Abstract
The role of P-selectin on polymorphonuclear leukocyte (PMN) adhesion-induced PMN elimination in the liver is unclear. Our objectives were to show the expression and distribution of P-selectin in rat liver, as well as to evaluate the changes in the modulation of the expression of P-selectin and its role in the accumulation and sequestration of PMNs. The intravenous administration of endotoxin markedly increased the expression of P-selectin on the venous and sinusoidal endothelial cells, as well as on the platelets trapped in the liver. Its expression peaked at 6 hours postinjection and was associated with a rapid increase in the aggregation and elimination of PMNs in the hepatic sinusoids. Combined treatment with an antibody to P-selectin or with low molecular weight heparin, a P-selectin antagonist, blocked the P-selectin, significantly reduced the arrest of PMNs, and delayed their removal in the liver. Pretreatment with gadolinium chloride inhibited phagocytosis of PMNs by the Kupffer cells, decreased the expression of P-selectin, and limited the hepatic accumulation of PMNs. Thus, P-selectin played a role in accumulation and elimination of PMNs from the liver. Results also suggest that activated Kupffer cells can modulate the expression of P-selectin in the liver and influence the homeostasis of PMNs in the circulation during acute inflammation.
RELATIVELY LITTLE is known about the modulation, kinetics, and role of P-selectin expression in the hepatic microvasculature. Using tissue extracts to analyze the upregulation of P-selectin synthesis1,2 cannot identify or localize the expression of P-selectin at the cellular level, nor can the intralobular heterogeneity of P-selectin expression on the endothelial cells be studied. The absence of endothelial P-selectin has been found to severely affect the homeostasis of polymorphonuclear leukocytes (PMNs), with the resulting neutrophilia caused, at least in part, by a delay in the removal of PMNs.3-5 However, these studies did not address the questions of how such homeostasis is maintained, where and how the increased PMNs are eliminated, or what the relationship between P-selectin expression and PMN sequestration might be. Unlike the microvasculature of other organs, the sinusoids in the liver contain Kupffer cells, which normally represent 70% to 80% of the tissue macrophages.6 However, a definitive role for the modulation of P-selectin expression and PMN adhesion and sequestration by Kupffer cells in the process of acute hepatitis remains unknown, although interactions between the leukocytes and endothelia have been reviewed.7 8 In addition, the role of platelets in the arrest and elimination of PMNs in vivo also remains to be investigated.
To investigate the death of PMNs and their elimination by the Kupffer cells, we previously developed an animal model that shows an increase in the number of circulating PMNs.9 This model shows that after an injection of lyophilized streptococci, the circulating PMNs undergo apoptosis and are phagocytosed by Kupffer cells in the liver. This observation remains unexplained because a detailed analysis of the causes of PMN aggregation in the liver has not been performed, and the expression and effects of P-selectin on the removal of PMNs have not been evaluated. Therefore, we decided to study further the expression of P-selectin in the hepatic lobules and its role in maintaining PMN homeostasis.
Methodically, we injected lipopolysaccharide (LPS) intravenously to produce an expression of P-selectin and an increase in number of PMNs in the circulation. We assumed that the resulting expression of P-selectin and excess of the PMNs provided visible and morphological evidence of P-selectin–induced PMN adhesion and sequestration in the liver. This approach enabled us to characterize the time course and magnitude of P-selectin expression, define the heterogeneous expression of P-selectin, and quantify and examine the localization and elimination of PMNs in the liver. Blocking the P-selectin and phagocytic activity of Kupffer cells enabled us to delineate the relative contribution of the P-selectin to PMN accumulation and Kupffer cells to PMN clearance. The current results lead us to believe the important role of P-selectin and the Kupffer cells in maintaining the homeostasis of PMNs in the circulation.
MATERIALS AND METHODS
Rats.
Seven- to 9-week-old male Wistar rats were obtained from Nippon Biological Supplies (Tokyo, Japan) and were bred in the animal facilities at Tokyo Medical and Dental University.
Antibodies and drugs.
Polyclonal antibodies to P-selectin and CD61 monoclonal antibodies (MoAbs) to platelet were obtained from PharMingen (San Diego, CA). To label the macrophage-related antigens (Ags), we used ED1 and ED2 MoAbs (Serotec, Kidlington, Oxford, UK). To label major histocompatibility complex (MHC) class II Ag, we used MRC OX6 MoAb (Cedarlane, Ontario, Canada). LPS, low molecular weight (LMW) heparin, an antagonist to P-selectin, and gadolinium chloride (GdCl3), an agent used to block the function of Kupffer cells, were all obtained from Sigma (St Louis, MO). Proteinase K and biotin-16-2′-deoxyuridine-5′-triphosphate (Biotin-16-dUTP) were obtained from Boehringer Mannheim (Mannheim, Germany). Terminal deoxynucleotidyl transferase (TdT) was obtained from GIBCO BRL (Gaithersburg, MD). Other Abs, including biotinylated goat antimouse Ig, biotinylated goat antirabbit Ig, goat Ig to mouse IgG, goat Ig to rabbit IgG, mouse peroxidase antiperoxidase (PAP) Ab complex, rabbit PAP Ab complex, and peroxidase-conjugated streptavidin were obtained from Dako (Glostrup, Denmark).
Experimental design.
In the first experiment, animals were injected with LPS (0.1 mg/kg intravenously [IV]) and perfusion-fixed at 1, 3, 6, 12, 24, and 48 hours postinjection. Control rats were either untreated (day 0) or injected with sterile saline at 1, 3, 6, 12, 24, and 48 hours. In the second experiment, animals were treated with anti–P-selectin Ab (1.5 mg/kg, IV) or LMW heparin (70-125 IU/mg, 30 mg/kg, IV) 10 minutes after the injection of LPS. Some animals were administered GdCl3(7.5 mg/kg, IV) 12 hours before the LPS injection. The liver of each animal was perfusion-fixed at 1, 3, 6, and 12 hours after the LPS injection. Each value is expressed as a mean ± standard deviation (SD) of three to five rats, and for some data, statistical analyses were performed using Student's t-test. A level ofP < .05 was considered significant.
Immunohistochemistry.
The liver of the control and treated animals was perfused through the portal vein with a peristaltic pump at a rate of 10 mL/min. Perfusion consisted of saline for 20 seconds followed by phosphate-buffered 4% paraformaldehyde for 2 minutes. After perfusion, slices of liver 3-mm thick were immersed in fixative for 6 hours at 4°C, then embedded in paraffin. Selected sections (4 μm) of the control and treated liver were immunostained by the streptavidin-biotin-peroxidase complex method. These sections were pretreated with 0.3% H2O2 in methanol to block endogenous peroxidase activity and were then incubated with normal goat serum (1:5 dilution). The sections were then incubated overnight with primary Abs (diluted 1:1000), rinsed in phosphate-buffered saline (PBS) containing 0.03% Triton X-100, incubated for 30 minutes with either biotinylated goat antimouse Ig (1:600 dilution) or biotinylated goat antirabbit Ig (1:800 dilution, for anti–P-selectin), rinsed again in PBS, and finally, incubated for 30 minutes with peroxidase-conjugated streptavidin (1:300 dilution). All Abs were diluted in 0.1 mol/L PBS containing 0.03% Triton X-100 and 1% bovine serum albumin (BSA), and all steps were performed at room temperature. Tissue peroxidase activity was visualized after a 5-minute exposure to 0.025% 3,3-diamino-benzidine tetrahydrochloride (DAB) in 0.05 mol/L Tris-HCl buffer (pH 7.4) containing 0.01% H2O2, or a 10-minute exposure to 3,3′,5,5′-tetramethylbenzidine in buffer. Some sections were counterstained with hematoxylin or nuclear fast red.
DNA TUNEL.
Paraffin sections 4-μm thick were collected on glass slides coated with poly-L-lysine. The nuclear DNA fragmentation of apoptotic cells was labeled in situ by the TUNEL method.10 Briefly, the sections were first deparaffinized and treated for 15 minutes with 20 μg/mL proteinase K in 0.1 mol/L Tris-HCl buffer (pH 7.4). After being rinsed with distilled water, the sections were treated for 5 minutes with 2% H2O2 to block endogenous peroxidases. The sections were again washed with distilled water, then incubated with 0.3 U/μL TdT and 0.04 nmol/μL biotinylated dUTP in TdT buffer at 37°C for 60 minutes. After another rinse with distilled water, the sections were incubated for 10 minutes with 2% BSA in 0.1 mol/L PBS (pH 7.4) and then for 30 minutes in peroxidase-conjugated streptavidin diluted 1:300 with PBS. Peroxidase activity in the sections was visualized after incubation in 0.025% DAB in 0.05 mol/L Tris-HCl (pH 7.4) containing 0.01% H2O2 for 5 minutes. For double-staining (TUNEL and ED1, ED2, OX-6, or anti–P-selectin Ab staining), the TUNEL-positive cells were stained brown or dark blue by adding 2 mL of 0.5% CoCl2 to the DAB solution. The sections were then processed by the PAP method in a manner similar to that described previously. In brief, sections treated with primary Ab were successively incubated with goat Ig to mouse IgG (diluted 1:100) or goat Ig to rabbit IgG (diluted 1:50) and mouse PAP complex (1:200 dilution) or rabbit PAP complex (1:100 dilution) before exposure to the DAB solution for visualization. All steps were performed at room temperature unless otherwise indicated.
Analysis of P-selectin expression.
The endothelial cells were separately analyzed in location of interlobular portal vein, periportal area, pericentral area and central vein. The staining intensity was rated negative (−), occasionally positive or weakly positive (+/−), positive (+), and strong positive (++) antigen staining by light microscopy.
Cell counting.
The number of PMNs was counted by a method described by Bouwens et al.11 For each animal, a total tissue area of approximately 4 mm2 (65 fields, 0.0625 mm2 per field) was counted from a random sample of three sections. The paraffin sections (4 μm) stained with the TUNEL method as well as the semithin sections (0.2 μm) stained with toluidine blue were analyzed by light microscopy at ×250. The number of PMNs per mm2 was counted.
RESULTS
Expression of P-selectin and PMN accumulation in the hepatic lobule after LPS injection.
Controls, both on day 0 or treated with saline, showed no difference among the two groups. Endothelial cells in the control liver showed no staining or, at best, slight anti–P-selectin Ab staining on the endothelial cells of periportal veins. PMNs were rare in sinusoids.
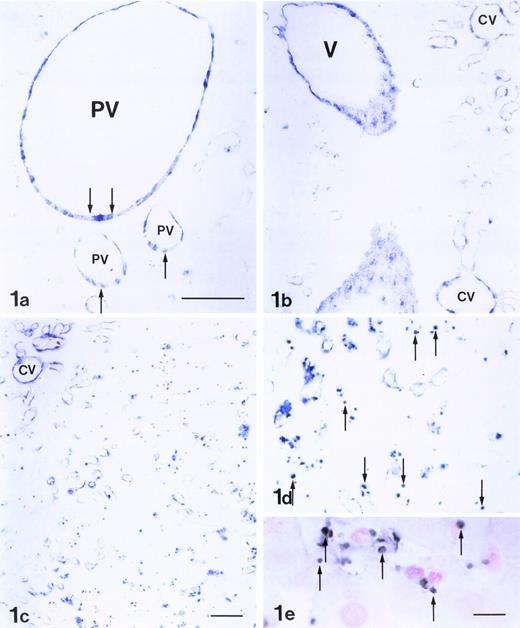
After LPS-treatment, the liver showed the expression of P-selectin on endothelial cells in the portal and sublobular veins by 1 hour, with extensive and strong expression observed in the lobule after 3 to 6 hours (Fig 1a and b). Its expression on hepatic venous and sinusoidal endothelial cells peaked at 6 hours (Fig1c). The expression of P-selectin varied within regions of the liver. The density of expression on the endothelial cells was ranked as follows: interlobular portal veins, sublobular veins, and central veins greater than pericentral area greater than periportal area. Many of the platelets that were trapped in the periportal and midzonal areas in density expressed P-selectin (Fig 1d). They were labeled by platelet marker, a MoAb to CD61 (F11) (Fig 1e). F11 MoAbs react with integrin β3 chain (CD61), which associates with integrin αIIb chain to form the gpIIb/IIIa (CD41/CD61) complex. Platelets were closely associated with the trapped PMNs (Fig 2a). No staining or only very weak staining could be found in arteries 6 hours after LPS injection (Fig2b). Serial sections showed that the areas of P-selectin expression correlated with the presence of PMNs in the intralobular area. Twelve hours after the LPS injection, weak P-selectin reactivity was restricted to the endothelia of portal, central, and large veins, and a few platelets were in the sinusoids. Twenty-four hours after the injection, little expression of P-selectin was found.
Expression of P-selectin in rat liver 3 to 6 hours after the administration of LPS. Endothelial cells of the large portal vein (PV) and its branchings showed intense reaction to anti–P-selectin Ab (arrows; a). The endothelia of sublobular veins (V) and central veins (CV) also expressed P-selectin (b). P-selectin was also present along the walls of sinusoids and veins. A strong Ab reaction in the endothelia of central veins, as well as the pericentral sinusoids, was observed (c). The Ab reaction was relatively weak in the endothelium of periportal sinusoids but strong in adherent platelets (arrows, d), which were labeled by MoAb CD61 to platelet (arrows; e). Bars, a, b, and d = 50 μm; c = 50 μm; e = 10 μm.
Expression of P-selectin in rat liver 3 to 6 hours after the administration of LPS. Endothelial cells of the large portal vein (PV) and its branchings showed intense reaction to anti–P-selectin Ab (arrows; a). The endothelia of sublobular veins (V) and central veins (CV) also expressed P-selectin (b). P-selectin was also present along the walls of sinusoids and veins. A strong Ab reaction in the endothelia of central veins, as well as the pericentral sinusoids, was observed (c). The Ab reaction was relatively weak in the endothelium of periportal sinusoids but strong in adherent platelets (arrows, d), which were labeled by MoAb CD61 to platelet (arrows; e). Bars, a, b, and d = 50 μm; c = 50 μm; e = 10 μm.
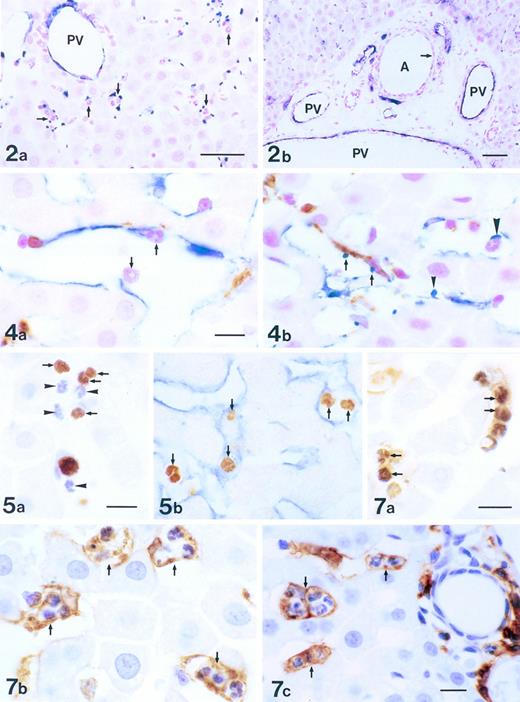
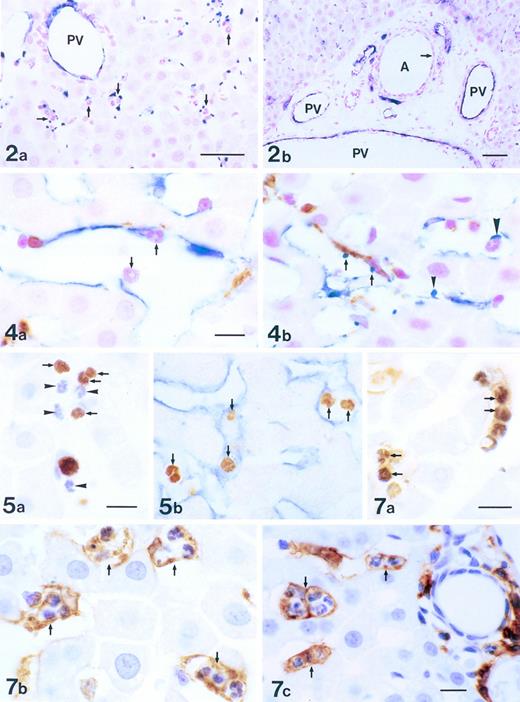
Localization of P-selectin in intralobular area of the liver 3 to 6 hours after the administration of LPS. Immunostaining was with anti–P-selectin Ab (blue) and counterstaining was by nuclear fast red (red). Many platelets expressing P-selectin were found in the periportal area, correlating closely with the presence of PMNs (arrows; a). P-selectin was expressed on the endothelial cells of portal veins (PV), but not on ones of the periportal arteries (A; arrow) in the periportal tract (b). Bars = 40 μm.
Localization of P-selectin in intralobular area of the liver 3 to 6 hours after the administration of LPS. Immunostaining was with anti–P-selectin Ab (blue) and counterstaining was by nuclear fast red (red). Many platelets expressing P-selectin were found in the periportal area, correlating closely with the presence of PMNs (arrows; a). P-selectin was expressed on the endothelial cells of portal veins (PV), but not on ones of the periportal arteries (A; arrow) in the periportal tract (b). Bars = 40 μm.
Double immunostaining of Kupffer cells and cells expressing P-selectin in the liver 6 hours after the administration of LPS. Endothelial cells and platelets (blue), Kupffer cells (brown), and PMNs (red) were labeled with anti–P-selectin Ab, ED2 Ab, and nuclear fast red, respectively. PMNs were adhering to endothelial cells of sinusoids (arrows; a). Platelets expressing P-selectin were in contact with Kupffer cells (arrows), endothelial cells (arrowhead), and PMNs (large arrowhead; b). Bars = 10 μm.
Double immunostaining of Kupffer cells and cells expressing P-selectin in the liver 6 hours after the administration of LPS. Endothelial cells and platelets (blue), Kupffer cells (brown), and PMNs (red) were labeled with anti–P-selectin Ab, ED2 Ab, and nuclear fast red, respectively. PMNs were adhering to endothelial cells of sinusoids (arrows; a). Platelets expressing P-selectin were in contact with Kupffer cells (arrows), endothelial cells (arrowhead), and PMNs (large arrowhead; b). Bars = 10 μm.
In situ detection of DNA fragmentation in PMNs in the liver 6 hours after the administration of LPS. The PMNs with DNA fragmentation were labeled brown (arrows) by the TUNEL method, whereas PMNs without DNA fragmentation were stained blue by hematoxylin (arrowheads; a). TUNEL-positive PMNs (brown, arrows) showed a close relationship with sinusoidal endothelial cells (blue) expressing P-selectin (b). Bar = 10 μm.
In situ detection of DNA fragmentation in PMNs in the liver 6 hours after the administration of LPS. The PMNs with DNA fragmentation were labeled brown (arrows) by the TUNEL method, whereas PMNs without DNA fragmentation were stained blue by hematoxylin (arrowheads; a). TUNEL-positive PMNs (brown, arrows) showed a close relationship with sinusoidal endothelial cells (blue) expressing P-selectin (b). Bar = 10 μm.
PMN-laden cells in the liver 6 hours after the administration of LPS. Double staining with TUNEL and ED1 Ab showed that numerous TUNEL-positive PMNs (dark brown, arrows) were trapped by ED1+ Kupffer cells (brown; a). Parallel with the phenomenon of phagocytosis (a), numerous PMN-laden Kupffer cells were stained with ED2 Ab (brown, arrows; b) and Ia Ag+PMN-laden cells were labeled with OX6 Ab (brown, arrows; c). Bars =10 μm.
PMN-laden cells in the liver 6 hours after the administration of LPS. Double staining with TUNEL and ED1 Ab showed that numerous TUNEL-positive PMNs (dark brown, arrows) were trapped by ED1+ Kupffer cells (brown; a). Parallel with the phenomenon of phagocytosis (a), numerous PMN-laden Kupffer cells were stained with ED2 Ab (brown, arrows; b) and Ia Ag+PMN-laden cells were labeled with OX6 Ab (brown, arrows; c). Bars =10 μm.
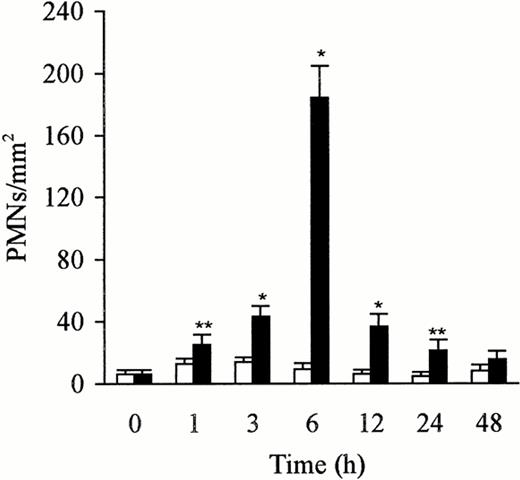
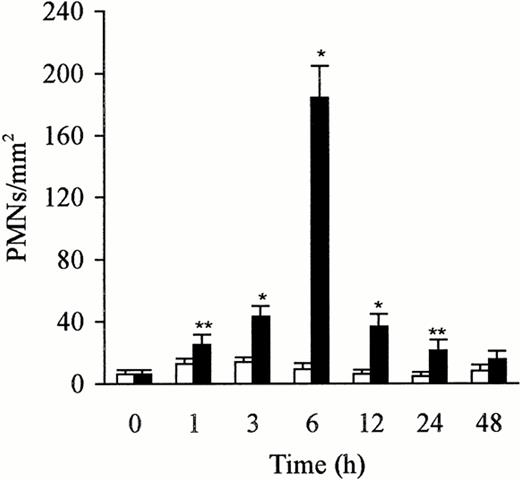
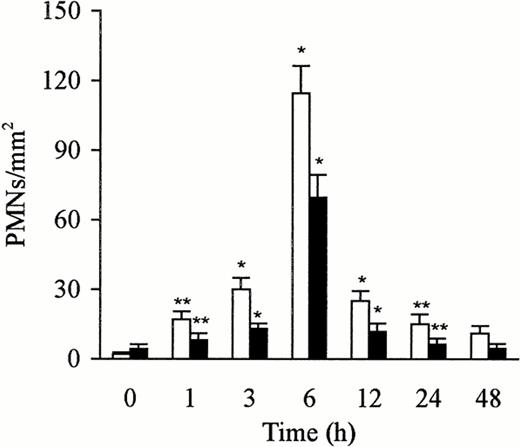
The kinetics of PMN accumulation in the liver has been evaluated by counting PMNs in 65 fields (0.0625 mm2 per field) from three sections per rat. The number of PMNs in the liver after treatment with sterile saline and LPS was counted on semithin section (0.2 μm). PMNs were identified and localized in the lumen of the sinusoids. The PMNs adhered to the endothelial cells, platelets, and Kupffer cells, or were phagocytosed by the Kupffer cells. The control liver exhibited few PMNs, but the number increased rapidly and peaked at 6 hours after the injection of LPS. This increase, compared with the untreated animals or animals treated with saline, was significant (P < .05 andP < .01). The number gradually decreased over a 12-hour period (Fig 3). The number of PMNs paralleled the increase or the decrease in the expression of P-selectin in the liver.
Number of PMNs in the liver after treatment with sterile saline (□) or LPS (▩). A slight increase of PMNs at 1 to 3 hours and a significant increase at 6 hours in the LPS-treated group were observed. The number of cells is expressed per mm2 of semithin section (0.2 μm). Data are expressed as mean ± SD. Bars represent SD. *P < .01, **P < .05 versus saline treatment. Five rats per group were examined.
Number of PMNs in the liver after treatment with sterile saline (□) or LPS (▩). A slight increase of PMNs at 1 to 3 hours and a significant increase at 6 hours in the LPS-treated group were observed. The number of cells is expressed per mm2 of semithin section (0.2 μm). Data are expressed as mean ± SD. Bars represent SD. *P < .01, **P < .05 versus saline treatment. Five rats per group were examined.
Expression of P-selectin and sequestration of PMNs.
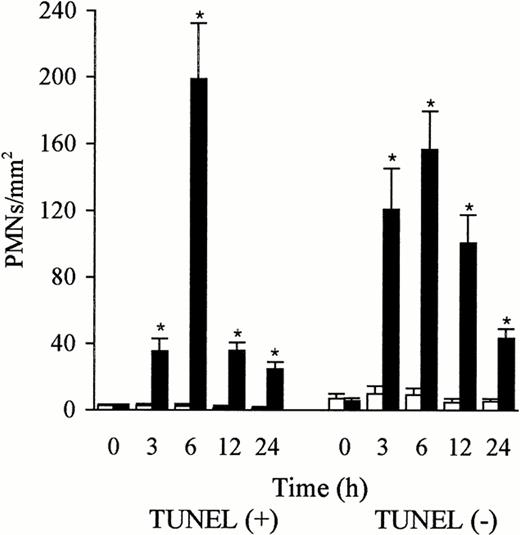
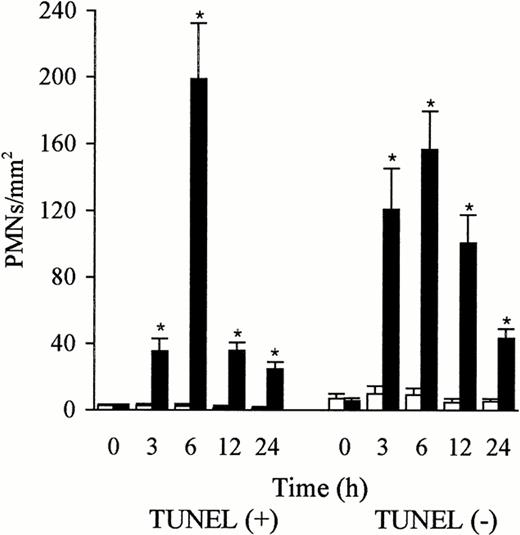
The PMNs in the sinusoidal lumen were seen to contact the endothelial cells (Fig 4a) and the platelets (Fig 4b) that expressed P-selectin. Platelets adhered to the endothelial cells, the Kupffer cells, and the PMNs (Fig 4b). PMNs with DNA fragmentation were labeled brown by TUNEL, whereas those without DNA fragmentation were counterstained blue by hematoxylin (Fig 5a). TUNEL-positive PMNs were in contact with the endothelial cells (blue) that expressed P-selectin (Fig 5b). The number of TUNEL-positive and TUNEL-negative PMNs were low in the control liver; however, the number of both increased rapidly and peaked 6 hours after the injection of LPS. The TUNEL-positive PMNs comprised more than 50% of the PMNs that had accumulated at 6 hours in the LPS-treated liver, and decreased also rapidly after 6 hours (Fig 6). This decrease, compared with the number of TUNEL-positive PMNs at 6 hours, was significant (P< .05). Numerous TUNEL-positive PMNs were phagocytosed by the macrophages/Kupffer cells that were labeled by ED1 (Fig 7a) or ED2 Ab 6 hours after the injection of LPS (Fig 7b). PMNs were also seen in the phagocytes labeled by OX6 Ab to MHC class II Ag (Fig 7c). The number of free and phagocytosed PMNs in the liver was evaluated on semithin sections (0.2 μm) and increased in parallel and reached a maximum 6 hours after the injection of LPS (Fig 8). This increase, compared with the untreated animals, was significant (P < .05 and P < .01).
Number of TUNEL-positive and TUNEL-negative PMNs in the liver after the administration of sterile saline (□) and LPS (▩). Few TUNEL-positive and TUNEL-negative PMNs were observed in the liver after sterile saline injection. However, the number of both TUNEL-positive and TUNEL-negative PMNs increased rapidly and peaked at 6 hours after LPS injection. The percentage of TUNEL positive PMNs exceeded 50% of the total (TUNEL-positive and TUNEL-negative PMNs) at 6 hours after the LPS injection. The number of cells is expressed per mm2 of TUNEL-stained paraffin sections (4 μm). Data are expressed as mean ± SD. Bars represent SD. *P < .01 versus saline treatment. Three rats per group were examined.
Number of TUNEL-positive and TUNEL-negative PMNs in the liver after the administration of sterile saline (□) and LPS (▩). Few TUNEL-positive and TUNEL-negative PMNs were observed in the liver after sterile saline injection. However, the number of both TUNEL-positive and TUNEL-negative PMNs increased rapidly and peaked at 6 hours after LPS injection. The percentage of TUNEL positive PMNs exceeded 50% of the total (TUNEL-positive and TUNEL-negative PMNs) at 6 hours after the LPS injection. The number of cells is expressed per mm2 of TUNEL-stained paraffin sections (4 μm). Data are expressed as mean ± SD. Bars represent SD. *P < .01 versus saline treatment. Three rats per group were examined.
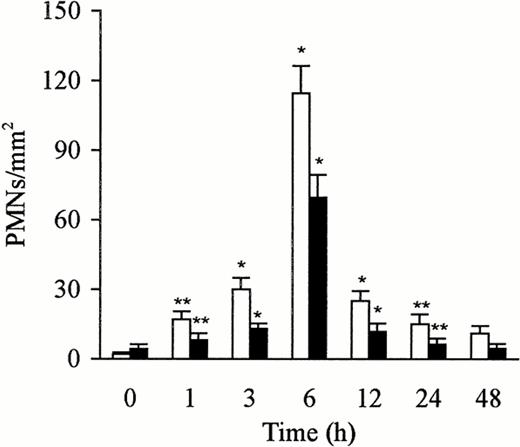
Number of free and phagocytosed PMNs in the liver after the administration of LPS. The number of free (□) and phagocytosed (▩) PMNs increased rapidly and reached maximal levels at 6 hours. The number of cells is expressed per mm2 of semithin section (0.2 μm). Data are expressed as mean ± SD. Bars represent SD. *P < .01, **P < .05 versus time 0 group. Five rats per group were examined.
Number of free and phagocytosed PMNs in the liver after the administration of LPS. The number of free (□) and phagocytosed (▩) PMNs increased rapidly and reached maximal levels at 6 hours. The number of cells is expressed per mm2 of semithin section (0.2 μm). Data are expressed as mean ± SD. Bars represent SD. *P < .01, **P < .05 versus time 0 group. Five rats per group were examined.
Blocking of P-selectin and depletion of Kupffer cells affected LPS-induced sequestration and elimination of PMNs in the liver.
Heparin chains containing four or more monosaccharide residues bind to P-selectin and inhibit the function of P-selectin. Pretreatment with an Ab to P-selectin or LMW heparin blocked the P-selectin, as observed by slight immunostaining in the hepatic lobule, positive staining was occasionally found on the endothelial cells of the portal veins and blocked the arrest of PMNs in the liver. Pretreatment with GdCl3 depleted the Kupffer cells, as observed by no immunostaining of ED2 to Kupffer cells, few phagocytosis of PMNs, and also weak staining of P-selectin (Table 1).
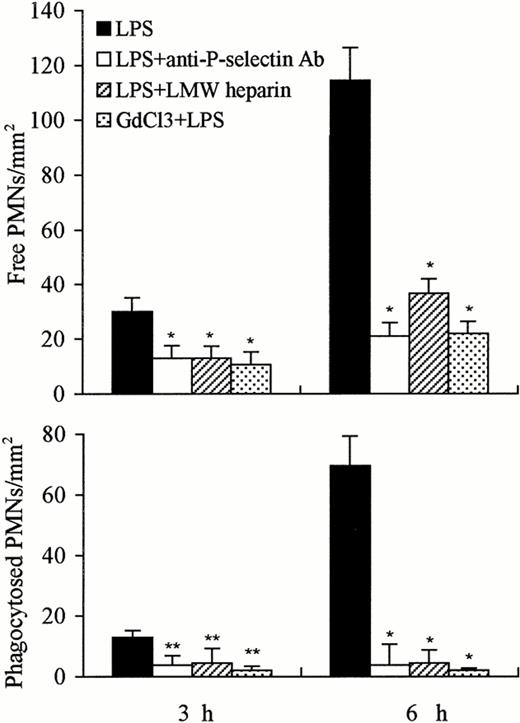
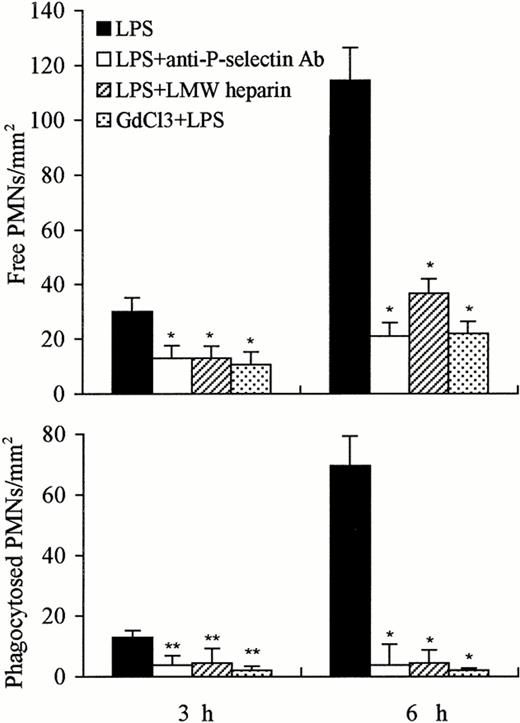
The number of free PMNs in the liver 3 or 6 hours after administration of LPS was significantly reduced by the concomitant administration of anti–P-selectin Ab or of LMW heparin or by pretreatment with GdCl3 compared with LPS treatment alone (P < .01). A significantly greater decrease was observed after concomitant treatment with anti–P-selectin Ab or GdCl3 versus that with LMW heparin (Fig 9). The number of phagocytosed PMNs observed in the liver 3 or 6 hours after the injection of LPS was also significantly decreased by the combined administration of anti–P-selectin Ab or of LMW heparin or by pretreatment with GdCl3. However, the decrease in the number of phagocytosed PMNs was greater at 6 hours than at 3 hours; GdCl3 pretreatment showed the greatest decrease (Fig 9).
Number of free and phagocytosed PMNs in the liver at 3 and 6 hours after treatment with LPS, LPS anti–P-selectin Ab, LPS LMW heparin, and GdCl3 LPS. The number of free and phagocytosed PMNs was reduced at 3 hours, and significant reduction was observed 6 hours after combined treatment with anti–P-selectin Ab or LMW heparin or GdCl3, compared with LPS treatment alone. The number of phagocytosed PMNs was near to 0 level 3 or 6 hours after combined treatment with GdCl3. The number of cells is expressed per mm2 of semithin section (0.2 μm). Data are expressed as mean ± SD. Bars represent SD. *P < .01, **P < .05 versus LPS treatment only. Three rats per group were examined.
Number of free and phagocytosed PMNs in the liver at 3 and 6 hours after treatment with LPS, LPS anti–P-selectin Ab, LPS LMW heparin, and GdCl3 LPS. The number of free and phagocytosed PMNs was reduced at 3 hours, and significant reduction was observed 6 hours after combined treatment with anti–P-selectin Ab or LMW heparin or GdCl3, compared with LPS treatment alone. The number of phagocytosed PMNs was near to 0 level 3 or 6 hours after combined treatment with GdCl3. The number of cells is expressed per mm2 of semithin section (0.2 μm). Data are expressed as mean ± SD. Bars represent SD. *P < .01, **P < .05 versus LPS treatment only. Three rats per group were examined.
DISCUSSION
We made three significant observations. First, we observed the heterogeneity expression of P-selectin on endothelia in the intralobular area of the liver after the administration of LPS. Second, a substantial number of PMNs and platelets were found in the liver, but PMNs eventually became apoptotic. Third, we showed that the adherent PMNs did not emigrate, but were phagocytosed by the Kupffer cells.
In most tissues, the maximal expression of P-selectin occurs 4 hours after the administration of LPS in vivo.1,12 However, in this study, P-selectin expression on venous and sinusoidal endothelial cells and platelets trapped in the liver peaked 6 hours after the injection of LPS. Because depletion of Kupffer cells by pretreatment with GdCl3 decreased intensity and shortened the time course of P-selectin expression, this prolonged expression may arise in part from the LPS-induced generation of tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) by the Kupffer cells,6,13 14 which acts to sustain the level of P-selectin expression on the endothelial cells in the liver. In addition, rather profound heterogeneous distribution of P-selectin expression was noted between the periportal and pericentral areas (pericentral area > periportal area), which suggests that the role of P-selectin in the adherence and migration of PMNs in the lobule may differ between the two areas. Significant regional differences in the intensity of P-selectin expression, with no expression on endothelia of lymph vessels or weak/part expression on that of arteries reflect that there were structural and functional heterogeneity in hepatic endothelia.
An interesting finding was the arrest of CD61+ platelets, which expressed P-selectin in the lobule mainly in the periportal and midzonal areas. The trapping of platelets with intense expression of P-selectin increased the concentration of P-selectin in the areas, although the expression of P-selectin was relatively weak on endothelia in the periportal area. Intravital microscopic observations have suggested that unstimulated as well as activated platelets roll on, but do not adhere to, the venous endothelium unless the integrity of the vessel wall is disrupted.15,16 However, activated platelets expressing P-selectin could preferentially bind the PMNs (Fig 2a) and roll together on the endothelial cells (Fig 4b, large arrowhead). In this way, each may recruit the other to the surface of stimulated endothelium. In addition, as shown by Wake et al,6 the Kupffer cell population is distributionally and functionally heterogeneous. When the liver lobule is divided in three regions from periportal to pericentral, the Kupffer cells are distributed in an approximate ratio of 4:3:2 as determined by morphometric and phagocytotic characterization. The distribution of arrested platelets is consistent with the areas of higher concentrations of Kupffer cells. Pretreatment with GdCl3 or the concomitant treatment with anti–P-selectin Ab or LMW heparin reduced the aggregation of platelets in the liver. Thus, it is reasonable that both the Kupffer cells and the expression of P-selectin on endothelial cells of sinusoids contribute to the sequestration of platelets in the liver. The concomitant aggregation of platelets to PMNs at sites of hemostasis and inflammation may contribute to the induction of vessel wall injury.17 On the other hand, P-selectin is important in the recruitment of PMNs to the sites of platelet deposition both in vivo and in vitro.18-20 The trapped platelets could promote the attachment of PMNs to the sinusoidal wall by slowing the PMNs so that platelet-opsonized PMN phagocytosis by Kupffer cells could occur. Furthermore, an activated monocyte-macrophage system can increase the rate of platelet removal,21 and as seen in this study, platelet P-selectin expression and platelet-PMN binding in the periportal and midzonal areas coincided with phagocytosis of PMN by the Kupffer cells there.
The results from perfused liver showed that a number of PMNs were TUNEL-positive (Fig 5 and 6), and comprised more than 50% of the PMNs that had accumulated at 6 hours in the LPS-treated liver; the number of TUNEL-positive PMNs fell off more rapidly than the that of TUNEL-negative cells. One explanation is that chromatin condensation and DNA fragmentation appear to occur within a few minutes, and the phagocytosis of apoptotic PMNs leads to their marked degradation, at which point, occurring also within minutes, they are no longer recognized as PMNs.22 As regards TUNEL-negative cells, there was a significant decrease, compared with the number at 6 hours after treatment, although there were some in the liver at a time when P-selectin staining of sinusoidal endothelial cells was negative. Coincidence of distribution of Kupffer cells and PMNs suggest that the Kupffer cells may also play a role in trapping PMNs in the liver. PMNs have been shown to die by apoptosis in vivo.9,14,23 The apoptotic PMNs show a reduced expression of L-selectin.24L-selectin has been reported to be expressed on the microvilli of leukocytes,25 and the loss of microvilli, a significant and early change in apoptotic PMNs,9 suggests that L-selectin is not important in accumulation of PMNs in the liver. Furthermore, P-selectin can tether the PMNs to the endothelial cells without PMN activation or the β2-integrin system.26,27LMW heparin reduces the influx of PMNs into the peritoneal cavity by binding to P- and L-selectin,28-31 which coincide with a report32 that blocking of the L- and P-selectin inhibits PMN migration into mouse peritoneum. In our experiments, combined treatment with Ab to P-selectin or LMW heparin resulted in a negative/weak staining of the P-selectin and blocking of PMN arrest in the liver. Thus, in this study, it appeared that the P-selectin on the endothelia and platelets mediated sequestration of PMNs in the liver.
The excessive accumulation of PMNs can damage tissues via the release of proteases and oxygen radicals.33 Therefore, the elimination of PMNs from the vasculature is important for stopping the development of lesions. In our study, the aggregation of PMNs by P-selectin–induced adhesion in the hepatic sinusoids was followed by phagocytosis of PMNs. These results suggest that phagocytosis of PMNs by Kupffer cells occurs when the PMNs transit through the hepatic sinusoids from portal to central veins. This elimination of PMNs could be caused by several factors. One explanation may be that the adhesion modulated by P-selectin was a prerequisite for phagocytosis by the Kupffer cells in this study. Furthermore, the PMNs were aging and undergoing apoptosis, a process that is closely followed in vivo by phagocytosis. These results are supported by our previous studies.9 14
The PMN-laden cells identified in this study were Kupffer cells and MHC class II Ag+ cells. The Kupffer cells were labeled by MoAbs to the macrophage-related Ag ED1 and ED2.9 The elimination of the Kupffer cells with GdCl3 caused a delayed removal of PMNs. MHC class II Ag+ cells were stained by MoAb OX6 to Ia-nonpolymorphic Ag. It was recently reported that MHC class II Ag+ phagocytes consist of activated monocytes and dendritic cells, and that dendritic cells exhibit phagocytic activity for particulates in vivo when they undergo a blood-lymph translocation via the hepatic sinusoids.34
The roles that adhesion molecules play in sinusoidal PMN accumulation remain unclear. Sequestration of PMNs in the hepatic vasculature during endotoxemia is independent of β2-integrins and intercellular adhesion molecule-1 (ICAM-1).33,35 A minimal role for selectins in the recruitment of leukocytes into the inflamed liver observed by Wong et al36 seems improbable, because expression of P- and E-selectin on normal and FMLP-treated animals was not determined. The results of this model showed that P-selectin was indeed required for the adhesion-induced elimination of PMNs. Results suggest that the adherent platelets on the walls of the hepatic sinusoids could act as receptors for the adhesion of flowing PMNs, or P-selectin might act as the first step in delaying the PMNs so that they become locally adherent to the endothelium via P-selectin–mediated binding. Margination, adhesion, and apoptotic changes of the flowing PMNs in hepatic sinusoids could, therefore, be a prerequisite for phagocytosis by the Kupffer cells. Our results also suggest that the Kupffer cells, together with the ability of phagocytosis and the modulation of P-selectin expression, appeared to play a role in the accumulation and homeostasis of PMNs in the circulatory system.
ACKNOWLEDGMENT
We are grateful to Prof Vinci Mizuhira and Katsuiku Hirokawa for their valuable suggestions and encouragement. We acknowledge the technical assistance of Akira Masuda and Yingli Yang.
Supported by Grant No. C08457002 from the Japanese Ministry of Education, Science, Sports and Culture.
Address reprint requests to Jialan Shi, MD, PhD, Division 1, Department of Anatomy, School of Medicine, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.