Abstract
Fas ligand/Fas receptor molecular interactions have been implicated as having an important function for the regulation of eosinophil apoptosis. The purpose of the present study was to investigate biochemical events triggered by the engagement of the Fas receptor in freshly isolated human and mouse eosinophils. Activation of the Fas receptor on eosinophils with the agonistic anti-Fas monoclonal antibody (MoAb) resulted in increased tyrosine phosphorylation of several intracellular proteins. The tyrosine kinase inhibitors lavendustin A and genistein inhibited Fas receptor-induced cell death in both human and mouse eosinophils in vitro and prevented, at least partially, Fas receptor-mediated resolution of eosinophilic inflammation in a mouse in vivo model of lung eosinophilia. In addition, in freshly purified human eosinophils, lavendustin A prevented anti-Fas MoAb-induced proteolytic cleavage of lamin B, suggesting that tyrosine kinases may amplify the proteolytic signaling cascade within interleukin-1β converting enzyme (ICE) family proteases. Moreover, the tyrosine kinase Lyn was identified as being involved in Fas receptor-mediated cell death. Collectively, these results demonstrate that tyrosine phosphorylation is an important step in the generation of the Fas receptor-linked transmembrane death signal in eosinophils and that Lyn participates in this pathway.
THE Fas RECEPTOR (CD95/APO-1) is a widely expressed 45-kD transmembrane protein.1,2 Fas is a member of the tumor necrosis factor (TNF)/nerve growth factor (NGF) receptor family, which mediates apoptosis in many but not all systems.3,4 It has been shown that Fas receptor/Fas ligand molecular interactions are critical for peripheral T-cell homeostasis.5-9 Therefore, it is possible that the Fas receptor contributes to the regulation of cell death in a multicellular organism to maintain correct cell numbers.
Functional Fas receptors have been recently described on human and murine eosinophils.10-13 These studies suggested that Fas receptor/Fas ligand molecular interactions are involved in the regulation of eosinophil apoptosis. Moreover, functional defects in Fas receptor-mediated eosinophil apoptosis may contribute to the chronic eosinophilic inflammation often observed in allergic and asthmatic patients. Therefore, it is important to identify the signaling pathways transduced via the Fas receptor that lead to induction of apoptosis in eosinophils.
Protein tyrosine kinases play a key role in the transduction of signals through cell surface receptors.14 Protein tyrosine phosphorylation has also been shown to be involved in Fas receptor transmembrane signaling in many cellular systems,15-20although it is controversial whether tyrosine kinase activation is necessary for Fas receptor-mediated apoptosis.20-23However, a role of tyrosine phosphorylation is supported by the observation that expression of the hematopoietic cell phosphatase is a prerequisite for Fas receptor-induced apoptosis in several lymphoid cell lines.24,25 Another protein-tyrosine phosphatase, FAP-1, has been shown to associate with the Fas receptor and to exert a negative influence on Fas receptor signal transduction.26
In this study, we show that activation of the Fas receptor on eosinophils results in increased protein-tyrosine phosphorylation of several cellular proteins. Blockers of tyrosine kinases inhibit Fas receptor-mediated cell death in both human and murine eosinophils. Moreover, we demonstrate that the proteolytic degradation of lamin B is prevented by inhibition of tyrosine kinase activity, suggesting that tyrosine kinases are part of a signaling pathway leading to amplification of the proteolytic cascade within the interleukin-1β converting enzyme (ICE) proteases family. We also show that the tyrosine kinase Lyn is functionally active within the death pathway initiated by anti-Fas monoclonal antibody (MoAb) treatment of these cells. In addition, the activation of Lyn does not appear to be dependent on early activition of ICE-like proteases. Taken together, these findings suggest that activation of tyrosine kinases, including Lyn, is important for Fas receptor-mediated transmembrane signaling events leading to eosinophil apoptosis.
MATERIALS AND METHODS
Antibodies.
Anti-Lyn MoAb and RC-20 Ab were obtained from Transduction Laboratories (Lexington, KY). Antilamin B MoAb was from Calbiochem-Novabiochem Corp (San Diego, CA). MoAb against antiphosphotyrosine (ptyr), clone 4G10, and Fas receptor, clone CH-11 (IgM), were from Immunotech (Bühlmann Laboratories AG, Basel, Switzerland). Control IgM and IgG2b MoAb were obtained from Dako (Zurich, Switzerland). Anti-Fas MoAb (IgG3, κ) was a kind gift of Dr P.H. Krammer (German Cancer Research Center, Heidelberg, Germany). This MoAb recognizes an epitope on the extracellular part of the Fas receptor.27 Anti-JNK Ab (C-17) was from Santa Cruz Biotechnology (Santa Cruz, CA). GST-fusion protein of c-Jun (GST-c-Jun, 1-79) was obtained from Biomol (Plymouth Meeting, PA). Goat antimouse horseradish peroxidase (HRP)-labeled secondary Ab was obtained from Amersham International (Amersham, Bucks, UK). Anti-CD16 MoAb microbeads were from Miltenyi Biotec (Bergisch-Gladbach, Germany). For the mouse experiments, antimouse Fas receptor MoAb (clone Jo2, Armenian Hamster IgG MoAb; Pharmingen, Palo Alto, CA) and irrelevant control Ab (Armenian Hamster IgG; Pharmingen) were used. Biotinylated antimouse B220, CD19, Thy1.2, CD4, CD8, and TER-119 MoAbs were also obtained from Pharmingen.
Purification of human blood eosinophils.
Human eosinophils were purified as previously described.9,13 28-31 Briefly, peripheral blood mononuclear cells (PBMC) were separated from peripheral blood of patients with moderate eosinophilia (8% to 16%) by centrifugation on Ficoll-Hypaque (Seromed-Fakola AG, Basel, Switzerland). The remaining cell population, mainly granulocytes and erythrocytes, was treated with erythrocyte lysis solution (155 mmol/L NH4Cl, 10 mmol/L KHCO3, and 0.1 mmol/L EDTA, pH 7.3). The resulting granulocyte population contained mainly neutrophils. To purify eosinophils, the granulocyte population was incubated with anti-CD16 MoAb microbeads. CD16+ neutrophils were depleted by passing the granulocytes through a magnetic cell separation (MACS; Miltenyi Biotec) with column type C and an attached 21-gauge needle in the field of a permanent magnet. The resulting cell populations contained 99% eosinophils as determined by staining with Diff-Quik (Baxter, Düdingen, Switzerland) and light microscopy.
Immunoprecipitation.
Human eosinophils (3 to 5 × 106/mL) were stimulated in CG medium (Vitromex GmbH, Vilshofen, Germany) in the absence of fetal calf serum (FCS) with 1 μg/mL anti-Fas MoAb (CH-11, IgM) or control IgM MoAb for the indicated times at 37°C. In some experiments, cells were pretreated with tyrosine kinase inhibitors at the indicated concentrations for 1 hour at 37°C before anti-Fas receptor stimulation. In other experiments, cells were pretreated with the ICE-inhibitor II (N-Acetyl-Tyr-Val-Ala-Asp-chloromethylketone; Ac-YVAD-cmk; Bachem, Bubendorf, Switzerland) at 100 nmol/L for 1 hour at 37°C before the addition of anti-Fas MoAb. The reaction was stopped by the addition of ice-cold phosphate-buffered saline (PBS) containing 0.5 mmol/L Na3VO4. Cells were immediately pelleted at 4°C and lysed with 1 mL of ice-cold 0.5% Triton X-100 lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 25 mmol/L KCl, 5 mmol/L MgCl2, 1 mmol/L EGTA, 1 mmol/L Na3VO4, 10 μg/mL pepstatin A, 18 μg/mL aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 1 μg/mL leupeptin, and 1 mmol/L benzamidine) on ice for 10 to 15 minutes. Insoluble material was removed by centrifugation at 4°C for 15 minutes at 15,800g. Cell lysates were precleared with 100 μL of Pansorbin (Calbiochem-Novabiochem; 10% vol/vol solution of fixed Staphylococcus aureus Cowan I, prewashed 3 times in lysis buffer without proteinase inhibitors) at room temperature for 1 hour or at 4°C overnight. Lysate supernatants were further incubated with 50 μL of packed Sepharose 4B (Pharmacia, Uppsala, Sweden) coupled to bovine serum albumin (BSA) at room temperature for 1 hour. Supernatants were transferred to 25 to 30 μL protein G-Sepharose (Pharmacia) that had been cross-linked with 4G10 MoAb, control IgG2b MoAb, anti-Fas IgG3 MoAb, or anti-Lyn MoAb overnight at 4°C and washed 4 times with lysis buffer. Tubes were rotated at 4°C overnight and immunocomplexes were washed 4 times with cold lysis buffer. The proteins were eluted by adding Laemmli sample buffer plus 0.1 mol/L dithiothreitol and heating at 95°C for 5 minutes before gel electrophoresis.
Gel electrophoresis and immunoblotting.
Electrophoresis was conducted in 10% sodium dodecyl sulfate (SDS) polyacrylamide gels, and the separated proteins were transferred to a polyvinylidene fluoride filter (Immobilon-P; Millipore AG, Volketswil, Switzerland). In the RC-20 experiments, the filters were incubated in blocking solution I (10 mmol/L Tris-HCl, pH 7.5, 100 mmol/L NaCl, 0.1% Tween 20, and 5% BSA; Sigma, Buchs, Switzerland). In the Fas receptor and Lyn experiments, the filters were incubated in blocking solution II (5% low fat milk, 10 mmol/L Tris-HCl, pH 7.5, 100 mmol/L NaCl, 0.1% Tween 20). These blocking steps were performed at room temperature for 1 hour. In the lamin B experiments, a new rapid immunodetection method without a blocking step was used according to the manufacturer's instructions. Filters were incubated with HRP-labeled RC-20 Ab or primary anti-Fas, anti-Lyn, or lamin B MoAb at room temperature for 1 to 2 hours. Binding of primary MoAb was detected by additional incubation of the filters with goat antimouse HRP-labeled secondary Ab at room temperature for 1 hour. Blots were developed by an enhanced chemiluminescence technique (ECL kit; Amersham) according to the manufacturer's instructions. Lamin B proteolysis was analyzed densitometrically using an automated scanner system supported by the NIH-image program.
Determination of JNK activation.
Determination of JNK activation was performed as previously described.32 Briefly, freshly purified human eosinophils were cultured in the presence or absence of 16 μg/mL lavendustin A for 1 hour at 37°C and then stimulated with anti-Fas MoAb for another 1 hour at 37°C. Cells were lysed on ice for 15 minutes with 1 mL of ice-cold 0.5% Triton X-100 lysis buffer (0.5% Triton X-100, 50 mmol/L Tris-HCl, pH 7.4, 25 mmol/L KCl, 5 mmol/L MgCl2, 1 mmol/L EGTA, 1 mmol/L Na3VO4, 1 μg/mL pepstatin A, 18 μg/mL aprotinin, 1 mmol/L PMSF, 1 μg/mL leupeptin, and 1 mmol/L benzamidine). Insoluble material was removed by centrifugation at 4°C for 15 minutes at 15,800g. Supernatants were incubated on ice for 1 hour with 1 μg/mL anti-JNK Ab. Forty microliters of protein A-Sepharose beads were added and tubes were rotated at 4°C for 1 hour. The bead pellets were washed, resuspended in 30 μL kinase buffer (20 mmol/L HEPES, pH 7.5, 2 mmol/L dithiothreitol, 5 mmol/L MgCl2, 10 μmol/L PMSF, 0.2 mmol/L Na3VO4, and 1 mmol/L benzamidine), and incubated with 20 μg GST-c-Jun (1-79) and 1 μL [γ-32P]ATP (Amersham) at 30°C for 20 minutes. The reaction was stopped by the addition of 40 μL 2× SDS, 2-ME sample buffer. The samples were heated to 95°C for 5 minutes. Electrophoresis was conducted in 15% SDS polyacrylamide gels, and phosphorylation of c-Jun was detected by autoradiography.
Antisense oligodeoxynucleotides.
Eosinophils (106/mL) were cultured in RPMI 1640 plus 10% FCS (complete culture medium) in the presence of phosphorothioate oligodeoxynucleotides (MWG-Biotech, Münchenstein, Germany) at 10 μmol/L. Sequences used were as follows: antisense, Lyn 5′-CATATTTCCCGCTCG-3′; and sense, Lyn 5′-CGAGCGGGAAATATG-3′. Treatment of eosinophils31 and neutrophils33 with phosphorothioate-derivatized Lyn antisense oligodeoxynucleotide for 6 hours has been previously shown to significantly reduce Lyn protein levels.
Determination of eosinophil death.
Human and mouse eosinophils (106/mL) were pretreated in the presence or absence of tyrosine kinase inhibitors (1 hour) or phosphorothioate oligodeoxynucleotides (6 hours). The following tyrosine kinase inhibitors (Life Technologies Trading AG, Basel, Switzerland) were used: genistein (10 to 50 μg/mL), herbimycin A (1 to 20 μg/mL), and lavendustin A (1 to 50 μg/mL). In other experiments, human eosinophils were preincubated with the ICE-inhibitor Ac-YVAD-cmk for 1 hour at the indicated concentrations. Cells were then cultured in complete culture medium in the presence or absence of 1 μg/mL (human eosinophils) or 0.1 μg/mL (mouse eosinophils) anti-Fas MoAb for the indicated times. Cell death of human eosinophils was assessed by uptake of 1 μmol/L ethidium bromide and flow cytometric analysis (EPICS XL; Coulter Corp, Hialeah, FL), as previously described.9,13,29,31 In the mouse experiments, eosinophil death was determined by trypan blue exclusion using a Neubauer chamber (Assistent GmbH, Sondheim, Germany) to enumerate the cells. We have previously demonstrated that the Jo2 anti-Fas MoAb induces apoptosis in mouse eosinophils under identical conditions.11
Determination of eosinophil apoptosis.
To determine whether eosinophil death was apoptosis, human eosinophils were morphologically examined. Cells were pretreated in the presence or absence of 16 μg/mL lavendustin A for 1 hour. Cells were then stimulated with 1 μg/mL anti-Fas IgM MoAb in complete culture medium for 8 hours. Cytospin preparations were made, stained with Diff-Quik, and photographed under a Zeiss Axioscope microscope (Oberkochen, Germany) at 1,000× magnifications.
Mice and in vivo experimental procedures.
Eosinophilic inflammation was elicited as previously described.11,34 35 Briefly, male Balb/c mice (16 to 20 g, approximately 6 weeks old) were immunized intraperitoneally with 10 μg of ovalbumin (OA; grade V; Sigma, St Louis, MO) in 4 mg of alum (Serva, Heidelberg, Germany) on days 0 and 14. Sham-immunized mice received 2 injections of alum alone. Seven to 10 days after the last immunization, animals were challenged with antigen by exposure to an aerosol of OA (50 mg/mL) for 20 minutes using a Devilbiss ultrasonic nebulizer whose particle size characteristics had been previously determined to produce 90% particles less than 10 μm in diameter. Seventy-two hours after allergen inhalation, mice were used for in vivo experiments or eosinophil purification. To determine the effects of tyrosine kinase inhibitors, mice were lightly anesthetized with 2.5% vol/vol enflurane (Parke-Davis, Frankfurt/Main, Germany) in air delivered into a 200-mL perspex narcosis chamber. During spontaneous breathing, 2× 10 μL aliquots of inhibitors (10 μg, total dose) or vehicle dimethyl sulfoxide (DMSO; 0.1% in PBS) were administered to the lungs via nares. Mice are obligate nose breathers, and it has been previously reported that this method effectively delivers greater than 90% of the complete drug volume to the lungs. After 1 hour, 35 μg/35 μL anti-Fas or control Ab was delivered to the lungs. Twenty-four hours after Ab administration, mice were deeply anesthetized with 0.3 mL of 14% wt/vol urethane (Fluka, Buchs, Switzerland), the trachea was cannulated, and the lungs were lavaged with 4× 0.3 mL aliquots of PBS at room temperature. Total cell counts on individual bronchoalveolar lavage (BAL) fluid aliquots were determined. Differential BAL cell counts on 500 cells were performed according to standard morphological criteria using cytospin preparations stained with Diff-Quik and light microscopy. All procedures conformed to international standards of animal care and welfare and were approved by the Federal Animal Health Department (Basel, Switzerland).
Purification of mouse lung eosinophils.
Mouse eosinophils were purified from BAL fluids of immunized and challenged mice using lectin affinity negative selection. Briefly, mice were deeply anesthetized, and the lungs were lavaged. BAL cells (106/mL) were incubated with 10 μg/mL biotinylated Bandierea (Griffonia) simplifora isolectin I (Vector Labs, Burlingame, CA), which preferentially binds alveolar macrophages and monocytes, together with biotinylated rat antimouse MoAb directed against the lymphocyte (B220, CD19, Thy1.2, CD4, CD8) and erythrocyte (TER-119) surface antigens. Biotin-bound cells were removed using streptavidin-coupled paramagnetic beads (Dynal, Hamburg, Germany). The resulting cell populations contained 98% to 99% eosinophils as determined by staining with Diff-Quik and light microscopy.
Statistical analysis.
Data are presented as the mean ± SEM. Statistical analysis was performed by using ANOVA followed by multiple comparison correctedt-test. A probability value of less than .05 was considered statistically significant.
RESULTS
Anti-Fas MoAb induces increased tyrosine phosphorylation in freshly isolated human eosinophils.
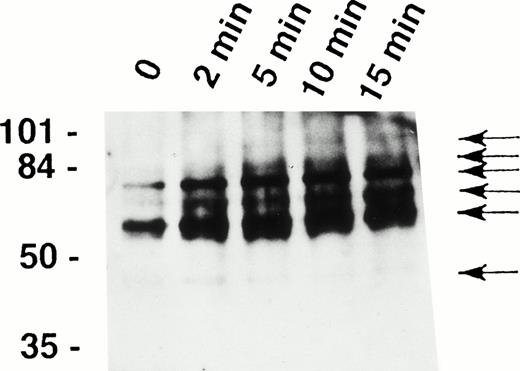
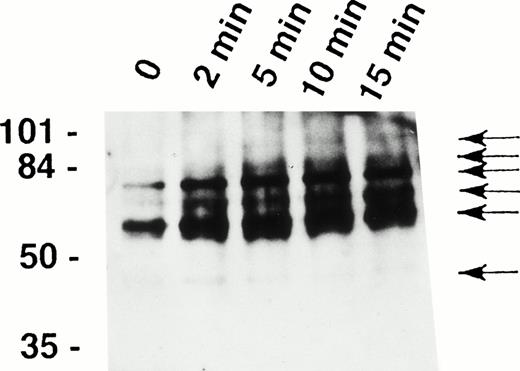
It has been recently shown that eosinophils express the Fas receptor10-13 and that granulocyte apoptosis is regulated by tyrosine phosphorylation.29,31 33 To determine whether tyrosine phosphorylation also participates in early signaling mediated by the Fas receptor in human eosinophils, we analyzed intracellular tyrosine phosphorylated proteins before and after stimulation of the cells with anti-Fas MoAb. As shown in Fig1, anti-Fas MoAb treatment was followed by increased tyrosine phosphorylation of proteins of approximately 48, 60, 72, 82, 88, and 98 kD within 2 minutes. Phosphorylation of the 48-kD protein peaked at 2 minutes and that of all other proteins at 5 to 10 minutes.
Fas receptor activation induces tyrosine phosphorylation of several cellular proteins in human eosinophils. Freshly isolated eosinophils were stimulated with anti-Fas MoAb for the indicated times. The cell lysates were immunoprecipitated with 4G10 MoAb, and the ptyr immunoprecipitates were analyzed by immunoblotting with a MoAb to ptyr, RC20. Increased tyrosine phosphorylation of 48-, 60-, 72-, 82-, 88-, and 98-kD proteins (arrows) was observed. The position of molecular size standards (left). Data are representative of three independent experiments.
Fas receptor activation induces tyrosine phosphorylation of several cellular proteins in human eosinophils. Freshly isolated eosinophils were stimulated with anti-Fas MoAb for the indicated times. The cell lysates were immunoprecipitated with 4G10 MoAb, and the ptyr immunoprecipitates were analyzed by immunoblotting with a MoAb to ptyr, RC20. Increased tyrosine phosphorylation of 48-, 60-, 72-, 82-, 88-, and 98-kD proteins (arrows) was observed. The position of molecular size standards (left). Data are representative of three independent experiments.
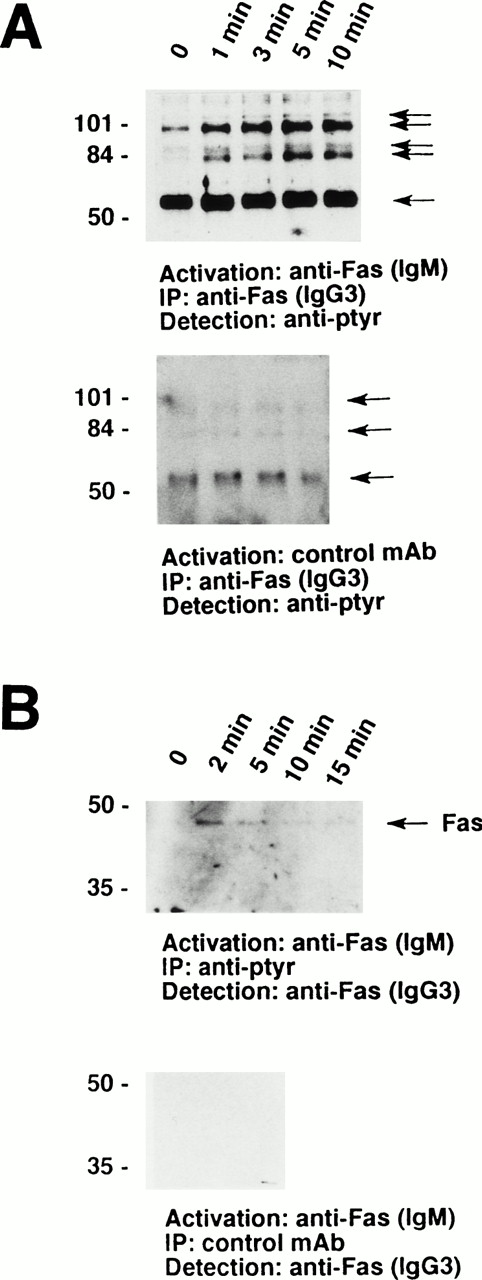
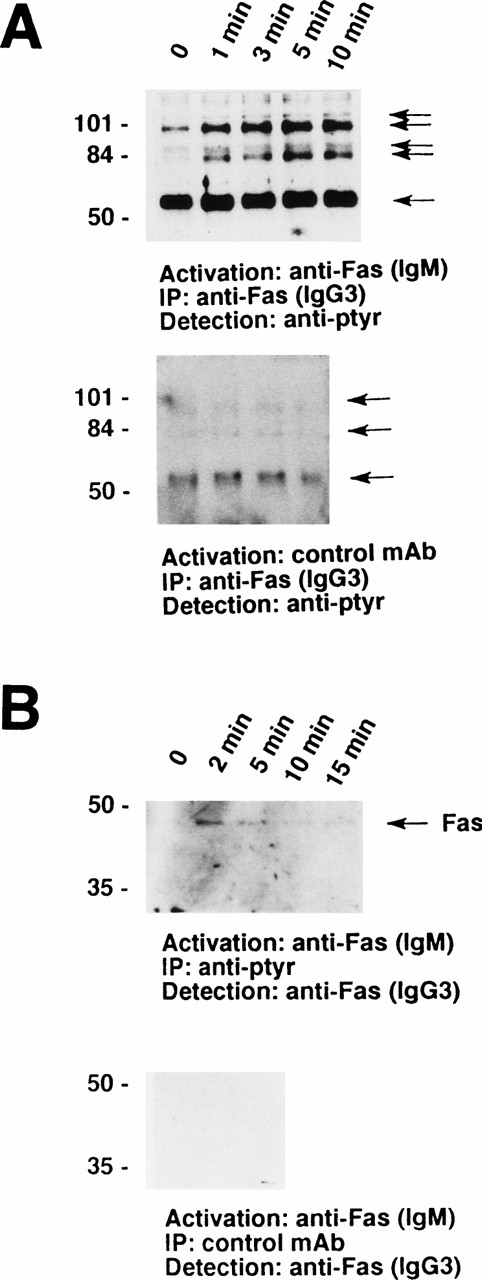
Furthermore, a series of tyrosine-phosphorylated proteins ranging from 56 to 110 kD coimmunoprecipitated with the Fas receptor. As demonstrated in Fig 2A, a prominant 56-kD protein appears to physically associate with the receptor in nonstimulated eosinophils. In addition, a 98-kD protein and, in some cases, a 82-kD protein associated with the receptor under these conditions. Moreover, 88- and 110-kD tyrosine-phosphorylated proteins inducibly coimmunoprecipitated with the Fas receptor. These phosphorylation events were best detected between 1 and 10 minutes after Fas receptor stimulation (Fig 2A, upper panel). In contrast, stimulation of the eosinophils with control MoAb did not result in increases in tyrosine phophorylation or in the appearance of 88- and 110-kD proteins (Fig 2A, lower panel). The Fas receptor could also be coimmunoprecipitated with anti-ptyr but not control MoAb (Fig 2B), further suggesting a physical association of the Fas receptor with tyrosine-phosphorylated proteins.
The Fas receptor is physically associated with a series of tyrosine-phosphorylated proteins in human eosinophils. (A) Cells (5 × 106) were stimulated with anti-Fas MoAb (IgM) for the indicated times. The cell lysates were immunoprecipitated with anti-Fas MoAb (IgG3). The immunoprecipitates were analyzed by immunoblotting with an MoAb to ptyr, RC20. (Upper panel) A prominent 56-kD protein and two additional tyrosine-phosphorylated proteins associated with the receptor in unstimulated cells. In addition, 88- and 110-kD tyrosine-phosphorylated proteins inducibly coimmunoprecipitated with the Fas receptor. (Lower panel) Stimulation of 3 × 106 eosinophils with control MoAb did not result in an increase in either the quantity or the degree of tyrosine phosphorylation of proteins associated with the Fas receptor. (B) Cells (3 × 106) were stimulated with anti-Fas MoAb (IgM) for the indicated times. The cell lysates were immunoprecipitated with 4G10 MoAb (upper panel) or control IgG2b MoAb (lower panel), and the immunoprecipitates were examined for the presence of Fas receptor protein. The Fas receptor inducibly coimmunoprecipitated with ptyr proteins (upper panel). The positions of molecular size standards for (A) and (B) are on the left. All data are representative of at least three independent experiments.
The Fas receptor is physically associated with a series of tyrosine-phosphorylated proteins in human eosinophils. (A) Cells (5 × 106) were stimulated with anti-Fas MoAb (IgM) for the indicated times. The cell lysates were immunoprecipitated with anti-Fas MoAb (IgG3). The immunoprecipitates were analyzed by immunoblotting with an MoAb to ptyr, RC20. (Upper panel) A prominent 56-kD protein and two additional tyrosine-phosphorylated proteins associated with the receptor in unstimulated cells. In addition, 88- and 110-kD tyrosine-phosphorylated proteins inducibly coimmunoprecipitated with the Fas receptor. (Lower panel) Stimulation of 3 × 106 eosinophils with control MoAb did not result in an increase in either the quantity or the degree of tyrosine phosphorylation of proteins associated with the Fas receptor. (B) Cells (3 × 106) were stimulated with anti-Fas MoAb (IgM) for the indicated times. The cell lysates were immunoprecipitated with 4G10 MoAb (upper panel) or control IgG2b MoAb (lower panel), and the immunoprecipitates were examined for the presence of Fas receptor protein. The Fas receptor inducibly coimmunoprecipitated with ptyr proteins (upper panel). The positions of molecular size standards for (A) and (B) are on the left. All data are representative of at least three independent experiments.
Tyrosine kinase blockers inhibit Fas receptor-mediated apoptosis in human eosinophils.
The role of protein tyrosine kinases in the transmembrane signaling cascade leading to eosinophil apoptosis was deduced from the ability of tyrosine kinase blockers to inhibit Fas-mediated apoptosis. As an initial control, we determined the effect of tyrosine kinase blockers on anti-Fas MoAb-induced levels of tyrosine phosphorylation. Eosinophils were pretreated with the inhibitors for 1 hour and then stimulated for the indicated times with anti-Fas MoAb. Genistein at 20 μg/mL and lavendustin A at 16 μg/mL prevented the inducible tyrosine phosphorylation of proteins after Fas receptor activation, suggesting that both compounds indeed inhibit tyrosine kinases in human eosinophils (data not presented).
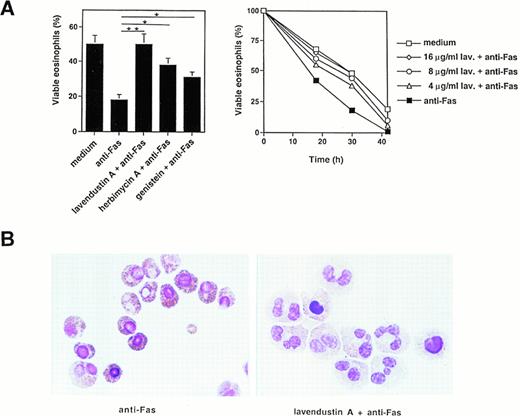
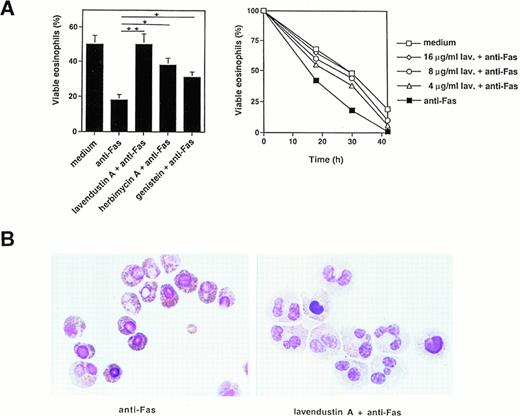
We then measured the effect of several tyrosine kinase inhibitors on Fas-mediated cell death and apoptosis. Pretreatment of freshly isolated human eosinophils with lavendustin A for 1 hour inhibited, in a dose-dependent manner, the death effect of anti-Fas MoAb treatment in these cells (Fig 3A, right panel). Optimal concentrations of lavendustin A (16 μg/mL) completely abrogated the specific cell death initiated by Fas receptor cross-linking (Fig 3A, left and right panel). Furthermore, the tyrosine kinase blockers genistein (20 μg/mL) and herbimycin A (4 μg/mL) inhibited Fas-mediated cell death by approximately twofold (Fig 3A, left panel). This inhibitory effect did not go beyond these values if higher concentrations of genistein or herbimycin A were used (data not presented). In addition, no cytotoxic effect of lavendustin A and genistein (up to 50 μg/mL) or herbimycin A (up to 20 μg/mL) was observed (data not presented).
Tyrosine kinase blockers inhibit Fas receptor-mediated apoptosis in human eosinophils. (A) (Left panel) Freshly isolated eosinophils were preincubated with lavendustin A (16 μg/mL), genistein (20 μg/mL), or herbimycin A (4 μg/mL) and then stimulated with anti-Fas MoAb for 30 hours. Lavendustin A completely, and the other two kinase blockers partially, abrogated Fas receptor-mediated eosinophil death. *P < .05; **P < .01. (Right panel) Dose-dependent effect of lavendustin A in a time course experiment. (B) Fas receptor-mediated cell death in human eosinophils is due to apoptosis. Untreated or lavendustin A-pretreated eosinophils were stimulated with anti-Fas MoAb. Cells were stained with Giemsa-May-Grünwald (Diff-Quik). Apoptotic eosinophils are characterized by reduced cell volume as well as nuclear condensation and fragmentation. The lavendustin A-pretreated cell populations demonstrated less apoptosis. Data are from six independent experiments.
Tyrosine kinase blockers inhibit Fas receptor-mediated apoptosis in human eosinophils. (A) (Left panel) Freshly isolated eosinophils were preincubated with lavendustin A (16 μg/mL), genistein (20 μg/mL), or herbimycin A (4 μg/mL) and then stimulated with anti-Fas MoAb for 30 hours. Lavendustin A completely, and the other two kinase blockers partially, abrogated Fas receptor-mediated eosinophil death. *P < .05; **P < .01. (Right panel) Dose-dependent effect of lavendustin A in a time course experiment. (B) Fas receptor-mediated cell death in human eosinophils is due to apoptosis. Untreated or lavendustin A-pretreated eosinophils were stimulated with anti-Fas MoAb. Cells were stained with Giemsa-May-Grünwald (Diff-Quik). Apoptotic eosinophils are characterized by reduced cell volume as well as nuclear condensation and fragmentation. The lavendustin A-pretreated cell populations demonstrated less apoptosis. Data are from six independent experiments.
We next investigated whether the observed cell death was apoptosis. Using analysis of the eosinophil morphology, we demonstrate that the type of cell death induced by activation of the Fas receptor is apoptotic and that lavendustin A inhibits Fas-mediated apoptosis (Fig3B). Apoptotic eosinophils are characterized by several features of apoptosis, including condensation of nuclear chromatin and reduced cell volume.36 Fas-induced apoptosis was visible 8 hours after Fas receptor stimulation (Fig 3B).
Tyrosine kinase blockers inhibit Fas receptor-mediated cell death in mouse eosinophils in vitro and resolution of eosinophilic inflammation of the airways in vivo.
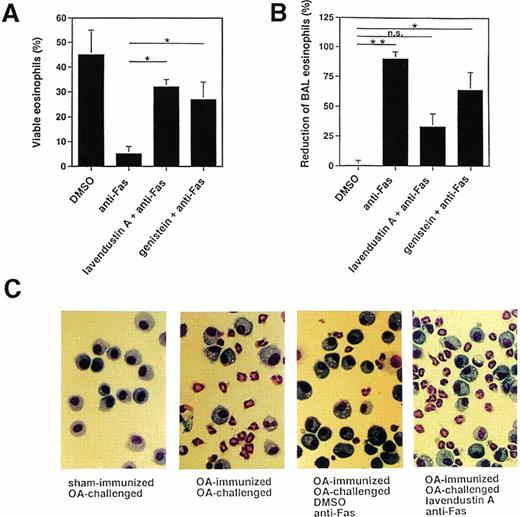
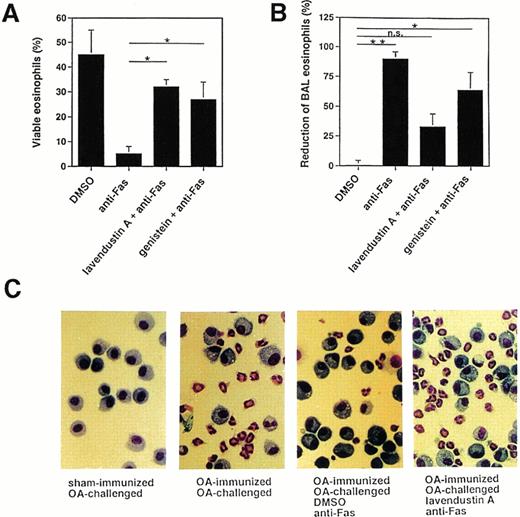
Because there are some discrepancies among the various reports regarding the role of tyrosine phosphorylation in Fas receptor signaling,15-26 we also investigated the effects of tyrosine kinase inhibitors on Fas receptor-mediated cell death in mouse eosinophils. As shown in Fig 4A, pretreatment of freshly purified mouse BAL eosinophils with 16 μg/mL lavendustin A or 20 μg/mL genistein significantly inhibited the death induced by anti-Fas MoAb treatment, suggesting that tyrosine kinase activation is also involved in the death process after activation of the Fas receptor in mouse eosinophils.
Tyrosine kinase blockers inhibit Fas receptor-mediated apoptosis in mouse eosinophils in vitro and in vivo. (A) Freshly purified BAL eosinophils were preincubated with lavendustin (16 μg/mL) or genistein (20 μg/mL) and then stimulated with anti-Fas MoAb for 24 hours. Both blockers significantly inhibited Fas receptor-mediated death. Data are from a total of 45 animals. *P < .05. (B) OA-immunized mice were challenged with antigen to cause lung eosinophilia. The lungs of these mice were pretreated with tyrosine kinase blocker for 1 hour and then with anti-Fas MoAb via the intranasal route. BAL was performed 24 hours later, and the numbers of eosinophils were determined. Anti-Fas MoAb-treated mice demonstrated significantly reduced BAL eosinophils. This treatment had no significant effect on BAL eosinophil numbers when mice were pretreated with lavendustin A. *P < .05; **P < .01. (C) Examples for BAL cytology showing disappearance of eosinophils as a consequence of anti-Fas MoAb treatment and abrogation of this effect by lavendustin A. Data are from 6 to 8 animals per condition.
Tyrosine kinase blockers inhibit Fas receptor-mediated apoptosis in mouse eosinophils in vitro and in vivo. (A) Freshly purified BAL eosinophils were preincubated with lavendustin (16 μg/mL) or genistein (20 μg/mL) and then stimulated with anti-Fas MoAb for 24 hours. Both blockers significantly inhibited Fas receptor-mediated death. Data are from a total of 45 animals. *P < .05. (B) OA-immunized mice were challenged with antigen to cause lung eosinophilia. The lungs of these mice were pretreated with tyrosine kinase blocker for 1 hour and then with anti-Fas MoAb via the intranasal route. BAL was performed 24 hours later, and the numbers of eosinophils were determined. Anti-Fas MoAb-treated mice demonstrated significantly reduced BAL eosinophils. This treatment had no significant effect on BAL eosinophil numbers when mice were pretreated with lavendustin A. *P < .05; **P < .01. (C) Examples for BAL cytology showing disappearance of eosinophils as a consequence of anti-Fas MoAb treatment and abrogation of this effect by lavendustin A. Data are from 6 to 8 animals per condition.
We have previously reported that allergen provocation of OA-immunized mice results in eosinophil infiltration of the lungs 72 hours later and that activation of the Fas receptor by anti-Fas MoAb leads to eosinophil apoptosis and the resolution of eosinophilic inflammation.11 Using this in vivo model, 72 hours after allergen provocation, the same tyrosine kinase inhibitors used in the in vitro systems were administered to the lungs via the intranasal route. Control mice received an equal volume of DMSO. One hour after inhibitor administration, anti-Fas or control Ab was administered to the lungs. BAL was performed 24 hours later and the cellular composition determined. As shown in Fig 4B and C (and previously reported11), anti-Fas MoAb treatment resulted in a resolution of the eosinophilic lung inflammation in control mice that had received DMSO. This effect has been shown to be due to the induction of eosinophil apoptosis.11 There was no change in the number of BAL eosinophils after control Ab treatment compared with before Ab administration (not presented). Moreover, pretreatment of the mice with 10 μg of tyrosine kinase inhibitor for 1 hour prevented, at least partially, the reduction of BAL eosinophil numbers induced by anti-Fas MoAb administration (Fig 4B and C). Thus, these data suggest that tyrosine kinase inhibitors exert similar blocking effects on Fas receptor-mediated cell death in human and mouse eosinophils.
Tyrosine kinase activation is required for Fas receptor-mediated lamin protease(s) and JNK activation in human eosinophils.
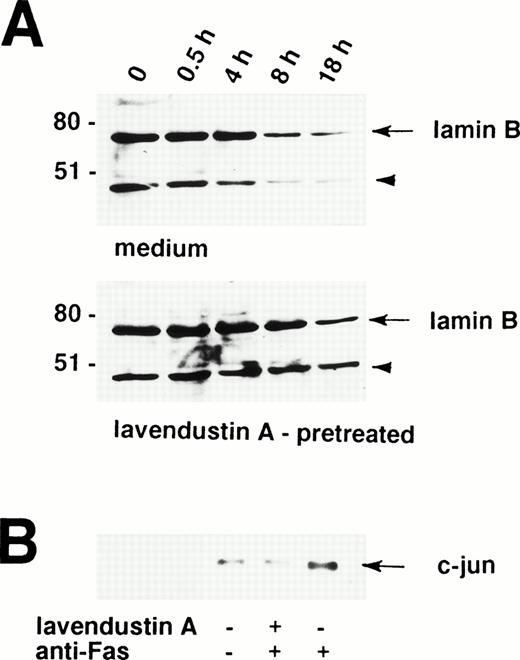
A critical event in the Fas receptor-mediated apoptotic program seems to be the activation of ICE-like proteases.37,38 The proteolytic cleavage of the nuclear lamins by ICE family members facilitates the nuclear events of apoptosis.39,40 For example, lamin cleavage seems to be required for packaging of the condensed chromatin into apoptotic bodies.41 Therefore, lamin cleavage is important for the nuclear events of apoptosis and constitutes an indirect marker for the activation of ICE family proteases.41,42 Because lavendustin A completely blocked the death signal via the Fas receptor in human eosinophils, we investigated the role of tyrosine kinases in ICE activation. To measure activation of ICE family proteases in lavendustin A-pretreated and untreated cells, we assessed Fas receptor-mediated proteolytic cleavage of lamin B. Lamin B is cleaved into a fragment of 46 kD, which can be visualized by Western blotting.39
As shown in Fig 5A, partial lamin B proteolysis is observed in freshly isolated human eosinophils, as demonstrated by a clearly visible 46-kD fragment. This implies that ICE proteases are active in eosinophils to facilitate spontaneous apoptosis. Treatment of human eosinophils with anti-Fas MoAb was followed by increased cleavage of lamin B clearly visible 8 hours upon stimulation (Fig 5A, upper panel). In contrast, when these cells were pretreated with lavendustin A before the anti-Fas MoAb stimulation, increased lamin B degradation did not occur before 18 hours (Fig 5A, lower panel). Moreover, as analyzed by densitometry, approximately 70% to 75% (compared with freshly isolated cells) of the lamin B was degraded in Fas receptor-activated eosinophils which have not been pretreated, whereas lamin B reduction was only about 20% to 25% in lavendustin A-pretreated cells after 18 hours (Fig 5A). In addition, increased lamin B degradation in untreated and lavendustin A-treated eosinophils also did not occur before 18 hours in culture (data not presented), indicating that lavendustin A prevents Fas receptor-mediated but not spontaneous lamin B cleavage in these cells.
Increased proteolytic cleavage of lamin B and phosphorylation of c-jun after stimulation of the Fas receptor are tyrosine kinase-dependent events in human eosinophils. (A) Freshly isolated lavendustin A (16 μg/mL) -pretreated and untreated eosinophils were stimulated with anti-Fas MoAb for the indicated times. The cell lysates were analyzed by immunoblotting with an antilamin B MoAb. A proteolytic fragment of lamin B (arrowhead) was already present in freshly purified eosinophils. After 8 hours, a reduction in the amount of lamin B was observed in untreated but not in lavendustin A-pretreated cells. The intensity (od-Bkg/mm2) of the lamin B signal was analyzed by densitometry. (Upper panel) 1,787 (100%), 1,626 (91%), 1,881 (105%), 775 (43%), and 496 (28%). (Lower panel) 1,459 (100%), 1,551 (106%), 1,375 (94%), 1,618 (111%), and 1,156 (79%). The position of molecular size standards (left). (B) Lavendustin A abrogated phosphorylation of c-Jun. Freshly isolated lavendustin A (16 μg/mL) -pretreated and untreated eosinophils were stimulated with anti-Fas MoAb for 60 minutes. The cell lysates were immunoprecipitated with an anti-JNK Ab, and phosphorylation of GST-c-Jun (1-79) was observed by an in vitro kinase assay. No increase in phosphorylation of c-Jun was found in lavendustin A-pretreated eosinophils, suggesting that tyrosine kinase(s) activation is essential for Fas receptor-mediated JNK activation. All data are from at least three independent experiments.
Increased proteolytic cleavage of lamin B and phosphorylation of c-jun after stimulation of the Fas receptor are tyrosine kinase-dependent events in human eosinophils. (A) Freshly isolated lavendustin A (16 μg/mL) -pretreated and untreated eosinophils were stimulated with anti-Fas MoAb for the indicated times. The cell lysates were analyzed by immunoblotting with an antilamin B MoAb. A proteolytic fragment of lamin B (arrowhead) was already present in freshly purified eosinophils. After 8 hours, a reduction in the amount of lamin B was observed in untreated but not in lavendustin A-pretreated cells. The intensity (od-Bkg/mm2) of the lamin B signal was analyzed by densitometry. (Upper panel) 1,787 (100%), 1,626 (91%), 1,881 (105%), 775 (43%), and 496 (28%). (Lower panel) 1,459 (100%), 1,551 (106%), 1,375 (94%), 1,618 (111%), and 1,156 (79%). The position of molecular size standards (left). (B) Lavendustin A abrogated phosphorylation of c-Jun. Freshly isolated lavendustin A (16 μg/mL) -pretreated and untreated eosinophils were stimulated with anti-Fas MoAb for 60 minutes. The cell lysates were immunoprecipitated with an anti-JNK Ab, and phosphorylation of GST-c-Jun (1-79) was observed by an in vitro kinase assay. No increase in phosphorylation of c-Jun was found in lavendustin A-pretreated eosinophils, suggesting that tyrosine kinase(s) activation is essential for Fas receptor-mediated JNK activation. All data are from at least three independent experiments.
Besides ICE family proteases, we also searched for other possible molecules involved within the signaling cascade transduced by cytoplasmic tyrosine kinases. Previously published work has suggested that JNK is a Fas receptor-activated kinase.23,32,43 44Therefore, phosphorylation of GST-c-Jun (1-79) was used to measure JNK activity by an immune-complex assay. As shown in Fig 5B, anti-Fas MoAb stimulation of eosinophils for 60 minutes induced phosphorylation of c-Jun. In contrast, this increase was abolished by pretreatment with lavendustin A. Together, these data suggest a link between tyrosine kinase(s), lamin protease(s), JNK activation, and apoptosis in Fas receptor signal transduction in freshly purified human eosinophils.
The tyrosine kinase Lyn is involved in Fas receptor-mediated death in human eosinophils.
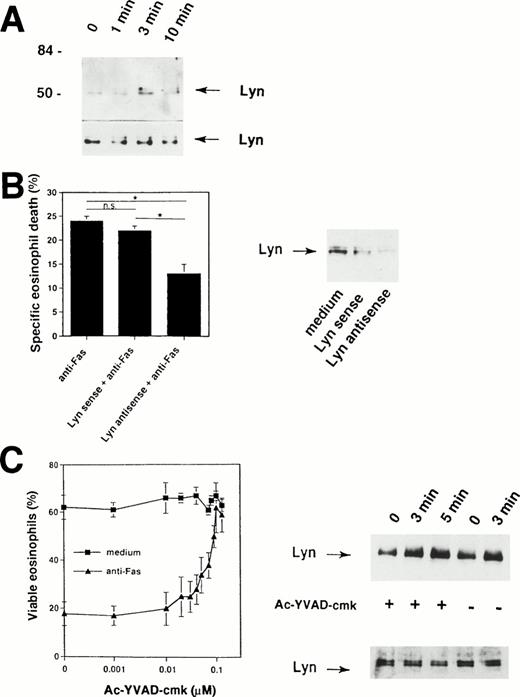
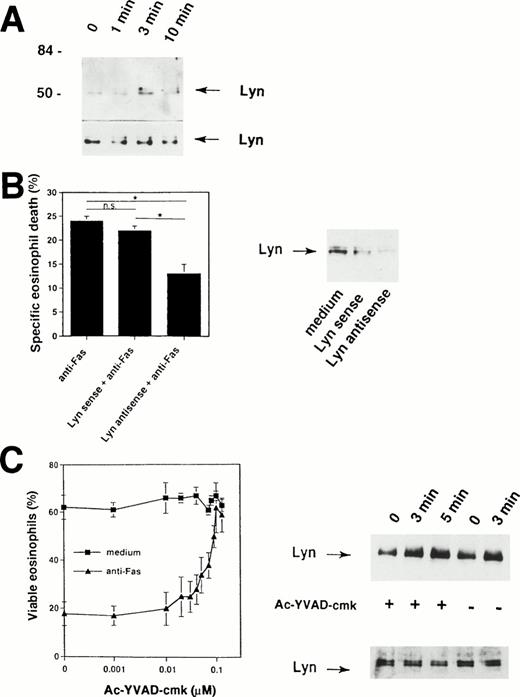
Because the Fas receptor does not contain an intrinsic kinase activity,1,2 our data suggested that cytoplasmic tyrosine kinases are activated rapidly after cross-linking of the Fas receptor. The prominent 56-kD protein that is physically associated with the Fas receptor (Fig 2A) could represent a member of the Src family of tyrosine kinases. It has been previously shown that a member of this family, the tyrosine kinase Lyn, is highly expressed in human eosinophils31,45,46 and neutrophils.33,47 48Therefore, we attempted to determine whether this tyrosine kinase is also involved in the Fas receptor signaling pathway. To do so, Lyn immunoprecipitates were examined. As shown in Fig 6A, Fas receptor stimulation by anti-Fas MoAb induced increases in tyrosine phosphorylation of Lyn within 3 minutes, suggesting activation of Lyn in human eosinophils after initiation of the death signal by receptor cross-linking.
Important role for Lyn tyrosine kinase in Fas receptor-mediated signaling in human eosinophils. (A) Freshly isolated eosinophils were stimulated with anti-Fas MoAb for the indicated times. The cell lysates were immunoprecipitated with an anti-Lyn MoAb and then analyzed by immunoblotting with a MoAb to ptyr, RC20. Increases in tyrosine phosphorylation of Lyn were observed within 3 minutes. The same Lyn immunoprecipitates were used for Western blotting with anti-Lyn MoAb to check for immunoprecipitation efficiency (bottom). The positions of molecular size standards (left). Data are representative of three independent experiments. (B) (Right panel) Eosinophils were cultured in the presence of 10 μmol/L Lyn antisense or sense oligodeoxynucleotides for 6 hours. Decreased Lyn expression in eosinophils cultured with Lyn antisense was observed. Lyn sense oligodeoxynucleotides had no or only little effects on Lyn protein expression. (Left panel) Cells were then stimulated with anti-Fas MoAb for 18 hours. Statistically significant inhibition of Fas receptor-mediated cell death was observed in Lyn antisense but not sense treated eosinophils. Data are from six independent experiments. *P < .05. (C) (Left panel) The ICE inhibitor Ac-YVAD-cmk blocked in a dose-dependent manner Fas receptor-mediated but not spontaneous eosinophils death. Purified eosinophils were cultured for 24 hours. Cell viability was assessed by uptake of 1 μmol/L ethidium bromide and flow cytometric analysis. Values are means ± SEM of three independent experiments. (Right panel) Ac-YVAD-cmk did not prevent activation of Lyn following Fas receptor cross-linking. Freshly isolated Ac-YVAD-cmk (0.1 μmol/L) -pretreated and untreated eosinophils were stimulated with anti-Fas MoAb for the indicated times. The cell lysates were immunoprecipitated with an anti-Lyn MoAb and then analyzed by immunoblotting with a MoAb to ptyr, RC20. Increases in tyrosine phosphorylation of Lyn were observed in both untreated and Ac-YVAD-cmk–treated eosinophils. The same Lyn immunoprecipitates were used for Western blotting with anti-Lyn MoAb to check for immunoprecipitation efficiency (bottom).
Important role for Lyn tyrosine kinase in Fas receptor-mediated signaling in human eosinophils. (A) Freshly isolated eosinophils were stimulated with anti-Fas MoAb for the indicated times. The cell lysates were immunoprecipitated with an anti-Lyn MoAb and then analyzed by immunoblotting with a MoAb to ptyr, RC20. Increases in tyrosine phosphorylation of Lyn were observed within 3 minutes. The same Lyn immunoprecipitates were used for Western blotting with anti-Lyn MoAb to check for immunoprecipitation efficiency (bottom). The positions of molecular size standards (left). Data are representative of three independent experiments. (B) (Right panel) Eosinophils were cultured in the presence of 10 μmol/L Lyn antisense or sense oligodeoxynucleotides for 6 hours. Decreased Lyn expression in eosinophils cultured with Lyn antisense was observed. Lyn sense oligodeoxynucleotides had no or only little effects on Lyn protein expression. (Left panel) Cells were then stimulated with anti-Fas MoAb for 18 hours. Statistically significant inhibition of Fas receptor-mediated cell death was observed in Lyn antisense but not sense treated eosinophils. Data are from six independent experiments. *P < .05. (C) (Left panel) The ICE inhibitor Ac-YVAD-cmk blocked in a dose-dependent manner Fas receptor-mediated but not spontaneous eosinophils death. Purified eosinophils were cultured for 24 hours. Cell viability was assessed by uptake of 1 μmol/L ethidium bromide and flow cytometric analysis. Values are means ± SEM of three independent experiments. (Right panel) Ac-YVAD-cmk did not prevent activation of Lyn following Fas receptor cross-linking. Freshly isolated Ac-YVAD-cmk (0.1 μmol/L) -pretreated and untreated eosinophils were stimulated with anti-Fas MoAb for the indicated times. The cell lysates were immunoprecipitated with an anti-Lyn MoAb and then analyzed by immunoblotting with a MoAb to ptyr, RC20. Increases in tyrosine phosphorylation of Lyn were observed in both untreated and Ac-YVAD-cmk–treated eosinophils. The same Lyn immunoprecipitates were used for Western blotting with anti-Lyn MoAb to check for immunoprecipitation efficiency (bottom).
To examine the specific role of Lyn in Fas receptor-mediated eosinophil death, we decreased the level of Lyn expression by antisense oligodeoxynucleotides. Antisense oligodeoxynucleotides corresponding to the Lyn tyrosine kinase have been previously used to inhibit Lyn protein expression in eosinophils,31neutrophils,33 and B-lineage lymphoma cells.49As previously reported31 33 and demonstrated in Fig 6B (right panel), eosinophils exposed to 10 μmol/L of phosphorothioate-derivatized Lyn antisense oligodeoxynucleotides for 6 hours expressed reduced Lyn protein, whereas Lyn sense oligodeoxynucleotides did not significantly alter Lyn protein levels.
The ability of antisense oligodeoxynucleotides to specifically decrease the expression of Lyn allowed exploration of the role of Lyn in Fas receptor-mediated cell death of eosinophils. Lyn antisense and sense oligodeoxynucleotides were added to freshly isolated human eosinophils 6 hours before the cells were exposed to anti-Fas MoAb. As shown in Fig6B (left panel), Lyn antisense oligodeoxynucleotides significantly reduced the ability of anti-Fas MoAb to induce eosinophil death. In contrast, Lyn sense oligodeoxynucleotides failed to inhibit Fas receptor-mediated death. These data suggest a role for Lyn in the apoptotic signaling pathway induced by activation of the Fas receptor in human eosinophils.
We next investigated whether ICE-like proteases activation is required for tyrosine kinase, in particular Lyn, activation. As demonstrated in Fig 6C (left panel), the ICE-inhibitor II Ac-YVAD-cmk completely abrogates Fas receptor-mediated death in human eosinophils. However, this inhibitor did not block tyrosine phosphorylation of Lyn after engagement of the Fas receptor (Fig 6C, right panel). These data suggest that Lyn activation does not depend on activation of ICE-like proteases.
DISCUSSION
Allergic diseases of the respiratory tract are associated with a marked eosinophilic inflammation.50-52 Several groups have studied the mechanisms of this eosinophil accumulation. For example, mechanisms of selective recruitment in tissue sites of allergic diseases have been suggested based on the identification of specific eosinophil chemotactic factors53-55 or on specific eosinophil adhesion to cytokine-activated endothelial cells.56-61 Moreover, cytokines such as interleukin-3 (IL-3), IL-5, granulocyte-macrophage colony-stimulating factor (GM-CSF), or interferon-γ (IFN-γ) dramatically increase the life-span of purified eosinophils by inhibiting their apoptotic cell death in vitro.29,31,62-67Furthermore, delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia has been recently demonstrated in nasal polyp tissues,68 suggesting that this process also occurs in vivo under certain pathological conditions. Thus, besides specific eosinophil adhesion and chemotaxis, inhibition of eosinophil apoptosis also contributes to the eosinophilia observed in patients with chronic eosinophilic disorders.
Besides cytokines, eosinophil apoptosis also seems to be regulated by at least one member of the TNF/NGF receptor superfamily, namely the Fas receptor.10-13 Cross-linking of the Fas receptor is associated with the induction of apoptosis in eosinophils even in the presence of eosinophil hematopoietins.10,11 These studies suggested that eosinophil apoptosis does not only occur passively after the withdrawal or absence of eosinophil survival factors, but can be actively induced via activation of the Fas receptor. Although it is still not clear whether cognate interactions between eosinophils and other cells expressing Fas ligand actually occur in vivo, it has been shown that eosinophils from eosinophilic donors often do not express functional Fas receptors, although Fas protein is normally expressed in these cells.13 Therefore, it is important to identify the signaling pathways that are transduced via the Fas receptor in eosinophils and to unravel the molecular mechanisms by which Fas receptor-mediated apoptosis can be inhibited.
Recently, there has been some progress in the understanding of the signal transduction pathways activated via the Fas receptor in cell lines expressing functional Fas receptors. A series of intracellular proteins (CAPs) inducibly coimmunoprecipitate with the Fas receptor to form a death-inducing signaling complex (DISC).69 CAP1 and 2 have been identified and represent FADD/MORT1.69-71Molecular characterization of CAP4 demonstrated that the molecule is an ICE-like protease, FLICE/MACH.72,73 Proteases of the ICE family play a key role within a protease cascade responsible for the structural changes of apoptosis.37 38 The identification of FLICE as a member of the DISC suggests a direct pathway where Fas receptor activation leads to triggering of the protease cascade.
In the present study, we have demonstrated that tyrosine phosphorylation is an important event involved in transmembrane signal transduction via the Fas receptor in human and mouse eosinophils. The Fas receptor is physically associated with a number of tyrosine-phosphorylated proteins, as shown by coimmunoprecipitation studies with Fas receptor. Also, the Fas receptor coimmunoprecipitated with tyrosine phosphorylated proteins, confirming this link. These data are in agreement with previously published work demonstrating increased tyrosine phosphorylation after Fas receptor cross-linking in several cellular systems.15,16,19,20 Moreover, phosphorylation of both tyrosine residues within the intracellular part of the human Fas receptor has been recently demonstrated.19 Interestingly, these two tyrosine residues are also present in the amino acid sequence predicted for the murine Fas receptor cDNA,2 consistent with the suggestion that these tyrosine residues are important for signal transduction.
Tyrosine kinase activation was also reported in response to stimulation of other members of the TNF/NGF receptor family. Extensive work has elucidated a role for tyrosine phosphorylation via CD40, a molecule that also belongs to this family of receptors and induces apoptosis under certain conditions.74 CD40 activation results in increases in tyrosine phosphorylation as well as activation of Lyn, Syk, Fyn, and Blk in B cells.75-77 Increased tyrosine phosphorylation has also been observed in TNF-treated neutrophils78 and endothelial cells.79Therefore, tyrosine phosphorylation appears to play a major role in Fas, TNF, and CD40 receptor-mediated transmembrane signaling.
Because activation of the Fas receptor stimulates eosinophil apoptosis10-13 and increases tyrosine phophorylation in human eosinophils (Figs 1, 2, and 6), we examined the effect of tyrosine kinase inhibitors on Fas receptor-mediated cell death. Because a direct involvement of tyrosine phosphorylation in Fas receptor-induced apoptosis is still controversial,20-23 we performed these experiments in both human and mouse systems. Among the tyrosine kinase blockers tested in this study, lavendustin A had the highest potency to inhibit Fas receptor-mediated death in both cultured human blood eosinophils and mouse lung eosinophils. To complement our studies of purified cell populations, we used an in vivo approach analyzing eosinophilic lung inflammation induced by allergen exposure in mice.11 Administration of anti-Fas MoAb resulted in a significant reduction in the number of eosinophils in the lung after 24 hours. In contrast, if the mice were pretreated with tyrosine kinase inhibitor, the resolution of eosinophilic inflammation was less prominent. Moreover, in this in vivo system, we also observed a similar potency of tyrosine kinase inhibitors compared with the in vitro experiments (lavendustin A > genistein). Taken together, these results strongly implicate tyrosine kinase activation as likely involved in the death response after cross-linking of the Fas receptor in eosinophils.
Recent studies suggested that there is a direct physical connection between the Fas receptor and the basal death machinery, the proteases of the ICE family.44,69-73 To determine whether tyrosine kinase activation contributes to the protease cascade initiated by receptor cross-linking, we observed the effects of inhibition of tyrosine kinases on lamin B cleavage. Lamins represent key substrates for ICE family proteases activated during the apoptotic process.39-42 Therefore, lamin B cleavage was used in this study as a marker to measure Fas receptor-mediated activation of ICE family members. We observed increased cleavage of lamin B in eosinophils after Fas receptor stimulation. This increased cleavage was prevented by lavendustin A, suggesting that tyrosine kinase(s) activation may be a prerequisite for full activation of the cascade of ICE family proteases.
Besides activation of tyrosine kinases and ICE family proteases, multiple other signaling events have been reported to be involved in Fas receptor-mediated apoptosis. For example, another signaling pathway may involve activation of Ras16 and, consequently, as demonstrated in this study, activation of JNK. Because lavendustin A completely blocked Fas receptor-mediated apoptosis, it is possible that amplification of the ICE family proteases cascade via tyrosine kinases, Ras, and JNK is essential for the apoptotic process in eosinophils. This assumption is further supported by very recent observations demonstrating that activation of CPP32-like proteases occurs distal to MAPK and JNK activation.80,81 Furthermore, it has been demonstrated that JNK activation correlates with cell death.23,32,43,44,80 82-84
The demonstration of tyrosine phosphorylation of the Fas receptor,19 which does not itself contain an intrinsic kinase activity,1,2 suggests that a cytoplasmic tyrosine kinase is associated with the receptor. The prominent tyrosine-phosphorylated 56-kD protein that coimmunoprecipitated with the Fas receptor provides evidence that at least one member of the Src family of tyrosine kinases may associate with, and act as signal tranducer for, this death receptor. Lyn has been previously demonstrated to be highly expressed in both neutrophils33,47,48 and eosinophils.31,45,46 To characterize the role of Lyn, Lyn immunoprecipitates were examined to assay tyrosine phosphorylation of the kinase. After exposure to anti-Fas MoAb, we observed a rapid increase in Lyn tyrosine phosphorylation, suggesting activation of this kinase. To specifically examine the possibility that Lyn participates in the death pathway after Fas receptor activation in eosinophils, we determined the effect of decreasing the level of expression of Lyn in this system. In agreement with earlier studies in granulocytes,31,33 Lyn antisense oligodeoxynucleotides significantly reduced Lyn protein expression after 6 hours. Similar to activated B cells from Lyn-deficient mice,18 we observed a reduced susceptibility to Fas receptor-mediated death in eosinophils partially lacking Lyn. These data suggest that Lyn is involved in the transmembrane death-signaling cascade via the Fas receptor in eosinophils.
Previous studies have suggested that Lyn is required for the prevention of apoptosis by cytokines in both eosinophils31 and neutrophils.33 Thus, Lyn emerges as a signaling molecule capable of inducing two mutually exclusive cellular functions, cell survival and cell death. Similar observations have been previously reported for other signaling molecules. For example, ceramide, a second messenger of the sphingomyelin pathway, can induce both mitogenesis and apoptosis.85 Moreover, a number of growth factors are known to have antiapoptotic effects on cells and many of them, including IL-5 in eosinophils, signal via Ras.45,46,86,87 However, activation of Ras was also seen in apoptotic pathways initiated by lymphokine deprivation88 or Fas receptor activation.16 Furthermore, not only signaling molecules but also cell surface receptors, including the Fas receptor itself, can signal both proliferation and apoptosis.89 Our results suggest that Lyn may have a similar ability to influence both cell survival and cell death pathways. However, it is currently unclear under which conditions Lyn activation is an antiapoptotic or proapoptotic event. A recent study provided evidence that both activation of the Lyn-Ras-Raf-1-MAP kinase and Jak-STAT pathways are obligatory events for cytokine-mediated delayed eosinophil apoptosis.90 Therefore, Lyn might only be able to facilitate proapoptotic activities under conditions in which Jak-STAT activation does not occur. Although further studies are needed to completely explain the striking phenomenon of transduction of both antiapoptotic and proapoptotic signals by Lyn, the data in this study further support an important role for this tyrosine kinase as a common element responsible for signal transduction after granulocyte stimulation with widely different agonists.48
Although it is clear that Fas receptor activation results in increased tyrosine phosphorylation, a requirement for tyrosine kinase activation for Fas receptor-mediated apoptosis is controversial, especially in T cells.20-23 In contrast, as this study demonstrates, tyrosine phosphorylation appears to modulate the functional death response not only in eosinophils but also in B cells18 and neutrophils.17 It has been suggested earlier that the efficacy of the death signal mediated by the Fas receptor may be dependent on a critical level of surface receptor expression.91 Because the level of Fas receptor surface expression is very low in eosinophils and neutrophils compared with T cells,13 it is possible that, in granulocytes, tyrosine kinase activation is required for optimal signal transduction via the Fas receptor. In contrast, an optimal interaction between the receptor and second messenger molecules might already be present in activated T cells and, therefore, the activation of tyrosine kinases might not be functionally relevant in these systems. Thus, in this model, the role of tyrosine kinase activation could be to decrease the threshold of needed receptor molecules per cell for induction of apoptosis in granulocytes and B cells. This assumption is supported by the fact that tyrosine kinase activation appears to be independent from ICE-like proteases, because an inhibitor of ICE, YVAD, completely blocked Fas receptor-mediated death, but did not abrogate the activation of Lyn in eosinophils.
Regardless of the exact mechanism, tyrosine kinase activation appears to increase the death response after Fas receptor cross-linking in eosinophils. It is still not clear under which circumstances tyrosine kinase activation is necessary or facilitates Fas receptor-mediated apoptosis. However, tyrosine kinase activation seems to be required for the signaling cascade within ICE family proteases after stimulation of the death receptor, at least in some cellular systems such as eosinophils.
ACKNOWLEDGMENT
The authors are grateful to all clinicians in Davos who provided blood samples from patients. We thank P.H. Krammer (Heidelberg, Germany) for affinity-purified anti-Fas MoAb (IgG3, κ) and M. Roth (Basel, Switzerland) for densitometry measurements.
Supported by Grant No. 32-49210.96 from the Swiss National Science Foundation.
Address reprint requests to Hans-Uwe Simon, MD, Swiss Institute of Allergy and Asthma Research, University of Zurich, Obere Strasse 22, CH-7270 Davos, Switzerland; e-mail: hus@siaf.unizh.ch.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.