Abstract
p38 MAP kinase (p38) and JNK have been described as playing a critical role in the response to a variety of environmental stresses and proinflammatory cytokines. It was recently reported that hematopoietic cytokines activate not only classical MAP kinases (ERK), but also p38 and JNK. However, the physiological function of these kinases in hematopoiesis remains obscure. We found that all MAP kinases examined, ERK1, ERK2, p38, JNK1, and JNK2, were rapidly and transiently activated by erythropoietin (Epo) stimulation in SKT6 cells, which can be induced to differentiate into hemoglobinized cells in response to Epo. Furthermore, p38-specific inhibitor SB203580 but not MEK-specific inhibitor PD98059 significantly suppressed Epo-induced differentiation and antisense oligonucleotides of p38, JNK1, and JNK2, but neither ERK1 nor ERK2 clearly inhibited Epo-induced hemoglobinization. However, in Epo-dependent FD-EPO cells, inhibition of either ERKs, p38, or JNKs suppressed cell growth. Furthermore, forced expression of a gain-of-function MKK6 mutant, which specifically activated p38, induced hemoglobinization of SKT6 cells without Epo. These results indicate that activation of p38 and JNKs but not of ERKs is required for Epo-induced erythroid differentiation of SKT6 cells, whereas all of these kinases are involved in Epo-induced mitogenesis of FD-EPO cells.
© 1998 by The American Society of Hematology.
MITOGEN-ACTIVATED protein (MAP) kinases form a large family of serine-threonine protein kinases conserved through evolution.1,2 In mammalian cells, four distinct MAP kinase cascades have been identified: extracellular signal-regulated kinases (ERK),3,4 c-Jun amino-terminal kinases (JNK) or stress-activated protein kinases (SAPK),5,6 p38 MAP kinase (p38) or cytokine suppressive anti-inflammatory drug binding protein,7,8 and Erk5/BMK.9 10 These cascades have become prototypes for the study of structurally related but functionally distinct pathways.
The classical MAP kinases (ERK1 and ERK2) are activated by a variety of cell growth and differentiation stimuli2,11,12 and play a central role in mitogenic signaling.13 The p38 and JNK cascades are primarily activated by various environmental stresses (osmotic shock, ultraviolet radiation, heat shock, x-ray radiation, hydrogen peroxide, and protein synthesis inhibitors) and by the proinflammatory cytokines tumor necrosis factor-α and interleukin-1 (IL-1).5-8,14-21 Cellular stresses and proinflammatory cytokines induce apoptotic cell death21; thus, it has been suggested that JNK and p38 have a role in apoptosis signals. On the other hand, stimulation of Fas results in only a modest activation of JNK,22 and CD40 ligand, which is known to counteract apoptosis in B cells, activates JNK.23 Mutational analysis of tumor necrosis factor-α receptor showed that JNK activation is not linked to apoptosis.24 JNK and p38 can also be activated by such mitogenic factors as epidermal growth factor and phorbol esters15 and by T-cell activation signaling.25Thus, JNK and p38 seem to act not only in apoptotic but also in mitogenic signalings.
Hematopoietic cytokines are known to be strong activators of ERK.26-31 Quite recently, various hematopoietic cytokines, interleukins, and colony-stimulating factors that regulate hematopoietic cell growth, survival, and differentiation were found to activate JNKs and p38 as well as ERKs.32-36 However, the roles of these distinct MAP kinases on hematopoiesis have not yet been determined. We therefore examined the functions of the MAP kinase family in erythropoietin (Epo)-induced erythroid differentiation of SKT6 cells, which can respond to Epo and induce hemoglobinization, and in Epo-dependent cell growth of FD-EPO cells, which grow dependently with Epo. Surprisingly, we found that activation of JNKs and p38 but not ERKs is essential for Epo-induced erythroid differentiation and that all MAP kinases are involved in Epo-dependent cell growth. We discuss here the possible functions of ERKs, JNKs, and p38 on hematopoietic cell growth and differentiation.
MATERIALS AND METHODS
Reagents.
Antibodies against mouse ERK1, ERK2, JNK1, JNK2, and p38 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). MAPKAP kinase-2 assay kit was purchased from Upstate Biotechnology (Lake Placid, NY). Human Epo (2.6 × 105 U/mg) was a gift from Kirin Brewery (Tokyo, Japan). SB203580 was a generous gift from Dr J. C. Lee (Smithkline Beecham Pharmaceuticals, King of Prussia, PA). PD98059 was from Calbiochem (La Jolla, CA). Phosphothioester oligonucleotides (S-oligos) were prepared and purified by BEX (Tokyo, Japan). Glutathione-S-transferase (GST)-human ATF-2 fusion protein (amino terminal domain corresponding to amino acids 1 to 96) was obtained from Santa Cruz Biotechnology and myelin basic protein (MBP) was from Sigma (St Louis, MO). GST-c-Jun was prepared as reported.33
Cell culture.
Epo-responsive mouse erythroleukemia SKT6 cells have been described37 and were cultured in Ham F-12 medium supplemented with 10% fetal calf serum. The Epo-dependent cell line FD-EPO was established as described previously33 and has been maintained in RPMI-1640 medium supplemented with 10% fetal calf serum and 1 U/mL of human Epo. SKT6 cells were induced to differentiate by the addition of 0.5 U/mL of recombinant human Epo followed by incubation for 4.5 days, and hemoglobin-positive cells were stained by 0.05% 2,7-diaminofluorene (DAF; Sigma).38 Various concentrations of antisense, sense, or scrambled S-oligos; p38-specific inhibitor SB203580 in dimethyl sulfoxide (DMSO); or MEK-specific inhibitor PD98059 in ethanol were mixed in the culture medium before the addition of 0.5 U/mL of Epo, and the effect on cell differentiation and/or cell growth was examined. The S-oligos were added again 24 hours after Epo stimulation. The effect on cell growth was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Antisense S-oligos used were as follows: GGCCTCTCCTGCGACATCTT for p38; TCACGCTTGCTTCTGCTCAT for JNK1; TCACATTTACTGTCGCTCAT for JNK2; and GCCGCCGCCGCCGCCAT for ERKs.39 As control, the corresponding sense S-oligos and scrambled S-oligos were used. Immunoblot analysis of the kinases was performed as described previously.33 34
In vitro protein kinase assay.
SKT6 cells were stimulated with or without 0.5 U/mL of Epo for up to 4.5 days and immediately lysed in a lysis buffer (50 mmol/L Tris, pH 7.5, 0.5% Nonidet P-40, 150 mmol/L NaCl, 100 mmol/L sodium fluoride, 10 mmol/L sodium pyrophosphate, 1 mmol/L EDTA, 2 mmol/L Pefabloc, 10 ng/mL leupeptin, and 10 ng/mL aprotinin). Insoluble material was then removed by centrifugation, and the precleared cell lysate was incubated with a specific antibody at 4°C for 2 hours. The immunocomplexes were bound to protein A-Sepharose at 4°C for 1 hour. The beads were washed five times with lysis buffer containing 0.1% Nonidet P-40. Immunoprecipitates with anti-p38 antibody, anti-JNK1 antibody, anti-JNK2 antibody, anti-ERK1 antibody, or anti-ERK2 antibody were mixed with the purified substrates (1 μg of GST-ATF-2 for p38, 3 μg of GST-c-Jun for JNKs, and 3 μg of MBP for ERKs) in 20 μmol/L ATP and 5 μCi of [γ-32P]ATP in 30 μL of kinase buffer A (25 mmol/L HEPES, pH 7.4, 25 mmol/L β-glycerophosphate, 25 mmol/L MgCl2, 0.1 mmol/L sodium orthovanadate, 2 mmol/L dithiothreitol [DTT]) for p38, in kinase buffer B (20 mmol/L HEPES, pH 7.6, 20 mmol/L MgCl2, 0.1 mmol/L sodium orthovanadate, 2 mmol/L DTT) for JNKs, or in kinase buffer C (60 mmol/L Tris, pH 8.0, and 60 mmol/L MgCl2) for ERKs. Incubation followed at 30°C for 30 minutes for p38, at 30°C for 20 minutes for JNKs, or at 25°C for 15 minutes for ERKs. The reactions were terminated by mixing with Laemmli sample buffer and boiling. The samples were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for p38 and JNKs or 12% SDS-PAGE for ERKs and autoradiographed. MAPKAP kinase-2 assay was performed as described in the MAPKAP kinase-2 assay kit manual (Upstate Biotechnology).
In-gel kinase assay.
In-gel kinase assays were performed using the modified method of Hibi et al.40 Immunoprecipitates with anti-JNK1 or anti-JNK2 antibody were resolved on a 10% SDS-polyacrylamide gel that was polymerized in the absence or presence of 50 μg/mL GST-c-Jun. After electrophoresis, the gel was washed twice for 30 minutes with 100 mL of 20% 2-propanol in 50 mmol/L HEPES, pH 7.6, to remove SDS. The gel was then washed twice for 30 minutes with 100 mL of buffer A (50 mmol/L HEPES, pH 7.6, 5 mmol/L β-mercaptoethanol) and then incubated in 200 mL of 6 mol/L guanidine in buffer A at 25°C for 1 hour. After washing several times for 1 hour each time with 100 mL of buffer A containing 0.05% Tween 20 at 4°C, the gel was incubated in kinase buffer containing 50 mmol/L ATP and 0.25 mCi/mL of [γ-32P]ATP at 30°C for 1 hour. Finally, the gel was washed several times with 100 mL of 5% trichloroacetic acid and 1% sodium pyrophosphate at 25°C, followed by drying and autoradiography.
Cell proliferation assay.
Cell proliferation was measured by a colorimetric assay using MTT (Sigma) originally developed by Mosmann.41 Exponentially growing cells (exactly 3 × 104) were plated on microtiter plates in 100 μL culture medium in the presence or absence of various concentrations of inhibitors, Epo, and antisense, sense, or scrambled S-oligos and were cultured at 37°C for 3 days. Ten microliters of 5 mg/mL of MTT in phosphate-buffered saline (PBS) was then added to each well and incubated at 37°C for an additional 4 hours, and 100 μL of 0.04 mol/L HCl in isopropanol was thoroughly mixed in. Optical densities were measured on a microplate reader using a test wavelength of 570 nm and a reference wavelength of 630 nm.
Analysis of DNA fragmentation.
DNA fragmentation was assayed by the modified method of Sellins and Cohen.42 SKT6 cells (1 × 106 cells) collected by centrifugation were washed once in PBS and suspended in a lysis buffer (10 mmol/L Tris, pH 7.5, 10 mmol/L EDTA, pH 8.0, 0.5% Triton X-100) for 10 minutes on ice. The suspension was centrifuged at 16,000 rpm for 20 minutes and the fragmented DNA was recovered from the supernatant, which was then subjected to digestion with ribonuclease A (0.5 mg/mL) for 1 hour at 37°C, followed by incubation with proteinase K (0.5 mg/mL) for 1 hour at 37°C. The sample was then extracted with isopropanol overnight at −20°C. DNA was precipitated and resuspended in 10 mmol/L Tris, pH 7.5, containing 1 mmol/L EDTA. Equal amounts of DNA were separated by 1.8% agarose gel electrophoresis, and DNA was visualized by ethidium bromide staining.
RESULTS
JNKs, p38, and ERKs are rapidly and transiently activated during Epo-induced erythroid differentiation.
It has been reported that Epo rapidly and transiently activates JNK1, JNK2, and p38 as well as ERK1 and ERK2 in Epo-dependent FD-EPO cells or BaF3/ER cells.30,33 34 These cells grow in response to Epo in a dose-dependent manner. In contrast, mouse erythroleukemia SKT6 cells can be induced to differentiate into hemoglobinized cells in response to Epo; these cells are not dependent on Epo to proliferate, and their proliferation is neither enhanced nor impaired by the presence of Epo. Therefore, we sought to learn whether the MAP kinase family can also be activated during Epo-induced erythroid differentiation of SKT6 cells.
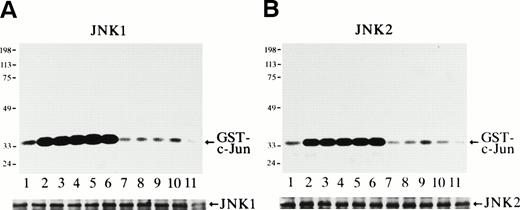
The protein kinase activity of JNK1 and JNK2 at various time points after Epo stimulation was measured with purified GST-c-Jun (molecular weight, 35 kD) as a substrate (Fig 1). It was clearly observed that Epo rapidly and transiently activated both JNK1 (Fig 1A) and JNK2 (Fig 1B). Both activities were seen in unstimulated cells, and a rapid and marked increase in the activities was detected within 3 minutes of Epo treatment (Fig 1A and B, lanes 2). These activities peaked at 5 to 60 minutes (Fig 1A and 1B, lanes 3 through 6) and returned to the basal levels by 3 hours after stimulation (Fig 1A and B, lanes 7). Immunoblot analysis of the immunoprecipitates showed that the amount of the immunoprecipitated JNK1 or JNK2 in each lane was similar (Fig 1A and B, lower panels).
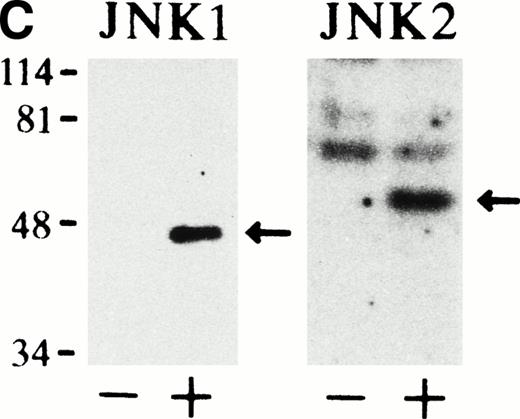
Epo rapidly and transiently activates JNK1 and JNK2 during Epo-induced erythroid differentiation. SKT6 cells were stimulated with Epo for 0, 3, 5, 15, 30, 60, and 180 minutes (lanes 1 through 7) and for 1, 2, 3, and 4.5 days (lanes 8 through 11). Activity of JNK1 (A) and JNK2 (B) was assayed in the immunoprecipitates with anti-JNK1– or anti-JNK2–specific antibody and GST-c-Jun as a substrate. Arrows indicate the phosphorylated GST-c-Jun (molecular weight, 35 kD). Lower panels show the immunoblots of the immunoprecipitated JNK1 or JNK2 in each lane. (C) In-gel kinase assay of JNK1 and JNK2. Cell lysates before (−) and 5 minutes after (+) Epo stimulation were used for in-gel kinase assay. Arrows indicate the position of JNK1 (molecular weight, 46 kD) and JNK2 (molecular weight, 55 kD).
Epo rapidly and transiently activates JNK1 and JNK2 during Epo-induced erythroid differentiation. SKT6 cells were stimulated with Epo for 0, 3, 5, 15, 30, 60, and 180 minutes (lanes 1 through 7) and for 1, 2, 3, and 4.5 days (lanes 8 through 11). Activity of JNK1 (A) and JNK2 (B) was assayed in the immunoprecipitates with anti-JNK1– or anti-JNK2–specific antibody and GST-c-Jun as a substrate. Arrows indicate the phosphorylated GST-c-Jun (molecular weight, 35 kD). Lower panels show the immunoblots of the immunoprecipitated JNK1 or JNK2 in each lane. (C) In-gel kinase assay of JNK1 and JNK2. Cell lysates before (−) and 5 minutes after (+) Epo stimulation were used for in-gel kinase assay. Arrows indicate the position of JNK1 (molecular weight, 46 kD) and JNK2 (molecular weight, 55 kD).
In-gel kinase assay with GST-c-Jun as a substrate confirmed that both JNK1 of 46 kD and JNK2 of 55 kD were indeed activated 5 minutes after Epo stimulation (Fig 1C). Furthermore, the anti-JNK1 and anti-JNK2 antibodies used here specifically immunoprecipitated JNK1 and JNK2, respectively, and did not cross-react with each other, as described previously.33
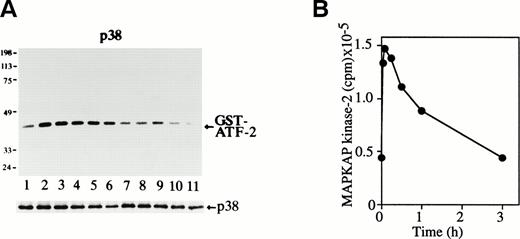
Possible p38 activation was next examined by in vitro p38 kinase assay. The purified GST-ATF-2 (molecular weight, 40 kD) was used as a substrate (Fig 2A). The p38 activity was seen in unstimulated cells (Fig 2A, lane 1), but it rapidly increased and reached the maximum level within 3 minutes of Epo treatment (Fig2A, lane 2). It remained at a high level until 30 minutes after stimulation (Fig 2A, lanes 2 through 5) and slowly returned to the basal level within 3 hours (Fig 2A, lane 7). Immunoblot analysis of the immunoprecipitates showed that the amount of the immunoprecipitated p38 in each lane was similar (Fig 2A, lower panel).
Epo rapidly and transiently activates p38 and MAPKAP kinase-2 during erythroid differentiation. (A) SKT6 cells were stimulated with Epo for 0, 3, 5, 15, 30, 60, and 180 minutes (lanes 1 through 7) and for 1, 2, 3 and 4.5 days (lanes 8 through 11). The p38 activity was measured in the immunoprecipitates with anti-p38–specific antibody and GST-ATF-2 as a substrate. Arrow indicates the phosphorylated GST-ATF-2 (molecular weight, 40 kD). The lower panel shows the immunoblots of the immunoprecipitated p38 in each lane. (B) The MAPKAP kinase-2 assay was performed in the immunoprecipitates of SKT6 cells in the presence of MAPKAP kinase-2 substrate peptide as a substrate at various time points after Epo stimulation, as indicated.
Epo rapidly and transiently activates p38 and MAPKAP kinase-2 during erythroid differentiation. (A) SKT6 cells were stimulated with Epo for 0, 3, 5, 15, 30, 60, and 180 minutes (lanes 1 through 7) and for 1, 2, 3 and 4.5 days (lanes 8 through 11). The p38 activity was measured in the immunoprecipitates with anti-p38–specific antibody and GST-ATF-2 as a substrate. Arrow indicates the phosphorylated GST-ATF-2 (molecular weight, 40 kD). The lower panel shows the immunoblots of the immunoprecipitated p38 in each lane. (B) The MAPKAP kinase-2 assay was performed in the immunoprecipitates of SKT6 cells in the presence of MAPKAP kinase-2 substrate peptide as a substrate at various time points after Epo stimulation, as indicated.
The p38 immunoprecipitated with anti-p38–specific antibody was immunoblotted with antiphosphotyrosine antibody 4G10, and it was found that the level of tyrosine phosphorylation of p38 was well correlated with its kinase activity (data not shown). Thus, it was concluded that Epo rapidly induces tyrosine phosphorylation and activation of p38 during erythroid differentiation of SKT6 cells.
We further examined the possible activation of one of the major substrates of p38, MAPKAP kinase-2,15 16 in Epo-stimulated SKT6 cells. As shown in Fig 2B, the kinase was rapidly and transiently activated, and this activity reached the maximal level 5 minutes after Epo stimulation and then returned to the basal level by 3 hours after stimulation. The activity profile of p38 is well correlated with that of MAPKAP kinase-2 activity; furthermore, inhibition of p38 by SB203580 blocked activation of MAPKAP kinase-2 (data not shown).
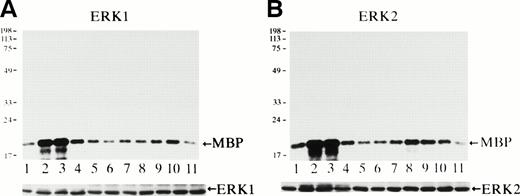
Activity of ERK1 and ERK2 was measured using MBP as a substrate at various time points after Epo stimulation (Fig 3). Both ERK1 (Fig 3A) and ERK2 (Fig3B) were rapidly activated within 3 minutes (Fig 3A and B, lanes 2), and their activities reached the maximum levels at 3 to 5 minutes (Fig3A and B, lanes 2 and 3) and then returned to the background levels by 15 to 30 minutes after stimulation (Fig 3A and B, lanes 4 and 5). The specific activation of both ERK1 of 44 kD and of ERK2 of 42 kD was confirmed by in-gel kinase assay (data not shown), and the immunoblot analysis of the immunoprecipitates indicated that the amount of the immunoprecipitated ERK1 or ERK2 in each lane was similar (Fig 3A and B, lower panels).
Epo rapidly and transiently activates both ERK1 and ERK2 during erythroid differentiation. SKT6 cells were stimulated with Epo for 0, 3, 5, 15, 30, 60, and 180 minutes (lanes 1 through 7) and for 1, 2, 3, and 4.5 days (lanes 8 through 11), and the activity of ERK1 (A) and ERK2 (B) was measured in the immunoprecipitates with each specific antibody and MBP as a substrate. Arrows indicate the phosphorylated MBP (molecular weight, 18 kD). The lower panels show the immunoblots of the immunoprecipitated ERK1 or ERK2 in each lane.
Epo rapidly and transiently activates both ERK1 and ERK2 during erythroid differentiation. SKT6 cells were stimulated with Epo for 0, 3, 5, 15, 30, 60, and 180 minutes (lanes 1 through 7) and for 1, 2, 3, and 4.5 days (lanes 8 through 11), and the activity of ERK1 (A) and ERK2 (B) was measured in the immunoprecipitates with each specific antibody and MBP as a substrate. Arrows indicate the phosphorylated MBP (molecular weight, 18 kD). The lower panels show the immunoblots of the immunoprecipitated ERK1 or ERK2 in each lane.
Taken together, these data clearly indicate that Epo induces rapid and transient activation of all MAP kinases examined, ERK1, ERK2, JNK1, JNK2, and p38, which suggests that some of these pathways may be involved in Epo-induced erythroid differentiation signals. Furthermore, whereas MAPKAP kinase-2 has been thought to act on apoptotic signal, this kinase may also play a role in Epo-induced erythroid differentiation.
Activation of p38 required for Epo-induced hemoglobinization.
We next examined the possible roles of these MAP kinase family members on Epo-induced erythroid differentiation. First of all, the effects of p38-specific inhibitor SB203580 (Fig 4) and MEK-specific inhibitor PD98059 (Fig 5) on Epo-induced hemoglobinization of SKT6 cells were examined. The percentage of hemoglobinized SKT6 cells after Epo stimulation varied between 40% and 70% from experiment to experiment. Thus, the percentage of hemoglobinized cells in the presence of Epo is defined as 100% as a control, and the inhibition or enhancement of SKT6 cell differentiation in the presence of the inhibitors is shown as a relative value against this control.
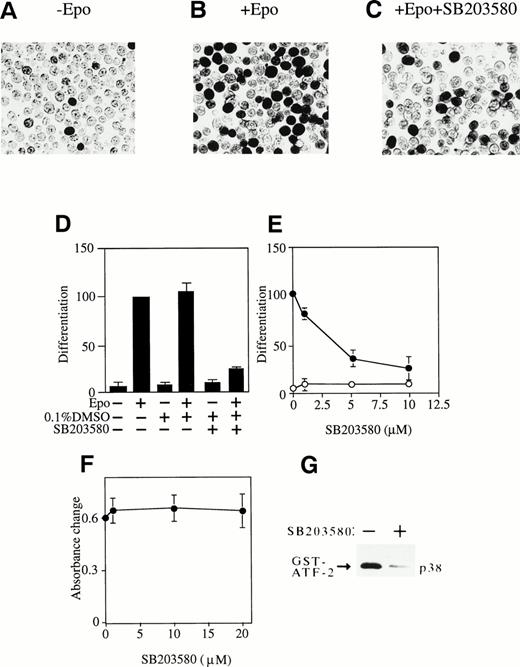
Activation of p38 is required for Epo-induced erythroid differentiation. (A, B, and C) DAF-staining of the hemoglobinized SKT6 cells with (B and C) or without (A) Epo stimulation in the presence (C) or absence (A and B) of 5 μmol/L of p38-specific inhibitor SB203580. (D) SKT6 cells were cultured in the presence (+) or absence (−) of Epo (0.5 U/mL) and SB203580 (10 μmol/L) dissolved in 0.1% DMSO, and the hemoglobinized cells were stained with DAF. The percentage of hemoglobinized cells in the presence of Epo is defined as 100% as a control, and the inhibition or enhancement of SKT6 cell differentiation in the presence of the inhibitor is shown as a relative value against the control. Values shown are the means of five experiments. (E) Dose-dependency of SB203580 in SKT6 cell differentiation in the presence (•) or absence (○) of Epo. (F) Dose-dependency of SB203580 in SKT6 cell growth. The cell growth was measured by MTT assay. (G) The p38 activity was measured in the presence (+) or absence (−) of SB203580 (10 μmol/L) 5 minutes after Epo stimulation.
Activation of p38 is required for Epo-induced erythroid differentiation. (A, B, and C) DAF-staining of the hemoglobinized SKT6 cells with (B and C) or without (A) Epo stimulation in the presence (C) or absence (A and B) of 5 μmol/L of p38-specific inhibitor SB203580. (D) SKT6 cells were cultured in the presence (+) or absence (−) of Epo (0.5 U/mL) and SB203580 (10 μmol/L) dissolved in 0.1% DMSO, and the hemoglobinized cells were stained with DAF. The percentage of hemoglobinized cells in the presence of Epo is defined as 100% as a control, and the inhibition or enhancement of SKT6 cell differentiation in the presence of the inhibitor is shown as a relative value against the control. Values shown are the means of five experiments. (E) Dose-dependency of SB203580 in SKT6 cell differentiation in the presence (•) or absence (○) of Epo. (F) Dose-dependency of SB203580 in SKT6 cell growth. The cell growth was measured by MTT assay. (G) The p38 activity was measured in the presence (+) or absence (−) of SB203580 (10 μmol/L) 5 minutes after Epo stimulation.
MEK-specific inhibitor PD98059 has no effect on Epo-induced erythroid differentiation of SKT6 cells. (A) SKT6 cells were cultured in the presence (+) or absence (−) of Epo and MEK-specific inhibitor PD98059 (50 μmol/L) dissolved in 0.1% ethanol, and the hemoglobinized cells were stained with DAF. (B) Dose-dependency of PD98059 in SKT6 cell differentiation in the presence (•) or absence (○) of Epo. The percentage of hemoglobinized cells in the presence of Epo is defined as 100% as a control. Values shown are the means of five experiments. (C) Dose-dependency of PD98059 on SKT6 cell growth. Cell proliferation was measured by MTT assay. (D) Activity of ERKs was blocked by PD98059. The ERK activity was assayed in the presence (+) or absence (−) of PD98059 (50 μmol/L) 5 minutes after Epo stimulation.
MEK-specific inhibitor PD98059 has no effect on Epo-induced erythroid differentiation of SKT6 cells. (A) SKT6 cells were cultured in the presence (+) or absence (−) of Epo and MEK-specific inhibitor PD98059 (50 μmol/L) dissolved in 0.1% ethanol, and the hemoglobinized cells were stained with DAF. (B) Dose-dependency of PD98059 in SKT6 cell differentiation in the presence (•) or absence (○) of Epo. The percentage of hemoglobinized cells in the presence of Epo is defined as 100% as a control. Values shown are the means of five experiments. (C) Dose-dependency of PD98059 on SKT6 cell growth. Cell proliferation was measured by MTT assay. (D) Activity of ERKs was blocked by PD98059. The ERK activity was assayed in the presence (+) or absence (−) of PD98059 (50 μmol/L) 5 minutes after Epo stimulation.
Figure 4A, B, and C shows the hemoglobinized SKT6 cells with (Fig 4B and C) or without (Fig 4A) Epo stimulation in the presence (Fig 4C) or absence (Fig 4A and B) of SB203580 (5 μmol/L). Surprisingly, the addition of SB203580 to SKT6 cell culture in the presence of Epo strongly blocked production of hemoglobinized cells (Fig 4C and D, lane 6) compared with the addition of Epo alone (Fig 4B and D, lane 2). The solvent used to dissolve SB203580 (0.1% DMSO) slightly stimulated Epo-induced hemoglobinization (Fig 4D, lane 4) compared with Epo alone (Fig 4D, lane 2). Neither DMSO (Fig 4D, lane 3) nor SB203580 alone (Fig4D, lane 5) induced erythroid differentiation. A dose-dependent experiment clearly demonstrated that SB203580 blocked Epo-induced differentiation in a dose-dependent manner (Fig 4E, •) and that the inhibitor alone had no effect on it (Fig 4E, ○). The MTT assay in the presence of various concentrations of SB203580 showed that the inhibitor has no effect on cell proliferation (Fig 4F). It was confirmed that SB203580 actually inhibited p38 activity to the basal level even 5 minutes after Epo stimulation, when it should be at the maximal level (Fig 4G). These results indicate that inhibition of p38 activity clearly blocks Epo-induced erythroid differentiation; in other words, activation of p38 induced by Epo is essential for erythroid differentiation of SKT6 cells.
The addition of PD98059 (50 μmol/L) to SKT6 cell culture in the presence or absence of Epo had no effect on hemoglobinization (Fig 5A, lanes 5 and 6) compared with the controls (Fig 5A, lanes 1 through 4), although PD98059 (50 μmol/L) completely blocked Epo-induced activation of both ERK1 and ERK2 even 5 minutes after Epo stimulation, at which both activities are maximum (Fig 5D). The phosphorylation of MEKs was also inhibited by the inhibitor (data not shown). The solvent used to dissolve PD98059 (0.1% ethanol) in the presence (Fig 5A, lane 4) or absence (Fig 5A, lane 3) of Epo had no effect on this assay. A dose-dependent experiment confirmed that even higher concentrations of PD98059 had no effect on erythroid differentiation in the presence (Fig5B, •) or absence (Fig 5B, ○) of Epo, whereas the dose-dependency of PD98059 on cell growth shows that 50 μmol/L of the inhibitor had little effect on it but over 100 μmol/L of the inhibitor exhibited some growth inhibitory effect on SKT6 cells (Fig 5C). Thus, the effect of PD98059 over 100 μmol/L may be nonspecific. It was therefore concluded, although unexpectedly, that ERKs may not be involved in Epo-induced erythroid differentiation of SKT6 cells.
The fact that inhibition of p38 activity strongly blocked Epo-induced hemoglobinization indicates that activation of p38 is required for Epo-induced erythroid differentiation in SKT6 cells. However, ERKs may not be involved in Epo-induced hemoglobinization of these cells. Because no specific inhibitor for JNK is available, the role of JNK could not be determined in this assay.
Activation of both p38 and JNKs essential for Epo-induced hemoglobinization.
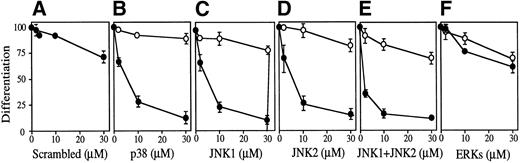
The possible involvement of JNKs during Epo-induced SKT6 cell differentiation was studied by applying antisense S-oligo strategy (Fig 6). In the presence of antisense S-oligo of p38, Epo-induced hemoglobinization was significantly inhibited in a dose-dependent manner, as expected (Fig 6B, •). Similarly, antisense S-oligo of JNK1 or JNK2 strongly blocked Epo-induced hemoglobinization in a dose-dependent manner (Figs 6C and D, •), and the addition of both of these antisense S-oligos synergistically inhibited it (Fig 6E, •). In contrast, antisense S-oligo of ERKs (commonly effective for both ERK1 and ERK2) exhibited only weak inhibition (Fig 6F, •), similar to that of sense S-oligos (Fig 6F, ○). The scrambled S-oligo (Fig 6A) and all sense S-oligos (Fig 6B through 6F, ○) had little effect, if any, on Epo-induced hemoglobinization. Figure 6G shows that all of these antisense S-oligos (30 μmol/L) specifically inhibited the expression of each corresponding kinase. These results demonstrated that inhibition of either JNK1, JNK2, or p38 resulted in the block of Epo-induced hemoglobinization of SKT6 cells; in other words, JNK1 and JNK2 as well as p38, but not ERKs, are required for Epo-induced hemoglobinization.
Activation of both p38 and JNKs is essential for Epo-induced differentiation. Various concentrations (0 to 30 μmol/L) of scrambled S-oligo (A), antisense S-oligo (•), or sense S-oligo (○) of p38 (B), JNK1 (C), JNK2 (D), a mixture of JNK1 and JNK2 (E), or ERKs (F) were mixed with SKT6 cells in the presence of Epo, and the hemoglobinized cells were stained with DAF after 4.5 days of culture. The percentage of DAF-positive cells in the presence of Epo is defined as 100%, and the inhibition of erythroid differentiation is shown as a relative value. Each point represents the mean of five replicates. (G) Antisense S-oligos specifically inhibit the expression of each corresponding kinase. The lysates of the cells treated (+) or untreated (−) with antisense S-oligos (30 μmol/L) indicated above were probed with specific antibody against each corresponding kinase shown left.
Activation of both p38 and JNKs is essential for Epo-induced differentiation. Various concentrations (0 to 30 μmol/L) of scrambled S-oligo (A), antisense S-oligo (•), or sense S-oligo (○) of p38 (B), JNK1 (C), JNK2 (D), a mixture of JNK1 and JNK2 (E), or ERKs (F) were mixed with SKT6 cells in the presence of Epo, and the hemoglobinized cells were stained with DAF after 4.5 days of culture. The percentage of DAF-positive cells in the presence of Epo is defined as 100%, and the inhibition of erythroid differentiation is shown as a relative value. Each point represents the mean of five replicates. (G) Antisense S-oligos specifically inhibit the expression of each corresponding kinase. The lysates of the cells treated (+) or untreated (−) with antisense S-oligos (30 μmol/L) indicated above were probed with specific antibody against each corresponding kinase shown left.
Forced activation of p38 induces erythroid differentiation of SKT6 cells without Epo stimulation.
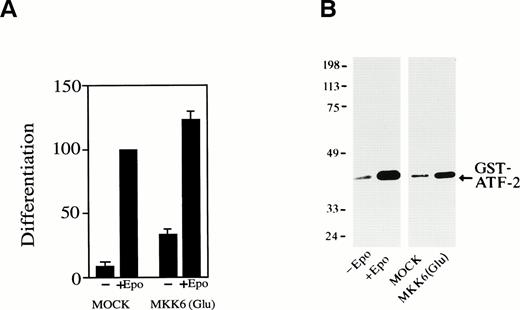
We next examined whether activation of p38 is enough to induce erythroid differentiation of SKT6 cells without Epo stimulation. MKK6(Glu), an activated form of MKK6, which can constitutively and specifically activate p38 as described previously,43 was expressed in SKT6 cells. Expression of MKK6(Glu) resulted in significant activation of p38 (Fig 7B, lane 4) compared with that of Epo stimulation (Fig 7B, lane 2) and production of hemoglobinized SKT6 cells without Epo stimulation (Fig7A, lane 3). In the presence of Epo, the expression of MKK6(Glu) (activation of p38) further enhanced Epo-induced differentiation (Fig7A, lane 4) compared with Epo-stimulated mock-transfectant (Fig 7A, lane 2). Transfection of vector alone had no effect on cell differentiation (Fig 7A, lanes 1 and 2). It was confirmed that no DNA fragmentation was detected in these MKK6(Glu)-expressing cells (data not shown). Thus, the activation of p38 is sufficient to induce erythroid differentiation to some extent without Epo stimulation. The gain-of-function mutant of neither JNKs nor their specific upstream kinases is yet available, so we could not examine the effect of forced activation of JNKs.
Forced activation of p38 induces erythroid differentiation without Epo stimulation. (A) SKT6 cells transfected with a gain-of-function MKK6 mutant MKK6(Glu) (lanes 3 and 4) or the vector (pCDNA3) alone (lanes 1 and 2) were cultured in the presence (+) or absence (−) of Epo, and the hemoglobinized cells were stained with DAF. The percentage of hemoglobinized cells of the mock-transfectants in the presence of Epo is defined as 100%. Each value represents the mean of six experiments. (B) MKK6(Glu) activates p38. The p38 activity in SKT6 cells (lanes 1 and 2), mock-transfected SKT6 cells (lane 3), or MKK6(Glu)-transfected SKT6 cells (lane 4) with (lane 2) or without (lanes 1, 3, and 4) Epo was measured in the immunoprecipitates with anti-p38–specific antibody and GST-ATF-2 as a substrate.
Forced activation of p38 induces erythroid differentiation without Epo stimulation. (A) SKT6 cells transfected with a gain-of-function MKK6 mutant MKK6(Glu) (lanes 3 and 4) or the vector (pCDNA3) alone (lanes 1 and 2) were cultured in the presence (+) or absence (−) of Epo, and the hemoglobinized cells were stained with DAF. The percentage of hemoglobinized cells of the mock-transfectants in the presence of Epo is defined as 100%. Each value represents the mean of six experiments. (B) MKK6(Glu) activates p38. The p38 activity in SKT6 cells (lanes 1 and 2), mock-transfected SKT6 cells (lane 3), or MKK6(Glu)-transfected SKT6 cells (lane 4) with (lane 2) or without (lanes 1, 3, and 4) Epo was measured in the immunoprecipitates with anti-p38–specific antibody and GST-ATF-2 as a substrate.
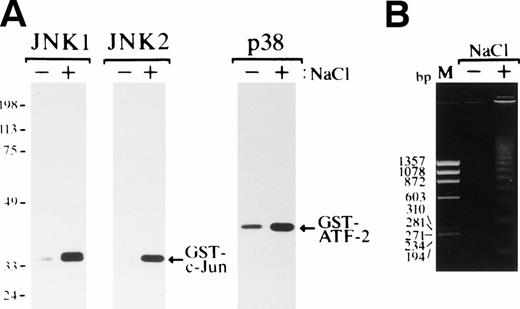
Exposure of SKT6 cells to osmotic shock induced activation of JNK1, JNK2, and p38 after 1 hour of incubation (Fig 8A), and DNA fragmentation was clearly observed after 24 hours of incubation (Fig 8B), as previously seen in various cell types.
Osmotic shock induces activation of JNKs and p38 and induces apoptotic cell death in SKT6 cells. (A) Activation of JNK1, JNK2, and p38. SKT6 cells were cultured in the presence (+) or absence (−) of 0.1 mol/L NaCl for 1 hour, and in vitro kinase assays of JNK1, JNK2, and p38 were performed in the immunoprecipitates with each specific antibody and GST-c-Jun or GST-ATF-2 as a substrate. (B) DNA fragmentation of the cells treated (+) or untreated (−) with 0.1 mol/L NaCl for 24 hours. M indicates the size marker (HaeIII digests of ▹X174 DNA).
Osmotic shock induces activation of JNKs and p38 and induces apoptotic cell death in SKT6 cells. (A) Activation of JNK1, JNK2, and p38. SKT6 cells were cultured in the presence (+) or absence (−) of 0.1 mol/L NaCl for 1 hour, and in vitro kinase assays of JNK1, JNK2, and p38 were performed in the immunoprecipitates with each specific antibody and GST-c-Jun or GST-ATF-2 as a substrate. (B) DNA fragmentation of the cells treated (+) or untreated (−) with 0.1 mol/L NaCl for 24 hours. M indicates the size marker (HaeIII digests of ▹X174 DNA).
Role of MAP kinases in Epo-dependent FD-EPO cells.
We previously reported that Epo similarly activates ERKs, p38, and JNKs in Epo-dependent cells.30,33 34 Therefore, we also examined the effects of SB203580 and PD98059 on Epo-dependent cell growth (Fig 9). Unexpectedly, the addition of SB203580 to FD-EPO cells clearly blocked cell proliferation in a dose-dependent manner, even in the presence of Epo (Fig 9A). Figure 9C shows that SB203580 (50 μmol/L) completely blocked p38 activity. The solvent used to dissolve the inhibitor (0.1% DMSO) had no effect on it (data not shown). Similarly, PD98059 also clearly inhibited Epo-dependent cell growth in a dose-dependent manner (Fig 9B). The activity of both ERK1 and ERK2 was completely inhibited by PD98059 (200 μmol/L; Fig 9D). The solvent used to dissolve PD98059 (0.1% ethanol) had no effect on this assay (data not shown).
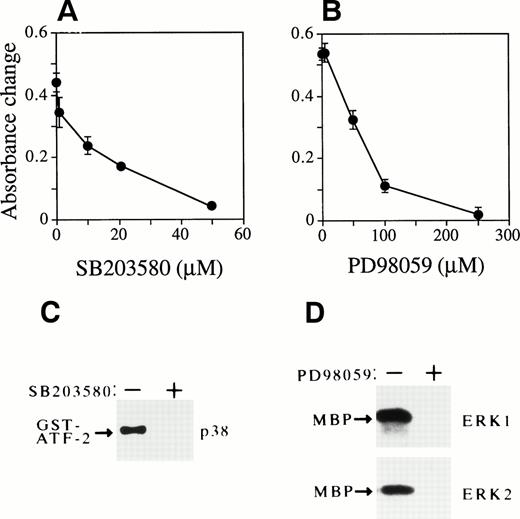
Activation of p38 and ERKs is required for Epo-dependent cell growth of FD-EPO cells. (A and B) Both SB203580 and PD98059 inhibit Epo-dependent cell growth. Various concentrations of SB203580 (A) or PD98059 (B) were mixed in FD-EPO cell culture, and the effect of the inhibitors on Epo-dependent cell growth was measured by MTT assay. (C) SB203580 blocked p38 activity. The p38 activity was assayed in the presence (+) or absence (−) of SB203580 (50 μmol/L) in Epo-stimulated FD-EPO cells. (D) PD98059 inhibited ERKs. The activity of ERKs was measured with (+) or without (−) PD98059 (200 μmol/L) in Epo-stimulated FD-EPO cells.
Activation of p38 and ERKs is required for Epo-dependent cell growth of FD-EPO cells. (A and B) Both SB203580 and PD98059 inhibit Epo-dependent cell growth. Various concentrations of SB203580 (A) or PD98059 (B) were mixed in FD-EPO cell culture, and the effect of the inhibitors on Epo-dependent cell growth was measured by MTT assay. (C) SB203580 blocked p38 activity. The p38 activity was assayed in the presence (+) or absence (−) of SB203580 (50 μmol/L) in Epo-stimulated FD-EPO cells. (D) PD98059 inhibited ERKs. The activity of ERKs was measured with (+) or without (−) PD98059 (200 μmol/L) in Epo-stimulated FD-EPO cells.
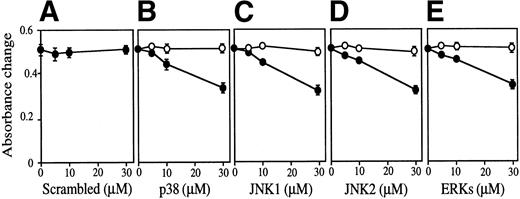
The effect of antisense S-oligos was examined on Epo-dependent FD-EPO cell growth (Fig 10). In the presence of either antisense S-oligo of p38, JNK1, JNK2, or ERKs weakly but clearly inhibited Epo-dependent cell growth of FD-EPO cells in a dose-dependent manner (Fig 10B through E, •), whereas sense S-oligo had no effect on cell growth (Fig 10B through E, ○). The scrambled S-oligo had no effect on it (Fig 10A). In the presence of these antisense S-oligos (30 μmol/L), little or no expression of the corresponding kinases was detected by immunoblot analyses (Fig 10F).
The MAP kinase family is essential for Epo-dependent cell growth. Various concentrations (0 to 30 μmol/L) of antisense S-oligo (•) or sense S-oligo (○) of p38 (B), JNK1 (C), JNK2 (D), a mixture of JNK1 and JNK2 (D), ERKs (E), or scrambled S-oligo (A) were mixed with FD-EPO cells in the presence of Epo, and the cell growth was measured by MTT assay. Values shown are the means of six experiments. (F) Immunoblot analyses of FD-EPO cells treated (+) or untreated (−) with antisense S-oligos (30 μmol/L) with specific antibody against each corresponding kinase.
The MAP kinase family is essential for Epo-dependent cell growth. Various concentrations (0 to 30 μmol/L) of antisense S-oligo (•) or sense S-oligo (○) of p38 (B), JNK1 (C), JNK2 (D), a mixture of JNK1 and JNK2 (D), ERKs (E), or scrambled S-oligo (A) were mixed with FD-EPO cells in the presence of Epo, and the cell growth was measured by MTT assay. Values shown are the means of six experiments. (F) Immunoblot analyses of FD-EPO cells treated (+) or untreated (−) with antisense S-oligos (30 μmol/L) with specific antibody against each corresponding kinase.
Exposure of FD-EPO cells to osmotic shock similarly induced activation of JNK1, JNK2, and p38 and consequently induced apoptotic cell death, as observed in SKT6 cells (Fig 8; data not shown).
Taken together, these results indicate that inhibition of p38, JNKs, or ERK blocks Epo-dependent cell growth in FD-EPO cells.
DISCUSSION
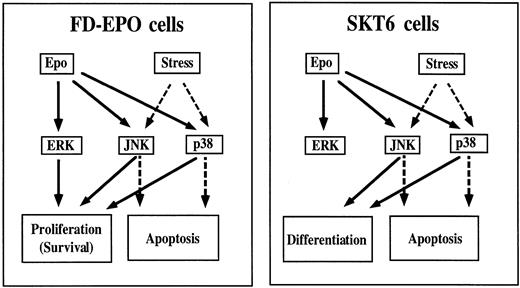
Figure 11 shows a schematic drawing summarizing the experimental results obtained here. In differentiation-inducible SKT6 cells, Epo rapidly and transiently activates all ERKs, JNKs, and p38. JNKs and p38 but not ERKs play a critical role in Epo-induced erythroid differentiation of SKT6 cells (Fig 11, right panel, solid lines), but JNKs and p38 play a part in apoptosis when the cells are exposed to environmental stresses (Fig 11, right panel, broken lines). However, in Epo-dependent FD-EPO cells, Epo similarly induces rapid and transient activation of ERKs, JNKs, and p38, but these kinases are all involved in cell proliferation (Fig 11, left panel, solid lines), whereas JNKs and p38 play a role in apoptotic signals when the cells are exposed to cellular stresses (Fig 11, left panel, broken line). Taken together, these findings indicate that JNKs and p38 may have a distinct function in different cell lineages and under different intracellular conditions, ie, whether the cell is to grow or to differentiate or whether the cell is to survive or to become apoptotic, even after the same extracellular stimulation (Epo in this case). Thus, the mechanism of how these kinase signaling cascades achieve a specific response remains to be elucidated.
Roles of the MAP kinase family in Epo-induced erythroid differentiation and proliferation. In differentiation-inducible SKT6 cells (right panel), JNKs and p38 function in erythroid differentiation (solid lines), but they have a role in apoptosis when the cells are exposed to environmental stresses (broken lines). However, in Epo-dependent FD-EPO cells (left panel), ERKs, JNKs, and p38 are all involved in cell proliferation (solid lines), whereas JNKs and p38 function in apoptotic signals when the cells are exposed to cellular stresses (broken lines).
Roles of the MAP kinase family in Epo-induced erythroid differentiation and proliferation. In differentiation-inducible SKT6 cells (right panel), JNKs and p38 function in erythroid differentiation (solid lines), but they have a role in apoptosis when the cells are exposed to environmental stresses (broken lines). However, in Epo-dependent FD-EPO cells (left panel), ERKs, JNKs, and p38 are all involved in cell proliferation (solid lines), whereas JNKs and p38 function in apoptotic signals when the cells are exposed to cellular stresses (broken lines).
It has been thought that ERK, p38, and JNK mediate different signals from different agonists or stimuli and are linked to different biological functions. Activation of the ERKs has been described as being involved in cell differentiation as well as cell proliferation.12 Nerve growth factor (NGF) treatment of pheochromocytoma PC12 cells induces ERK activation associated with neurite outgrowth and cessation of cell division, whereas the treatment of PC12 cells with EGF induces ERK activation and cell proliferation.12 In NIH3T3 cells, activation of the ERK pathway induces cell proliferation, and overexpression of the gain-of-function mutant of MEK induces morphological transformation of the transfected cells.44,45 In contrast, it was recently reported that ERK is not necessary for, but antagonizes, 3T3-L1 adipocytic differentiation.46 However, in hematopoietic cells, the roles of the ERKs have not been clearly defined. It was demonstrated that the ERK pathway controls the differentiation of immature thymocytes downstream of the pre–T-cell receptor.47,48 Recently, it was also shown that activation of ERK pathway is required for megakaryocytic differentiation.49-51 We showed here that activation of ERKs is essential for Epo-dependent cell growth but not for Epo-induced erythroid differentiation. Therefore, it seems likely that the ERKs play a distinct role in cell differentiation and/or proliferation, depending on the cell lineage, the extracellular stimuli, and/or the intracellular conditions. An alternative explanation is that these transformed cell lines might not be simply representative of the normal physiology.
It has been well documented that JNKs and p38 have an important function in apoptotic signaling. In PC12 cells, activation of p38 and JNK and concurrent inhibition of ERK are critical for induction of apoptosis.52 Overexpression of MEKK1 or ASK1 in Swiss 3T3 cells, REF52 fibroblasts or, COS7 cells induces apoptotic cell death.53,54 However, these notions are not applicable to all cellular responses in a variety of cell types. It was recently reported that JNK and p38 are activated by the stimulation of the hematopoietic cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, thrombopoietin, and Epo.32-36Hematopoietic cytokines are known to provide not only the signals to proliferate but also the signals to suppress apoptosis.55In fact, JNK and p38 were recently indicated to be involved in cell survival and/or cell growth rather than apoptosis in hematopoietic cells. The upstream kinase of JNK and p38, SEK1, reportedly mediates survival signals in T-cell development.56 More recently, it was also reported that T-cell proliferation in response to IL-2 and IL-7 requires p38 activation.57 In this study, we demonstrated that the activation of JNKs and p38 is required for Epo-induced erythroid differentiation of SKT6 cells and for cell proliferation of FD-EPO cells. Therefore, JNKs and p38 have distinct functions (ie, induction of apoptosis, suppression of apoptosis, cell survival, cell proliferation, and cell differentiation) depending on the cell lineage, the extracellular stimuli, and the intracellular conditions. It is thus critical to clarify how these various cellular responses producing opposite effects are regulated by the same signaling pathways.
It has been reported that JNK is essential for cell differentiation. GTPase-deficient G proteins induce persistent activation of JNK and PC12 cell differentiation, and overexpression of c-Jun induces neurite outgrowth.58 In P19 carcinoma cells, ectopic expression of c-Jun leads to differentiation of P19 cells,59 and overexpression of the dominant negative form of JNK blocks the induction of differentiation by retinoic acid.60Differentiation of WEHI-3B(D+) myelomonocytic leukemia cells is reportedly also induced by ectopic expression of c-Jun.61 Furthermore, the expression and phosphorylation of heat shock protein hsp28, which is known as one of the target molecules of p38, are correlated with hemin-induced K562 cell differentiation into erythroid-like cells.62 These reports are consistent with our findings described here, and it is obvious that JNK and p38 pathways are involved in cell differentiation of at least some cell lineages.
JNKs and p38 are coordinately regulated, although to a different extent, by proinflammatory cytokines, environmental stresses, and hematopoietic cytokines.15,19,33-36 We also showed that JNKs and p38 are coordinately activated and function in Epo-induced erythroid differentiation. We saw no clear differences between them, although their activation profiles differ. The two of them may thus play overlapping roles in erythroid differentiation. JNKs and p38 are responsible for the phosphorylation and activation of transcription factors necessary for the stress responses, including c-Jun, ATF-2, Max, and CHOP.63-65 These substrates of the MAP kinase family are more or less specific for each MAP kinase,15indicating that they may have particular functions in response to various stimuli. Thus, the target molecules of JNKs and p38 and the role of JNKs and p38 signaling pathways need to be identified in hematopoietic cytokine-induced growth and differentiation systems.
ACKNOWLEDGMENT
The authors thank Drs E. Nishida and T. Moriguchi (Kyoto University, Kyoto, Japan), M. Hibi and R. Fukunaga (Osaka University, Osaka, Japan), and S. Hirai (Yokohama City University, Yokohama, Japan) for valuable discussions and thank J.S. Lee (Smithkline Beecham Pharmaceuticals) for SB203580 and Kirin Brewery for Epo.
Supported in part by Special Grants for Promotion of Research from The Institute of Physical and Chemical Research (RIKEN) and by grants from the Ministry of Education, Science and Culture of Japan.
Address reprint requests to Kazuo Todokoro, PhD, Tsukuba Life Science Center, The Institute of Physical and Chemical Research (RIKEN), 3-1, Koyadai, Tsukuba, Ibaraki 305-0074, Japan; e-mail:todokoro@rtc.riken.go.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.