Abstract
We recently showed that a retrovirally transduced prolactin receptor (PrlR) efficiently supports the differentiation of wild-type burst-forming unit erythroid (BFU-e) and colony-forming unit erythroid (CFU-e) progenitors in response to prolactin and in the absence of erythropoietin (Epo). To examine directly whether the Epo receptor (EpoR) expressed by wild-type erythroid progenitors was essential for their terminal differentiation, we infected EpoR−/−progenitors with retroviral constructs encoding either the PrlR or a chimeric receptor containing the extracellular domain of the PrlR and intracellular domain of EpoR. In response to prolactin, both receptors were equally efficient in supporting full differentiation of the EpoR−/− progenitors into erythroid colonies in vitro. Therefore, there is no requirement for an EpoR-unique signal in erythroid differentiation; EpoR signaling has no instructive role in red blood cell differentiation. A synergistic interaction between EpoR and c-kit is essential for the production of normal numbers of red blood cells, as demonstrated by the severe anemia of mice mutant for either c-kit or its ligand, stem cell factor. We show that the addition of stem cell factor potentiates the ability of the PrlR to support differentiation of both EpoR−/− and wild-type CFU-e progenitors. This synergism is quantitatively equivalent to that observed between c-kit and EpoR. Therefore, there is no requirement for an EpoR-unique signal in the synergistic interaction between c-kit and EpoR.
© 1998 by The American Society of Hematology.
SIGNALING BY THE erythropoietin receptor (EpoR) is essential for the production of red blood cells (RBCs). Disruption of either the Epo or EpoR genes in mice leads to embryonic lethality due to a severe deficiency in adult-type erythropoiesis.1-3 The fetal livers of Epo−/− or EpoR−/− mice contain normal numbers of committed burst-forming unit erythroid (BFU-e) and colony-forming unit erythroid (CFU-e) progenitors, but these fail to differentiate into RBCs. Therefore, there is an essential requirement for EpoR signaling during differentiation of committed CFU-e progenitors.
We recently showed that wild-type BFU-e and CFU-e progenitors infected with a retroviral construct encoding the nonhematopoietic prolactin receptor (PrlR) respond to prolactin by differentiating into RBCs in vitro.4 By comparing the PrlR with a chimeric receptor containing the extracellular domain of PrlR and intracellular domain of EpoR, we showed that the cytoplasmic domains of PrlR and EpoR are equally efficient in supporting erythroid differentiation. There was no difference in ligand sensitivity, colony number, morphology, or degree of hemoglobinization between progenitors expressing either of the two receptors.
These findings raised the intriguing possibility that there is no requirement for an EpoR-unique signal during erythroid differentiation and, therefore, that EpoR signaling has no instructive role in erythropoiesis. However, this conclusion could not be drawn definitively, because the above experiments were performed in wild-type erythroid progenitors expressing the EpoR. An Epo-induced instructive signal derived from the EpoR may have been delivered in vivo before harvesting of erythroid progenitors.
Alternatively, although Epo was absent from the medium, it remained possible that the EpoR was nevertheless able to generate a unique instructive signal in an Epo-independent manner. Such a signal might arise in a number of different ways. First, the receptor tyrosine kinase c-kit associates with and phosphorylate the EpoR in response to stem cell factor and in the absence of Epo.5,6EpoR phosphorylation by c-kit does not require EpoR dimerization and is therefore distinct to its manner of activation by Epo.7 Indeed, in the absence of stem cell factor, there is a substantial reduction in the number of differentiated RBCs, raising the possibility that the c-kit/EpoR interaction delivers unique, essential signals for erythroid differentiation.8,9A second example of Epo-independent EpoR activation is provided by gp55, the envelope protein of Friend spleen focus-forming virus (SFFV). When coexpressed with EpoR in hematopoietic cells it renders them factor independent. It has been suggested that an as yet unknown endogenous protein homologous to gp55 may similarly activate EpoR.10 Finally, there may be sufficient basal activity of the unliganded EpoR for it to generate an instructive signal.
To examine directly whether the EpoR expressed by wild-type erythroid progenitors was essential for their terminal differentiation, we looked at the ability of the PrlR to rescue EpoR−/−erythroid progenitors. We found that the PrlR fully supported differentiation of EpoR−/− progenitors into RBCs. Therefore, there is no requirement for an EpoR-unique instructive signal during erythroid differentiation.
Both in vivo and in vitro, maximal effects of the EpoR in erythroid differentiation require the simultaneous activation of c-kit. c-kit alone does not support differentiation of erythroid progenitors; however, it functionally potentiates EpoR signaling, resulting in a significant quantitative increase in the number of RBC progeny. This is shown by the anemia of mice mutant at the W(White-spotting) and Sl (Steel) loci.8,9 Although c-kit interacts synergistically with many hematopoietic cytokine receptors,9,11 the only hematopoietic phenotypic manifestations in the W mouse are in the mast cell and erythroid lineages. This raised the possibility that its interaction with EpoR might be relatively unique. As noted, c-kit directly associates with and phosphorylates the EpoR5,6; box 1 and extended box 2 domains of EpoR are essential for the potentiation of its signaling by c-kit in erythroid cell lines.5 12Having found that the PrlR was quantitatively as efficient as EpoR in supporting differentiation of EpoR−/−progenitors, we asked whether, like the EpoR, it too was dependent on c-kit activation for maximal effect. We show that this is indeed the case. Therefore, the synergistic interaction of EpoR with c-kit does not require an EpoR-unique signal or EpoR-unique domain.
MATERIALS AND METHODS
Retroviral constructs.
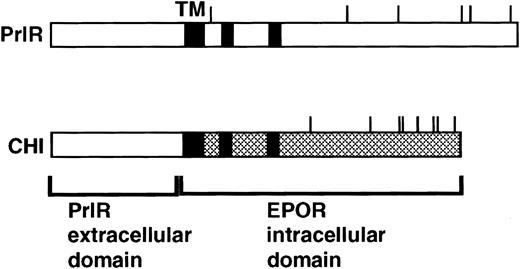
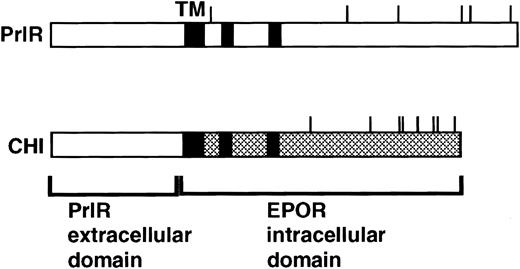
CHI4 (Fig 1) contains the extracellular domain of the rabbit prolactin receptor and membrane-spanning and intracellular domains of the murine EpoR. PrlR encodes the rabbit prolactin receptor. Both receptors were expressed in the retroviral expression vector MSCV.13
PrlR and CHI, a chimeric PrlR-EpoR receptor.16 CHI consists of PrlR extracellular domain and EpoR transmembrane (TM) and cytoplasmic domains. Shaded rectangles represent regions of homology with the cytokine receptor superfamily (box 1, box 2). Cytoplasmic tyrosines are marked with a line.
PrlR and CHI, a chimeric PrlR-EpoR receptor.16 CHI consists of PrlR extracellular domain and EpoR transmembrane (TM) and cytoplasmic domains. Shaded rectangles represent regions of homology with the cytokine receptor superfamily (box 1, box 2). Cytoplasmic tyrosines are marked with a line.
Transducing retroviruses.
VE23 ecotropic packaging cells4 were transiently transfected using the calcium-phosphate method with MSCV retroviral constructs each encoding the desired receptor. Culture supernatants were collected at 48 hours and either immediately frozen or used for infection.
Mice, fetal liver cell infection, and transduced receptor expression.
Male mice (mixed 129C/C57BL/6 background) heterozygous for the mutant EpoR allele1 were backcrossed with either Balb/cJ or Balb/CBYJ females. Heterozygote F1 and F2 mice were selected by Southern blot analysis on EcoRV-digested genomic DNA, using the cytoplasmic domain of the erythropoietin receptor as a probe. Heterozygote mice were crossed and fetal liver cells obtained from pregnant females on day 12.5 of gestation.
Equal numbers of EpoR−/− and wild-type or heterozygote littermate fetal livers were processed in each experiment. EpoR−/− embryos were identified visually by their extreme pallor and rudimentary fetal liver. The correct identification of the EpoR−/− embryos was monitored by setting up a control Epo-containing culture in each experiment. In all cases there were no Epo-dependent erythroid colonies in the EpoR−/− cultures. Retroviral infections were performed as described.4 Briefly, fetal liver cells were incubated with viral supernatants in the presence of 4 μg/mL polybrene for 4 hours. We previously showed that CHI and PrlR are equally well expressed on the surface of fetal liver cells by fluorescence-activated cell sorter (FACS) analysis 36 hours after infection using the M110 monoclonal antibody directed against the extracellular domain of PrlR.4 In the present studies about 10% of the fetal liver cells were infected.
CFU-e cultures and colony scoring.
After infection, cells were washed and resuspended in semisolid methylcellulose medium containing either 10% plasma-derived serum (PDS) or serum-free medium as described.4 Growth factors were added to the methylcellulose medium as indicated in the text. Ovine-prolactin (National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD) was added at 500 ng/mL. Recombinant rat stem cell factor (Amgen, Thousand Oaks, CA) was added at 100 ng/mL. Recombinant human Epo (Amgen) was used at 2 U/mL. Hemoglobinized CFU-e colonies were scored on day 3 after staining with diaminobenzidine.
RESULTS
The prolactin receptor rescues EpoR−/−progenitors.
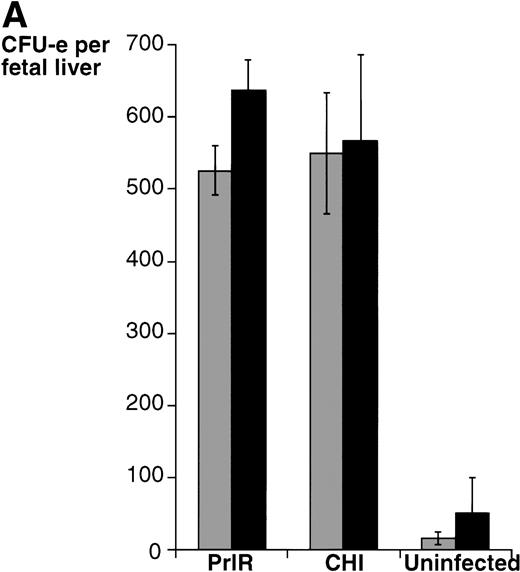
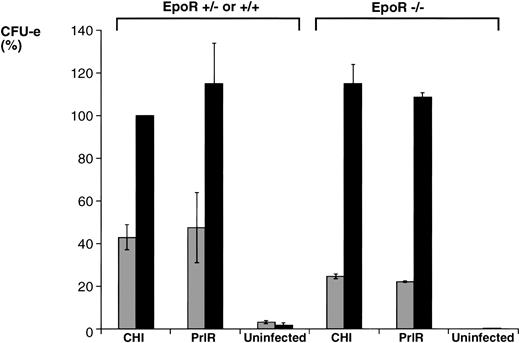
The PrlR and a chimeric receptor, CHI, containing the PrlR extracellular domain and EpoR membrane-spanning and intracellular domains (Fig 1), are as efficient as the EpoR in mediating differentiation of wild-type fetal liver CFU-e progenitors in vitro. Also, retrovirally transduced CHI and PrlR are expressed equally well on the surface of fetal liver cells by FACS analysis using an anti-PrlR monoclonal antibody; on average, approximately 10% of fetal liver cells express the retrovirally transduced receptors.4 The study in Fig 2 confirms our previous finding that, in response to prolactin, both PrlR and CHI support full differentiation of wild-type CFU-e progenitors.4 There is no significant difference in the sensitivity to prolactin, CFU-e colony numbers, colony morphology, and degree of hemoglobinization between CHI and the PrlR.4 Figure 2A also shows that a similar result is obtained in EpoR−/− progenitors. CHI and PrlR give rise to similar numbers of CFU-e colonies in EpoR−/− fetal liver cells in response to prolactin. Also, the number of colonies for either receptor per EpoR−/− fetal liver is similar to the number of colonies seen in wild-type or heterozygote fetal livers, consistent with previous observations that EpoR−/− fetal livers contain normal numbers of CFU-e progenitors.1 The size, morphology, and degree of hemoglobinization of the EpoR−/− colonies supported by PrlR were not significantly different from CHI-supported colonies (Fig 2B). Therefore, the cytoplasmic domains of PrlR and EpoR are equally efficient in mediating differentiation of erythroid progenitors in the absence of the wild-type EpoR.
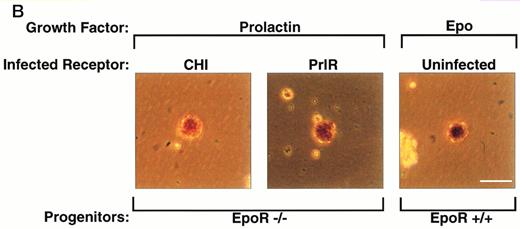
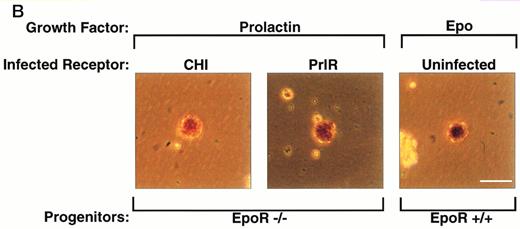
PrlR is as efficient as CHI in supporting EpoR−/− CFU-e colony formation. (A) Each determination is the mean ± SEM of four independent experiments. Fetal liver cells were cultured in methylcellulose medium containing 500 ng/mL ovine prolactin, 100 ng/mL rat SCF (rSCF), and 10% PDS. Between 3 and 7 EpoR−/− livers or livers from wild-type littermates were used per experiment. In the presence of Epo, an average of 5,000 CFU-e colonies per wild-type fetal liver, and 12 CFU-e colonies per EpoR−/− fetal liver were obtained. Wild-type and EpoR−/− fetal livers contained an average of 2.5 × 105 and 5 × 104 nucleated cells per liver, respectively. (▧), EpoR−/−; (▪), EpoR+/− or +/+. (B) EpoR−/− CFU-e colonies supported by PrlR are qualitatively similar to EpoR−/− CFU-e colonies supported by CHI or wild-type CFU-e colonies differentiating in response to Epo. Scale bar: 100 μm.
PrlR is as efficient as CHI in supporting EpoR−/− CFU-e colony formation. (A) Each determination is the mean ± SEM of four independent experiments. Fetal liver cells were cultured in methylcellulose medium containing 500 ng/mL ovine prolactin, 100 ng/mL rat SCF (rSCF), and 10% PDS. Between 3 and 7 EpoR−/− livers or livers from wild-type littermates were used per experiment. In the presence of Epo, an average of 5,000 CFU-e colonies per wild-type fetal liver, and 12 CFU-e colonies per EpoR−/− fetal liver were obtained. Wild-type and EpoR−/− fetal livers contained an average of 2.5 × 105 and 5 × 104 nucleated cells per liver, respectively. (▧), EpoR−/−; (▪), EpoR+/− or +/+. (B) EpoR−/− CFU-e colonies supported by PrlR are qualitatively similar to EpoR−/− CFU-e colonies supported by CHI or wild-type CFU-e colonies differentiating in response to Epo. Scale bar: 100 μm.
The PrlR efficiently replaces EpoR in a synergistic interaction with c-kit.
The essential function of c-kit in erythropoiesis is thought to be due to its ability to quantitatively potentiate the action of the EpoR. Our finding that, in the presence of stem cell factor (SCF), PrlR efficiently rescues EpoR−/− CFU-e progenitors (Fig 2) raised the possibility that PrlR may functionally interact with c-kit in a manner analogous to the EpoR.
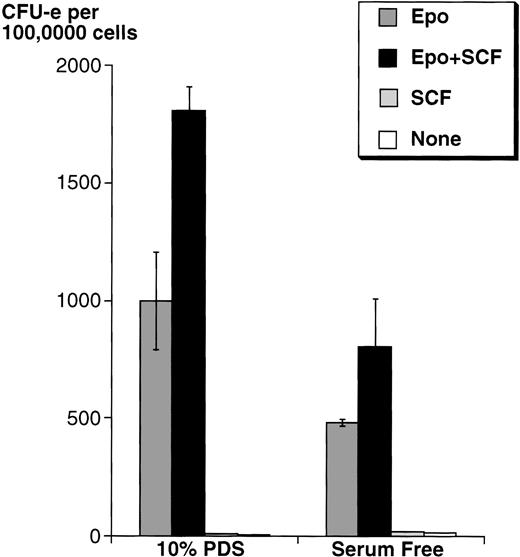
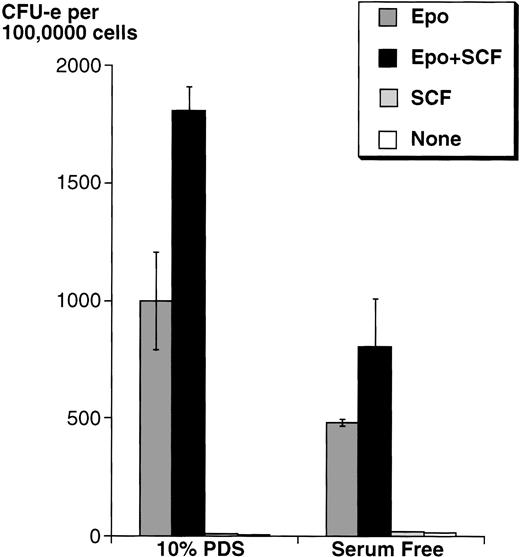
Because serum contains SCF, we first studied the effects of adding SCF on wild-type mouse fetal liver CFU-e progenitors in either serum-free medium or in medium containing 10% PDS. In both cases, in the presence of a saturating Epo concentration (2 U/mL), SCF increased the number of fetal liver CFU-e progenitors able to give rise to differentiated colonies by approximately twofold, although the absolute number of colonies was higher in medium with serum (Fig 3).
SCF potentiates the ability of Epo to support wild-type fetal liver CFU-e colony formation. Fetal liver cells were cultured in methylcellulose containing Epo (2 U/mL) and/or rSCF (100 ng/mL). The methylcellulose medium was either serum free, or contained 10% PDS. Results are the mean ± SD of triplicate samples.
SCF potentiates the ability of Epo to support wild-type fetal liver CFU-e colony formation. Fetal liver cells were cultured in methylcellulose containing Epo (2 U/mL) and/or rSCF (100 ng/mL). The methylcellulose medium was either serum free, or contained 10% PDS. Results are the mean ± SD of triplicate samples.
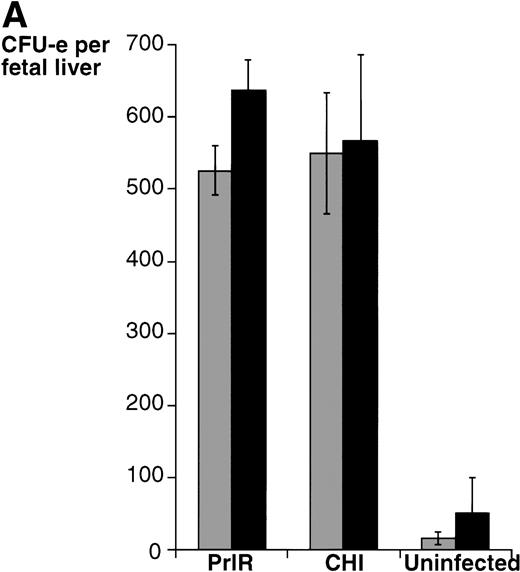
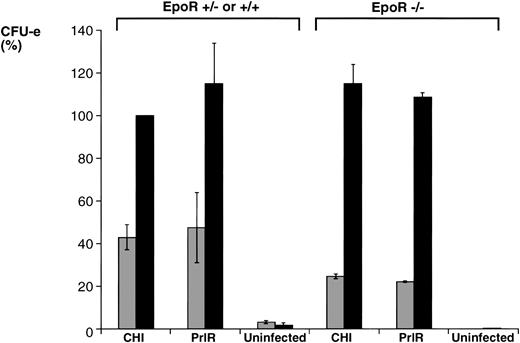
We proceeded to examine the effects of exogenously added SCF in this medium, and determined the effect of SCF on prolactin-dependent differentiation of progenitors infected with either PrlR or CHI. In progenitors derived from EpoR−/− animals and infected with either receptor, addition of SCF to the culture increased the number of CFU-e colonies by 4.5- to 5-fold. In progenitors derived from wild-type littermate animals and infected with either receptor, SCF increased the number of colonies 2- to 2.5-fold (Fig 4), similar to the effect of SCF seen in wild-type progenitors cultured in Epo (Fig 3). There was no significant quantitative difference between PrlR-infected and CHI-infected progenitors in their response to SCF, in either EpoR−/− or wild-type animals (Fig 4). Therefore, PrlR is fully efficient at substituting for EpoR in a synergistic interaction with c-kit essential for supporting CFU-e differentiation.
SCF potentiates the CFU-e response to prolactin in EpoR−/− and wild-type progenitors infected with either PrlR or CHI. EpoR−/− fetal liver cells or wild-type or heterozygous littermate fetal liver cells were infected with retrovirus encoding CHI or PrlR, and cultured in either the presence (▪) or absence (▧) of SCF (100 ng/mL), in methylcellulose containing 500 ng/mL ovine-prolactin and 10% PDS. Results in each experiment were expressed as percent of colonies obtained for wild-type fetal liver cells infected with CHI and cultured in the presence of prolactin and SCF. Each determination is the mean ± SD of three independent experiments. The mean colony number per fetal liver was 540 ± 220 for PrlR-infected cells and 660 ± 198 for CHI-infected cells.
SCF potentiates the CFU-e response to prolactin in EpoR−/− and wild-type progenitors infected with either PrlR or CHI. EpoR−/− fetal liver cells or wild-type or heterozygous littermate fetal liver cells were infected with retrovirus encoding CHI or PrlR, and cultured in either the presence (▪) or absence (▧) of SCF (100 ng/mL), in methylcellulose containing 500 ng/mL ovine-prolactin and 10% PDS. Results in each experiment were expressed as percent of colonies obtained for wild-type fetal liver cells infected with CHI and cultured in the presence of prolactin and SCF. Each determination is the mean ± SD of three independent experiments. The mean colony number per fetal liver was 540 ± 220 for PrlR-infected cells and 660 ± 198 for CHI-infected cells.
DISCUSSION
EpoR does not play an instructive role in erythroid differentiation.
Our principal finding is that a nonhematopoietic receptor, the PrlR, can rescue EpoR−/− erythroid progenitors in vitro. The cytoplasmic domain of the PrlR was as efficient as the cytoplasmic domain of EpoR in supporting the full differentiation of EpoR−/− CFU-e progenitors, with no significant quantitative or qualitative difference in the resulting erythroid colonies. In EpoR−/− progenitors the PrlR was also efficiently able to substitute for the EpoR in a synergistic interaction with c-kit, which is essential for normal erythropoiesis. This confirms and expands our previous finding that a retrovirally transduced PrlR can support the differentiation of wild-type CFU-e progenitors.4 It directly shows for the first time that there is no EpoR-unique function required for erythroid differentiation. Instead, the essential role of EpoR signaling in RBC differentiation must be supportive. The signals generated by EpoR are presumably “generic” and may also be induced by other cytokine receptors. Although there is little sequence homology between the cytoplasmic domains of EpoR and PrlR, both receptors activate a very similar set of downstream signaling molecules, including JAK2 and STAT5; these are also activated by many other cytokine receptors.14-18
Therefore, the specificity of EpoR action in erythropoiesis is a result of its unique expression by erythroid progenitors. The unique outcome of EpoR signaling is presumably a result of the unique cellular environment in the erythroid progenitors in which it acts. The precise functions supported by these “generic” EpoR signals are still to be fully determined, but include anti-apoptotic19 as well as mitogenic20 effects, functions common to many cytokine receptors. The unique cellular context of a committed progenitor may also allow a nonunique signal to result in tissue-specific gene induction.
Supportive signaling by other cytokine receptors.
Recent reports suggest that our findings regarding the lack of an instructive role for EpoR in differentiation may be extended to other cytokine receptors. Mice mutant for the interleukin-7 (IL-7) receptor show severe lymphocyte deficiency; transgenic expression of bcl-2 in T cells of these mice rescued T-cell lymphocyte development and reversed the lymphopenic phenotype.21,22 Similarly, the low macrophage and osteoclast cell numbers found in the op/op(macrophage colony-stimulating factor [M-CSF] deficient) mouse can be restored by transgenic expression of bcl-2 in macrophage progenitors.23 Therefore, the essential function of these cytokine receptors is to ensure survival of progenitors, while lineage commitment and maturation events occur by other means. In contrast, transgenic expression of bcl-2 in the erythroid lineage did not result in Epo independence.24 Taken together with the results presented here, it may indicate that the signals generated by EpoR, although not unique, are not exclusively concerned with progenitor cell survival. Alternatively, additional non–bcl-2 anti-apoptotic pathways may be operating in erythroid cells.
The absence of instructive signaling by lineage-restricted cytokine receptors raises the question of how lineage commitment is determined. We cannot exclude a role for as yet unknown extracellular factors in this process. Alternatively, lineage commitment may occur stochastically, as first proposed by Till and others.25-28The expression of lineage-restricted cytokine receptors would be a manifestation of lineage commitment rather than its cause, and would allow specific cytokines to selectively rescue and amplify the progenitors of a particular lineage according to physiological need. Support for this comes from experiments in which expression of an activated EpoR or M-CSF receptor by pluripotent progenitors did not bias differentiation in favor of the lineages to which they are physiologically restricted; instead, it caused an overall increase in differentiated cell numbers.27
The role of c-kit in erythroid differentiation.
The activation of c-kit by SCF plays a unique role in erythropoiesis. The principal effect of c-kit is quantitative: RBCs are formed in its absence, but are fewer in number, as shown by the macrocytic anemia of mice mutant at the W (White-spotting) and Sl (Steel) loci, respectively.8,9The severity of the different allelic forms of W mice correlates with the degree of impairment of kinase activity of the mutant c-kit.29-31 c-kit exerts its effects at the BFU-e and CFU-e stages of erythropoiesis: 80% to 90% of BFU-e and CFU-e progenitors express c-kit,32 fetal livers ofW mice contain significantly reduced numbers of CFU-e progenitors,8 and although the number of adult marrow CFU-e in W/Wv mice is normal, they have a markedly reduced response to Epo.33 In vitro, c-kitpotentiates the proliferative and anti-apoptotic effects of EpoR, a synergistic interaction that had been shown in primary erythroid progenitors as well as erythroid cell lines.6,11,12,34 35
c-kit is widely expressed and interacts synergistically with many other cytokine receptors, including those for IL-3, IL-6, IL-7, granulocyte-macrophage CSF (GM-CSF), and G-CSF.10,11However, the hematopoietic deficiencies in W mice are confined to the erythroid and mast cell lineages, raising the possibility that the synergistic interaction of c-kit with the EpoR might be relatively unique. The precise molecular mechanism of this synergistic interaction is not known, but may involve direct association of the two receptors, as well as phosphorylation of the EpoR by c-kit.5,6 Box 1 and the extended box 2 domains of EpoR are essential for the potentiation of its signaling by c-kit in erythroid cell lines.5 12
The relatively greater SCF dependence of EpoR−/− CFU-e progenitors when compared to wild-type littermate progenitors (Fig 4) had been previously seen in EpoR−/− progenitors rescued by retrovirally transduced EpoR.7 It may be due to a requirement for simultaneous signaling by the two receptors. In wild-type animals, but not in EpoR−/− animals, some simultaneous activation of c-kit and EpoR in vivo may have preceded harvesting of progenitors, allowing a greater number of CFU-e cells to subsequently mature in vitro in the absence of SCF.
Our finding that PrlR can quantitatively replace the EpoR in its synergistic interaction with c-kit in both wild-type and EpoR−/− progenitors suggests that this interaction does not require an EpoR-unique domain or signal. The unique outcome of c-kit signaling in the erythroid lineage cannot be explained by a unique molecular interaction with the EpoR. Conversely, c-kit’s association with and phosphorylation of EpoR5 6 may turn out to be features of its synergistic interactions with other cytokine receptors.
ACKNOWLEDGMENT
We thank Drs Stefan Constantinescu and Saghi Ghaffari for discussion and comments on the manuscript.
Supported by Grant No. HL 32262 from The National Institutes of Health, by a grant from Amgen Corporation, and in part by a Howard Hughes postdoctoral fellowship for physicians (M.S.).
Address reprint requests to Harvey F. Lodish, PhD, Whitehead Institute for Biomedical Research, Nine Cambridge Center, Cambridge, MA 02142; e-mail: lodish@wi.mit.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.