate family members, and normal control were also run on the same gel as indicated by the pedigree. The position of heteroduplexes (between normal and 3-bp deleted strands) and the length of homoduplexes (both normal and mutant alleles) are indicated. The genetic transmission of the 3-bp deletion is shown. The symbols for male (□) and female (○) are hatched according to the alleles present.
Heteroduplex analysis of amplified exon 8 DNA. The mixture of the patient's and a normal subject's amplified DNA was run on the first lane on the right. Amplified normal exon 8 DNA was run as a control (second from right). The unmixed amplified DNA from the patient, her immediate family members, and normal control were also run on the same gel as indicated by the pedigree. The position of heteroduplexes (between normal and 3-bp deleted strands) and the length of homoduplexes (both normal and mutant alleles) are indicated. The genetic transmission of the 3-bp deletion is shown. The symbols for male (□) and female (○) are hatched according to the alleles present.
Direct sequencing of amplified exon 8 DNA. The normal and mutated sequences are given on the left of the ladder. The missing nucleotides AAT between the positions 1030 and 1032 are shown in bold. The asterisk (*) indicates the position of the deletion.
Direct sequencing of amplified exon 8 DNA. The normal and mutated sequences are given on the left of the ladder. The missing nucleotides AAT between the positions 1030 and 1032 are shown in bold. The asterisk (*) indicates the position of the deletion.
The cosegregation of the 3-bp deletion (AAT1030-1032) in the A-subunit gene and the (AAAG)n STR alleles in the Thai family. The photograph shows the electrophoretic separation of the (AAAG)n PCR products from each family member. Repeat alleles have been numbered 1 and 2 exclusively in this family and do not relate to the number of repeats. The symbols for male (□) and female (○) are hatched according to the mutation alleles present.
The cosegregation of the 3-bp deletion (AAT1030-1032) in the A-subunit gene and the (AAAG)n STR alleles in the Thai family. The photograph shows the electrophoretic separation of the (AAAG)n PCR products from each family member. Repeat alleles have been numbered 1 and 2 exclusively in this family and do not relate to the number of repeats. The symbols for male (□) and female (○) are hatched according to the mutation alleles present.
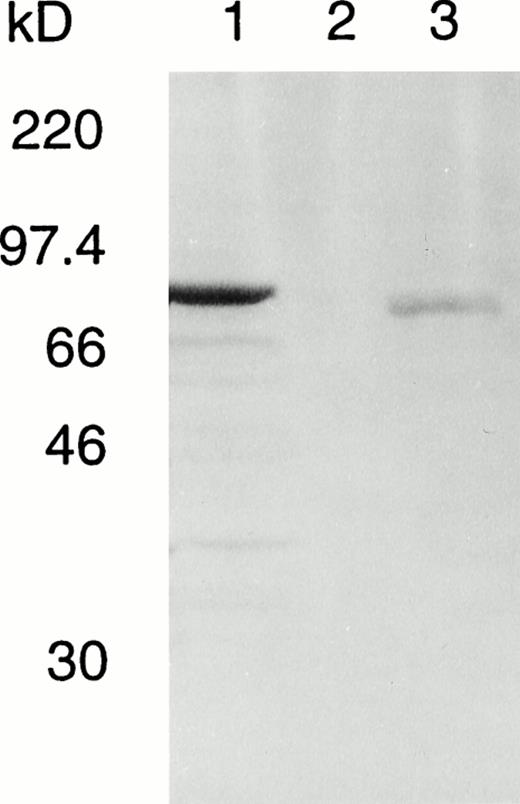
To elucidate whether this inframe deletion leads to a loss of catalytic activity or protein instability, we have introduced the deletion into an A-subunit cDNA and expressed the recombinant protein in S cerevisiae. An immunodetectable band was observed in the crude lysate of yeast expressing the mutant protein (Fig5); however, this was far weaker than the level of expression obtained for the normal enzyme in parallel cultures since the amount of mutant culture lysate applied to the gel was about four times that of the normal control.
Western blot of normal and deleted A subunits in fresh lysates of S cerevisiae AH22. Total yeast lysate protein was prepared from S cerevisiae transformed with lane 1, pGal181/wild-type factor XIII A-subunit cDNA (1.5 μg); lane 2, negative control vector pGal181 (1.5 μg); and lane 3, pGal181/Asn 344–deleted factor XIII A-subunit cDNA (6 μg). The samples were subjected to SDS-PAGE, blotted onto nitrocellulose membrane, and developed with antiserum to human factor XIII A subunit.
Western blot of normal and deleted A subunits in fresh lysates of S cerevisiae AH22. Total yeast lysate protein was prepared from S cerevisiae transformed with lane 1, pGal181/wild-type factor XIII A-subunit cDNA (1.5 μg); lane 2, negative control vector pGal181 (1.5 μg); and lane 3, pGal181/Asn 344–deleted factor XIII A-subunit cDNA (6 μg). The samples were subjected to SDS-PAGE, blotted onto nitrocellulose membrane, and developed with antiserum to human factor XIII A subunit.
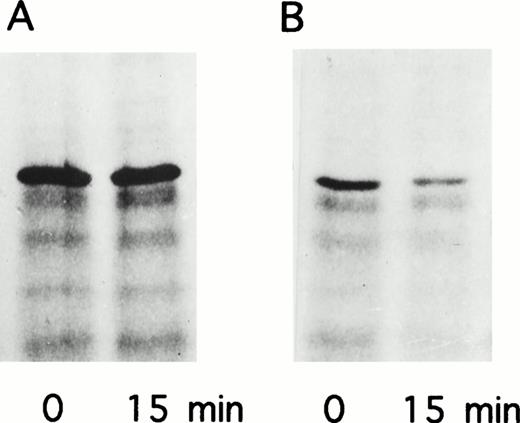
RNA hybridization analysis (Northern blot) revealed that mRNAs for both wild-type and mutant factor XIII A subunit were present in equal abundance in cells grown in galactose but were absent from cells grown in glucose, consistent with induction from the GAL1 promoter and no change in mRNA stability caused by the mutation (Fig6). The transglutaminase activity of the mutant enzyme determined by 14C putrescine incorporation into α casein was not significantly different to the negative control of yeast lysate without the recombinant A-subunit plasmid (Table2). Attempts to purify the mutant protein via a C-terminal 6xHis tag and immobilized metal affinity chromatography were not successful. Thus, it was not possible to obtain a specific activity for the mutant protein. The failure of this purification appeared to be due to instability of the mutant protein as normal A subunit can be readily prepared by that procedure. A pulse-chase experiment was performed to evaluate the stability of the recombinant protein. After 15 minutes there was significant degradation of the mutant enzyme and no change in the normal enzyme (Fig7). Thus, the mutant protein appears to be both catalytically inactive and unstable.
RNA hybridization analysis of factor XIII A-subunit expression in yeast. (A) Ethidium bromide stained gel; (B) hybridized membrane. Total RNA was prepared from S cerevisiae AH22 transformed with negative control vector pGal181 (lanes 1, 4), pGal181/wild-type factor XIII A-subunit cDNA (lanes 2, 5), or pGal181/Asn344–deleted factor XIII A-subunit cDNA (lanes 3 through 6) and grown either in YPGal (induced; lanes 1 through 3) or YPD (repressed; lanes 4 through 6). Hybridization with a factor XIII A-subunit probe and chemiluminescent detection were performed as described in Materials and Methods. RNA size standards (Promega) on the left are in nucleotides.
RNA hybridization analysis of factor XIII A-subunit expression in yeast. (A) Ethidium bromide stained gel; (B) hybridized membrane. Total RNA was prepared from S cerevisiae AH22 transformed with negative control vector pGal181 (lanes 1, 4), pGal181/wild-type factor XIII A-subunit cDNA (lanes 2, 5), or pGal181/Asn344–deleted factor XIII A-subunit cDNA (lanes 3 through 6) and grown either in YPGal (induced; lanes 1 through 3) or YPD (repressed; lanes 4 through 6). Hybridization with a factor XIII A-subunit probe and chemiluminescent detection were performed as described in Materials and Methods. RNA size standards (Promega) on the left are in nucleotides.
Pulse-chase analysis of recombinant factor XIII A subunits expressed in yeast. (A) pGal181/wild-type factor XIII A subunit. (B) pGal181/Asn344–deleted factor XIII A subunit. The cells were metabolically labeled for 10 minutes followed by a 15-minute chase.
Pulse-chase analysis of recombinant factor XIII A subunits expressed in yeast. (A) pGal181/wild-type factor XIII A subunit. (B) pGal181/Asn344–deleted factor XIII A subunit. The cells were metabolically labeled for 10 minutes followed by a 15-minute chase.
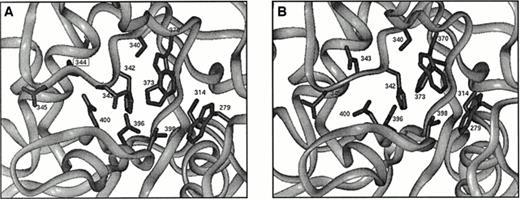
The Asn344 deletion occurs within the core domain of the enzyme. Figure8A shows the relative position of this deletion with respect to the active center of the A subunit. Comparison of the wild-type structure to that of the minimized structure shows good agreement with an overall root mean square (rms) deviation of about 0.7 Å. This measure of structural deviation takes the square root of the average squared Euclidian distance between superimposed atoms from both structures. The rms deviation for the residues from 340-349 show a similar value, and all side chains have similar orientations. In contrast, comparison of the wild-type structure with that of the minimized deletion mutant shows an rms deviation of about 1.8 Å for residues 340 to 343 and 345 to 349. The largest change involves the complete reorientation of Asp 343 (Fig 8B), which now points away from the proposed catalytic triad. This residue is conserved among transglutaminase sequences that possess the Cys314, His373, and Asp396 triad. Further, it normally forms a hydrogen bond with Arg11 in the activation peptide.51 The location of Asp343, directly in the proposed active site, suggests that its reorientation as a consequence of the Asn344 deletion would be detrimental to the enzyme.
(A) Illustration of the active site in the core domain of factor XIII A subunit. Side chains of active site residues Cys314, His373, Asp396, and Trp279, Ser340, His342, Asp343, Asp345, Trp370, Thr398, and Gln400 are shown along with the side chain of Asn344, which is deleted in the patient studied here. (B) As for Fig8A but showing a possible configuration for the mutant enzyme. Asp343 has a completely different orientation that is expected to be detrimental for enzyme activity.
(A) Illustration of the active site in the core domain of factor XIII A subunit. Side chains of active site residues Cys314, His373, Asp396, and Trp279, Ser340, His342, Asp343, Asp345, Trp370, Thr398, and Gln400 are shown along with the side chain of Asn344, which is deleted in the patient studied here. (B) As for Fig8A but showing a possible configuration for the mutant enzyme. Asp343 has a completely different orientation that is expected to be detrimental for enzyme activity.
DISCUSSION
Since the publication of the first reports of mutations in the A subunit of factor XIII in 1992,21 22 a number of additional mutations have been identified. Among those mutations, six deletions in the A-subunit gene have been reported (Table3). Surprisingly, all of them cause frameshifts resulting in the premature termination of translation. In this study we have identified the first inframe deletion in the factor XIII A-subunit gene. The resulting protein has a normal sequence but lacks Asn344 (which is coded by the missing AAT1030-1032). The effect of the deletion on the enzyme's expression and activity has been investigated by in vitro mutagenesis, and its effect on enzyme structure has been predicted by the computer modeling of the deleted enzyme.
The result of the Western blot analysis indicates that the deletion of Asn344 significantly lowers the level of expressed enzyme in yeast lysates. Because the mutant A subunit mRNA is stable, it is possible to conclude that the deleted protein might be expressed at a normal level but is sensitive to degradation. It is notable that although immunodetectable protein can be observed in lysate of yeast expressing the mutant enzyme, its activity is not significantly different to that of the negative control lysate. This implies that the deleted enzyme definitely loses its transglutaminase activity. The instability of the mutant protein prevented its successful purification and characterization in greater detail. These results are in agreement with data derived directly from the patient. Activity measurement of the patient's plasma found less than 5% of normal activity. In addition, a Western blot of the patient's plasma revealed the presence of only a trace band of A subunit. Because this minimal amount of plasma A subunit in the patient could be residual normal A subunit remaining from prior replacement therapy, it appears that the mutant enzyme prematurely degrades and has a short lifespan in the circulation.
The Asn344 residue is directly adjacent to residues associated with the active center. The Asn344 deletion occurs on a loop and might otherwise leave the molecule largely unperturbed but for the fact that other residues on this loop are directly associated with the active center. It has been proposed that His342 and Asp343 guide the lysyl substrate residue into the active site.51 These two residues occur adjacent, in sequence as well as in the structure, to Asn344 and will almost certainly be reoriented as a result of a deletion at position 344. Figure 8B shows a possible configuration of these residues that are also immediately juxtaposed to the proposed catalytic triad: Cys314, His373, and Asp396. As a consequence, a deletion occurring at position 344 would be expected to have a profound impact on enzymatic activity. Such prediction is in agreement with the absence of transglutaminase activity in the lysate of yeast expressing the deleted enzyme and the absence of fibrin cross-linking activity in the plasma of this patient. In addition, this Asn344 deletion could have a serious effect on the stability of the enzyme.
Because both parents of the patient are heterozygous for the same AAT1030-1032 deletion, it implies that they may have a common ancestor despite the apparent absence of consanguinity in this family. An extensive family study could reveal further information about the origin of this deletion. As this deletion does not change any restriction cleavage site, the best method for its identification is the direct electrophoresis of amplified exon 8 DNA. Under the conditions described here the 3-bp deletion can be readily resolved. In addition, further study of this family using the linked polymorphic short tandem repeat element in the 5′ flanking sequence40revealed the mode of inheritance of this deletion among the immediate family members (Fig 4). Although the inheritance of the STR allele 1 from the patient's father is equivocal, the deletion allele in the patient's brother is clearly inherited from the father. Analysis of the inheritance of the linked STR alleles could be readily applied for the prenatal diagnosis of the deletion in this family.
Two of the previously reported deletions in the A-subunit gene, AATT1385-1388 in exon 1131 and AG at the intron B/exon 3 boundary,22 occurred within repetitive sequence and might be explained by the slipped strand mispairing mechanism.52 However, the mechanism causing the AAT1030-1032 deletion in this study and another four previously identified deletions (a deletion/insertion in exon 3, a 13 bp deletion in exon 3, a 1-bp deletion in exon 2, and the entire deletion of exon 5)26,30,32 35 are still not clear.
In conclusion we have identified the first inframe deletion in the factor XIII A-subunit gene. The resulting enzyme has a deletion of Asn344. This deletion occurs in the catalytic core domain, and computer modeling suggests that it will have a profound effect on catalytic activity and may influence stability. The deleted enzyme has been expressed in yeast and shown to have negligible activity and appears to be prematurely degraded, thus confirming the structural predictions. The effects of the deletion identified in this study confirm the significance of the amino acid residues surrounding the catalytic area in maintaining the structure, stability, and activity of the enzyme.
G.C. is a Postdoctoral Fellow of the Australian Research Council. R.T.B. holds a QEII Fellowship from the Australian Research Council.
Address reprint requests to Philip G. Board, PhD, Molecular Genetics Group, John Curtin School of Medical Research, Australian National University, GPO 334, Canberra, Australian Capital Territory, 2601, Australia.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.