Abstract
Plasminogen-activator inhibitor type I (PAI-1), the primary inhibitor of urinary-type plasminogen activator, is thought to play an important role in the control of stroma invasion by both endothelial and tumor cells. Using an in vitro angiogenesis model of capillary extension through a preformed monolayer, in conjunction with in situ hybridization analysis, we showed that PAI-1 mRNA is specifically induced in cells juxtaposed next to elongating sprouts. To further establish that PAI-1 expression is induced as a consequence of a direct contact with endothelial cells, coculture experiments were performed. PAI-1 mRNA was induced exclusively in fibroblasts (L-cells) contacting endothelial cell (LE-II) colonies. Reporter gene constructs driven by a PAI-1 promoter and stably transfected into L-cells were used to establish that both mouse and rat PAI-1 promoters mediate apposition-dependent regulation. This mode of PAI-1 regulation is not mediated by plasmin, as an identical spatial pattern of expression was detected in cocultures treated with plasmin inhibitors. Because endothelial cells may establish direct contacts with fibroblasts only during angiogenesis, we propose that focal induction of PAI-1 at the site of heterotypic cell contacts provides a mechanism to negate excessive pericellular proteolysis associated with endothelial cell invasion.
© 1998 by The American Society of Hematology.
A PROTEOLYTIC CASCADE of zymogen activation triggered by plasminogen activation appears to be a fundamental component in many situations of cellular invasion. Urokinase- and tissue-type plasminogen activators (uPA, tPA) convert plasminogen to plasmin, a serin protease capable of degrading (either directly or indirectly through the activation of other zymogens) most of the major components of the extracellular matrix (ECM).1-3 The pericellular nature of uPA-triggered proteolysis is explained by the fact that pro-uPA is converted to its active form upon interaction with a high-affinity cell-surface receptor.4-6 Uneven cellular distribution of uPA receptors (uPAR) may also polarize proteolysis to particular regions of cell-cell contacts or to the leading edge of migrating cells.7-9
Plasminogen activators (PAs) have been implicated as mediators of extracellular proteolysis during angiogenesis. During angiogenesis, quiescent endothelial cells are induced to locally degrade their basement membrane and to form new blood vessels by sprouting into the surrounding stroma. PAs are induced in endothelial cells upon stimulation with angiogenic factors like vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), and a large body of evidence implicates PAs in angiogenesis-associated matrix degradation and acquisition by endothelial cells of invasive properties.10-14
Excessive matrix degradation, however, is incompatible with efficient cellular migration.15 Likewise, the maintenance of a certain degree of ECM integrity is an essential requirement for capillary morphogenesis.16 17 Therefore, means must exist that will protect the stroma from adverse proteolysis during endothelial cell invasion. Natural PA inhibitors (PAIs) are thought to act as natural balancers of PA-mediated pericellular proteolysis.
PA inhibitor type 1 (PAI-1) is a member of the serpin family of protease inhibitors that reacts specifically with tPA and uPA.2,18 PAI-1 is expressed by various types of cells, including endothelial cells.19 PAI-I may accumulate within the tissue environment due to its sequestration and stabilization by the ECM.20-22 Upon binding of PAI-1 to uPAR-bound uPA, the complex (which otherwise is active on the cell surface for several hours) is rapidly internalized and degraded.23 24 Through the clearance of active proteolytic complexes from the surface of invading cells, PAI-1 keeps the extent of ECM degradation in check.
Little is known about the regulation of PAI-1 in vivo. To assign a role for PAI-1 in the restraint of pericellular proteolysis, it is necessary to invoke that PAI-1 is locally induced at the invasion site and to propose a mechanism how a tissue senses invasion and, in response, upregulates PAI-1 expression.
The present study addresses these issues in the context of an in vitro angiogenesis model and heterotypic cell cultures. It provides evidence supporting the supposition that PAI-1 is specifically induced at the invasion site as a result of heterotypic cell contacts.
MATERIALS AND METHODS
Cell cultures.
Cell lines used were mouse endothelial cells derived from lung capillaries (LE-II cells)25 and mouse fibroblasts (L-cells). Cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 4.5 g/L glucose, 2 mmol/L glutamine, 2 μg/mL streptomycin, 20 U/mL penicillin, and 10% fetal calf serum (FCS).
When indicated, the following materials were added to the medium:ε-Amino-n-Caproic Acid (A-2504; Sigma Chemical Company, St Louis, MO), Soybean Trypsin Inhibitor (03-048; Beit Haemek - Biological Industries), Tranexamic Acid (A-6516; Sigma), Trasylol (aprotinin; Bayer), and D-mannose 6-phosphate (M3655; Sigma).
In vitro angiogenesis in aorta explant cultures.
Explants of rat aorta rings (1 mm long) were grown in tissue-culture chamber slides (Lab-Tek) in DMEM supplemented with 4.5 g/L glucose, 2 mmol/L glutamine, 2 mg/mL streptomycin, 20 μ/mL penicillin, and 10% FCS, essentially as described by Diglio et al.26 Outgrowth of aortic cells, mostly muscle cells, during the first 2 weeks was followed by the formation of vascular-like sprouts extending from the aorta segment. Cultures were fixed after 3 weeks and processed for immunohistochemistry and in situ hybridization analysis.
Immunohistochemistry.
Aorta explant cultures were fixed with cold methanol and stained with rabbit anti-human von Willebrand factor (vWF) antibody (A 082;DAKO, Glostrup, Denmark) diluted 1:250 in phosphate-buffered saline (PBS). An immunoperoxidase procedure was used to detect the primary antibody using VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions.
In situ hybridization.
Aorta explant cultures and heterotypic cell cocultures (both seeded directly on glass slides) were subjected to in situ hybridization as previously described.27 DNA fragments used as templates for synthesis of specific complementary RNAs (cRNAs) were a 1.1-kbEcoRI-BglII fragment derived from the coding and 3′ noncoding regions of the mouse uPA cDNA28 and a 2.4 Xho I–EcoRI fragment derived from the coding and 3′ noncoding regions of the mouse PAI-1 cDNA clone mr1.29 cDNAs were subcloned onto the polylinker of a PBS vector (Stratagene, La Jolla, CA) and were linearized by digestion with the appropriate restriction endonuclease to allow synthesis of a35S-labeled cRNA in either the antisense or sense orientation (using T3 or T7 RNA polymerase). The RNA probe was fragmented by mild alkaline treatment before the hybridization step.
Promoter constructs and stable transfectants.
A mouse PAI-1 promoter-LacZ reporter gene was constructed by fusing a 1.3-kb fragment of mouse PAI-1 promoter (excised with HindIII from plasmid pH 1.6 [generously provided by Dr M. Cole, Princeton, NJ]) upstream to a LacZ-coding region (also containing a nuclear localization signal and SV40-derived polyadenylation signal). A control plasmid, composed of LacZ gene driven by a ubiquitous promoter, was constructed by replacing the PAI-1 promoter with a 180-bp-long promoter of the ribosomal protein S1630 (a kind gift of Dr O. Meyuhas, The Hebrew University). A 2.4-kb rat PAI-1 promoter31 and shorter fragments thereof were fused to a CAT reporter gene (generously provided by Dr T.D. Gelehrter, University of Michigan).
L-cells were cotransfected with the test plasmid and a plasmid encoding a neomycin-resistance, using the poly-L-ornithine/dimethylsulfoxide (DMSO) method. Stably tranfected clones were selected for use in coculture experiments with LE-II cells. Cells expressing β-Galactosidase (β-Gal) activity were identified using the substrate X-gal (5-bromo-4-chloro-3-indolyl-β-galactoside). Chloramphenicol acetyltransferase (CAT) activity in cell extracts was determined by a standard procedure using a14C-chloramphenicol substrate, resolution of products by silica gel chromatography, and relative quantification of reaction products using a Fuji BAS 1000 image analyzer (Fuji).
Staining of fixed cells for β-Gal activity.
Cells were fixed in 50 mmol/L phosphate buffer (pH 7.4) containing 0.2% glutaraldehyde, 2% formaldehyde, and 2 mmol/L MgCl2at room temperature for 5 minutes. The cells were rinsed three times in 50 mmol/L phosphate buffer (pH 7.4) containing 2 mmol/L MgCl2 and 0.02% Nonidet P-40 at room temperature for 20 minutes each. Cells were stained in 50 mmol/L phosphate buffer (pH 7.4) containing 0.5 mg/mL X-gal, 5 mmol/L potassium ferocyanide, 5 mmol/L potassium ferricyanide, and 2 mmol/L MgCl2 at 37°C.
RESULTS
In cultured cells, PAI-1 is expressed in various cell types and is often produced by the same cells that produce uPA.13 Little is known, however, regarding the nature of PAI-1–expressing cells in the context of natural invasive processes. In situ analysis of natural angiogenic processes (neovascularization of the maternal decidua and corpus luteum) has shown that whereas uPA is produced by sprouting endothelial cells, PAI-1 is preferentially expressed in nearby nonendothelial cells (decidual cells and lutein cells, respectively).27 Notably, PAI-1 was not uniformly expressed in the surrounding tissue but seemed to be more abundant in the vicinity of forming capillaries, suggesting that induction of PAI-1 may represent a tissue response to cellular invasion.
In situ analysis of natural angiogenesis, however, is short of providing sufficient resolution to identify cellular contacts of PAI-1–expressing cells. Therefore, we resorted to in situ analyses of an in vitro angiogenesis model. To gain better insight into PAI-1 regulation as a function of cellular contexts, we also analyzed native as well as genetically manipulated heterotypic cell cocultures.
PAI-1 expression is induced in cells juxtaposed to capillary sprouts in an aorta explant system.
Rat aortic segments were maintained in culture as explants for up to 3 weeks.26,27 Following initial outgrowth of aortic cells, mostly muscle cells,26 vascular sprouts started to grow radiating from the aorta segment and extending on and through the established cell sheet. Figure 1A shows a phase-contrasted image of the culture, depicting the tips of sprouts in the process of extension through the preformed cell monolayer. Figure1B shows a region of the aorta explant culture immunostained with the endothelial cell–specific marker vWF, to highlight the network of branching endothelial cell sprouts. The advantage of this system over a similar system of vascular sprouting, induced by embedding the aorta segment in a three-dimensional fibrin gel,32 is that it allows one to examine interactions between vascular sprouts and nonendothelial cells that they contact. Specifically, it enables one to identify changes in gene expression resulting from encounter with invading endothelial cell sprouts.
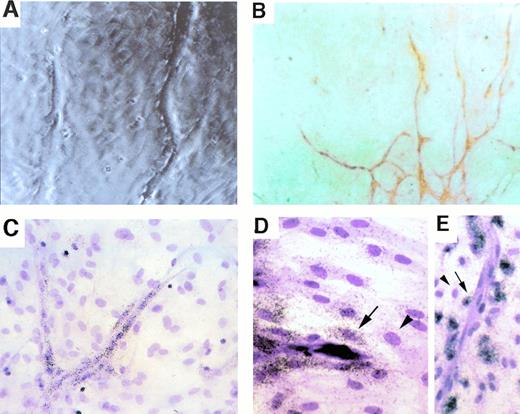
Spatial relationship between uPA-expressing cells and PAI-expressing cells during capillary sprouting in vitro. Cultured rat aortic ring explants, displaying branching capillary-like sprouts, are shown 3 weeks after initial culture. (A) A phase-contrasted micrograph showing the tips of sprouts and the preformed monolayer through which sprouts extend. (B) Immunostaining with vWF antibodies. vWF-positive cells are organized in cords, indicating that all cells seen as surrounding the cords in (A) and (C through E) are nonendothelial cells. (C) In situ hybridization with a uPA-specific probe. Note that expressing cells are endothelial cells forming the cords. (D and E) In situ hybidization with a PAI-1–specific probe. Note that strongest hybridization signals are in cells closest to cords (highlighted by arrows) and that fibroblasts residing more distally show much weaker hybridization (arrowheads). Magnification: (A, C, and E) ×200 original magnification; (B) ×100 original magnification; (D) ×300 original magnification.
Spatial relationship between uPA-expressing cells and PAI-expressing cells during capillary sprouting in vitro. Cultured rat aortic ring explants, displaying branching capillary-like sprouts, are shown 3 weeks after initial culture. (A) A phase-contrasted micrograph showing the tips of sprouts and the preformed monolayer through which sprouts extend. (B) Immunostaining with vWF antibodies. vWF-positive cells are organized in cords, indicating that all cells seen as surrounding the cords in (A) and (C through E) are nonendothelial cells. (C) In situ hybridization with a uPA-specific probe. Note that expressing cells are endothelial cells forming the cords. (D and E) In situ hybidization with a PAI-1–specific probe. Note that strongest hybridization signals are in cells closest to cords (highlighted by arrows) and that fibroblasts residing more distally show much weaker hybridization (arrowheads). Magnification: (A, C, and E) ×200 original magnification; (B) ×100 original magnification; (D) ×300 original magnification.
Aorta explant cultures were fixed and hybridized in situ with either a uPA-specific probe or a PAI-1–specific probe. Whereas expression of uPA was confined to the endothelial cell sprouts (Fig 1C), PAI-1 expression was mostly detected in cells adjacent to the uPA-expressing cells (Fig 1D and E). Note that the highest level of PAI-1 expression is detected in cells juxtaposed to the capillary sprout and that expression drops precipitously in cells residing further away from the capillary. These findings suggested that PAI-1 expression is specifically upregulated in cells in close proximity, and likely also in direct contact with invading capillaries.
PAI-1 expression in a heterotypic coculture is induced only in fibroblasts juxtaposed to endothelial cells.
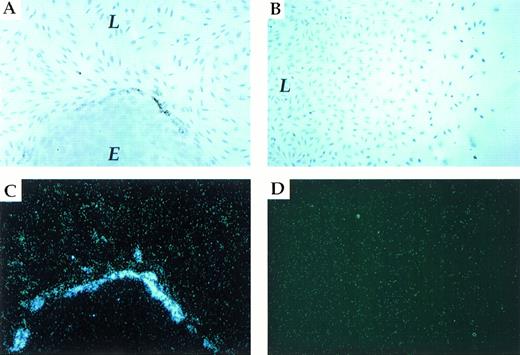
To establish that PAI-1 expression is induced as a result of heterotypic cell contact, we used a coculture system composed of an established endothelial cell line (LE-II) and a fibroblastic cell line (L-cells). As shown in Fig 2A, colonies of LE-II cells (round colonies of cobblestone-like cells) are easily distinguishable from L-cell fibroblasts among which they are interspersed. In situ hybridization with a PAI-1–specific probe was used to identify cells, either fibroblasts or endothelial cells, that have upregulated PAI-1 mRNA expression above the basal level of expression. As shown in Fig 2A and C, expression of PAI-1 mRNA was upregulated exclusively in fibroblasts juxtaposed to endothelial cells. Strikingly, fibroblasts positioned only a single row away from endothelial cells expressed only a low level of PAI-1 mRNA (L-cells are known to express a constitutive, low level of PAI-1 mRNA and protein,33 and the low level of expression in LE-II cells was barely detectable due to a relatively short exposure time).
L-cells/LE-II cells coculture. Induction of endogenous PAI-1 expression in fibroblasts contacting endothelial cells. (A and C) (brightfield and darkfield, respectively) show in situ hybridization of L-fibroblast cells (L)/ LE-II endothelial cells (E) cocultured with a PAI-1–specific probe. (B and D) (brightfield and darkfield, respectively) show in situ hybridization of a L-fibroblast cell culture displaying a gradient of cell density.
L-cells/LE-II cells coculture. Induction of endogenous PAI-1 expression in fibroblasts contacting endothelial cells. (A and C) (brightfield and darkfield, respectively) show in situ hybridization of L-fibroblast cells (L)/ LE-II endothelial cells (E) cocultured with a PAI-1–specific probe. (B and D) (brightfield and darkfield, respectively) show in situ hybridization of a L-fibroblast cell culture displaying a gradient of cell density.
To rule out that induction of PAI-1 mRNA was due to the localization of the expressing subpopulation at the edge of the fibroblastic sheet (ie, due to fewer homotypic cell-cell contacts), the following experiment was performed. Small coverslips were initially placed on the glass slides before the seeding of L-cells (to preclude cell growth in certain areas), and cells were grown to a saturation density. Coverslips were then removed, and partial filling of cell-free areas was allowed to take place during a further incubation for 3 days. As shown in Fig 2B and D, no difference with respect to PAI-1 expression could be detected between cells in confluent regions and cells residing at the edges of the cellular sheet. These results strongly suggest that PAI-1 is induced as a result of heterotypic cell-cell contacts.
Apposition-dependent regulation of PAI-1 expression is mediated by PAI-1 promoter sequences.
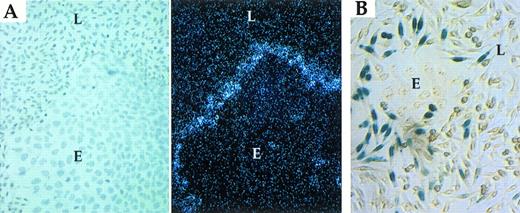
To determine whether apposition-dependent induction of PAI-1 is dictated by the PAI-1 promoter, reporter gene constructs were used. A DNA fragment containing approximately 1.3 kb of the murine PAI-1 promoter sequences was ligated to a β-Gal reporter gene containing a nuclear localization signal (NLS; to improve visualization of β-Gal–expressing cells). Stably transfected L-cell colonies were selected, transformed clones were cocultured with LE-II cells, and confluent cocultures were examined for the distribution of β-Gal–positive cells. As shown in Fig3A, β-Gal activity was confined to a subpopulation of transformed L-cells distinguished by proximity to endothelial cells, ie, a pattern of expression similar to that of the endogenous PAI-1 gene (Fig 2). As a control, when expression of β-Gal was driven by an irrelevant promoter (promoter of the ribosomal protein S16), a uniform pattern of expression in all transformed L-cells was detected (Fig 3B). This finding indicates that apposition-dependent regulation of PAI-1 expression is mediated by PAI-1 promoter sequences.
PAI-1 promoter directs expression of a β-Gal reporter to fibroblasts juxtaposed to endothelial cells. (A) Fibroblasts transfected with a PAI promoter-LacZ plasmid. (B) Fibroblasts transfected with an L16 promoter-LacZ plasmid. See Materials and Methods for details. L-fibroblasts (L); LE-II endothelial cells (E).
PAI-1 promoter directs expression of a β-Gal reporter to fibroblasts juxtaposed to endothelial cells. (A) Fibroblasts transfected with a PAI promoter-LacZ plasmid. (B) Fibroblasts transfected with an L16 promoter-LacZ plasmid. See Materials and Methods for details. L-fibroblasts (L); LE-II endothelial cells (E).
Mechanistic aspects of apposition-dependent PAI-1 regulation.
Rifkin and coworkers have shown that plasmin, generated by activated PAs, converts latent transforming growth factor-β (TGF-β) to its active form and that TGF-β induces, in turn, expression of PAI-1. Their studies further suggested that activation of latent TGF-β is achieved when two different cell types, at least one of which produces latent TGF-β, are cocultured.34 35 We therefore wished to determine whether induction of PAI-1 at the sites of heterotypic cell contact represents a TGF-β–mediated feedback response to plasmin production.
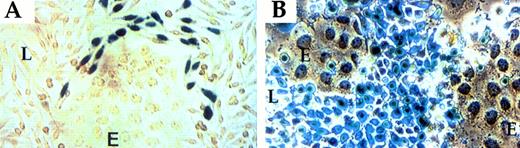
To address this question, we performed a series of experiments similar to those illustrated in Figs 2 and 3, except that different inhibitors of the PA system were added and were present throughout the period of coculture growth. Inhibitors used were the same as those used previously to show the TGF-β link34,35 and included the following: ε-Amino-n-Caproic Acid (a general inhibitor of serine proteases), Soybean Trypsin Inhibitor (a general inhibitor of serine proteases), Tranexamic Acid (inhibits binding of plasminogen and plasmin to the cell surface), and Trasylol (Aprotinin; inhibits plasmin, including cell-bound plasmin). In addition, we examined the effect of mannose 6-phosphate (Man-6-P) on PAI-1 induction (exogenous Man-6-P is known to compete with natural Man-6-P residues of latent TGF-β for binding to a cell surface receptor [of the cation-independent Man-6-P/IGFII type] and, hence, to inhibition of its activation36). All of these treatments had no effect on PAI-1 induction or on the size and distribution pattern of PAI-1–expressing cells. Representative examples are shown in Fig 4 with respect to both the endogenous PAI-1 gene and a transfected reporter gene driven by PAI-1 promoter. These findings suggest that apposition-dependent regulation of PAI-1 is not plasmin mediated. To further rule out a role for soluble plasmin, experiments were also performed using a serum-free medium (devoid of plasminogen or plasmin) and L-cells and LE-II cells adapted for growth in a serum-free medium. An identical pattern of PAI-1 mRNA induction was observed, reinforcing the notion that plasmin activity is not involved (data not shown).
Apposition-dependent regulation of PAI-1 is unaffected by inhibitors of the PA system and effectors of latent TGF-β activation. (A) In situ hybridization of L-fibroblast cells (L)/LE-II endothelial cells (E) grown in the presence of 100 mmol/L mannose-6-phosphate (brightfield, left and darkfield, right). (B) β-Gal activity in fibroblasts (L) transfected with a PAI promoter-LacZ plasmid and cocultured with LE-II cells (E) in the presence of 200 mg/mL Soybean Trypsin Inhibitor.
Apposition-dependent regulation of PAI-1 is unaffected by inhibitors of the PA system and effectors of latent TGF-β activation. (A) In situ hybridization of L-fibroblast cells (L)/LE-II endothelial cells (E) grown in the presence of 100 mmol/L mannose-6-phosphate (brightfield, left and darkfield, right). (B) β-Gal activity in fibroblasts (L) transfected with a PAI promoter-LacZ plasmid and cocultured with LE-II cells (E) in the presence of 200 mg/mL Soybean Trypsin Inhibitor.
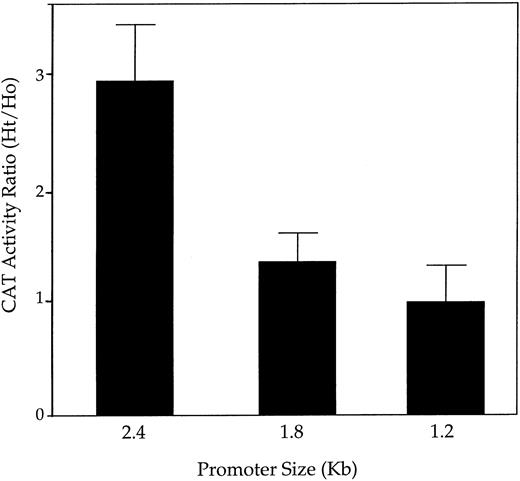
To grossly map regulatory domains required for PAI-1 induction by heterotypic cell contacts, 5′ truncated versions of the rat PAI-1 promoter were linked to a CAT reporter gene, and clones of transfected L-cells were obtained. CAT activity was quantified in extracts of selected L-cell clones and was compared with the activity found in extracts of the same L-cell clones that have been cocultured with LE-II endothelial cells. The heterotypic/homotypic ratio of CAT activity was taken as a measure of the extent of PAI-1 induction due to heterotypic contacts. As shown in Fig 5, a 2.4-kb rat PAI-1 promoter directed CAT expression in heterotypic cultures to significantly higher levels than in homotypic cultures (also take into account that the apparent level of stimulation is an underestimate because CAT activity measured is averaging for the entire coculture, whereas the fraction of L-cells contacting endothelial cells is relatively small). Deletion of approximately 600 bp of distal promoter sequences resulted in the loss of most of the increase in CAT activity, and deletion of additional 600 bp completely abolished the coculture effect. These results suggested that regulation of PAI-1 mRNA expression by heterotypic cell contact is mediated by regulatory elements located in a 5′ distal promoter region.
The 5′ upstream region of rat PAI-1 promoter is required for induction in a heterotypic coculture. A series of 5′ deletions of the 2.4-kb rat PAI-1 promoter31 were cloned upstream of a CAT reporter gene and transfected into L-cells. For each construct several transfected clones were selected and grown either alone or after mixing with LE-II endothelial cells (in a 1:1 ratio). Cultures were grown for 5 days (ie, at least 1 day after reaching confluence) and then obtained and analyzed for CAT activity. A comparison was made between the heterotypic cocultures (Ht) and an equal number of cells of the respective L-cell clone to which LE-II cells were added (both obtained from individually grown cultures) (Ho). Results are expressed as an Ht/Ho ratio and are the average of six different clones for each construct.
The 5′ upstream region of rat PAI-1 promoter is required for induction in a heterotypic coculture. A series of 5′ deletions of the 2.4-kb rat PAI-1 promoter31 were cloned upstream of a CAT reporter gene and transfected into L-cells. For each construct several transfected clones were selected and grown either alone or after mixing with LE-II endothelial cells (in a 1:1 ratio). Cultures were grown for 5 days (ie, at least 1 day after reaching confluence) and then obtained and analyzed for CAT activity. A comparison was made between the heterotypic cocultures (Ht) and an equal number of cells of the respective L-cell clone to which LE-II cells were added (both obtained from individually grown cultures) (Ho). Results are expressed as an Ht/Ho ratio and are the average of six different clones for each construct.
To determine whether PAI-1 expression is induced in all circumstances of heterotypic cocultures or, alternatively, that only particular heterotypic cell contacts are conducive for PAI-1 induction, we also examined by in situ hybridization the pattern of PAI-1 expression in heterotypic cultures composed of L-cells fibroblast and epithelial cells (a human carcinoma MLS line). In contrast to endothelial cells cocultures, we could not detect elevated levels of PAI-1 expression in fibroblasts juxtaposed to the epithelial cells (data not shown). This finding suggests that not all heterotypic cell contacts are conducive for PAI-1 induction.
DISCUSSION
By virtue of focusing proteolysis close to the cell surface, PAs play an important role in clearing a path for invading cells. Because PAs trigger a degradative cascade that is further amplified through the activation of matrix metalloproteinases, an efficient way to limit matrix degradation may include additional mechanism(s) to a regulated PA expression. A tightly controlled pericellular proteolysis is particularly important during certain processes of physiological angiogenesis where the tissue is invaded by a large number of endothelial cell sprouts (eg, during neovascularization of the corpus luteum). In these highly invasive processes matrix degradation is restricted to provide a sufficient ECM milieu around capillary sprouts (which is necessary for proper capillary morphogenesis). PAI-1, by virtue of its ability to clear active uPA complexes from cell surfaces,23 is situated in an excellent position for the role of protecting the interstitial tissue from excessive degradation.
Previous work has shown that PAI-1 mRNA and protein are produced by a variety of cultured cell types and that high concentrations of PAI-1 are constitutively expressed in a number of mouse tissues. A widespread expression of PAI-1 may reflect the facts that the PA system may also play a role in cellular functions other than cellular invasion (notably in control of fibrinolysis37), that PAI-1 might be required to counteract both tPA and uPA, and that PAI-1 might interact with both cell-bound and diffusible forms of PAs. Therefore, to assign a regulatory role for PAI-1 in uPA-mediated proteolysis associated with cellular invasion and, in particular, to examine the proposition that PAI-1 may shield cells in the immediate surrounding of invasive endothelium from excessive degradation, it is necessary to elucidate the spatial relationship between uPA-producing cells and PAI-1–producing cells in the context of natural invasive processes. Clearly, the fact that PAI-1 is sequestered in the ECM underlying its producer cell in a PA-accessible form9 38 is consistent with this role.
Data presented here, showing that PAI-1 induction is confined to within a single cell distance from endothelial cells (eg, Fig 2), strongly suggest that a heterotypic cell-cell contact is required for PAI-1 induction. This requirement for a cell-cell contact was further supported by findings that daily exchanges of media conditioned by LE-II and L-cells had no effect on PAI-1 expression in either cell type grown alone (data not shown).
Experiments using a PAI-1 promoter–reporter gene construct have shown that a PAI-1 promoter confers an apposition-dependent expression on a heterologous gene, and a preliminary promoter deletion analysis of the rat PAI-1 promoter has shown that a far upstream region (from −2.4 kb to −1.2 kb) is required for this novel mode of PAI-1 regulation. Although this analysis is preliminary, it argues against the notion that restricting PAI-1 induction to cells contacting endothelial cells reflects a juxtacrine response to endothelial cell-bound TGF-β, as TGF-β regulatory elements were mapped to a more proximal promoter region.39 A more detailed analysis is required to determine whether any of the putative regulatory elements previously mapped to this region31 or, alternatively, a yet unidentified regulatory element mediates the cell contact regulation of PAI-1.
A possible mechanism for apposition-dependent PAI-1 induction is a feedback response in which the protease plays a direct role. One example for a protease-inducing expression of its cognate inhibitor is the proteolytic enzyme elastase, which regulates the synthesis of its inhibitor, α1-proteinase inhibitor.40 More relevantly, it was shown that tPA increases steady-state levels of PAI-1 mRNA in HUVEC endothelial cells.41 Interestingly, further studies have shown that induction of PAI-1 mRNA expression by tPA also takes place with a protease devoid of enzymatic activity.42 Thus, the triggering molecule could be a cell-bound PA, but not necessarily with the involvement of plasmin. The latter possibility is consistent with our findings that induction of PAI-1 in fibroblasts juxtaposed to endothelial cells is independent of plasmin activity.
A fundamental difference between a quiescent endothelium and endothelial cells engaged in angiogenesis is that, whereas the first is enveloped in a basement membrane (BM), the latter are set free of the BM constraints following angiogenic factor-induced matrix degradation and, hence, are accessible to contact cells of the interstitial tissue. We speculate that transient interactions between endothelial cells and cells that under nonangiogenic circumstances do not contact the endothelium, trigger a signaling pathway that culminates in PAI-1 induction. Such a mechanism would provide an efficient way to sense endothelial cell invasion and is consistent with the observations that PAI-1 is induced only during endothelial cell invasion and only in cells juxtaposed to invading endothelial cells.
Supported by the Cooperation Program in Cancer Research of the Deutsches Krebsforschungszentrum (DKFZ) and Israel's Ministry of Science and Arts.
Address reprint requests to Eli Keshet, PhD, Department of Molecular Biology, Hebrew University, Hadassah Medical School, Jerusalem 91120, Israel.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.