To the Editor:
In a recent article, Leith et al of the Southwest Oncology Group1 investigated the prognostic value of cytogenetics and MDR1 in patients with previously untreated acute myeloid leukemia (AML; de novo + secondary) and age greater than 55 years. The investigators concluded that AML in the elderly is characterized by an increased frequency of unfavorable karyotypic abnormalities and MDR1 expression, both of which independently contribute to poor outcomes. We would like to focus on some points of this report that, in our opinion, deserve further elucidation and contribute our own results.
(1) In this SWOG trial, patients were presumably selected according to their performance status, but no data are given about noneligible cases. It is essential to verify whether these patients had biological characteristics similar to those actually recruited to rule out selection biases. In our series of 159 consecutive patients greater than 60 years of age with AML, only 101 were deemed suitable for aggressive chemotherapy. It is noteworthy that eligible patients had a median age of 67 years, as compared with a median age of 71 years of those who received conservative or supportive therapy only. On the other hand, the biological characteristics, most notably MDR expression and cytogenetic patterns, did not exhibit significant differences between the two groups.2
(2) To assess whether unfavorable cytogenetics and MDR1 expression really varied with age, the investigators should have compared the frequencies of these variables with those of consecutive individuals less than 55 years of age with AML treated in the same institutions in the same lap of time. In our studies, we found no significant differences in the frequency distribution of the MDR1 phenotype and abnormal karyotypes between the elderly population and younger adults with AML admitted at the S. Eugenio University Hospital of Rome between January 1987 and June 1993. However, when looking at favorable and unfavorable cytogenetic patterns, a significant difference between age groups was observed (P = .002).2 3
(3) Although intrinsic differences in the biology of the disease are important in partly explaining the poorer prognosis observed in the elderly, there is considerable evidence in the literature to suggest that age-related host factors, particularly increased susceptibility to the stress of infectious episodes, play a relevant role.2,4-7 In our study, the overall complete remission (CR) rate was 52.3%, decreasing from 65.3% in individuals 60 to 67 years of age to 37.2% in the group 68 to 79 years of age (P = .007), a difference determined essentially by a reduced ability to cope with infections.2 Conversely, in the SWOG study, the major determinant of induction treatment failure was resistant disease. However, it should be underlined that the CR rate (45%) is among the lowest reported in recent trials, being significantly affected by the poor outcome of secondary AMLs. Because most de novo and secondary AMLs share similar features, resistant disease in patients with secondary AMLs may not be determined by abnormal cytogenetics and MDR1 expression alone, but by the presence of other biological abnormalities. This corroborates the notion that secondary AML is a distinct disease entity and that experimental protocols different from those of de novo AML should be adopted.8 9
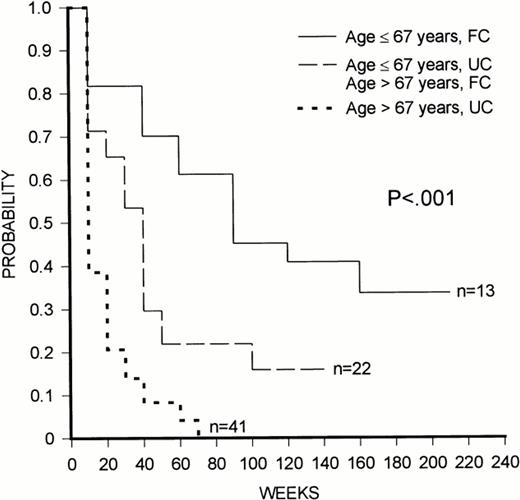
(4) It is relevant that, in the SWOG study, the multivariate analysis showed two independent prognostic factors for overall survival, ie, unfavorable cytogenetics and age, that were also identified in our own series, whereas we did not confirm the prognostic value of the white blood cell count found by the SWOG group. In our study, patients could be stratified into discrete groups with different prognosis according to their age and cytogenetic pattern (Fig 1). Patients ≤67 years of age with favorable cytogenetics had a good prognosis, those either greater than 67 years of age with favorable karyotype or ≤67 years of age with unfavorable karyotype had an intermediate prognosis, whereas individuals with 67 years of age with unfavorable cytogenetics had a poor outlook. Previously, we have also demonstrated that stratification into age groups significantly enhances the prognostic value of the MDR1 phenotype, although the most discriminant cut-off point in that study was age 45 years.10
Kaplan-Meier plot of overall survival duration according to age and cytogenetic pattern. FC, favorable cytogenetics; UC, unfavorable cytogenetics.
Kaplan-Meier plot of overall survival duration according to age and cytogenetic pattern. FC, favorable cytogenetics; UC, unfavorable cytogenetics.
(5) It is notorious that data from the literature are extremely variable and highly dependent on methodological factors. By using two separate primary monoclonal antibodies (C219 and JSB1), the same procedure of cell fixation-permeabilization, and histogram subtraction analysis, cytofluorimetric detection of the multidrug resistance P-glycoprotein varied from 43% (C219) to 73% (JSB1) in 158 patients with newly diagnosed AML.10 This might account for the differences in frequency distribution between the various studies and for the different prognostic impact of this variable. The lower MDR expression in the M4 and M5 FAB categories reported in the SWOG paper is peculiar. In our series, we found strict correlations between C219 negativity and M3 subtype and between JSB1 positivity and M0-M4-M5 subtypes.10 Methodological factors or age-related differences might also explain the considerable discrepancy between the percentage of cases with karyotypic abnormalities (86.6%) in our study2 as compared with the SWOG report (55%).
(6) Although the use of chronological age as a differentiating parameter is controversial and has brought about a whole variety of cut-off points, in a time when allogeneic bone marrow transplantation has been extended to patients up to 60 years of age and high-dose chemotherapy with peripheral blood stem cell or autologous bone marrow support is used in patients aged 60 to 70 years,11 the definition of elderly applied to individuals greater than 55 years of age does not seem entirely appropriate.
In conclusion, AML in elderly patients is not a homogenous disease and careful evaluation of clinical and biological features of single individuals is essential for a judicious treatment planning. Certain subgroups of patients can be defined who are characterized by age, cytogenetics, MDR1 phenotype, and secondary AML, who are likely to fail conventional standard chemotherapy. These patients, as well as those who are not eligible for aggressive chemotherapy because of a poor performance status, may better be served by alternative treatment approaches that have yet to be developed.
Sensitive and Specific Assessment of MDR1 Is Essential to Determine Prognostic Impact in AML
We have read the letter by Stasi et al and would like to offer the following comments to each of the points raised.
(1) The Southwest Oncology Group (SWOG) does not collect data on patients seen at our cooperating institutions who are not entered onto SWOG clinical trials, in this case, SWOG 9031. We are gratified to learn that, according to the experience of Stasi et al, the biologic characteristics (most notably MDR expression and cytogenetic patterns) did not differ between patients receiving induction chemotherapy and those receiving only supportive care.
(2) We are very surprised that Stasi et al did not find a significant difference in the frequency of MDR1 expression between younger versus older AML patients in their single institutional study. We have now examined the incidence of MDR1 expression (using the MDR1-specific antibodies MRK16 and MM4.17) and functional dye efflux (assessing rhodamine efflux and its inhibition by the MDR1-specific inhibitor cyclosporine or PSC833) in more than 1,500 cases of AML using multiparameter flow cytometric techniques in a single reference laboratory. In our experience, the frequency of MDR1 expression and functional drug efflux increases dramatically with age. In our initial study,1-1 we reported an MDR1 incidence of 71% in de novo and 77% in secondary AML cases arising in individuals greater than 55 years of age. These results have now been confirmed in a second ongoing study (SWOG 9333) in which the frequency of MDR1 expression in elderly patients is 73%. In contrast, in both a retrospective (SWOG 8600) and a prospective (SWOG 9500) study of younger AML patients using identical laboratory techniques, we find the incidence of MDR1 expression to be only 25% to 35%.
(3) The lower 45% overall complete remission (CR) rate reported for SWOG 9031 was likely due in part to the inclusion of patients with secondary AML in our study. If we limit our analysis to the type of patients reported by Stasi et al (patients who were 60 to 79 years of age with de novo AML), then we achieved a CR rate of 54%, highly similar to the rate of 52% reported by Stasi et al. Secondly, we agree that older patients may be less able to cope with infection, which is why in SWOG 9031, we studied the use of granulocyte colony-stimulating factor (G-CSF) support after induction chemotherapy. Although G-CSF accelerated myeloid recovery, it did not increase the complete remission (CR) rate. However, it is often difficult to distinguish the cause of remission induction failure and, particularly, to determine if patients who die with infection after prolonged neutropenia would have survived had they had more responsive leukemia and entered into a more rapid CR. For years, physicians have assumed that older patients cannot cope with infection. However, the 81% CR rate seen in SWOG 9031 in elderly patients with leukemia characterized by a lack of MDR1 expression and favorable or intermediate cytogenetics argues that this inability to cope may more reflect the resistant nature of the underlying disease.1-1 Finally, we agree that “… resistant disease in patients with secondary AMLs may not be determined by abnormal cytogenetics and MDR expression alone, but by the presence of other biologic abnormalities.” As indicated in our multiple logistic regression analysis,1-1 a patient with secondary AML has a lower probability of achieving CR than does a patient with de novo AML who is otherwise comparable, ie, who has the same level of MDR1 expression and the same cytogenetic status (favorable, intermediate, or unfavorable). This implies that there must be some other biological mechanism related to disease onset that operates separately from MDR1 and cytogenetics.
(4) We are surprised that Stasi et al failed to find peripheral white blood cell count as a prognostic factor, because in five consecutive SWOG clinical trials, each reporting more than 250 patients, the peripheral white blood cell count was in every case a significant prognostic factor for achievement of CR. The magnitude of the effect of peripheral white blood cell count is not immense; therefore, large numbers of patients may be necessary to see the effect. This may explain why Stasi et al failed to see this relationship.
(5) We completely agree that many studies of the incidence and clinical significance of MDR1 expression in leukemia (and other cancers) previously reported in the literature are fraught with numerous methodologic problems and frequently lack sufficient sensitivity and specificity. In particular, the use of the C219 antibody as reported by Stasi et al and many others is extremely problematic, because this antibody lacks specificity for MDR1 detection.1,2 The C219 antibody cross-reacts with the related MDR2 gene and other unrelated cytoplasmic epitopes. In fact, we have found that C219 will frequently stain more differentiated myelomonocytic leukemias that in fact lack MDR1 transcripts using specific reverse transcription-polymerase chain reaction techniques. Thus, for the most specific assessment of MDR1, it is essential to use only MDR1-specific antibodies and to correlate MDR1 protein expression with a functional assay.1-3 1-4 This specific and sensitive approach has been used in all of our laboratory assays, and we consistently detect lower levels of MDR1 expression in FAB M4 and M5 AML cases as compared with the FAB M0, M1, and M2 subgroups.
(6) We whole-heartedly agree with Stasi et al that the definition of patients greater than 55 years of age as elderly is inappropriate, particularly as our own age increases. However, the median age of our patients registered to SWOG 9031 was 68 years.