Abstract
Thrombopoietin (TPO) has been used in preclinical myelosuppression models to evaluate the effect on hematopoietic reconstitution. Here we report the importance of dose and dose scheduling for multilineage reconstitution after myelosuppressive total body irradiation (TBI) in mice. After 6 Gy TBI, a dose of 0.3 μg TPO/mouse (12 μg/kg) intraperitoneally (IP), 0 to 4 hours after TBI, prevented the severe thrombopenia observed in control mice, and in addition stimulated red and white blood cell regeneration. Time course studies showed a gradual decline in efficacy after an optimum within the first hours after TBI, accompanied by a replacement of the multilineage effects by lineage dominant thrombopoietic stimulation. Pharmacokinetic data showed that IP injection resulted in maximum plasma levels 2 hours after administration. On the basis of the data, we inferred that a substantial level of TPO was required at a critical time interval after TBI to induce multilineage stimulation of residual bone marrow cells. A more precise estimate of the effect of dose and dose timing was provided by intravenous administration of TPO, which showed an optimum immediately after TBI and a sharp decline in efficacy between a dose of 0.1 μg/mouse (4 μg/kg; plasma level 60 ng/mL), which was fully effective, and a dose of 0.03 μg/mouse (1.2 μg/kg; plasma level 20 ng/mL), which was largely ineffective. This is consistent with a threshold level of TPO required to overcome initial c-mpl–mediated clearance and to reach sufficient plasma levels for a maximum hematopoietic response. In mice exposed to fractionated TBI (3 × 3 Gy, 24 hours apart), IP administration of 0.3 μg TPO 2 hours after each fraction completely prevented the severe thrombopenia and anemia that occurred in control mice. Using short-term transplantation assays, ie, colony-forming unit–spleen (CFU-S) day 13 (CFU-S-13) and the more immature cells with marrow repopulating ability (MRA), it could be shown that TPO promoted CFU-S-13 and transiently depleted MRA. The initial depletion of MRA in response to TPO was replenished during long-term reconstitution followed for a period of 3 months. Apart from demonstrating again that MRA cells and CFU-S-13 are separate functional entities, the data thus showed that TPO promotes short-term multilineage repopulating cells at the expense of more immature ancestral cells, thereby preventing pancytopenia. The short time interval available after TBI to exert these effects shows that TPO is able to intervene in mechanisms that result in functional depletion of its multilineage target cells shortly after TBI and emphasizes the requirement of dose scheduling of TPO in keeping with these mechanisms to obtain optimal clinical efficacy.
© 1998 by The American Society of Hematology.
THROMBOPOIETIN (TPO), the ligand for the cytokine receptor c-mpl, has been cloned and characterized in 19941-3 and shown to be the physiological regulator of platelet production by generating mice deficient for either TPO or c-mpl.4,5 Administration of pharmacological doses of TPO to normal mice and nonhuman primates resulted in dose-dependent increases in platelets, far exceeding that observed after administration of other growth factors.1,2,6,7 These observations have led to the pharmaceutical development of TPO as a therapeutic to counteract thrombopenic states, such as those resulting from myelosuppression due to cancer treatment. In myelosuppression models, TPO effectively alleviated the nadir for platelets and accelerated recovery to normal values.6,8-11 In several of those models, daily administration during the pancytopenic phase resulted in an overshoot in platelet counts to supranormal values.6,8,10,11 Although in normal experimental animals the response to TPO was dominant along the megakaryocytic lineage, in myelosuppression models multilineage effects have been shown such as stimulation of erythroid recovery,8,11-14 acceleration of immature progenitor cell reconstitution in bone marrow,8,15 and augmentation of the responses to granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF).8,16 We and others have shown that a single dose of TPO administered 24 hours after total body irradiation (TBI) was as effective in alleviating the nadir for thrombocytes as daily dosing,13,16-18 thereby reducing the need for thrombocyte transfusions in myelosuppressed nonhuman primates and accelerating recovery to normal platelet counts. The effects on red cell regeneration and immature bone marrow progenitor cells were retained with single dosing of TPO.13 16 These results showed that the more conventional dose schedules that have come into use for growth factors such as GM-CSF and G-CSF were less appropriate for TPO.
Careful design of growth factor treatment protocols requires preclinical experiments to gain insight into the mechanisms of action and in the optimal dose and dose schedule to achieve maximum therapeutic benefit and reductions in the occurrence of side effects, as well as to prevent unnecessary treatment, thereby reducing costs. Because a delay in the administration of TPO after cytoreductive treatment proved to be detrimental to its efficacy,18 19 we examined in detail the time dependence of TPO efficacy in the initial 12 hours after myelosuppressive TBI, with special emphasis on the response of immature bone marrow cells and their progeny, to reveal mechanisms of TPO action on immature repopulating cells important for efficacy.
MATERIALS AND METHODS
Animals.
Female (C57BL × CBA)F1 (BCBA) mice, approximately 12 weeks of age, were bred at the Experimental Animal Facility of Erasmus University (Rotterdam, The Netherlands) and maintained under specific pathogen-free conditions. Housing, experiments, and all other conditions were approved by an ethical committee in accordance with legal regulations in The Netherlands.
Experimental design.
TBI was administered at day 0 using two opposing 137Cs sources (Gammacell 40; Atomic Energy of Canada, Ottawa, Canada) at a dose rate between 0.92 and 0.94 Gy/min as described.20Doses used were 6 Gy for single dose irradiation and a fractionated dose of 9 Gy in three equal doses with 24-hour intervals. Mice were bled only once; for each data point a random experimental group of three mice was killed. All parameters were collected for individual mice.
Test drug.
Recombinant full length murine TPO produced by Chinese hamster ovary cells (Genentech Inc, South San Francisco, CA) was used throughout the experiments, diluted in phosphate-buffered saline/0.01% Tween 20, and administered intraperitoneally (IP) or intravenously (IV) in a volume of 0.5 mL. The dose of TPO used was 0.3 μg/mouse (12 μg/kg, based on a mean body weight of 25 g at the time of irradiation) unless otherwise indicated. We have previously shown that this dose was effective in a similar model for myelosuppression.13
Hematologic examinations.
After ether-anesthesia, the mice were bled by retro-orbital puncture and killed by cervical dislocation. Blood was collected in EDTA tubes. Complete blood cell counts were measured using a Sysmex F-800 hematology analyzer (Toa Medical Electronics Co, LTD, Kobe, Japan).
Phenotypic analysis of white blood cells.
For phenotypic analysis blood was collected in EDTA tubes. Samples from three mice that received the same treatment were pooled to yield sufficient numbers of cells. Red blood cells were removed by incubating whole blood in lysing solution (8.26 g ammonium chloride/1.0 g potassium bicarbonate and 0.037 g EDTA/L) for 10 minutes at 4°C. After lysing, cells were washed twice with Hanks’ buffered Hepes solution (HHBS) containing 0.5% (vol/vol) bovine serum albumin (BSA; Sigma, St Louis, MO), 0.05% (wt/vol) sodium azide, and 0.45% (wt/vol) glucose (Merck, Darmstadt, Germany) (HBN). The cells were resuspended in 50 μL HBN containing 4% (vol/vol) normal mouse serum to prevent nonspecific binding of the monoclonal antibodies (MoAbs). To detect neutrophilic granulocytes and monocytes the MoAb ER-MP20 (rat IgG2a)21 was added in a volume of 50 μL. ER-MP20 bright cells are monocytes (corresponding with Mac-1–positive cells22) and cells staining intermediate with ER-MP20 are granulocytes (corresponding with Gr-1–positive cells22). To detect lymphocytes the anti-CD4 MoAb YTS 191 and the anti-CD8 MoAb YTS 16923 (a kind gift from Dr H. Waldmann, Department of Pathology, Cambridge University, UK) were added at a concentration of 2 μg/mL. Cells and MoAbs were incubated for 30 minutes on ice. After two washes the cells were incubated with a fluorescein-labeled goat-anti-rat MoAb. After another two washes, the cells were labeled with propidium iodide and measured by flow cytometry. Ungated list mode data were collected for 10,000 events and analyzed using Lysis II software (Becton Dickinson, Mountain View, CA).
TPO levels.
Data for characterization plasma TPO pharmacokinetics were generated at Genentech Inc as previously described.24 In short, mice were injected IP with 125I-rmTPO either with a single dose of 0.9 μg/mouse (36 μg/kg) or with three doses of 0.3 μg/mouse (12 μg/kg) separated by 24 hours. Citrated blood was collected immediately after dosing and at intervals thereafter (n = 3 mice per time point), centrifuged at 2,950g for 10 minutes, plasma obtained, and TCA-precipitable radioactivity determined. Pharmacokinetic parameters were estimated after converting trichloroacetic acid–precipitable cpm/mL and fitting the data of concentration versus time to a two-compartment model with first order absorption using nonlinear least-squares regression analysis (WIN-NONLIN; Statistical Consultants, Lexington, KY). Area under the concentration time curves, maximum concentration, terminal half-lives, and clearance (mL/h/kg) were calculated using coefficients and exponents obtained from the model fits.
Colony assays.
Serum-free methylcellulose cultures were used in this study.25-27 Appropriate numbers of bone marrow cells were suspended in Dulbecco’s modified Eagle’s medium obtained from GIBCO (Life Technologies LTD, Paisley, Scotland) supplemented with the amino acids L-alanine, L-asparagine, L-aspartic acid, L-cysteine, L-glutamic acid, and L-proline (Sigma); vitamin B12, biotin, Na-pyruvate, glucose, NaHCO3, and antibiotics (penicillin and streptomycin) at an osmolarity of 300 mOsm/L; supplemented with 1% BSA (Fraction V, Sigma), 2 × 10−6 mol/L iron-saturated human transferrin (Intergen Company, New York, NY), 10−7mol/L Na2SeO3 (Merck), 10−4 mol/L β-mercapto-ethanol (Merck), linoleic acid (Merck), and cholesterol (Sigma), both at a final concentration of 1.5 × 10−5mol/L and 10−3 g/L nucleosides (cytidine, adenosine, uridine, guanosine, 2′-deoxycytidine, 2′deoxyadenosine, thymidine, and 2′deoxyguanosine obtained from Sigma) and 0.8% methylcellulose (Methocel A4M Premium Grade; Dow Chemical Co, Barendrecht, The Netherlands). The cultures were plated in 35-mm Falcon 1008 Petri dishes (Becton Dickinson Labware) in 1-mL aliquots.25
Granulocyte/macrophage colony formation was stimulated by a saturating concentration of macrophage colony-stimulating factor (M-CSF) purified from pregnant mouse uteri extract as described before,28 29supplemented with 100 ng/mL murine stem cell factor (SCF; Immunex Corporation, Seattle, WA) and 10 ng/mL murine interleukin-3 (IL-3; R&D, Minneapolis, MN). Granulocyte/macrophage colony-forming unit (GM-CFU) colonies were counted after 7 days of culture. Burst-forming unit–erythroid (BFU-E) growth was stimulated by 100 ng/mL SCF and 4 U/mL human erythropoietin (EPO; Behringwerke, Marburg, Germany), titrated to an optimal concentration. Colonies were counted after 10 days of culture. The culture medium of the erythroid progenitors also contained hemine (bovine, type I; Sigma) at a concentration of 2 × 10−4 mol/L.
Megakaryocyte progenitor cells (CFU-Meg) were cultured in 0.375% agar cultures. Colony formation was stimulated by 100 ng/mL SCF, 10 ng/mL IL-3, and 10 ng/mL murine TPO (Genentech Inc). After 10 days, the cultures were dried, stained for acetylcholinesterase-positive cells, and counted.30-32 All cultures were grown in duplicate at 37°C in a fully humidified atmosphere with 10% CO2 in the air. Colony numbers are expressed as a number per femur or per spleen and represent the mean ± SD of individual mice.
The spleen colony assay.
This assay was performed as described by Till and McCulloch.33 Briefly, mice were injected with one fifth of the cell content of a femur in HHBS one day after TBI. Thirteen days later, mice were killed, and spleens were excised and fixed in Tellyesniczky’s solution (64% ethanol, 5% acetic acid, and 2% formaldehyde) in H2O. Colony numbers were expressed as a number per donor femur ± SD. The cells giving rise to the spleen colonies were designated as day 13 CFU-spleen (CFU-S), shortly CFU-S-13.
Marrow repopulating ability (MRA).
Bone marrow from mice irradiated with 9 Gy TBI (in 3 equal fractions, each separated by 24 hours) was collected 24 hours after the last fraction of TBI, and 10 lethally irradiated recipient mice were injected with the cellular content of one femur of control mice or mice treated with 0.3 μg of TPO 2 hours after each fraction of TBI. After 13 days the bone marrow of recipient mice were assayed for the presence of GM-CFU.34 MRA is expressed as the number of GM-CFU per recipient femur. Data from two independent experiments with similar results were pooled. At the time points of 1 and 3 months and for normal mice, the standard number of 105 bone marrow cells was injected.
Statistics.
SDs were calculated and are given in the text and the figures on the assumption of a normal distribution. The significance of a difference was calculated by one-way analysis of variance followed by a nonpaired Student’s t-test using StatView (Abacus Concepts Inc, Berkeley, CA). The SD of CFU-S-13 was calculated on the assumption that crude colony counts are Poisson distributed. Differences in repopulating abilities were evaluated using Fisher’s exact test. All colony assays were performed in duplicate for individual mice. The results of the colony assays are expressed as the mean ± SD per femur or spleen for at least three mice per group.
RESULTS
Efficacy of TPO after 6 Gy TBI.
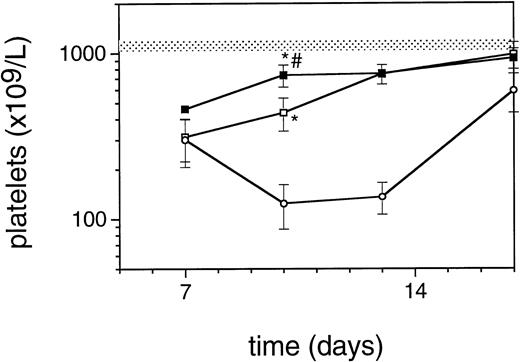
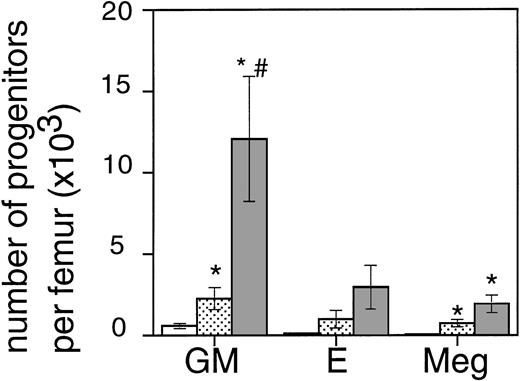
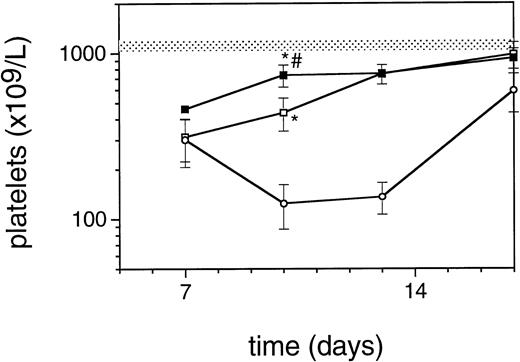
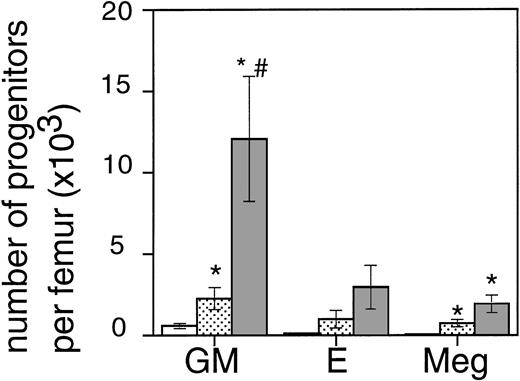
Exposure of mice to a TBI dose of 6 Gy resulted in severe bone marrow suppression and impaired blood cell production for a period of about 3 weeks. The nadir for thrombocytes occurred around day 10 (Fig1). A single dose of TPO administered 24 hours after TBI was effective in alleviating the thrombocyte nadir. Platelet counts 10 days after irradiation were 465 ± 142 × 109/L (n = 15) compared with 144 ± 62 × 109/L for control mice (n = 14), (Fig 1 and Table1). TPO injected 2 hours after exposure was significantly more effective, thrombocyte levels 10 days after TBI being 739 ± 165 × 109/L (n = 15,P < .0001). White blood cell regeneration was not influenced by TPO administered 24 hours after TBI; counts at day 10 were 0.4 ± 0.1 × 109/L, similar to 0.4 ± 0.1 × 109/L for control mice (Table 1). However, if TPO was administered 2 hours after TBI, white blood cell counts 10 days after TBI were 1.1 ± 0.4 × 109/L. For red blood cells the results were: 7.2 ± 0.5 × 1012/L for control mice, 7.5 ± 0.5 × 1012/L for the 24-hour mice, and 9.0 ± 0.6 × 1012/L for mice treated 2 hours after TBI (Table 1). Clearly, administration earlier than 24 hours after TBI resulted in an accelerated multilineage peripheral blood reconstitution in contrast to the lineage dominant effect of TPO when administered 24 hours after TBI. As is well known from previous observations in mice and nonhuman primates,9,15 the multilineage effects originated from accelerated reconstitution of progenitor cells along the neutrophil, erythroid, and megakaryocytic lineages measured in bone marrow of mice 7 days after irradiation. Femoral GM-CFU, BFU-E, and CFU-Meg reached consistently higher numbers in the treatment group compared with the placebo controls. (Fig 2). The results in the 24-hour treatment group were in between those of the 2-hour and control groups, although the differences were significant only for GM-CFU due to the large variance as a result of exponential reconstitution of progenitor cells at this time interval after TBI. The higher number of femoral GM-CFU in the 24-hour treatment group compared with control numbers was not reflected in accelerated leukocyte regeneration 10 days after TBI (Fig 3), which is consistent with our previous observation in rhesus monkeys that the TPO effect on GM progenitors may require administration of G- or GM-CSF for peripheral blood manifestation.8 16
Regeneration pattern of platelets in mice irradiated with 6 Gy TBI and treated with 0.3 μg/mouse of TPO 24 hours after TBI (□), 2 hours after TBI (▪), or placebo (○), data from one representative experiment, n = 3 per data point. The shaded area represents the mean platelet counts ±SD of 19 normal mice (1123 ± 89). *P < .0001 compared with control mice;#P < .0001 compared with mice treated 24 hours after TBI.
Regeneration pattern of platelets in mice irradiated with 6 Gy TBI and treated with 0.3 μg/mouse of TPO 24 hours after TBI (□), 2 hours after TBI (▪), or placebo (○), data from one representative experiment, n = 3 per data point. The shaded area represents the mean platelet counts ±SD of 19 normal mice (1123 ± 89). *P < .0001 compared with control mice;#P < .0001 compared with mice treated 24 hours after TBI.
The effect of 0.3 μg TPO 24 hours or 2 hours after TBI on the regeneration of bone marrow progenitors after 6 Gy TBI in mice. GM, GM-CFU; E, BFU-E; and Meg, CFU-Meg per femur at day 7 after TBI (means ± SE). The open bar (□) represents control mice (n = 6), the lightly shaded bar (▧) represents mice treated with TPO 24 hours after TBI (n = 6), and the dark shaded bar (▧) represents mice treated with TPO 2 hours after TBI (n = 6). *Significant compared with control mice (P < .03),#significant compared with mice treated with TPO 24 hours after irradiation (P < .03).
The effect of 0.3 μg TPO 24 hours or 2 hours after TBI on the regeneration of bone marrow progenitors after 6 Gy TBI in mice. GM, GM-CFU; E, BFU-E; and Meg, CFU-Meg per femur at day 7 after TBI (means ± SE). The open bar (□) represents control mice (n = 6), the lightly shaded bar (▧) represents mice treated with TPO 24 hours after TBI (n = 6), and the dark shaded bar (▧) represents mice treated with TPO 2 hours after TBI (n = 6). *Significant compared with control mice (P < .03),#significant compared with mice treated with TPO 24 hours after irradiation (P < .03).
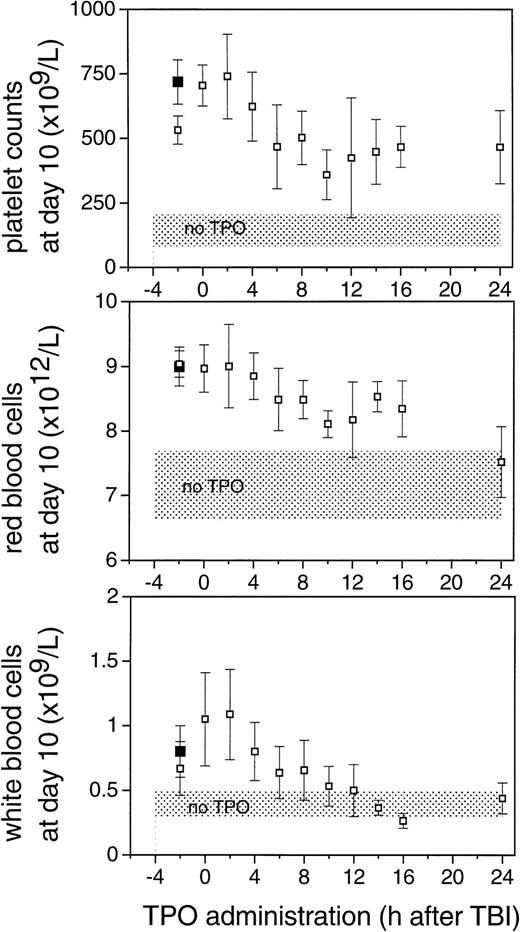
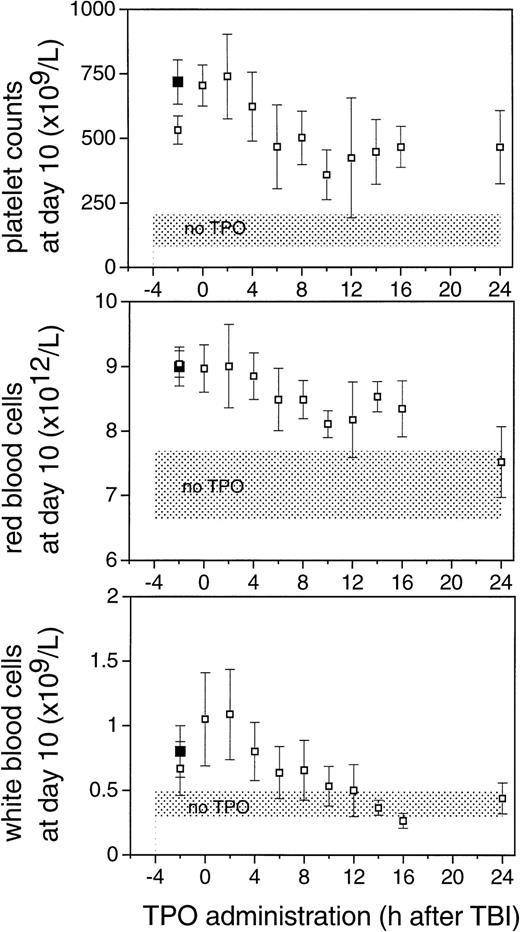
The effect of the time of administration of 0.3 μg TPO IP on peripheral blood cell counts 10 days after 6 Gy TBI. Values are means ± SD. Shaded areas represent the mean ± SD of 14 control mice. The closed squares (▪) are counts from mice treated with 30 μg of mTPO 2 hours before irradiation.
The effect of the time of administration of 0.3 μg TPO IP on peripheral blood cell counts 10 days after 6 Gy TBI. Values are means ± SD. Shaded areas represent the mean ± SD of 14 control mice. The closed squares (▪) are counts from mice treated with 30 μg of mTPO 2 hours before irradiation.
To establish the optimal time point for TPO administration, mice were injected IP with a single dose of TPO at various intervals before or after 6 Gy TBI. Based on Fig 1, the time dependence of the effect of TPO treatment was evaluated 10 days after TBI. The effect of TPO was consistently optimal if TPO was administered IP 0 or 2 hours after TBI (Fig 3). Progressively lower counts were observed when TPO was injected at later time points; platelet counts at day 10 after TBI did not significantly differ between mice treated 6 hours or later after TBI, whereas the difference between later groups and the 0- and 2-hour groups were highly significant (P < .003).
A similar time dependence was observed for white and red blood cell counts. For the white blood cells, TPO administration 10 hours or later after TBI resulted in counts not significantly different from control mice while being significantly lower than the 0- and 2-hour treatment groups. The effect on white blood cell regeneration could be attributed to differences in neutrophilic granulocytes and monocytes. Flow cytometric analysis of peripheral blood cells 10 days after irradiation performed in two experiments revealed a major increase in the numbers of ER-MP20–positive granulocytes and monocytes in the TPO treated mice, whereas the number of CD4/CD8-positive T cells was similar in TPO treated mice compared with controls (data not shown), thus showing the myeloid nature of the white blood cell response. Also for red blood cells a gradual decline in effectiveness of TPO with later administration of TPO was observed. We noted that there was no difference between the effects of TPO administration at 0 hours and at 2 hours after TBI, whereas TPO administration 2 hours before TBI resulted in consistently lower levels of platelets and white blood cells at day 10. The hypothesis that pharmacological levels of TPO in the first hours after TBI are required was initially evaluated by administration of a very high dose of TPO before TBI to examine whether an efficacy could be reached similar to that obtained by TPO early after irradiation. Administration of 30 μg TPO IP 2 hours before TBI was as effective as 0.3 μg IP 2 hours after TBI (Table 1 and Fig 3). The dose of 0.3 μg/mouse IP 2 hours before irradiation was significantly less effective compared with the 30-μg dose in alleviating the thrombocyte nadir (P = .03) (Table 1 and Fig3).
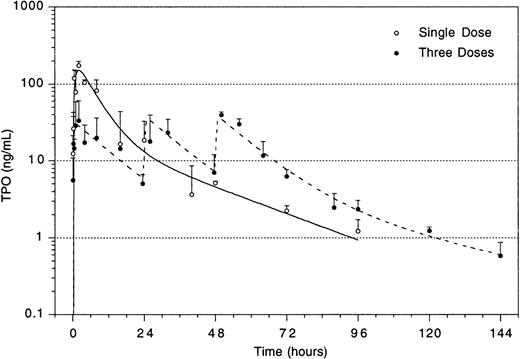
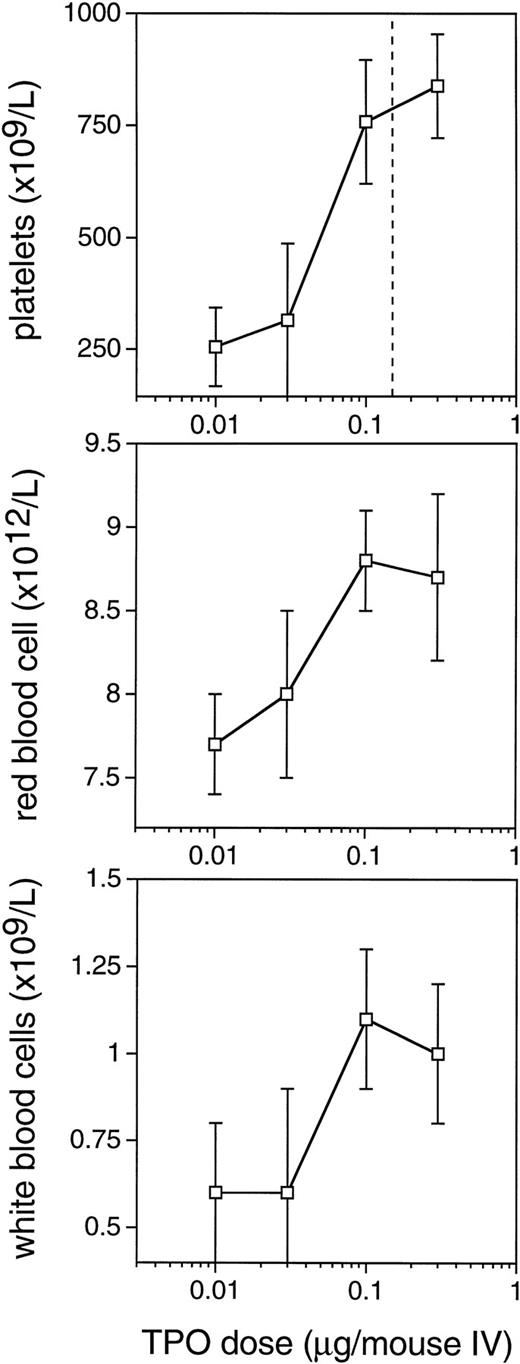
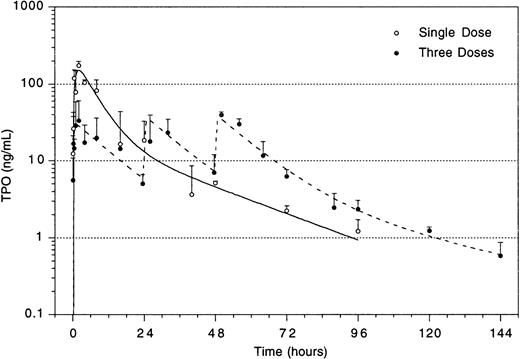
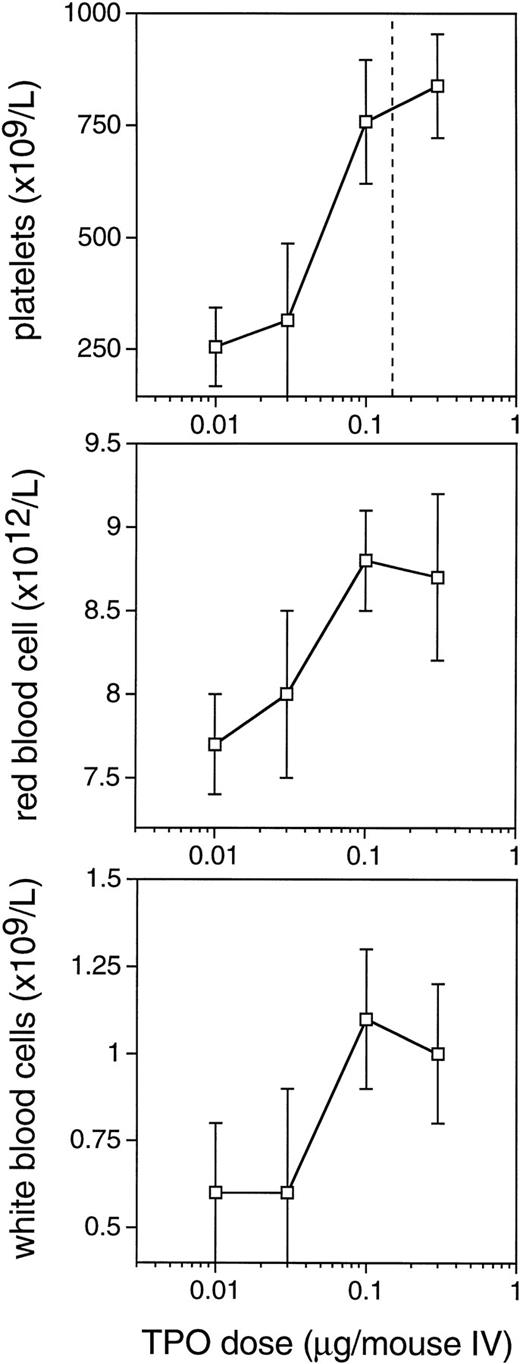
The improved efficacy of TPO when administered early and the decline in the efficacy at time points later than 4 hours after TBI led us to speculate that relatively high levels of TPO in the first 2 hours after administration would be of decisive importance for its efficacy. A pharmacokinetic analysis of plasma levels after IP injection of 0.3 μg TPO revealed that peak levels of 29 ng/mL were reached 2 to 2.5 hours after administration, with a terminal half-life of 35 hours and a clearance of 27 mL/h/kg. After the maximum value, there was an initial steep decline followed by a slower wash-out phase (Fig4). On the basis of these data, in combination with those of Fig 3, we postulated that the multilineage efficacy of TPO was dependent on a threshold level of TPO within the first few hours after TBI. The time course and the plasma level required were accurately assessed using IV administration of TPO (Table2 and Fig 5). IV administration also showed that early treatment (0 and 2 hours after TBI) was considerably more effective in stimulation of platelet level regeneration than treatment 24 hours after TBI. The multilineage effects seen with early IP treatment were also observed with early IV administration, and were similarly lost if TPO was administered 24 hours after TBI (Table 2). Lowering the dose of TPO to 0.1 μg/mouse did not affect the results, but at 0.03 μg/mouse or lower efficacy was lost, thus demonstrating a threshold TPO level required to achieve optimal multilineage efficacy (Fig 5).
Plasma level of TPO after IP administration of 3 doses of 0.3 μg/mouse separated by 24 hours each (dotted lines), and of a single dose of 0.9 μg/mouse (solid line). For explanation, see text.
Plasma level of TPO after IP administration of 3 doses of 0.3 μg/mouse separated by 24 hours each (dotted lines), and of a single dose of 0.9 μg/mouse (solid line). For explanation, see text.
Dose-response curves for the multilineage effect of TPO administered IV immediately after 6 Gy TBI. Peripheral blood cell counts 10 days after 6 Gy TBI. Values are means ± SD, n = 6 for the doses of 0.01, 0.03, and 0.1 μg and n = 12 for the dose 0.3 μg/mouse. The vertical line in the upper panel represents the dose level of 0.15 μg/mouse, which results in 50% saturation of c-mpl on platelets. The placebo control levels coincide with the level at which the horizontal axis is set.
Dose-response curves for the multilineage effect of TPO administered IV immediately after 6 Gy TBI. Peripheral blood cell counts 10 days after 6 Gy TBI. Values are means ± SD, n = 6 for the doses of 0.01, 0.03, and 0.1 μg and n = 12 for the dose 0.3 μg/mouse. The vertical line in the upper panel represents the dose level of 0.15 μg/mouse, which results in 50% saturation of c-mpl on platelets. The placebo control levels coincide with the level at which the horizontal axis is set.
Efficacy of TPO after fractionated irradiation.
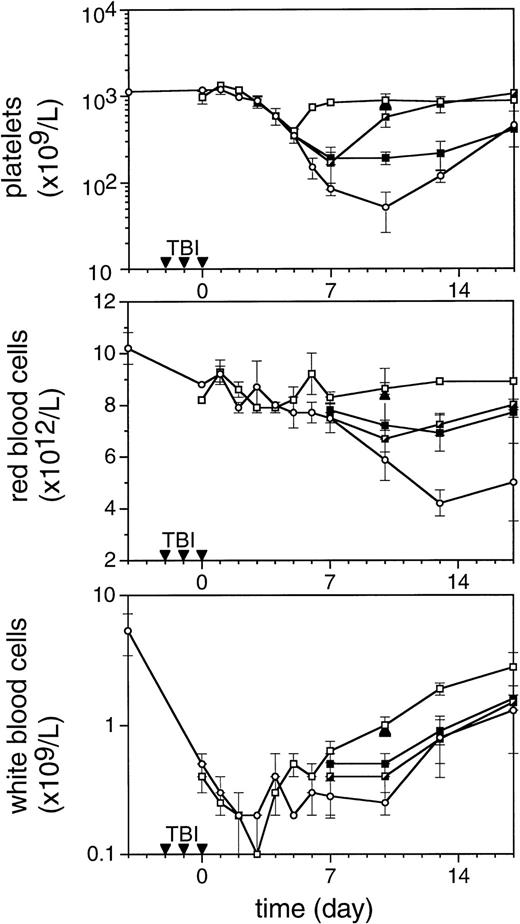
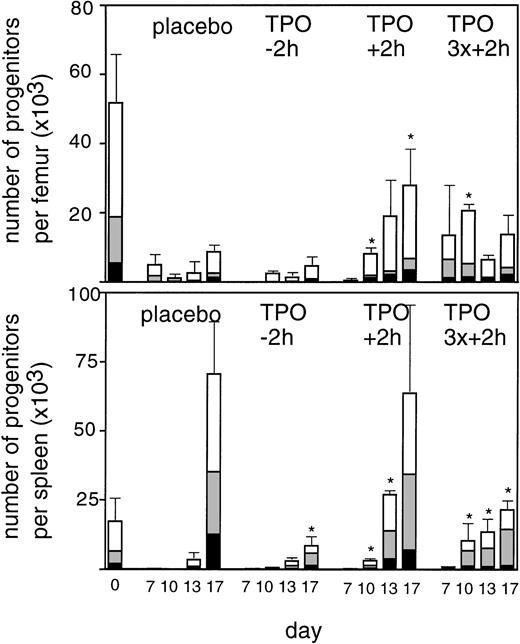
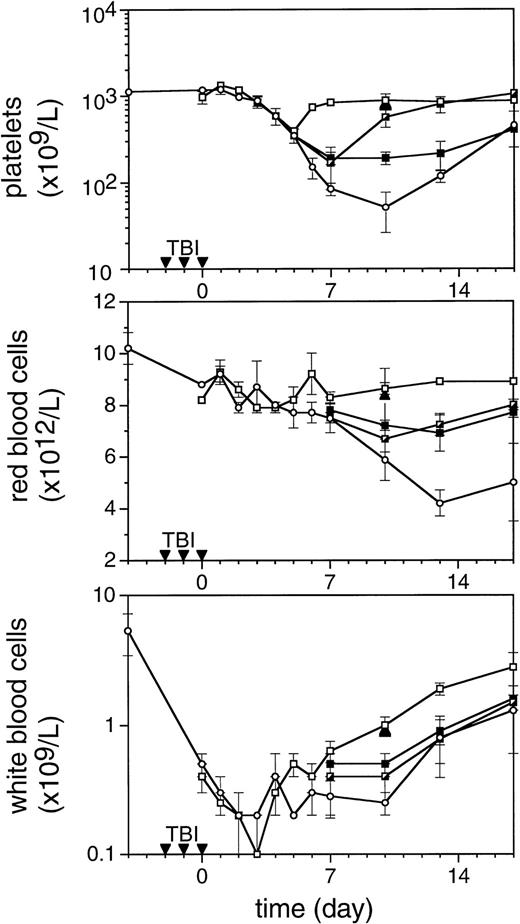
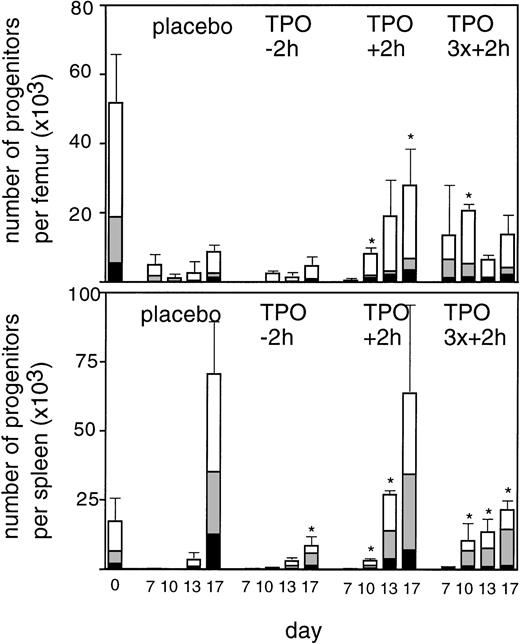
To enable assessment of immature hematopoietic cells by short-term transplantation assays, which is technically not feasible shortly after 6 Gy TBI due to the very low frequency of residual immature cells, we amplified the TPO response by adjusting the model by fractionation of a total dose of 9 Gy TBI in three equal doses separated by 24 hours. This regimen induced a slightly more profound pancytopenia than the single dose of 6 Gy TBI (Fig 6). Fractionated TBI would also more closely resemble the protracted cytotoxic insult administered to chemotherapy patients. Groups of mice received either no TPO, or were treated with 0.9 μg TPO 2 hours after the last fraction of TBI, 0.3 μg TPO 2 hours after each fraction of TBI, or 0.9 μg TPO 2 hours before the first fraction of TBI. Pharmacokinetic analysis of repetitive IP administration (three doses of 0.3 μg/mouse, each separated by 24 hours) did not reveal accumulation of TPO plasma levels. The dose of 0.9 μg/mouse resulted in higher peak plasma levels (151 ng/mL) without affecting the terminal half-life or clearance (Fig 4). TPO administered 2 hours after each fraction of radiation completely prevented thrombopenia (Fig 6 and Table3). This schedule did not prevent the severe reduction in neutrophils, but accelerated their recovery to normal values. Platelet counts and red and white blood cells were significantly lower in the group treated with 0.9 μg TPO 2 hours after the last fraction compared with 0.3 μg TPO 2 hours after each fraction. Administration of TPO 2 hours before the first fraction in a dose of 0.9 μg/mouse was largely ineffective. The effect of TPO on blood cell regeneration was also reflected at the level of progenitors of different blood cell lineages (Fig 7). Placebo mice and mice treated with 0.9 μg/mouse 2 hours before TBI displayed very low levels of progenitors even at day 13 after TBI, before progenitor cell reconstitution resulted in a large overshoot, especially manifest in the spleen 3 weeks after TBI. In contrast, the schedule optimal for blood cell regeneration (2 hours after each fraction) also led to a rapid normalization of progenitor cells in the spleen without the characteristic large overshoot in the placebo controls (P = .01 at day 17) and in the mice treated with the suboptimal TPO dose schedule. In the fractionated radiation model, administration of 30 μg of TPO 2 hours before the first radiation fraction was as effective as TPO administered 2 hours after each dose of radiation (Table 3 and Fig 6).
The effect of TPO on hematopoietic regeneration after 9 Gy TBI in mice. The mice were irradiated with three fractions (▾) of 3 Gy with 24-hour intervals. In the upper panel platelet regeneration is depicted, in the middle panel red blood cell regeneration is shown, and in the lower panel white blood cell regeneration is shown. (□), mice treated with three doses of 0.3 μg/mouse of mTPO IP 2 hours after each fraction of TBI; (┌), mice treated with 0.9 μg IP 2 hours after the last fraction of TBI (day 0); (▪), mice treated with 0.9 μg IP 2 hours before the first fraction of TBI; (○), control mice. Data are given as means ± SD, three mice per data point per group. The closed triangles (▴) are counts from mice treated with 30 μg of mTPO IP 2 hours before irradiation (n = 3).
The effect of TPO on hematopoietic regeneration after 9 Gy TBI in mice. The mice were irradiated with three fractions (▾) of 3 Gy with 24-hour intervals. In the upper panel platelet regeneration is depicted, in the middle panel red blood cell regeneration is shown, and in the lower panel white blood cell regeneration is shown. (□), mice treated with three doses of 0.3 μg/mouse of mTPO IP 2 hours after each fraction of TBI; (┌), mice treated with 0.9 μg IP 2 hours after the last fraction of TBI (day 0); (▪), mice treated with 0.9 μg IP 2 hours before the first fraction of TBI; (○), control mice. Data are given as means ± SD, three mice per data point per group. The closed triangles (▴) are counts from mice treated with 30 μg of mTPO IP 2 hours before irradiation (n = 3).
Regeneration of progenitor cells in bone marrow (upper panel) and spleen (lower panel) at 7, 10, 13, and 17 days after the last fraction of TBI in mice irradiated with three fractions of 3 Gy with 24 hour intervals. (▪), GM-CFU per femur or spleen; (▧), BFU-E; (□), CFU-Megs per femur or spleen. Placebo, control mice; TPO-2h, mice treated with 0.9 μg TPO 2 hours before the first fraction of TBI; TPO+ 2h, mice treated with 0.9 μg 2 hours after the last fraction of TBI; TPO3x+2h, mice treated with three doses of 0.3 μg/mouse 2 hours after each fraction of TBI, three mice per data point. SDs were calculated for the sum of the individual colonies per mouse. *Significantly different from time matched controls (P < .05), **(P < .005).
Regeneration of progenitor cells in bone marrow (upper panel) and spleen (lower panel) at 7, 10, 13, and 17 days after the last fraction of TBI in mice irradiated with three fractions of 3 Gy with 24 hour intervals. (▪), GM-CFU per femur or spleen; (▧), BFU-E; (□), CFU-Megs per femur or spleen. Placebo, control mice; TPO-2h, mice treated with 0.9 μg TPO 2 hours before the first fraction of TBI; TPO+ 2h, mice treated with 0.9 μg 2 hours after the last fraction of TBI; TPO3x+2h, mice treated with three doses of 0.3 μg/mouse 2 hours after each fraction of TBI, three mice per data point. SDs were calculated for the sum of the individual colonies per mouse. *Significantly different from time matched controls (P < .05), **(P < .005).
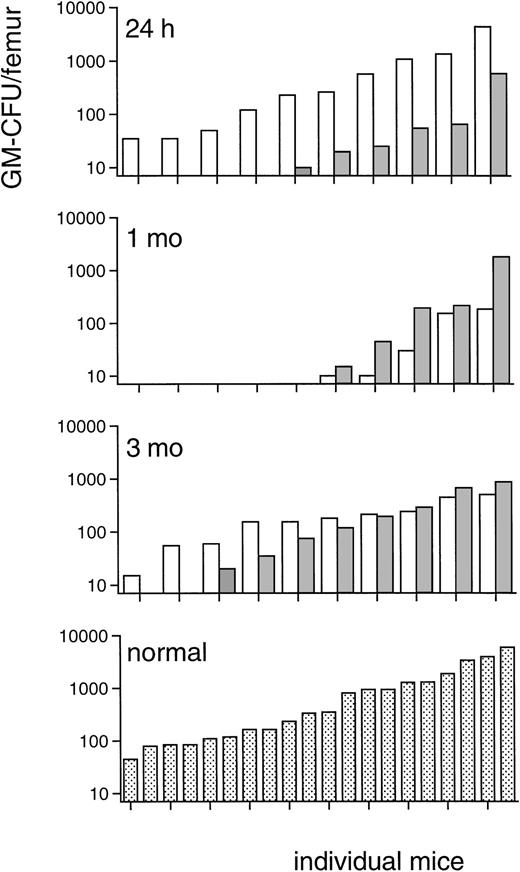
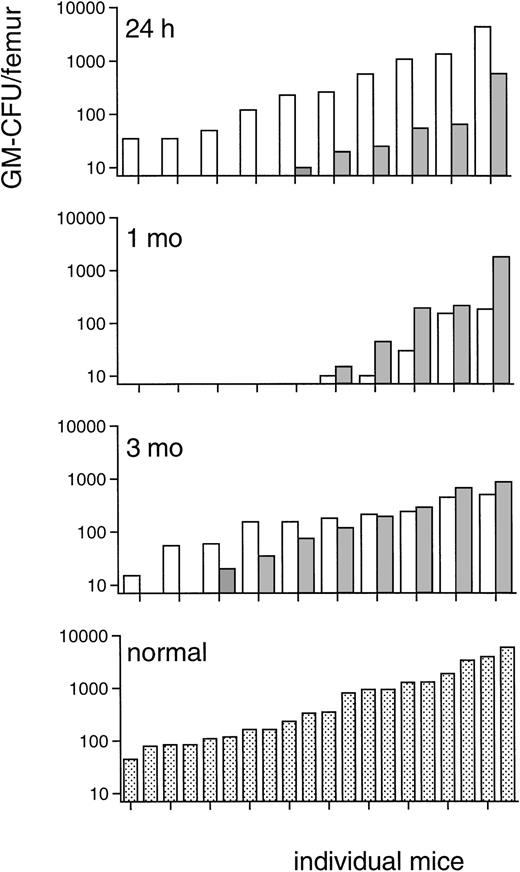
Because the progenitor cell data of irradiated TPO-treated mice in this fractionated TBI regimen indicated a prominent effect of TPO on immature bone marrow BM cells, CFU-S-13 were enumerated, the MRA of bone marrow of the TPO-treated mice assessed, and progenitor cells assayed 24 hours after the last TBI fraction (Table4). The numbers of CFU-S-13 of mice treated with TPO 2 hours after each dose of irradiation were approximately 14-fold those of the placebo control mice (Table 4). The progenitor cell content of the bone marrow 24 hours after TBI was also significantly increased (Table 4), GM-CFU numbers per femur approximately 4-fold, BFU-E numbers 65-fold, and CFU-Meg 20-fold those of placebo control mice, which grossly corresponds to the efficacy of TPO treatment along those lineages. The MRA of TPO-treated mice, defined as secondary GM-CFU in the bone marrow of lethally irradiated recipients, was more than one order of magnitude reduced in the TPO-treated mice compared with placebo-treated controls (Fig8). This result showed that the TPO-stimulated increase of CFU-S-13 and progenitor cells occurred at the expense of the more immature MRA cells, which were proportionally depleted. Although day 13, which is the time of the peripheral blood count nadirs at which white blood cells were undetectable in both groups of lethally irradiated recipients, is not the most suitable time interval to study the impact of this shift among the immature bone marrow cells on peripheral blood cell reconstitution, the difference in repopulating ability was also clearly reflected in the erythrocyte as well as the thrombocyte counts of the lethally irradiated recipients used for the MRA assay (measured in one of the two experiments from which the upper panel of Fig 8 was derived). The 10 recipients of bone marrow from TPO-treated mice reached erythrocyte counts all exceeding 4.4 × 1012/L (4.7 ± 0.3), and all but 1 reached thrombocyte counts of more than 10 × 109/L, as opposed to the 8 (of 10) surviving recipients of bone marrow from placebo-treated mice that had erythrocyte counts of 3.4 ± 0.9 × 1012/L and thrombocyte counts of 5.5 ± 2.8 × 109/L. These differences are highly significant (P = .002 andP = .006, respectively; Fisher’s exact test), thus illustrating the importance of spleen-repopulating cells in early reconstitution of peripheral blood counts in mice. Because a depletion of immature stem cells, such as measured by the MRA assay, could be potentially deleterious in the clinical setting, the effect of TPO on bone marrow progenitor cells was also evaluated after a more prolonged interval. Peripheral blood count and progenitor cell content of both spleen and femurs were evaluated 1 and 3 months after the 3 × 3 Gy irradiation protocol. Peripheral blood counts did not differ between TPO- or placebo-treated mice at 1 and 3 months, and also the femoral and spleen content of GM-CFU, BFU-E, and CFU-Meg was not different between both groups at either time point (data not shown). At these time intervals, we also measured the MRA of the bone marrow of the TPO- versus placebo-treated mice (Fig 8). MRA cells were similar in both groups of mice at 1 month after irradiation although still depleted compared with normal levels. At 3 months after irradiation, MRA cells in both groups had returned to subnormal levels, again without a difference between the recipient groups. On this basis, we concluded that the initial depletion of MRA of TPO-treated mice became replenished during long-term hematopoietic reconstitution from cells with, by definition, long-term repopulating ability. The protracted nature of MRA reconstitution, which has not been documented before, is noteworthy.
Marrow repopulating ability: GM-CFU, ranked in ascending order, on day 13 in femurs of recipients of bone marrow of mice treated with 0.3 μg TPO IP 2 hours after each fraction of 3 Gy TBI (▧) in comparison with those of recipients of control marrow (□) of mice that did not receive TPO. Upper panel: a total of 25 mice were injected with bone marrow (the content of 1 femur, 24 hours after TBI) of TPO-treated mice of which only 5 mice had more than 10 GM-CFU per femur, and 17 (surviving of 20 injected) mice with bone marrow of placebo-treated mice of which 10 had more than 10 GM-CFU per femur. Results from two experiments. This difference is highly significant (P = .01, Fisher’s exact test). Upper middle panel: 10 mice were injected with the standard 105 bone marrow cells from each of the groups 1 month after TBI, of which in each recipient group 9 mice survived and 5 mice had more than 10 GM-CFU per femur. Lower middle panel: 10 mice were injected with the standard 105 bone marrow cells from each of the groups 3 months after TBI, of which in the recipient group of the TPO-treated mice 1 mouse died before day 13 and 1 mouse had less than 10 GM-CFU/femur. Lower panel: 20 mice were injected with the standard 105bone marrow cells from normal, untreated donors for comparison.
Marrow repopulating ability: GM-CFU, ranked in ascending order, on day 13 in femurs of recipients of bone marrow of mice treated with 0.3 μg TPO IP 2 hours after each fraction of 3 Gy TBI (▧) in comparison with those of recipients of control marrow (□) of mice that did not receive TPO. Upper panel: a total of 25 mice were injected with bone marrow (the content of 1 femur, 24 hours after TBI) of TPO-treated mice of which only 5 mice had more than 10 GM-CFU per femur, and 17 (surviving of 20 injected) mice with bone marrow of placebo-treated mice of which 10 had more than 10 GM-CFU per femur. Results from two experiments. This difference is highly significant (P = .01, Fisher’s exact test). Upper middle panel: 10 mice were injected with the standard 105 bone marrow cells from each of the groups 1 month after TBI, of which in each recipient group 9 mice survived and 5 mice had more than 10 GM-CFU per femur. Lower middle panel: 10 mice were injected with the standard 105 bone marrow cells from each of the groups 3 months after TBI, of which in the recipient group of the TPO-treated mice 1 mouse died before day 13 and 1 mouse had less than 10 GM-CFU/femur. Lower panel: 20 mice were injected with the standard 105bone marrow cells from normal, untreated donors for comparison.
DISCUSSION
The present analysis shows that a single administration of TPO is capable of counteracting radiation-induced pancytopenia mediated through its effect on immature multilineage repopulating cells and their direct progeny, thereby promoting peripheral blood reconstitution of thrombocytes, erythrocytes, and granulocytes. Multilineage efficacy was critically dependent on time relative to TBI and a relatively high dose of TPO. In particular, the first few hours after TBI appeared to be important to elicit the multilineage response to TPO. Mechanisms involved included prevention of depletion of multilineage cells during the first 24 hours after TBI, a preferential stimulation of spleen-repopulating cells with short-term peripheral blood reconstituting ability at the transient expense of marrow-repopulating cells, and a threshold dose of TPO to overcome initial c-mpl–mediated clearance.
The mechanism by which TPO makes multilineage cells available for accelerated hematopoietic reconstitution remains to be further elucidated, as does their apparent functional depletion as a function of time after TBI in the absence of TPO. Multilineage TPO responsiveness declined sharply as a function of time after TBI, leaving a lineage dominant thrombopoietic response when administered 24 hours after TBI. This indicates that in the absence of TPO, multilineage TPO responsive cells are rapidly depleted or become inaccessible. The loss of the multilineage TPO response may have diverse and complex causes, which include apoptosis or radiation-induced cell death, differentiation along other hematopoietic lineages, inhibition mediated by cytokines produced in response to radiation injury, or inaccessibility of the immature cells for TPO due to stromal reactions to radiation. TPO has been shown to prevent apoptosis of immature hematopoietic cells35 and this might be a prime candidate mechanism to explain the short time interval available for optimally effective TPO intervention. In vitro, TPO does not confer a proliferative response to immature hematopoietic cells, but does so strongly in the presence of suitable other factors, eg, Kit ligand.36-42 The mechanisms involved might therefore also include activation of one or more cofactors required for the strong proliferative response observed in the present study. We also do not exclude that the effect of TPO on multilineage cells is augmented by the release of various cytokines by megakaryocytes43 44subsequent to stimulation by TPO, although the time frame observed makes such a mechanism not likely.
The lack of a leukocyte response 10 days after 6 Gy TBI in the presence of significantly more GM-CFU at 7 days in the 24-hour treatment group is noteworthy. The neutrophil response to TPO has been variable throughout the reported studies.6,9,11,45,46 We observed in a myelosuppression model in rhesus monkeys that TPO-stimulated GM-CFU reconstitution was not reflected in an increase in peripheral blood granulocytes which, however, could be brought out by coadministration of G- or GM-CSF.8,16 Thus, without exogenous CSFs, the myeloid progenitor cell effect of TPO after myelosuppression may remain unnoticed at the peripheral blood cell level, to which the short survival of circulating neutrophils might also contribute. These observations are fully consistent with the mouse data presented here. It is not inconceivable that some myelosuppression regimens may result in sufficiently high endogenous CSF levels for a significant neutrophil response to exogenous TPO alone, as has been observed in some studies.9,11 45
To establish the TPO dose level needed, a dose titration was performed using IV administration immediately after irradiation. The doses of 0.3 and 0.1 μg/mouse (12 and 4 μg/kg, respectively) gave identical results, whereas 0.03 (1.2 μg/kg) and 0.01 μg/mouse were largely ineffective. This observation is interpreted as evidence for a threshold level of TPO required for optimal efficacy. It was previously shown13,24 that IV injection of 125I-rmTPO into mice results in an initial sharp decline in plasma levels, followed by steady state clearance approximately 3 hours after IV injection.24 A lower dose does not influence the terminal half-life.13 After IV bolus injection, the initial rapid decline in plasma TPO levels is due to binding of TPO to c-mplon platelets and on cells in the spleen,24 whereas the slower terminal decline is likely related to uptake and clearance by c-mpl on platelets, spleen cells, and megakaryocytes, as well as nonspecific mechanisms.24,47 The initial binding and uptake of TPO to c-mpl is concentration dependent and becomes saturated at higher doses, leading to greater plasma TPO levels.48 This relationship between TPO pharmacokinetics and c-mpl levels has recently been studied in normal mice, which showed that an IV dose of approximately 6 μg/kg (≈0.15 μg/mouse) was needed to obtain an occupancy of 50% of c-mplsites (indicated in Fig 5). Doses greater than 6 μg/kg began to saturate this specific clearance mechanism, whereas lower doses failed to reach 50% receptor occupancy.48 This is consistent with a threshold level of TPO needed to overcome initial c-mpl–mediated clearance and to result in sufficient plasma TPO levels to achieve a maximal hematopoietic response. Doses larger than 6 μg/kg (0.15 μg/mouse) may not provide any greater efficacy. These data are consistent with the findings presented in Fig 5, showing that the hematopoietic recoveries after IV doses of 0.3 and 0.1 μg/mouse (12 and 4 μg/kg, respectively) were not different, whereas a dose of 0.03 μg/mouse (1.2 μg/kg) was suboptimal in preventing myelosuppression. Based on the previous IV pharmacokinetic data comparing TPO plasma levels in c-mpl knockout and normal mice,24 it was inferred that 40% of the exogenous TPO binds to c-mpl on platelets and 60% is available as free TPO in the plasma. Assuming a plasma volume of 1 mL in a 25-g mouse, the suboptimal dose of 0.03 μg/mouse results in a maximum level of 20 ng/mL and the effective dose of 0.1 μg/mouse in 60 ng/mL. Consequently, the minimum effective or threshold plasma level is in between those levels.
Fractionation of the dose of radiation and appropriate dosing of TPO was thought to amplify the TPO effect on immature cells to enable short-term transplantation assays. Such an approach would also be more representative of clinically used radiation regimens and of the protracted nature of cytoreductive treatment by means of chemotherapy. Also after fractionated TBI (3 × 3 Gy, 24 hours apart), the optimal dose and dose scheduling of TPO derived from the 6 Gy experiments, ie, 0.3 μg/mouse, 2 hours after each TBI fraction, prevented thrombopenia and promoted erythrocyte and leukocyte reconstitution. We noted that also the femoral and spleen progenitor cells (Fig 7) normalized rapidly and lacked the late overshoot in the spleen characteristic of the placebo controls. Because the range of stimulation by TPO was not lineage specific, we postulated that the effect was mediated by stimulation of multilineage cells. After transplantation of bone marrow into lethally irradiated recipients, the number of CFU-S-13 is a measure for relatively immature repopulating stem cells,49associated with the initial, short-term wave of hematopoietic reconstitution, which lasts for several months.50 By this assay it was shown that the multilineage effect of TPO administered 2 hours after TBI is mediated through stimulation of these immature cells and is already manifest 24 hours after the last fraction of TBI. The number of secondary in vitro clonogenic progenitors in the bone marrow of such recipients is a measure of the MRA of the graft, the primary cells being closely associated with those that provide sustained hematopoiesis after bone marrow transplantation.34 MRA, measured by enumeration of GM-CFU numbers in the bone marrow of mice injected with cells from TPO treated mice, was one to two orders of magnitude less than that in control mice. The increase of CFU-S-13 and the concomitant decrease of cells with MRA most likely indicates recruitment of multilineage short-term repopulating cells from a more immature ancestral population. This is the more conceivable from the magnitude of this effect (14-fold for CFU-S), which suggests three to four cell doublings. Because these occurred during the 3 days that elapsed from the first TPO administration to the time of the measurement, 24 hours after the last fraction of TBI, this would be in close agreement with the doubling time established previously27 for such immature cell populations during hematopoietic reconstitution. The decline in marrow-repopulating cells with the concomitant increase of spleen-repopulating cells could also be considered as a shift in homing pattern among these immature cells. To date, there is no evidence to suggest that such a shift may occur without cell divisions, whereas the ancestral position of the marrow-repopulating cells relative to the spleen colony-forming cells has been well documented.34,51 52
The depletion of MRA cells in the TPO-treated mice, measured 24 hours after TBI, was transient. Peripheral blood cell regeneration 1 and 3 months after three fractions of 3 Gy was not different in mice treated with TPO compared with controls and neither were the femoral and spleen in vitro colony-forming cell numbers. Assessment of the MRA at the same time intervals also did not show differences between the TPO-treated mice and the placebo group. We interpret these observations as replenishment of the MRA cells from a more ancestral cell population with, by definition, long-term repopulating ability, consistent with a model in which MRA cells are a transitory population intermediate to stem cells with long-term repopulating ability and the spleen-repopulating cells measured by the CFU-S-13 assay. To date, the effect of TPO treatment on long-term repopulating cells has not been quantitatively documented. Recently, it was reported that transplantation of bone marrow from TPO-treated, 3.5-Gy irradiated donor mice facilitated the 90-day survival of the recipient mice.53 However, this survival effect had become established already at the short-term hematopoietic reconstitution parameter of 30-day survival (in practice already within 17 days), closely associated50 to the CFU-S-13 assay used in the present study. Using such an experimental design to establish TPO effects on long-term repopulating cells would require a genetic marker capable of distinguishing between donor and recipient cells along multiple hematopoietic lineages.
In addition, we showed that TPO is effective if administered at a very high dose shortly before myelosuppressive TBI. The dose used, 30 μg/mouse (1.2 mg/kg), suprasaturates the c-mpl–mediated clearance mechanism (which makes pharmacokinetic measurements futile) and is not recommended for clinical use. We did not so far establish empirically the minimum effective dose required for prophylactic TPO administration, but such a dose could be derived on the assumption of maintaining plasma TPO levels of approximately 60 ng/mL in the first hours after TBI as calculated above. However, the observation has relevance for the further development of efficacious dose and dose scheduling regimens, especially in conjunction with chemotherapy, as well as in the area of radiation protection. The present study did not address propensity to hemorrhage and prevention of mortality, but rather was directed at mechanisms important to optimize efficacy. The benefit of TPO treatment shortly after much higher (“supralethal”) doses of TBI on survival and prevention of bleeding will be published separately.54
In vivo studies on TPO efficacy have yielded various results, in that in normal animals usually only a platelet response was obtained,6,7,55-59 whereas in myelosuppressed animals multilineage responses was the prevailing pattern,8,9,11-13,15,16,53,60,61 and after transplantation of limited numbers of stem cells no response was obtained at all.62 On the basis of this heterogeneity, it can be assumed that the response to exogenous TPO is determined by multiple factors. We already pointed out the importance of cotreatment with G-CSF or GM-CSF to make the TPO effect on GM-CFU reconstitution manifest in neutrophil numbers in the peripheral blood. The present study also identifies time relative to myelosuppression and dose of exogenous TPO as pivotal factors. In addition, the difference between normal and myelosuppressed animals indicates that the TPO response of immature cells might be dependent on the presence or activation of one or more cofactors. As already pointed out, TPO by itself does not induce in vitro a proliferative response in immature bone marrow cells, but does so strongly in synergy with, eg, Kit ligand.36-42Identification of the cofactor(s) which operate in irradiated or otherwise myelosuppressed animals to generate a proliferative response to TPO administration might therefore be highly relevant as well to improve the clinical TPO response.
Irrespective of the mechanisms involved, the optimal efficacy of TPO if administered within 2 hours after cytoreductive treatment places emphasis on the importance of dose and dose scheduling in clinical protocols of cancer treatment and/or after bone marrow transplantation. Indeed, the initial clinical experience did not demonstrate a major effect similar to those observed in the experiments described here.63-66 It is concluded that dosing similar to what has become conventional based on the G-CSF and GM-CSF experience is not optimal for TPO and that its efficacy can be substantially improved by achieving relatively high TPO levels shortly after cytoreductive treatment. This was attributable to the dual target cell nature of the TPO response, of which the important multilineage component is functionally depleted shortly after radiation exposure, and to the identified threshold plasma level of TPO required to overcome its initial c-mpl–mediated clearance.
ACKNOWLEDGMENT
The authors thank Els van Bodegem, Dorinde Kieboom-Pluimes, Hannie Busking-van der Lelie, Eric Stefanich, Tauri Senn, and Melinda Marian for technical assistance; and Dr A.W. Wognum for assistance with flow cytometric analysis.
Supported in part by The Netherlands Cancer Foundation Koningin Wilhelmina Fonds, the Dutch Organization for Scientific Research NWO, and Contracts of the Commission of the European Communities.
Address reprint requests to Gerard Wagemaker, PhD, Institute of Hematology, H Ee 1314, Erasmus University Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands; e-mail: wagemaker@hema.fgg.eur.nl.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.