Abstract
The receptors for interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-5 share a common signaling subunit βc. However, in the mouse, there is an additional IL-3 signaling protein, βIL-3, which is specific for IL-3. We have previously reported that IL-3 abrogates the lymphoid potentials of murine lymphohematopoietic progenitors and the reconstituting ability of hematopoietic stem cells. We used bone marrow cells from βc- and βIL-3–knock-out mice to examine the relative contributions of the receptor proteins to the negative regulation by IL-3. First, we tested the effects of IL-3 on lymphohematopoietic progenitors by using lineage-negative (Lin−) marrow cells of 5-fluorouracil (5-FU)-treated mice in the two-step methylcellulose culture we reported previously. Addition of IL-3 to the combination of steel factor (SF, c-kit ligand) and IL-11 abrogated the B-lymphoid potential of the marrow cells of both types of knock-out mice as well as wild-type mice. Next, we investigated the effects of IL-3 on in vitro expansion of the hematopoietic stem cells. We cultured Lin−Sca-1–positive, c-kit–positive marrow cells from 5-FU–treated mice in suspension in the presence of SF and IL-11 with or without IL-3 for 7 days and tested the reconstituting ability of the cultured cells by transplanting the cells into lethally irradiated Ly-5 congenic mice together with “compromised” marrow cells. Presence of IL-3 in culture abrogated the reconstituting ability of the cells from both types of knock-out mice and the wild-type mice. In contrast, addition of GM-CSF to the suspension culture abrogated neither B-cell potential nor reconstituting abilities of the cultured cells of wild-type mice. These observations may have implications in the choice of cytokines for use in in vitro expansion of human hematopoietic stem cells and progenitors.
© 1998 by The American Society of Hematology.
INTERLEUKIN-3 (IL-3) supports the development of multiple hematopoietic lineages by interacting with multipotential and lineage-committed progenitors in culture.1-3 Studies in our laboratory indicated that IL-3, as a single factor supports proliferation of the progenitors after they exit from the cell-cycle dormant state (G0).3,4 IL-3 also synergizes with IL-6,5 IL-11,6,7 granulocyte colony-stimulating factor (G-CSF),8 leukemia inhibitory factor,9 thrombopoietin (TPO),10,11 and steel factor (SF, c-kit ligand)12 13 in triggering cell divisions of the multipotential progenitors in G0.
In contrast to the positive regulation of myeloid lineages, IL-3 seems to exert negative effects on the early stages of lymphopoiesis. In our laboratory, we have established a two-step methylcellulose culture assay for murine lymphohematopoietic progenitors and characterized their cytokine requirement.14 SF-based cytokine combinations supported the proliferation and differentiation of lymphohematopoietic progenitors whereas addition of IL-3 to the permissive cytokine combinations abrogated the B-lymphoid potential of the progenitors.15 We subsequently observed that T-cell16 and natural killer–cell17 potential of the progenitors is also inhibited by IL-3. These observations raised the possibility that IL-3 may be a stage-specific negative regulator and that it may suppress the earliest process of hematopoiesis, ie, self-renewal of the stem cells. This hypothesis was confirmed later by our observation that IL-3 abrogates reconstituting ability of hematopoietic stem cells with long-term engraftment capability.18
Both mouse and human IL-3 receptors are heterodimers consisting of α and β subunits.19-22 The high-affinity receptors for human IL-3, granulocyte-macrophage CSF (GM-CSF), and IL-5 share common β subunit (βc).19-22 The α subunits are specific for each cytokine and bind their ligand with low affinity. Whereas there is only one type of human β subunit, βc, mouse has two closely related β subunits, βc and βIL-3.23-25 They have 56% homology with human βc. Like human βc, mouse βc is the common β subunit of the receptors for mouse IL-3, GM-CSF, and IL-5. Although βIL-3 has extensive sequence homology with mouse βc (91% at the amino acid level), βIL-3 does not form a high-affinity receptor with mouse IL-5 or mouse GM-CSF α receptors. When transfected into mouse T-cell line, CTLL-2, both βc and βIL-3 interacted equally well with the α subunit of mouse IL-3 receptor and transmitted proliferation signals in the presence of IL-3.25 Independently, investigators in two laboratories26-28 reported generation and analysis of the mice lacking either of the two β receptor genes. Mice lacking the βc showed pulmonary alveolar proteinosis-like disease, a phenotype similar to that of GM-CSF–deficient mice.29,30 The number of peripheral blood eosinophils of βc-deficient mice was markedly reduced, and the mice failed to produce eosinophils in response to parasitic infections because of absence of transduction of IL-5 signals.26 The colony-forming ability of the bone marrow cells from βc- or βIL-3–deficient mice was also examined.26-28 Cells from βc-deficient mice did not respond to GM-CSF or IL-5 but responded normally to IL-3. In contrast, cells from βIL-3–deficient mice responded normally to IL-3, GM-CSF, or IL-5. These results clearly show the unique redundancy of mouse IL-3 receptor system, which is not present for the human IL-3 receptor. In this study, we used cells from βc and βIL-3–gene knock-out mice to examine the relative contributions of βc and βIL-3 to the negative regulation of the early B lymphopoiesis and the long-term reconstituting ability of stem cells.
MATERIALS AND METHODS
Cytokines.
Purified recombinant murine IL-3 and GM-CSF were purchased from R&D Systems (Minneapolis, MN). Purified recombinant murine SF was obtained from Immunex (Seattle, WA). Purified recombinant human IL-6 was a gift from M. Naruto of Toray Industries (Kamakura, Japan). Purified recombinant human IL-7 was a gift from Sterling Winthrop Inc (Collegeville, PA). Purified recombinant human IL-11 was a gift from P. Schendel, Genetics Institute (Cambridge, MA). Purified recombinant human TPO was prepared by the Cytokine Production Group of Kirin Brewery (Takasaki, Japan). Recombinant human FLT3/FLK-2 ligand (FL) was provided by S.D. Lyman of Immunex. Purified recombinant human erythropoietin (EPO) was provided by the Genetics Institute Clinical Manufacturing Group (Cambridge, MA). Recombinant human G-CSF was a gift from A. Shimosaka of Kirin Brewery, Co, Ltd. Unless otherwise specified, the concentrations of cytokines used were as follows: IL-3, 10 ng/mL; SF, 100 ng/mL; IL-6, 100 ng/mL; IL-7, 200 U/mL; IL-11, 100 ng/mL; TPO, 100 ng/mL; FL, 100 ng/mL; EPO, 2 U/mL; G-CSF, 100 ng/mL; GM-CSF, 160 ng/mL.
Monoclonal antibodies (MoAbs).
Hybridoma D7 (anti–Ly-6A/E [anti–Sca-1]; rat immunoglobulin G [IgG]2a) was a gift from P. Kincade of Oklahoma Medical Research Foundation (Oklahoma City, OK). MoAb ACK4 (anti–c-kit; rat IgG2a) was provided by S.I. Nishikawa of Kyoto University (Kyoto, Japan). Hybridoma RB6-8C5 (anti-mouse granulocytes; rat IgG2b) was provided by R.L. Coffman of DNAX (Palo Alto, CA). MoAb TER119 (anti-erythrocytes; rat IgG2b) was a gift from T. Kina of Kyoto University. Hybridomas 14.8 (anti-B220; rat IgG2b), M1/70.15.11.5 (anti-macrophages; rat IgG2b), GK1.5 (anti-CD4; rat IgG2b), and 53-6.72 (anti-CD8; rat IgG2a) were purchased from American Type Culture Collection (Rockville, MD). 53-2.1 (Biotin-conjugated-anti-Thy-1.2; rat IgG2a), RA3-6B2 (Biotin-conjugated-anti-CD45R/B220; rat IgG2a), RB6-8C5 (Biotin-conjugated-anti-Gr-1; rat IgG2b), and M1/70 (Biotin-conjugated-anti-Mac-1; rat IgG2b) were purchased from Pharmingen (San Diego, CA). AL1-4A2 (fluorescein isothiocyanate [FITC]-conjugated anti-Ly-5.2; mouse IgG1) and A20-1.7 (FITC-conjugated anti-Ly-5.1; mouse IgG1) were provided by H. Fleming of Emory University.
Cell preparations.
Cells from 10- to 15-week-old male and female βc-deficient,26 βIL-3–deficient,26 and wild-type littermates (C57B1/6)26 were used in clonal cultures and transplantation experiments. 5-fluorouracil (5-FU; Adria Laboratories, Columbus, OH) was administered intravenously through the tail vein at 150 mg/kg body weight, and bone marrow cells were obtained 2 days later. Cells prepared from pooled femurs and tibiae were washed twice and then subjected to density gradient separation by using Nycodenz (Accurate Chemical and Scientific Corp, Westbury, NY) solution. Cells with densities ranging from 1.063 g/mL to 1.077 g/mL were collected.31 Cells reacting to a cocktail of lineage-specific rat MoAbs (RB6-8C5, 14.8, M1/70.15.11.5, GK1.5, TER119, and 53-6.72) were removed twice by using immunomagnetic beads (Dynabeads M-450 coupled to sheep anti-rat IgG; DYNAL, Great Neck, NY). The resulting lineage-negative cells (Lin−) were treated with normal rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) at 20 μg/106 cells to prevent nonspecific binding of MoAbs to Fc receptors, and were then stained with FITC-conjugated rat MoAb D7 (anti-Sca-1)32 and biotin-conjugated rat MoAb ACK4 (anti–c-kit).33 Cells were washed twice before staining with streptavidin-conjugated R-phycoerythrin (PE) (Jackson ImmunoResearch Laboratories). Both FITC-conjugated rat IgG2a and biotin-labeled rat IgG2a (Caltag Laboratories, San Francisco, CA) were used as isotype controls. Sca-1+ c-kit+ cells were collected by sorting on FACS Vantage (Becton Dickinson Immunocytometry Systems).
Two-step methylcellulose culture for lymphohematopoietic progenitors.
Four thousand Lin− cells or 100 Lin−Sca-1+ c-kit+ cells of 5-FU–treated mice were plated in 35-mm suspension culture dishes (Falcon, Lincoln Park, NJ) containing α-medium (ICN, Irvine, CA), 1.2% 1,500-cp methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% (vol/vol) fetal calf serum (FCS) (Intergen, Purchase, NY), 1% deionized Fraction V bovine serum albumin (BSA) (Sigma Chemical, St Louis, MO), 1 × 10−4mol/L 2-mercaptoethanol (2-ME) (Sigma), and designated cytokines. Dishes were incubated at 37°C in a humidified atmosphere flushed with 5% CO2. On the designated day of incubation, 20 colonies were picked, pooled, and washed and 1/20 of the pooled cells were plated in secondary methylcellulose culture containing SF and IL-7. The number of pre–B-cell colonies was counted on day 10 of the secondary culture by using criteria previously described.14
Suspension and clonal cell cultures.
Two hundred Lin− Sca-1+ c-kit+cells were incubated in each well of a 6-well plate (Falcon) in 5 mL suspension culture. The culture medium contained α-medium, 20% (vol/vol) FCS, 1% deionized BSA, 1 × 10−4 mol/L 2-ME, and designated cytokines. On day 7 of incubation, aliquots were analyzed for colony formation and in vivo reconstituting capabilities. Clonal culture was performed in 35-mm suspension culture dishes containing α-medium; 1.2% 1,500-cp methylcellulose; 30% FCS; 1% BSA; and 1 × 10−4 mol/L 2-ME, SF, IL-3, IL-6, FL, TPO, and EPO. Colonies were scored on day 8 of incubation by in situ observation of the plates on an inverted microscope according to the criteria described previously.34 Megakaryocyte colonies were scored when the colony contained four or more megakaryocytes. Abbreviations for colony types are as follows: GM, granulocyte/macrophage colonies; GEM, granulocyte/erythrocyte/macrophage colonies; GMM, granulocyte/macrophage/megakaryocyte colonies; GEMM, granulocyte/erythrocyte/macrophage/megakaryocyte colonies34; Meg, megakaryocyte colonies.
In vivo reconstitution experiments.
In studies of knock-out mice, 10- to 12-week-old male C57Bl/6-Ly-5.1 mice were administered with single 850-cGy total-body irradiation via a 4 × 106 V linear accelerator. After irradiation of the recipient mice, freshly sorted Lin− Sca-1+c-kit+ marrow cells (Ly-5.2 cells) from female wild-type, βc −/−, and βIL-3 −/− mice were injected into the tail vein of the recipients together with 4 × 105“compromised” marrow cells of male C57B1/6-Ly-5.1 mice. “Compromised” cells had been subjected to two previous rounds of transplantation and regeneration in male mice.35 Cells cultured in suspension were also tested for reconstituting capabilities after 7 days' incubation with designated cytokines; all of the cells in each well were injected into male C57B1/6-Ly-5.1 mice together with “compromised” cells. Peripheral blood was obtained from the retro-orbital venous plexus using heparin-coated micropipettes (Drummond Scientific Co, Broomall, PA) 2, 4, and 6 months after transplantation. Red blood cells were lysed by 0.15 mol/L NH4Cl. The samples were then used for flow cytometric analysis of donor-derived cells by staining with FITC-conjugated anti–Ly-5.2 (AL1-4A2). In studies of GM-CSF effects, we used C57B1/6-Ly-5.1 male mice as donors and C57B1/6-Ly-5.2 female mice as recipients. The lineage phenotype of the donor cells at 6 months posttransplantation was determined by staining with biotin-conjugated anti–Thy-1.2, biotin-conjugated anti-CD45R/B220, biotin-conjugated anti–Gr-1, and biotin-conjugated anti–Mac-1. For indirect staining of cells with biotin-conjugated antibodies, cells were first incubated with biotin-conjugated antibodies, then followed by staining with streptavidin-conjugated PE.
RESULTS
Effects of IL-3 on lymphohematopoietic progenitors.
The results of the studies of lymphohematopoietic progenitors are presented in Table 1. Regardless of the origin of the cells, colonies supported by the combination of SF and IL-11 possessed B-cell potential. Addition of IL-3 to the combination of SF and IL-11 strongly inhibited the B-cell potential of the primary colonies of both types of knock-out mice as well as wild-type mice. As we reported previously,14 15 the number of primary colonies was unaffected by IL-3. These results indicated that βc and βIL-3 are redundant regarding signal transduction in negative regulation of early B lymphopoiesis by IL-3.
Effects of IL-3 on expansion of total cells and progenitors.
Next we studied the effects of IL-3 on the expansion of cells and colony-forming cells by plating 200 enriched cells in 7-day suspension culture in the presence of 100 ng/mL SF and 100 ng/mL IL-11 with or without 100 ng/mL IL-3. As shown in Table2, the combination of SF and IL-11 increased the total cell counts by 335- to 600-fold, total colony-forming units (total CFU) by 172- to 402-fold, CFU-GEMM by 26- to 43-fold, and CFU-Meg by 6- to 19-fold, as compared with freshly enriched cells. Addition of IL-3 resulted in about 30-fold enhancement of total cell counts and several-fold increase in the total CFU, CFU-GEMM, and CFU-Meg. These results confirmed that the myelostimulatory effects of IL-3 are transduced by both βc and βIL-3.
Effects of IL-3 on long-term repopulating cells (LTRC).
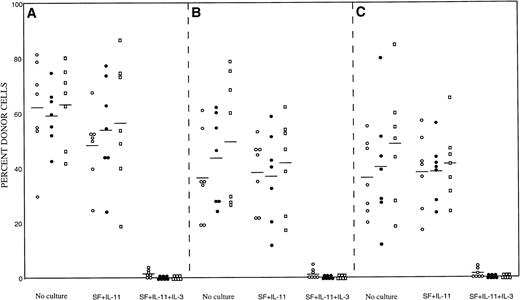
We then tested the in vivo reconstituting ability of the cultured cells. The suspension cultures were initiated with 200 Lin− Sca-1+ c-kit+ cells in the presence of 100 ng/mL SF and 100 ng/mL IL-11 with or without 100 ng/mL IL-3. After 7 days of incubation, cells in a well were obtained and injected into a lethally irradiated Ly-5.1 recipient. As a control group, we also transplanted 200 freshly prepared Lin−Sca-1+ c-kit+ cells from wild-type, βIL-3 −/−, and βc −/− mice. The results of the analyses of peripheral blood nucleated cells are presented in Fig1. The enriched cells from all mice incubated with SF and IL-11 had similar reconstituting ability as freshly prepared cells. In contrast, incubation in the presence of IL-3 significantly reduced the reconstituting abilities of the cells of all types of mice. A few repopulating cells survived in SF, IL-11, and IL-3 at 2 months posttransplantation, as evinced by the appearance of small percentages of donor cells in five, three, and three mice out of a total of seven in Fig 1A, B, and C, respectively. However, reconstitution was absent at 4 and 6 months posttransplantation. These observations confirmed that the negative effects of IL-3 are directed only to LTRC and that the effects are transduced by both βc and βIL-3.
Repopulating abilities of freshly sorted bone marrow cells and cultured cells. (A) Mice transplanted with wild-type cells. (B) Mice transplanted with βIL-3 −/− cells. (C) Mice transplanted with βc −/− cells. (○), 2 months posttransplantation; (•), 4 months posttransplantation; (□), 6 months posttransplantation.
Repopulating abilities of freshly sorted bone marrow cells and cultured cells. (A) Mice transplanted with wild-type cells. (B) Mice transplanted with βIL-3 −/− cells. (C) Mice transplanted with βc −/− cells. (○), 2 months posttransplantation; (•), 4 months posttransplantation; (□), 6 months posttransplantation.
At 6 months posttransplantation, the proportions of donor blood nucleated cells in each of T-cell, B-cell, and myeloid (granulocyte and monocyte/macrophage) compartments were determined (Table3). Multi-lineage cells were detected in the peripheral blood of engrafted recipients. An example of analysis of a mouse transplanted with βc −/− cells cultured with SF and IL-11 is shown in Fig 2.
An example of hematopoietic reconstitution by βc −/− cells cultured with SF and IL-11. Nucleated blood cells of a recipient mouse were analyzed using flow cytometry 6 months after transplantation. Thy-1.2+ cells, B220+cells, and Gr-1+ Mac-1+ cells of donor (Ly-5.2) origin are seen.
An example of hematopoietic reconstitution by βc −/− cells cultured with SF and IL-11. Nucleated blood cells of a recipient mouse were analyzed using flow cytometry 6 months after transplantation. Thy-1.2+ cells, B220+cells, and Gr-1+ Mac-1+ cells of donor (Ly-5.2) origin are seen.
Effects of GM-CSF.
The observation that the βc chain transduces the negative effects of IL-3 on the stem cells and lymphohematopoietic progenitors raised the possibility that βc may also transduce negative signals of GM-CSF. Earlier we reported that, in addition to IL-11, IL-6 and G-CSF can interact with SF in stimulating proliferation of cell cycle dormant progenitors3,12,13 and the lymphohematopoietic progenitors.14,15 In the next two experiments, we tested the effects of GM-CSF and IL-3 in variable concentrations on the progenitors and stem cells using cells from wild-type mice. Specifically, we tested the effects of addition of these cytokines to the cultures supported by SF and one of IL-11, IL-6, and G-CSF. The results are presented in Table 4. As reported previously,14 15 the primary colonies supported by SF plus IL-11, SF plus IL-6, and SF plus G-CSF possessed B-lymphoid potential. Addition of IL-3 at as low as 1 ng/mL concentration completely abrogated the B-cell potential of the primary colonies. In contrast, addition of GM-CSF in concentrates ranging from 10 to 1,000 ng/mL failed to suppress the lymphohematopoietic progenitors.
We then tested the effects of GM-CSF on repopulating abilities of the bone marrow cells. The enriched marrow cells of Ly-5.1 wild-type mice were cultured in suspension for 1 week under permissive cytokine conditions with or without additional GM-CSF and transplanted to lethally irradiated Ly-5.2 recipients. As presented in Table5, GM-CSF did not negatively affect the repopulating abilities of cultured cells.
DISCUSSION
There is significant current interest in hematology/oncology fields regarding in vitro expansion of hematopoietic stem cells and progenitors.36-51 A number of investigators have already shown that it is possible to increase the number of hematopoietic progenitors in culture by using combinations of early-acting cytokines.36-51 Because of the well-known myelopoietic effects of IL-3, the majority of preclinical protocols for murine37-43 and human36,44-51 cells included IL-3. We previously noted negative effects of IL-3 on the ability of cultured cells to engraft the marrow of recipient mice.18Our observation was in agreement with the report from Peters et al52 that suspension culture of murine marrow cells in the presence of IL-3, IL-6, IL-11, and SF results in impairment of the engrafting capability of the cultured cells.
The negative effects of IL-3 observed in murine models may be relevant to in vitro manipulation of human stem cells. Ten patients with advanced cancers were transplanted with peripheral blood progenitors that had been expanded in cultures containing IL-3.53Recently, Williams et al54 reported transplantation of peripheral blood CD34+ cells expanded in liquid culture with PIXY321 (the fusion product of IL-3 and GM-CSF) to 8 patients with advanced breast cancer. No information was provided in these reports regarding long-term effects on the recipient's hematopoiesis. Donahue et al55 studied transduction of glucocerebrosidase genes to primate CD34+ Thy-1+ cells using 7-day culture in the presence of IL-3, IL-6, and SF.55 After autologous transplantation, provirus was detected at all time points in both B-cell and T-cell lineages, but long-term gene transfer was not observed in the granulocyte population.55 It is possible that IL-3 abrogated the long-term reconstitution capability of the cultured primate stem cells.
Both βc and βIL-3 transduced the negative signals of IL-3. βc and βIL-3 have been shown to have redundant functions. Coexpression of one of the two β proteins and IL-3 receptor α protein in an IL-2–dependent mouse T-cell line, CTLL-2, resulted in identical, high-affinity binding for mouse IL-3 and IL-3–dependent proliferation of the cell line.25 Neither βc-null nor βIL-3–null mice showed apparent hematopoietic defects.26 However, careful analysis of the βIL-3–null mice revealed hyporesponsiveness of the hematopoietic progenitors to IL-3, indicating probable quantitative differences between βc and βIL-3.28
Although receptors for IL-3 and GM-CSF share common signal-transducing protein βc, neither lymphohematopoietic progenitors nor long-term reconstituting cells were negatively affected by GM-CSF. These observations are in agreement with the previous reports on the differences between IL-3 and GM-CSF. Earlier, we documented that GM-CSF is significantly weaker than IL-3 in support of murine56and human57 blast cell colony formation. Recently, McKinstry et al58 reported that GM-CSF receptor is not expressed by mouse Rhodamine123loLin−Ly6A/E (Sca-1)+ c-kit+ cells that are highly enriched for long-term repopulating cells. These observations together with our observations presented in this paper indicate that the α protein of GM-CSF receptor is expressed by neither the stem cells nor the most primitive progenitors. Further, these observations may have a significant implication in “in vitro expansion” of human stem cells and progenitors. It is possible that the use of GM-CSF may be preferable to IL-3 in cytokine mixtures because GM-CSF may not abrogate long-term reconstituting stem cells while it supports expansion of some multipotential progenitors.
ACKNOWLEDGMENT
We thank Dr Haiqun Zeng for assistance in cell sorting; Dr Pamela N. Pharr and Anne G. Leary for assistance in preparation of this manuscript; and the staff of Radiation Oncology Department of the Medical University of South Carolina for assistance in irradiation of mice.
Supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs; NIH grants DK32294 and DK/HL48714; and a contribution from Amgen.
Address reprint requests to Makio Ogawa, MD, PhD, Ralph H. Johnson Medical Center, 109 Bee St, Charleston, SC 29401-5799.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.