Abstract
CD36 is an 88-kD glycoprotein involved in the cytoadherence ofPlasmodium falciparum–parasitized erythrocytes (PE) to endothelial cells. The molecular mechanisms involved in CD36-dependent cytoadherence were examined by expressing three CD36 homologues (human, murine, and rat) in COS-7 cells and observing their PE-binding characteristics over a pH range of 6.0 to 7.4 and following iodination of these receptors. PE binding to human CD36 was pH dependent, with peak binding at pH 6.8 to 7.0, and binding was unaffected by iodination. In contrast, PE adherence to murine and rat CD36 was insensitive to changes in pH, and iodination significantly reduced binding. We further show that the differences observed in the binding phenotype of human and rodent CD36 can be attributed to a single residue. Site-directed mutagenesis of the histidine at position 242 of human CD36 to tyrosine (found in rodent CD36) conferred the binding phenotype of rodent CD36 onto human CD36. Furthermore, substitution of the tyrosine at position 242 of rat CD36 for histidine conferred the binding phenotype of human CD36 onto rat CD36. These findings suggest that residue 242 is part of, or important to the conformation of, the PE-binding domain of CD36.
© 1998 by The American Society of Hematology.
PLASMODIUM FALCIPARUM malaria is the world’s most important parasitic infection accounting for an estimated 300 million cases and 1.5 to 2.7 million deaths annually.1 The central pathophysiologic event in falciparum malaria is the sequestration of parasitized erythrocytes (PE) in the microvascular beds of vital organs resulting in the disruption of local circulation and the manifestations of severe malaria.2 Sequestration of infected erythrocytes is mediated by specific ligand-receptor interactions. A number of endothelial cell receptors have been implicated in this interaction, including intercellular cell adhesion molecule-1 (ICAM-1),3vascular cell adhesion molecule (VCAM),4E-selectin,4,5 chondroitin sulphate A,6,7thrombospondin,8 and CD36.9 Although the interaction of wild isolates of P falciparum with many of the implicated endothelial cell receptors is variable, almost all natural populations preferentially bind to CD36,10 even under conditions of shear stress.11
CD36 is an 88-kD cell surface glycoprotein that is expressed on monocytes, macrophages, endothelial cells, platelets, erythroid precursors, and breast epithelium.12,13 CD36 together with lysosomal integral membrane protein II (LIMP II), CD36 and LIMP II analogous-1 (CLA-1),14 class B scavenger receptor 1(SR-B1),15 and epithelial membrane protein (EMP)16 constitute a novel gene family. CD36 is composed of a single polypeptide chain and, based on hydropathy analysis,17,18 is predicted to contain transmembrane domains at both the carboxyl and amino termini with evidence of palmitoylation of the cysteine residues at both termini.19Although little is definitively known about the structure and function of CD36, many roles have been ascribed to it, including an endothelial cell and macrophage receptor for P falciparum–infected erythrocytes20; a receptor for collagen type I and IV or thrombospondin21; an interaction with platelet agglutinating protein p3722; a receptor for the uptake of the oxidatively modified low-density lipoprotein23; a receptor for neutrophils undergoing apoptosis24; a cell marker in endothelial cancer25; and a receptor that participates in signaling via a physical association with thesrc family of tyrosine kinases.26
Although it is known that the CD36 receptor family binds a variety of ligands, the molecular mechanisms underlying these various interactions are poorly understood. The objective of this study was to characterize the molecular basis for the interaction of PEs with CD36. We examined the binding of human PEs to CD36 from human (hCD36),27murine (mCD36),28 and rat (rCD36),18 which share over 90% sequence homology at the amino acid level. mCD36 has previously been shown to participate in the binding and uptake of oxidized low-density lipoprotein (ox LDL),28 and rCD36 (or FAT) has been shown to mediate the transport of long-chain fatty acids.18,29 Both mCD36 and rCD36 are not recognized by OKM5, a cytoadherence inhibiting monoclonal antibody.30However, we have recently shown that both mCD36 and rCD36 support PE binding. Human, murine, and rat CD36 possess six conserved cysteine residues located in a 90 amino acid cysteine-rich region (residues 243-333). Cysteine replacement mutagenesis studies in our laboratory have shown that five of these six cysteine residues are essential for avid cytoadherence, suggesting that this cysteine-rich region represents a discrete domain important for CD36-mediated adhesion of PEs (unpublished data).
In this study, we show that PE binding to human CD36 is pH dependent but unaffected by iodination of the receptor. In contrast, PE binding to murine and rat CD36 is pH-independent but inhibited by iodination. We examined the amino acid sequences of the homologues to determine which divergent residues may be responsible for the different binding characteristics observed. We then show that a single amino acid substitution within the previously identified cysteine-rich domain can confer the binding phenotype of murine or rat CD36 onto human CD36 and the binding phenotype of human CD36 onto rat CD36.
MATERIALS AND METHODS
Chemicals and antibodies.
RPMI 1640 and fetal bovine serum were from Life Technologies (Mississauga, Ontario, Canada). COS-7 cells were purchased from ATCC (Rockville, MA). pGEM7 was from Promega (Madison, WI). pcDNAI/amp was from Invitrogen (San Diego, CA). DEAE-dextran, chloroquine, dimethylsulfoxide (DMSO), and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG were from Sigma (St Louis, MO). Iodobeads were from Pierce (Rockford, IL). Anti-CD36 monoclonal antibodies no. 25, no. 30, and no. 32 were the kind gift of Dr C. Ockenhouse (WRAIR, Washington, DC). cDNAs were the kind gifts of Dr B. Seed (hCD36; Harvard University, Cambridge, MA), Dr G. Endemann (mCD36; Scios Nova Inc, Mountain View, CA), and Dr N. Abumrad (rCD36; SUNY, Syracuse, NY).
Cultures.
P falciparum cultures of the ITG clone were grown in A+ blood and serum obtained by venupunture of volunteers. Cultures were maintained by the method of Trager and Jensen31 using RPMI 1640 supplemented with 10% human serum and 50 μmol/L hypoxanthine.
Site-directed mutagenesis.
Segments of hCD36 DNA containing nucleotides −210 to 549 and 550 to 1872, and the complete rCD36 cDNA were subcloned into pGEM7, and single-stranded template was generated by the method of Kunkel.32 The codon for the histidine residue at position 242 and the codon for phenylalanine at position 97 of hCD36 were mutagenized to a codon for tyrosine, and the tyrosine residue at position 242 of rCD36 was mutagenized to a codon for histidine, using the appropriate templates. The presence of the mutations was verified via the dideoxy chain termination method using the Sequenase PCR product sequencing kit (United States Biochemical Corporation, Cleveland, OH). The mutated hCD36 fragment was then subcloned back into pcDNAI/amp such that the entire 5′ to 3′ untranslated sequence was restored. The mutated rCD36 cDNA was also subcloned back into pcDNAI/amp.
Transient transfection.
Plasmid DNA for transfection was prepared using Qiagen columns (Qiagen, CA) according to manufacturer’s instructions. COS-7 cells were plated at 30% confluency in 12-well culture plates and incubated at 37°C in 5% CO2 overnight. The cells were then transiently transfected using DNA (1 μg/mL) in RPMI plus DEAE-dextran (0.2 mg/mL) and 100 μmol/L chloroquine for 2 hours at 37°C in 5% CO2, followed by exposure to 10% DMSO in RPMI for 3 minutes.32 The DMSO solution was removed, and RPMI with 10% fetal bovine serum was added. Cell surface expression was confirmed, 72 hours post transfection, by immunofluoresence using a pool of monoclonal antibodies (no. 25, no. 30, and no. 32) to human CD36 that recognize hCD36, rCD36, and the human and rat CD36 mutants. Expression of murine CD36, which is not recognized by the pool of monoclonal antibodies, was inferred by ability to cytoadhere. The level of cell surface expression of human and rat CD36 and all CD36 mutants was quantitated by use of confocal microscopy and fluorescence-activated cell sorter (FACS) analysis.
Cytoadherence assays.
Seventy-two hours after transfection, COS-7 cells were fixed with 4% formalin in phosphate buffer saline (PBS) for 10 minutes and were then rinsed three times with Bis Tris saline (BTS; 25 mmol/L Bis Tris, 135 mmol/L NaCl, pH 6.8). P falciparum–infected erythrocytes were pelleted, resuspended in BTS and diluted to 5% hematocrit and 5% parasitemia in a total volume of 300 μL of BTS per well. The sample was kept in suspension by continuous agitation using a 3D Rotator (Lab Line, Melrose Park, IL) and was incubated with the target cells for 90 minutes. Nonadherent cells were removed by aspiration, the well was rinsed with 400 μL aliquots of BTS with agitation, and the process was repeated until no erythrocytes were present between COS-7 cells. Adherence was quantitated by direct microscopic observation of COS-7 cells. The number of adherent erythrocytes per transfected cell (defined as supporting the adherence of at least 3 PEs) was counted for at least 40 representative COS-7 cells per well. Transfection efficiency of COS-7 cells was between 40% and 60%. Transfected COS-7 cells did not support adherence of uninfected red cells. All cytoadherence assays were performed in triplicate and repeated at least three times. The results are presented as the mean with standard deviation of a typical experiment performed in triplicate.
In cytoadherence assays to determine the optimum pH binding, the pH of BTS was adjusted using concentrated NaOH or HCl. The iodination studies were done at pH 6.833 using fixed cells. The transfected COS-7 cells were fixed with 4% formalin and then subjected to either no additions; the addition of 10 mmol/L NaI in phosphate-buffered saline (PBS); the addition of an iodobead in PBS; or the addition of both 10 mmol/L NaI and a single iodobead in PBS. After a 5-minute incubation at room temperature with gentle agitation, the bead was removed and the wells were thoroughly washed three times with BTS before use in the adhesion assays.
Statistical analysis.
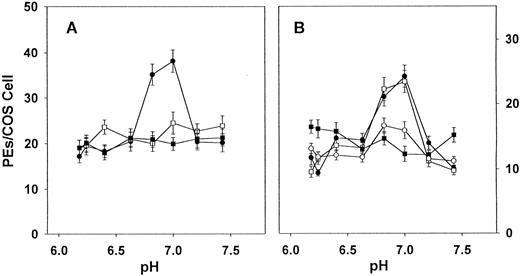
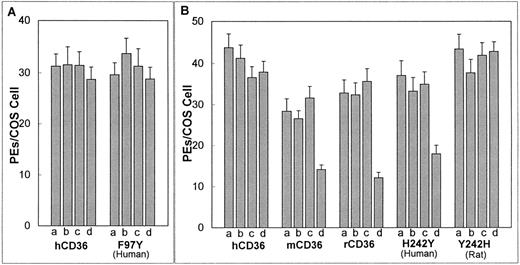
Analysis of variance (ANOVA) was used to determine whether pH is a factor in PE cytoadherence to CD36 (Fig 1A and B). t-Test analysis was performed to examine differences in cytoadherence levels between homologues, mutants, and between control and treated wells in the iodination experiments (Figs 1 and2).
Effect of pH on the cytoadherence of PEs to hCD36, mCD36, rCD36, H242Y, and Y242H. Adhesion assays were performed using transiently transfected COS-7 cells expressing human CD36, murine CD36, rat CD36, H242Y (human CD36 with residue 242 altered to tyrosine), or Y242H (rat CD36 with residue 242 altered to histidine). Infected blood suspended in BTS (pH adjusted by HCl or NaOH) to a 5% hematocrit and 5% parasitemia was added and incubated under continuous agitation for 90 minutes. After washing, adherence was quantitated microscopically by counting the number of adherent erythrocytes per transfected COS cell for at least 40 representative COS-7 cells per data point. Values indicated are for (A) human CD36 (•), rat CD36 (▪), and H242Y (□) and for (B) human CD36 (•), rat CD36 (▪),mouse CD36 (○), and Y242H (□). The values are mean values with standard deviations represented by error bars. Similar results were obtained in three independent experiments. Cytoadherence to human CD36 and Y242H is significantly pH dependent (P < .005, by ANOVA).
Effect of pH on the cytoadherence of PEs to hCD36, mCD36, rCD36, H242Y, and Y242H. Adhesion assays were performed using transiently transfected COS-7 cells expressing human CD36, murine CD36, rat CD36, H242Y (human CD36 with residue 242 altered to tyrosine), or Y242H (rat CD36 with residue 242 altered to histidine). Infected blood suspended in BTS (pH adjusted by HCl or NaOH) to a 5% hematocrit and 5% parasitemia was added and incubated under continuous agitation for 90 minutes. After washing, adherence was quantitated microscopically by counting the number of adherent erythrocytes per transfected COS cell for at least 40 representative COS-7 cells per data point. Values indicated are for (A) human CD36 (•), rat CD36 (▪), and H242Y (□) and for (B) human CD36 (•), rat CD36 (▪),mouse CD36 (○), and Y242H (□). The values are mean values with standard deviations represented by error bars. Similar results were obtained in three independent experiments. Cytoadherence to human CD36 and Y242H is significantly pH dependent (P < .005, by ANOVA).
Effect of CD36 iodination on the cytoadherence of PEs. Adhesion assay was as in Fig 1 with all assays performed at pH 6.8. Iodination was performed by subjecting the transfected COS-7 cells to no additions (a); addition of 10 mmol/L NaI in PBS (b); addition of an iodobead (c); or the addition of both 10 mmol/L NaI and an iodobead in PBS (d). Results represent the mean with standard deviation indicated by error bars. Similar results were obtained in three independent experiments. Values indicated are for (A) human CD36 and F97Y (human CD36 with residue 97 substitute for tyrosine) and for (B) human CD36, murine CD36, rat CD36, H242Y (human CD36 with residue 242 substituted for tyrosine), and Y242H (rat CD36 with residue 242 substituted for histidine). Iodination significantly reduces the level of cytoadherence to murine CD36, rat CD36, and H242Y (P < .01,t-test).
Effect of CD36 iodination on the cytoadherence of PEs. Adhesion assay was as in Fig 1 with all assays performed at pH 6.8. Iodination was performed by subjecting the transfected COS-7 cells to no additions (a); addition of 10 mmol/L NaI in PBS (b); addition of an iodobead (c); or the addition of both 10 mmol/L NaI and an iodobead in PBS (d). Results represent the mean with standard deviation indicated by error bars. Similar results were obtained in three independent experiments. Values indicated are for (A) human CD36 and F97Y (human CD36 with residue 97 substitute for tyrosine) and for (B) human CD36, murine CD36, rat CD36, H242Y (human CD36 with residue 242 substituted for tyrosine), and Y242H (rat CD36 with residue 242 substituted for histidine). Iodination significantly reduces the level of cytoadherence to murine CD36, rat CD36, and H242Y (P < .01,t-test).
RESULTS
Altering pH.
It has been observed that the interaction of human PEs with target cells bearing a mixed population of receptors has a notable pH dependence,34 and studies using CHO cell lines transfected with human CD36 or ICAM-1 suggest that it is the CD36 contribution to cytoadherence that is pH dependent.33 To determine whether the CD36 homologues, mCD36 and rCD36, also show this pH dependency, we tested the level of cytoadherence to all three homologues over a pH range of 6.0 to 7.4. hCD36 showed pH-dependent binding (P < .001, by ANOVA), with peak binding at pH 6.8 to 7.0. In contrast, no pH optimum was observed for mCD36 and rCD36 (Fig 1A and B).
Iodination of CD36.
Tyrosine residues are converted to di-iodotyrosine in the presence of iodide and an oxidizing agent (iodogen). To test whether iodination of tyrosines present in CD36 has an effect on cytoadherence of PEs, human, murine, and rat CD36 were iodinated before their interaction with PEs. Iodination did not affect cytoadherence of PEs to hCD36 (Fig 2A and B), but this procedure significantly reduced binding of PEs to murine and rat CD36 (P < .01, by t-test; Fig 2B).
Mutagenesis of hCD36 and rCD36.
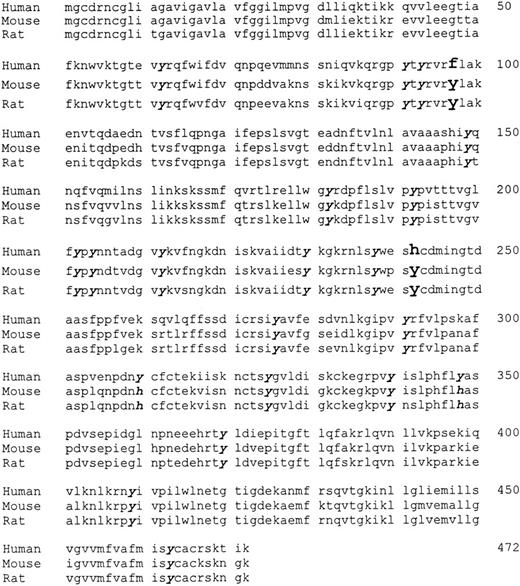
The pH and iodination results suggested that one or more histidines might be involved in the human CD36-PE interaction, whereas one or more tyrosines might be involved in the murine and rat CD36-PE interaction. A comparison of the aligned sequences of the three homologues (Fig 3; GenBank M24795, L23108, L19658) indicated that tyrosines at position 97 and 242 in the murine and rat CD36 are replaced by phenylalanine and histidine, respectively, in the human sequence. The histidine at position 242 is the only histidine in hCD36 that is not conserved in mCD36 and rCD36. To determine if these residues are participating in the CD36-PE interaction, site-directed mutagenesis of hCD36 and rCD36 was performed to change the phenylalanine at position 97 in hCD36 to a tyrosine (to produce mutant F97Y), the histidine at position 242 of hCD36 to a tyrosine (to produce mutant H242Y), and the tyrosine at position 242 of rCD36 to a histidine (to produce mutant Y242H).
Predicted amino acid sequences of human, murine, and rat CD36. Tyrosine residues found in rodent CD36 that are not present in human CD36 are indicated in enlarged and bolded script, and conserved tyrosine residues are in bolded italicized script. GenBank accession numbers are M24795 (hCD36),27L23108(mCD36),28L19658 (rCD36).18
Predicted amino acid sequences of human, murine, and rat CD36. Tyrosine residues found in rodent CD36 that are not present in human CD36 are indicated in enlarged and bolded script, and conserved tyrosine residues are in bolded italicized script. GenBank accession numbers are M24795 (hCD36),27L23108(mCD36),28L19658 (rCD36).18
Cell surface expression of the three mutants was equal to that of human and rat CD36 as determined by confocal microscopy and FACS analysis (data not shown). All three mutants supported avid cytoadherence of PEs. As was observed in the case of native hCD36, PE binding to F97Y was not reduced following iodination of this mutant (Fig 2A). In contrast, cytoadherence of PEs to H242Y was significantly reduced upon iodination (P < .01, by t-test; Fig 2B). Cytoadherence to native rCD36 is reduced following iodination; however, PE binding to the mutant Y242H was not affected by iodination (Fig 2B).
The ability of PEs to bind to H242Y and Y242H over a pH range was determined. Although H242Y is only a single residue different from hCD36, PE binding to H242Y was pH-independent, similar to rodent CD36 (Fig 1A). Binding of PEs to Y242H, which differs from rCD36 also by a single residue, became pH dependent (P < .005, by ANOVA; Fig1B) resembling hCD36. At the pH optimum of 7.0, binding to hCD36 was significantly greater than to rCD36 (P < .001,t-test) or to the human mutant, H242Y (P < .001,t-test) but was not significantly different from the rat mutant Y242H. At the same pH optimum, binding to rCD36 was equivalent to H242Y but was significantly less than to Y242H (P < .001,t-test).
DISCUSSION
Human, murine, and rat CD36 show over 90% identity at the amino acid level. However, these homologues display different binding characteristics to P falciparum–infected erythrocytes under varying pH conditions and following iodination. These observations suggest that minor sequence differences might account for the observed binding disparities. The iodination process primarily affects tyrosines; therefore, we looked for sites in the homologues sequences where murine and rat CD36 had tyrosine-related substitutions. This only occurred in two sites, residues 97 and 242. We mutagenized residue 97 in hCD36 from a phenylalanine to a tyrosine but observed no decrease in PE cytoadherence upon iodination. This suggests that residue 97 is not responsible for the iodination sensitivity observed in rodent CD36.
The rodent sequences have a tyrosine at position 242, which is substituted by a histidine in hCD36. This is the only histidine present in the human sequence that is not conserved in rodent CD36. To determine if the presence of this histidine affected the CD36-PE interaction, we replaced the histidine at position 242 in hCD36 with the tyrosine found in rodent CD36. This substitution conferred iodination sensitivity to hCD36, indicating that the tyrosine at position 242 is responsible for the decrease in cytoadherence observed upon iodination of rodent CD36. When the same mutant was tested for PE cytoadherence over a pH range of 6.0 to 7.4, the pH-dependent binding observed with native hCD36 was absent. Thus, a single residue substitution at position 242 conferred the binding characteristics of rodent CD36, iodination sensitivity, and pH insensitivity onto human CD36.
To confirm the importance of residue 242 in the CD36-PE binding interaction, we replaced the tyrosine found in position 242 of rCD36 to the histidine found in hCD36. This single residue change to rCD36 (to produce mutant Y242H) conferred pH sensitivity to Y242H, with a pH optimum similar to hCD36. In addition, in contrast to native rCD36, PE binding to Y242H was unaffected by iodination. Thus, the reciprocal substitution at residue 242 of rCD36 (to the hCD36 equivalent), conferred the human CD36-binding phenotype onto rat CD36.
The process of iodination converts a tyrosine residue to a di-iodotyrosine. Iodine atoms are a sizable electronegative addition to the residue that might sterically interfere with the approach of the PE ligand and thus potentially reduce binding. Although steric interference might result in the complete loss of PE binding, the results seen in both rodent CD36 and the mutant H242Y may be due to incomplete iodination of all CD36 receptors present on the COS cells, allowing for some binding to occur. It may also suggest that if residue 242 is a contact residue it is not the only residue involved in binding the PE ligand and that preventing the interaction of the ligand with residue 242 reduces the efficiency but does not completely abrogate binding.
Previous studies have shown that cytoadherence to hCD36 is a pH-dependent process. Cytoadherence to C32 amelanotic melanoma cells (which express CD36) and to CHO-CD36 cells have pH optimums of 6.9 and 6.6 to 6.8, respectively.33,34 We have shown that PE adherence to hCD36 expressed on COS-7 cells also displays pH dependence, with optimum adherence occurring at pH 6.8 to 7.0, similar to optimums previously reported. Substitutions at position 242 of hCD36 or rCD36 modify the effect of pH on binding, indicating that histidine 242 is responsible for the pH effect. The observation that cytoadherence to hCD36 shows a significant increase around pH 6.8 to 7.0 is of interest given that human erythrocytes infected with late trophozoites/schizonts sequester preferentially in postcapillary venules of malaria patients where the surrounding microenvironment is of lower than physiological pH due to higher CO2 and lactate levels.2
Although all three CD36 homologues support avid cytoadherence of PEs, we have observed that murine and rat CD36 consistently bind lower levels of PEs compared with hCD36 using standard assay conditions. Using FACS and confocal microscopy analysis we determined that the lower binding avidity of rodent CD36, as compared with human CD36, cannot be attributed to differences in cell surface expression. Cytoadherence assays in our lab are generally performed at pH 6.8 where the hCD36 binding pH optimum is observed; thus, the pH sensitivity of hCD36 may be responsible for the binding avidity differences observed between human and rodent CD36. It is of note that the binding efficiency of rCD36 is increased to that of hCD36 levels when the tyrosine at position 242 is substituted for a histidine. Conversely, the binding efficiency of hCD36 is decreased to rCD36 levels when residue 242 is changed from a histidine to a tyrosine. Because altering residue 242 eliminates the pH effect observed in hCD36, the avidity difference between human and rodent CD36 can be attributed to residue 242.
Previous studies have used epitope mapping of cytoadherence-inhibiting monoclonal antibodies and recombinant CD36 fragments to define domains that may participate in cytoadherence.21,30 It has been suggested that the CD36 epitope of the cytoadherence inhibiting monoclonal antibody OKM5 and the CD36-ligand binding domain overlap.30 We have observed that OKM5’s recognition of the hCD36 mutant H242Y does not differ from that of native hCD36, under control conditions and after iodination of both proteins as determined by FACS analysis (data not shown). These observations suggest that the OKM5 epitope and the PE-binding domain on CD36 are distinct, because iodination of H242Y reduces PE binding but not OKM5 binding. The cytoadherence inhibitory properties of OKM5 may be due to steric interference of the approaching PE ligand rather than direct competition for the binding domain. The present study examined CD36 homologues and mutants in the context of the cell membrane and examined the effect of subtle amino acid substitutions on a cell-cell interaction. We believe that this system represents a more direct and physiologically relevant assessment of cytoadherence and one that more closely reflects the in vivo situation. Our findings do not directly refute previously implicated domains but suggest that the domain involved in cytoadherence may be discontinuous.
In summary, we present evidence that a single amino acid substitution at position 242 of CD36 can modify the characteristics of the P falciparum–infected erythrocyte-CD36 interaction, suggesting that the P falciparum binding domain includes or is influenced by this residue. These findings are directly supported by cysteine replacement mutagenesis studies in our laboratory that indicate that this residue lies within a discrete cysteine-rich domain of CD36, region 243 to 333, which is essential for PE cytoadherence (unpublished data, January 1998). Ultimately these observations may facilitate the rational design of interventions to prevent or reverse sequestration. Further study to assess whether CD36 mutants still support other receptor functions such as the binding and uptake of oxLDL and apoptotic neutrophils may help to determine whether these ligands share or have unique binding sites on CD36.
ACKNOWLEDGMENT
CD36 clones were kindly provided by Dr B. Seed (human), Dr G. Endemann (mouse), and Dr N. Abumrad (rat).
Supported by the Medical Research Council of Canada (MT-13721), The World Health Organization TDR program (TDR 920223), and the Heart and Stroke Foundation of Canada (NA-3391). K.C.K. was supported in part by a Career Scientist Award from the Ontario Ministry of Health.
Address reprint requests to Kevin C. Kain, MD, Tropical Disease Unit, EN G-224, The Toronto Hospital, 200 Elizabeth St, Toronto, Canada, M5G 2C4; e-mail: kkain@torhosp.toronto.on.ca.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.