Abstract
Hodgkin's disease is a common malignancy of the lymphoid system. Although the scarce Hodgkin and Reed-Sternberg (HRS) tumor cells in involved tissue synthesize major histocompatibility complex (MHC) class II and costimulatory molecules such as CD40 or CD86, it is unclear whether these tumor cells are operational antigen-presenting cells (APC). We developed an immunofluorescence-based assay to determine the number of MHC class II molecules present on the surface of single living HRS cells. We found that in fresh Hodgkin's disease lymph node biopsies, a subset of HRS cells express a substantial number of surface MHC class II molecules that are occupied by MHC class II–associated invariant chain peptides (CLIP), indicating deficient loading of MHC class II molecules with antigenic peptides. Cultured Hodgkin's disease–derived (HD) cell lines, however, were found to express few MHC class II molecules carrying CLIP peptides on the cell surface and were shown to generate sodium dodecyl sulphate (SDS)-stable MHC class II αβ dimers. In addition to showing deficient MHC class II antigen presentation in a subset of HRS cells, our results show that the widely used HD-cell lines are not ideal in vitro models for the disease. The disruption of MHC class II–restricted antigen presentation in HRS cells could represent a key mechanism by which these tumor cells escape immune surveillance.
HODGKIN'S DISEASE is one of the most common malignancies in young adults and is characterized by the presence of the scarce Hodgkin and Reed-Sternberg (HRS) tumor cells within a large population of nonmalignant bystander cells, predominantly CD4+ T cells.1 The lineage derivation of HRS cells is still controversial. A recently proposed scenario suggests that HRS cells derive from B-lineage cells,2 which subsequently adopt features of dendritic cells.3 Consistent with this model of HRS tumor cell development is a phenotype that resembles that of a “professional” antigen-presenting cell (APC). Like all APC, HRS cells as well as Hodgkin's disease–derived (HD) cell lines express major histocompatibility complex (MHC) class II together with costimulatory molecules, such as CD40 and CD86.4 It is, however, unclear whether CD4+ T cells recognize MHC class II peptide complexes on the surface of HRS cells in vivo.5
Under physiological conditions, engagement of T-cell receptors by MHC class II peptide complexes (signal 1)6 results in upregulation of CD40 ligand on the surface of the CD4+ T cell.7 Subsequent triggering of CD40 by CD40 ligand activates the APC by induction of costimulatory molecules, such as CD86, which deliver a second signal (signal 2) back to the CD4+ T cell.8 Signal 1 and signal 2 lead to full CD4+ T-cell activation and cytokine production.9 APC that have been activated by CD4+ T cells can activate CD8+ cytotoxic T cells, which subsequently develop the capacity to kill the APC.10 How HRS tumor cells can escape elimination by effector T cells in spite of having an APC phenotype has intrigued investigators for years.4
For an APC to deliver signal 1 to a CD4+ T cell, MHC class II molecules must be loaded with a complex array of endogenously generated antigenic peptides that displace CLIP, a peptide derived from MHC class II-associated invariant chain. Invariant chains are escort proteins that assemble with newly synthesized MHC class II α and β chains in the endoplasmic reticulum to form a nonameric complex11 in which part of the invariant chain luminal domains occupy the MHC class II peptide-binding grooves. After exit from the endoplasmic reticulum, the MHC class II nonamers are transported to specialized lysosomes, called MHC class II compartments,12 by means of targeting motifs within the invariant chain transmembrane domains and cytoplasmic tails.13 The subsequent degradation of invariant chains is incomplete, leaving intact those luminal peptide stretches (CLIP peptide, residues 80-107) that occupy the MHC class II peptide-binding grooves.14 The resulting MHC class II CLIP complexes are a substrate for HLA-linked gene products, called HLA-DM, which catalyze the exchange of CLIP for antigenic peptides.15 In mutant cells that fail to synthesize HLA-DM proteins, MHC class II molecules that are trafficked to the surface remain associated with CLIP peptides.16 Because HRS cells are not readily isolated from fresh tissue, APC and costimulatory functions have only been examined in cultured HD cells. For example, L428, one of the first established HD-cell lines, was shown to have the capability of activating CD4+ T cells in the mixed lymphocyte reaction.17
In the present study, we asked whether MHC class II molecules on the surface of HRS cells in fresh tumor tissue present endogenously processed antigenic peptides. Using a set of specific monoclonal antibodies (MoAbs) in combination with a quantitative immunofluorescence-based assay, we determined the membrane density of MHC class II molecules expressed on the surface of single living HRS cells. We found that in fresh Hodgkin's disease lymph node biopsies, a fraction of HRS cells expressed high levels of MHC class II CLIP complexes on the surface, which points to a defect in loading of MHC class II molecules with antigenic peptides. Interestingly, HD-cell lines consistently expressed low levels of MHC class II CLIP complexes on the surface, showing intact loading and presentation of antigenic peptides in these cells. Taken together, our observations show that the MHC class II antigen presentation pathway is disrupted in a proportion of HRS cells. HRS cells that express high levels of surface MHC class II CLIP complexes are not likely to activate CD4+ helper T cells, which could explain why HRS cells are not rapidly eliminated by CD8+ cytotoxic T cells.18 Surface appearance of high levels of MHC class II CLIP complexes could therefore represent a selection process by which some HRS tumor cells escape immune surveillance.
MATERIALS AND METHODS
Lymph Nodes
Diagnostic lymph node biopsy specimens were obtained from a 33-year-old man, a 38-year-old woman (nodular sclerosis subtypes), and a 30-year-old man (lymphocyte-rich classical subtype). The biopsy specimens were placed in Hanks' Balanced Salt Solution (HBSS; GIBCO-BRL, Life Technologies Ltd, Paisley, UK) containing 2% fetal bovine serum (FBS; complete HBSS) and disaggregated into a single-cell suspension using a Medimachine (Dako Ltd, Bucks, UK). After resuspension in complete HBSS, the viable cell fraction was recovered by flotation on Lymphoprep density medium (Nycomed Pharma AS, Oslo, Norway). The cells were washed twice in complete HBSS and either cryopreserved or used immediately for immunofluorescent staining.
Cell Lines and Antibodies
Cell lines.
The Burkitt's lymphoma–derived and Epstein-Barr virus (EBV)–transformed human B-lymphocyte cell lines RAJI and DAUDI were from the American Type Culture Collection (ATCC; Rockville, MD). The EBV-negative B-lymphocyte cell line BJAB19 was obtained from the Beatson Institute for Cancer Research (Glasgow, UK). The nodular sclerosis HD-cell lines L428 and HO, derived from pleural effusion and lymph node tissue, respectively, were a gift from David Jones (University of Southampton, UK). The nodular sclerosis HD-cell line HDLM2 and the mixed cellularity HD-cell line KMH2, both derived from pleural effusions, were a gift from Hans Drexler (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany). The T-cell lymphoblastic lymphoma–derived cell line SUPT1 was obtained from the MRC AIDS Reagent Project (Herts, UK). The acute T-cell leukemia–derived cell line JJHAN, a subclone of the cell line JURKAT, was a gift from Michael Steel (University of St Andrews, Fife, UK). All cells were cultured in RPMI 1640 medium containing L-glutamine (GIBCO-BRL) supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin (complete RPMI medium).
Antibodies.
HRS4, a mouse MoAb that recognizes the CD30 antigen20 was purchased from Coulter Electronics Ltd (Luton, UK). BU27 is a mouse MoAb that recognizes assembled, dimeric forms of HLA-DP, HLA-DQ, and HLA-DR gene products and was obtained from The Binding Site (Birmingham, UK). CerCLIP.1 is a mouse MoAb that recognizes CLIP peptides in association with MHC class II molecules16 and was a gift from Peter Cresswell (Yale University, New Haven, CT). Control mouse IgG, phycoerythrin (PE)-, and fluorescein (FITC)-conjugated goat anti-mouse IgG were purchased from Dako Ltd. PE-conjugated mouse IgG and PE-conjugated anti-CD40 MoAb were from Coulter Electronics Ltd.
Flow Cytometry
For certain experiments, cells were cultured in the presence of 1.5 mmol/L leupeptin (Sigma-Aldrich Company Ltd, Dorset, UK). For indirect fluorescent labeling, aliquots of 106 cells were washed once in ice-cold phosphate-buffered saline containing 3% FBS, 0.1% bovine serum albumin, and 0.1% sodium azide (phosphate azide buffer [PAB]). Washed cells were resuspended in 100 μL PAB and incubated for 1 hour at 4°C with either 10 μL of purified mouse IgG (0.1 μg/μL), 10 μL of BU27, or 10 μL of CerCLIP.1 crude mouse ascitic fluid. The cells were then washed in PAB again, resuspended in 100 μL PAB, and further incubated for 30 minutes at 4°C with 5 μL FITC-conjugated goat anti-mouse IgG (0.5 μg/μL). Titration experiments showed that the amounts of primary and secondary antibodies used here were well above the saturation levels of 106cells (data not shown). After two additional washing steps, the cells were fixed in PAB containing 1% paraformaldehyde (PFA; Sigma-Aldrich Company Ltd). For direct fluorescent labeling, aliquots of 106 cells were incubated under saturating conditions for 1 hour at 4°C with either 10 μL PE-conjugated mouse IgG (2 μg/μL) or 10 μL PE-conjugated anti-CD40 IgG (2 μg/μL) in a total volume of 100 μL PAB. Labeled cells were washed twice and fixed in PAB containing 1% PFA. Samples were analyzed on an EPICSR Elite instrument (Coulter Electronics Ltd).
Immunofluorescence Microscopy
Aliquots of 106 cells were resuspended in complete RPMI medium containing 5 μg/mL Hoechst 33258 (Sigma-Aldrich Company Ltd) and incubated for 90 minutes at 37°C. The cells were then incubated with the same primary antibodies and under the same conditions as described for flow cytometric analysis. After washing in PAB, cells were resuspended in 100 μL PAB and incubated for 30 minutes at 4°C with 5 μL of PE-conjugated goat anti-mouse IgG (0.5 μg/μL). Fixed cells were transferred onto glass slides using a Shandon CytospinR2 cytocentrifuge (Life Sciences International Ltd, Basingstoke, UK), mounted with DakoRFluorescence Mounting Medium (Dako Ltd), and examined with a Leitz Laborlux K microscope (Leitz Instruments Ltd, Luton, UK). To determine the number of MHC class II molecules on the surface of single cells, synthetic beads with a known number of bound MoAbs (DAKO QIFIKITR, Dako Ltd) were labeled with a PE-conjugated goat anti-mouse antibody under saturating conditions and processed along with fluorescently labeled cells. Briefly, a mixture of labeled (4.2 × 105 bound MoAb molecules per bead) and unlabeled (no MoAbs bound) synthetic beads (approximately 106 in total) were resuspended in a total volume of 100 μL PAB, incubated for 30 minutes at 4°C with 5 μL PE-conjugated goat anti-mouse IgG (0.5 μg/μL), washed twice with PAB, and resuspended in PAB containing 1% PFA. Fluorescence intensities of individual cells and beads were determined by densitometric analysis of exposed HP5 Plus negatives (HA West, Glasgow, UK) using a Kodak RFS 2035 scanner (HA West) in combination with Adobe Photoshop 3.0 (Adobe Systems Inc, Edinburgh, UK) and Molecular AnalystR 1.5 (Bio-Rad Laboratories Ltd, Hemel Hempstead, UK) software.
Western Blotting
Cells were lysed in 50 mmol/L Tris-HCl pH 6.8, 2% sodium dodecyl sulfate (SDS), 10% glycerol, 0.1% bromophenol blue (nonreducing sample buffer), and left at room temperature for 30 minutes. The lysates were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE)21 on 10% acrylamide gels, and proteins were transferred to Immobilon-P membranes (Millipore, Watford, UK). The membranes were subsequently probed with the MoAb BU27 and labeled bands revealed using the VECTASTAINR ABC system (Vector Laboratories, Bretton, UK).
RESULTS
Intact MHC Class II Antigen Presentation in HD Cell Lines
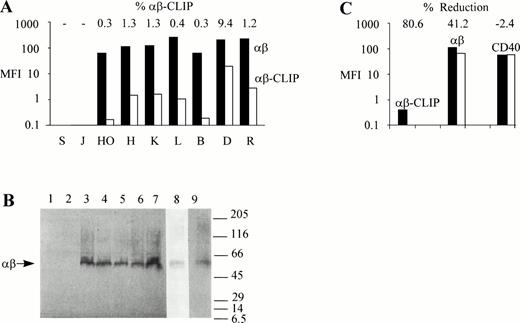
To study MHC class II antigen processing and presentation in Hodgkin's disease, we first compared surface expression of MHC class II αβ dimers in HD-cell lines with those in human B- and T-cell lines. Flow cytometric analysis showed that MHC class II αβ dimers were expressed at high levels on the surface of the HD-cell lines, HO, HDLM2, KMH2, and L428 (Fig 1A), with 102-to 103-fold differences in the mean fluorescence intensity (MFI) values between cells labeled with the MHC class II–specific MoAb, BU27, and cells labeled with control mouse IgG. Similarly, high levels of MHC class II surface expression were found in the human B-cell lines, BJAB,19 DAUDI, and RAJI (Fig 1A). No MHC class II molecules could be detected on the surface of the human T-cell lines, SUPT1 and JJHAN (Fig 1A). As judged by the intensity of the fluorescent staining, MHC class II surface expression was somewhat higher in L428, DAUDI, and RAJI cells when compared with the other MHC class II–positive cell lines used.
Intact MHC class II antigen presentation in HD-cell lines. (A) Surface expression of MHC class II αβ dimers and MHC class II αβ–CLIP complexes in HD-cell lines. SUPT1 (S), JJHAN (J), HO, (HO), HDLM2 (H), KMH2 (K), L428 (L), BJAB (B), DAUDI (D), and RAJI (R) cells were either incubated with mouse IgG, CerCLIP.1, or BU27 MoAbs. Bound MoAbs were labeled with FITC-conjugated goat anti-mouse secondary antibodies and the samples processed for flow cytometric analysis. Bars indicate MFI values (MFI) of MHC class II αβ–CLIP complexes (αβ-CLIP, white bars) and MHC class II αβ dimers (αβ, black bars) after subtraction of MFI values obtained from fluorescent staining using mouse IgG as a primary reagent. Shown on top are the relative amounts of MHC class II αβ–CLIP complexes expressed as a percentage of the total amount of surface expressed MHC class II αβ dimers (% αβ-CLIP). (B) Formation of SDS-stable MHC class II αβ dimers in HD-cell lines. 106 SUPT1 (lane 1), JJHAN (lane 2), HO (lane 3), L428 (lane 4), BJAB (lane 5), DAUDI (lane 6), RAJI (lane 7), HDLM2 (lane 8), and KMH2 cells (lane 9) were lysed in presence of SDS under nonreducing conditions and lysates analyzed by SDS-PAGE and Western blotting using the anti-MHC class II antibody, BU27, as a probe. This antibody reacts only with MHC class II αβ dimers. It does not react with either free MHC class II α or β chains. The positions of molecular weight markers (expressed as 10-3 × Mr) are shown on the right. The symbol, αβ, shown on the left, points to the SDS-stable MHC class II αβ dimers (∼60 kD). HDLM2 (lane 8) and KMH2 cell lysates (lane 9) were run on separate gels because the background produced by the MoAb, BU27, was lower in HDLM2 and higher in KMH2 cells than in the other cell lines used. (C) Leupeptin inhibits surface expression of MHC class II αβ–CLIP complexes in L428 cells. L428 cells were cultured in the absence (black bars) or presence (white bars) of 1.5 mmol/L leupeptin for 24 hours. The cells were stained with the same primary (BU27, αβ; CerCLIP.1, αβ-CLIP) and secondary reagents as described in (A). As a negative control for the effect of leupeptin on the surface expression of MHC class II molecules, cells were labeled with a PE-conjugated anti-CD40 antibody or with a PE-conjugated mouse IgG. Shown are MFI values obtained with the PE-conjugated anti-CD40 antibody (CD40) after subtraction of MFI values generated by the PE-conjugated mouse IgG. The relative reductions of cell surface fluorescence after leupeptin treatment are indicated on top (% Reduction).
Intact MHC class II antigen presentation in HD-cell lines. (A) Surface expression of MHC class II αβ dimers and MHC class II αβ–CLIP complexes in HD-cell lines. SUPT1 (S), JJHAN (J), HO, (HO), HDLM2 (H), KMH2 (K), L428 (L), BJAB (B), DAUDI (D), and RAJI (R) cells were either incubated with mouse IgG, CerCLIP.1, or BU27 MoAbs. Bound MoAbs were labeled with FITC-conjugated goat anti-mouse secondary antibodies and the samples processed for flow cytometric analysis. Bars indicate MFI values (MFI) of MHC class II αβ–CLIP complexes (αβ-CLIP, white bars) and MHC class II αβ dimers (αβ, black bars) after subtraction of MFI values obtained from fluorescent staining using mouse IgG as a primary reagent. Shown on top are the relative amounts of MHC class II αβ–CLIP complexes expressed as a percentage of the total amount of surface expressed MHC class II αβ dimers (% αβ-CLIP). (B) Formation of SDS-stable MHC class II αβ dimers in HD-cell lines. 106 SUPT1 (lane 1), JJHAN (lane 2), HO (lane 3), L428 (lane 4), BJAB (lane 5), DAUDI (lane 6), RAJI (lane 7), HDLM2 (lane 8), and KMH2 cells (lane 9) were lysed in presence of SDS under nonreducing conditions and lysates analyzed by SDS-PAGE and Western blotting using the anti-MHC class II antibody, BU27, as a probe. This antibody reacts only with MHC class II αβ dimers. It does not react with either free MHC class II α or β chains. The positions of molecular weight markers (expressed as 10-3 × Mr) are shown on the right. The symbol, αβ, shown on the left, points to the SDS-stable MHC class II αβ dimers (∼60 kD). HDLM2 (lane 8) and KMH2 cell lysates (lane 9) were run on separate gels because the background produced by the MoAb, BU27, was lower in HDLM2 and higher in KMH2 cells than in the other cell lines used. (C) Leupeptin inhibits surface expression of MHC class II αβ–CLIP complexes in L428 cells. L428 cells were cultured in the absence (black bars) or presence (white bars) of 1.5 mmol/L leupeptin for 24 hours. The cells were stained with the same primary (BU27, αβ; CerCLIP.1, αβ-CLIP) and secondary reagents as described in (A). As a negative control for the effect of leupeptin on the surface expression of MHC class II molecules, cells were labeled with a PE-conjugated anti-CD40 antibody or with a PE-conjugated mouse IgG. Shown are MFI values obtained with the PE-conjugated anti-CD40 antibody (CD40) after subtraction of MFI values generated by the PE-conjugated mouse IgG. The relative reductions of cell surface fluorescence after leupeptin treatment are indicated on top (% Reduction).
We next examined whether HD-cell lines were capable of generating the processing intermediate MHC class II αβ-CLIP. As shown in Fig 1A, surface MHC class II αβ–CLIP complexes were detectable in HO, HDLM2, KMH2, and L428 cells as well as in BJAB, DAUDI, and RAJI cells, but not in the MHC class II–negative T-cell lines, SUPT1 and JJHAN, which excludes the possibility of an MHC class II αβ–CLIP staining artifact due to different preparations of control mouse IgG and CerCLIP.1 MoAb. In all MHC class II–positive cell lines examined, surface localized MHC class II αβ–CLIP complexes accounted for about 1% or less of the total surface pool of MHC class II molecules, except for DAUDI cells where surface expression of MHC class II αβ–CLIP complexes reached approximately 10% (Fig 1A). These observations show that HD-cell lines possess an operational proximal MHC class II pathway, including correct synthesis, assembly, and intracellular trafficking of MHC class II molecules with subsequent limited proteolysis of invariant chains and the formation of MHC class II αβ–CLIP processing intermediates.
To show that the majority of MHC class II molecules expressed by HD-cell lines are complexed to peptides other than CLIP, we examined the SDS stability of MHC class II molecules in these cell lines. MHC class II αβ dimers that are empty or associated with either invariant chains22 or CLIP peptides16dissociate in the presence of SDS at room temperature. Upon acquisition of antigenic peptides, MHC class II αβ dimers undergo a structural change that renders them resistant to SDS at room temperature.22 Therefore, compact SDS-stable MHC class II αβ dimers migrate as a 60 kD complex when subjected to SDS-PAGE.22 Aliquots of the cell lines described in the legend to Fig 1A were solubilized in nonreducing sample buffer, containing 2% SDS, and left at room temperature for 30 minutes before analysis by SDS-PAGE and Western blotting. As shown in Fig 1B, the B-cell lines BJAB (lane 5), DAUDI (lane 6), and RAJI (lane 7), as well as the HD-cell lines HO (lane 3), L428 (lane 4), HDLM2 (lane 8), and KMH2 (lane 9) were capable of generating MHC class II αβ dimers that, under nonreducing conditions, were stable in SDS, migrating with an apparent molecular weight of approximately 60 kD. SUPT1 (lane 1) and JJHAN cells (lane 2), which do not express MHC class II molecules, were included as negative controls. These observations show the capability of HD-cell lines to generate and present peptides from intracellular and/or extracellular sources like other cultured professional APC, such as BJAB, DAUDI, or RAJI cells, and are indicative of an intact MHC class II pathway downstream of the formation of MHC class II αβ–CLIP intermediates. To further exclude an MHC class II αβ–CLIP staining artifact, we cultured L428 cells, which express little MHC class II αβ–CLIP complexes on the cell surface, in the presence of the serine-cystein protease inhibitor, leupeptin, a peptide analogue that interferes with the formation of MHC class II αβ–CLIP complexes by inhibiting the proteolytic processing of invariant chains.23 Leupeptin also blocks cell surface transport of accumulating MHC class II αβ dimers associated with NH2-terminal invariant chain processing intermediates by an unknown mechanism.24 As shown in Fig 1C, L428 cells that had been cultured in the presence or absence of 1.5 mmol/L leupeptin for 24 hours and that had been subsequently surface labeled with CerCLIP.1 MoAb produced MFI values of approximately 0.1 and 0.4, respectively. Therefore, 80% of all MHC class II αβ–CLIP complexes disappeared from the surface of L428 cells after leupeptin treatment. The leupeptin-induced reduction of the total cell surface pool of MHC class II αβ dimers within the same time period was approximately 40% (Fig 1C), reflecting a long lifespan of MHC class II molecules in L428 cells. Leupeptin had no effect on the surface expression of the tumor necrosis factor receptor protein, CD40, which is expressed at high levels in L428 cells (Fig 1C). As determined by flow cytometric forward and side scatter analysis, the size and granularity of the cells did not change in presence of leupeptin even after prolonged incubation periods of up to 72 hours (data not shown), indicating that the reduction of MHC class II molecules and the disappearance of MHC class II αβ–CLIP complexes from the cell surface was not caused by the cytotoxicity of the drug. Thus, the data shown in Fig 1C show the validity of MHC class II αβ–CLIP detection by immunofluorescent surface staining.
Deficient MHC Class II Antigen Presentation in a Subset of HRS Cells
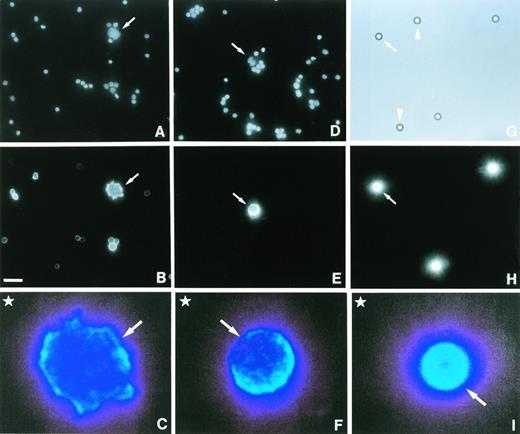
Having shown intact MHC class II antigen presentation in cultured HD cells, we next examined HRS tumor cells in diseased lymph node biopsies. Because HRS tumor cells are not readily isolated from involved tissue, we used a morphological approach to obtain more information on the functionality of the MHC class II pathway in these cells. Fresh lymph node tissue was disaggregated into a single-cell suspension, and the viable cell fraction was cultured in complete RPMI medium containing the DNA-binding fluorescent dye, Hoechst 33258, to visualize nuclear size and shape, which are both defining criteria of HRS tumor cells.25 As shown in Fig 2, Hoechst staining clearly identified HRS tumor cells (A and D, arrows) within the large population of nonmalignant bystander cells. Bystander cell nuclei were approximately 5 μm in diameter and often brightly stained (Fig 2A and D). The size of HRS cell nuclei, which were usually dimly stained, ranged from 5 μm to over 10 μm (Fig. 2A and D, arrows). Expression of the CD30 antigen20 by HRS cells was confirmed using cell-surface fluorescent labeling with the anti-CD30 MoAb, HRS4 (data not shown). Surface labeling of MHC class II αβ dimers showed bright staining of HRS cells (Fig 2B, arrow) and somewhat weaker staining of most of the MHC class II–positive surrounding lymphocytes (Fig 2A and B).
Deficient MHC class II antigen presentation in a subset of HRS cells. Fresh lymph node tissue from a patient with nodular sclerosis Hodgkin's disease was disaggregated into a single-cell suspension and the viable cell fraction incubated for 90 minutes at 37°C with 5 μg/mL Hoechst 33258 (A and D) and stained with either an MHC class II αβ dimer-specific antibody (BU27) (B) or an antibody that recognizes CLIP bound to MHC class II molecules (CerCLIP.1) (E). Cell suspensions with surface-bound MoAbs or a mixture of synthetic beads with (G, H, I, arrows) or without (G, arrowheads) bound mouse IgG2a molecules (4.2 × 105 per bead) were fluorescently labeled with a PE-conjugated goat anti-mouse secondary antibody. Samples were processed for fluorescence microscopy and visualized by illumination with either ultraviolet (A, D), green (B, E, H), or white light (G). Enlarged images of labeled HRS cells (C, F, arrows) and synthetic beads (I, arrow) were used for densitometric analysis. Here, examples are shown in false colors to visualize the relative membrane densities of MHC class II molecules. A long wavelength (light blue) represents a high membrane density. Densities in the upper left-hand corner (C, F, I, stars) were used for background subtraction. Notice that a proportion of bystander lymphocytes express MHC class II αβ dimers (B) but not MHC class II αβ–CLIP complexes (E). Bar in (B) is 20 μm. Magnifications in (A, B, D, E, G, H) are the same.
Deficient MHC class II antigen presentation in a subset of HRS cells. Fresh lymph node tissue from a patient with nodular sclerosis Hodgkin's disease was disaggregated into a single-cell suspension and the viable cell fraction incubated for 90 minutes at 37°C with 5 μg/mL Hoechst 33258 (A and D) and stained with either an MHC class II αβ dimer-specific antibody (BU27) (B) or an antibody that recognizes CLIP bound to MHC class II molecules (CerCLIP.1) (E). Cell suspensions with surface-bound MoAbs or a mixture of synthetic beads with (G, H, I, arrows) or without (G, arrowheads) bound mouse IgG2a molecules (4.2 × 105 per bead) were fluorescently labeled with a PE-conjugated goat anti-mouse secondary antibody. Samples were processed for fluorescence microscopy and visualized by illumination with either ultraviolet (A, D), green (B, E, H), or white light (G). Enlarged images of labeled HRS cells (C, F, arrows) and synthetic beads (I, arrow) were used for densitometric analysis. Here, examples are shown in false colors to visualize the relative membrane densities of MHC class II molecules. A long wavelength (light blue) represents a high membrane density. Densities in the upper left-hand corner (C, F, I, stars) were used for background subtraction. Notice that a proportion of bystander lymphocytes express MHC class II αβ dimers (B) but not MHC class II αβ–CLIP complexes (E). Bar in (B) is 20 μm. Magnifications in (A, B, D, E, G, H) are the same.
We next asked whether some of the MHC class II molecules on the surface of HRS or bystander cells were occupied by the MHC class II–associated invariant chain peptide, CLIP. Fluorescent surface labeling using the MoAb, CerCLIP.1, showed high levels of MHC class II αβ–CLIP complexes on the surface of a proportion of HRS cells (Fig 2E, arrow), whereas bystander cells were consistently negative or only weakly positive (Fig 2D and E). Cell-surface–localized MHC class II αβ–CLIP complexes were detectable on approximately half of all HRS cells (data not shown). Our observations suggest that a fraction of HRS cells has a significantly reduced capability of loading and presenting MHC class II–restricted antigenic peptides.
To determine to what extent MHC class II antigen presentation is compromised in HRS cells, we next calculated the number of surface-localized MHC class II αβ dimers or alternatively MHC class II αβ–CLIP complexes in these cells. To this end, a mixture of synthetic beads with either a high number of bound MoAbs or with no MoAbs bound were fluorescently labeled along with the single-cell suspensions, using a PE-conjugated goat anti-mouse secondary antibody. Fluorescently labeled samples were then exposed to photographic film, and the developed negatives were subjected to densitometric analysis as described in Materials and Methods. In the example shown in Fig 2, the differences in fluorescence intensities of either labeled HRS cells (C and F) or labeled synthetic beads (I) are visualized using false color analysis. After subtraction of background values (Fig 2C, F, and I, star in upper left-hand corner), the calculated mean densities of stained cells and stained beads together with the known number of MoAb molecules bound to the beads were then used to calculate the number of MoAbs bound to the surface of HRS cells. The calculated mean densitometric value derived from 10 analyzed fluorescently labeled synthetic beads, representing approximately 0.4 × 106MoAb molecules, was used to calculate the number of surface-bound BU27 and CerCLIP.1 MoAbs on individual HRS cells. Analysis of the HRS cells depicted in Fig 2C and F yielded numbers of approximately 1.2 × 106 surface-bound BU27 MoAb and 0.7 × 106surface-bound CerCLIP.1 MoAb, respectively. These results show that in a subset of HRS cells in diseased lymph node tissue, a high number of surface-localized MHC class II αβ dimers carry CLIP peptides, indicating a loading and presentation deficiency of antigenic peptides in these cells.
Quantitation of MHC Class II αβ Dimers and MHC Class II αβ–CLIP Complexes Expressed on the Surface of HRS Cells
Having shown that a subset of HRS cells express high levels of MHC class II αβ–CLIP complexes on the surface (Fig 2F), we next calculated the plasma membrane density of MHC class II αβ dimers and compared it with the density of MHC class II αβ–CLIP complexes. Membrane densities are more informative than the total number of molecules expressed on the cell surface because the size of HRS cells can vary significantly. For example, the mean surface densities of the two HRS cells shown in Fig 2C and F were 1.0 × 103 MHC class II αβ dimers and 1.1 × 103 MHC class II αβ–CLIP complexes per μm2 cell surface area, respectively. The mean density of the fluorescently labeled synthetic bead shown in Fig 2I is approximately 1.4 × 103 IgG2a molecules per μm2 surface area.
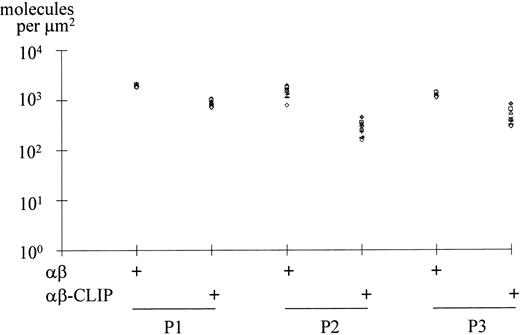
To generalize our findings, we compared three different diagnostic lymph node biopsies, of which two were of nodular sclerosis subtype (Fig 3, P1 and P2) and one of lymphocyte-rich classical subtype (Fig 3, P3). Photographic negatives with the 10 most brightly stained HRS cells were selected and subjected to densitometric analysis. The mean densities of MHC class II αβ dimers and MHC class II αβ–CLIP complexes were expressed as the number of molecules per μm2 surface area. As shown in Fig3, HRS cells from cases P1 and P3 expressed on average 1.9 and 1.2 × 103 MHC class II αβ dimers per μm2 membrane area, respectively. The average membrane densities of MHC class II αβ–CLIP complexes were 0.9 and 0.5 × 103 MHC class II αβ–CLIP complexes per μm2, respectively. Therefore, every second surface localized MHC class II αβ dimer carried the CLIP peptide. HRS cells from case P2 expressed an average number of 1.4 × 103MHC class II αβ dimers per μm2 surface area. The average number of MHC class II αβ–CLIP complexes per μm2 was 0.3 × 103; thus, one in five surface-localized MHC class II molecules was associated with CLIP in this case. Our observations suggest that a disturbed MHC class II antigen presentation pathway in a proportion of HRS tumor cells may be a general phenomenon in Hodgkin's disease.
Quantitation of MHC class II αβ dimers and MHC class II αβ–CLIP complexes expressed on the surface of HRS cells. Diagnostic lymph node biopsies from two cases of nodular sclerosis Hodgkin's disease (P1, P2) and one case of lymphocyte-rich classical Hodgkin's disease (P3) were disaggregated into single-cell suspensions and the cells fluorescently labeled with BU27 (αβ) and CerCLIP.1 (αβ-CLIP) MoAbs as described in the legend to Fig 2. For each staining, the 10 most intensely stained HRS cells were selected and used for densitometric analysis. The membrane densities of MHC class II αβ dimers and MHC class II αβ–CLIP complexes are expressed as the number of molecules per μm2 plasma membrane area.
Quantitation of MHC class II αβ dimers and MHC class II αβ–CLIP complexes expressed on the surface of HRS cells. Diagnostic lymph node biopsies from two cases of nodular sclerosis Hodgkin's disease (P1, P2) and one case of lymphocyte-rich classical Hodgkin's disease (P3) were disaggregated into single-cell suspensions and the cells fluorescently labeled with BU27 (αβ) and CerCLIP.1 (αβ-CLIP) MoAbs as described in the legend to Fig 2. For each staining, the 10 most intensely stained HRS cells were selected and used for densitometric analysis. The membrane densities of MHC class II αβ dimers and MHC class II αβ–CLIP complexes are expressed as the number of molecules per μm2 plasma membrane area.
DISCUSSION
Hodgkin's disease is an unusual type of cancer in which the number of nonmalignant bystander cells in tumor tissue exceeds the number of the malignant HRS cells by two or three orders of magnitude. The scarcity of HRS cells in diseased lymph node tissue and the difficulty of isolating them have hampered attempts to study the biology of HRS tumor cells. HRS tumor cells have the molecular signature of a professional APC4 and are probably B-cell derived.2 To obtain more information on the integrity of the MHC class II antigen-presentation pathway in HRS tumor cells, we tested whether MHC class II molecules on the cell surface present endogenously processed antigenic peptides.
In professional APC, exchange of CLIP peptides for endogenous self or exogenous foreign peptides precedes transport of MHC class II molecules to the plasma membrane, hence MHC class II αβ–CLIP intermediates are predominantly localized to intracellular compartments of the endocytic pathway.16 However, some of the MHC class II αβ–CLIP complexes are trafficked to the plasma membrane of professional APC,26 and the extent to which these intermediates appear on the surface seems to depend on the HLA alleles expressed by these cells (A. Rudensky, University of Washington, Seattle, personal communication, January 1997). We speculated that the formation of MHC class II αβ–CLIP intermediates in HD-cell lines could be shown by surface fluorescent labeling alone without the need to permeabilize the cells. This is of practical importance because intracellular staining protocols include a fixation step with PFA before permeabilization and are therefore not suitable in combination with the anti–MHC class II αβ–CLIP antibody, CerCLIP.1, which recognizes a conformation-dependent epitope that is lost during PFA fixation (data not shown).
We first examined HD-cell lines in comparison with human B-cell lines for the formation and surface expression of MHC class II–CLIP complexes (Fig 1). With the exception of DAUDI cells, all HD- and B-cell lines used in this study showed that only about 1% of all plasma-membrane–localized MHC class II molecules were occupied by CLIP peptides (Fig 1A, percent αβ-CLIP). Having shown that HD-cell lines possess an operational proximal MHC class II pathway that generates MHC class II–CLIP intermediates, we subsequently tested whether these intermediates would be further processed to give rise to SDS-stable MHC class II αβ dimers associated with endogenously generated antigenic peptides. We found SDS-stable MHC class II complexes in HD-cell lines as well as in B-cell lines (Fig 1B), which shows an intact MHC class II pathway distal to the formation of MHC class II αβ–CLIP intermediates, excluding the possibility that any of the cell lines tested are deficient in MHC class II antigen processing and presentation.
One caveat of using HD-cell lines as an in vitro model for the disease lies in the fact that cell lines represent the outgrowth of a single parent tumor cell. HD-cell lines are therefore not representations of the heterogeneous tumor cell mass in the Hodgkin's lymphoma patients from whom the cell lines were derived. To date, only 15 HD-cell lines have been established,27 representing 15 transformed parent cells from different cases of Hodgkin's disease. In addition, it is uncertain whether the parent cells that gave rise to the cell lines were bona fide HRS cells. For instance, the outgrowth of EBV-transformed lymphoblastoid cell lines from cultured Hodgkin's lymphoma specimens is a frequent occurrence. Moreover, these lymphoblastoid cell lines are not easily distinguishable from HD-cell lines harboring the EBV genome. Although all but one of the HD-cell lines are not infected with EBV, it remains uncertain whether these cell lines are derived from HRS tumor cells because of the possibility that bystander cells harboring another unidentified transforming virus could give rise to cell lines in vitro. Therefore, data obtained from cultured HD cells must be complemented with data obtained from fixed tissue sections or, ideally, fresh biopsy material, to determine the biological significance of the observations obtained from cell cultures.
Thus, we next determined the levels of expression of MHC class II αβ–CLIP complexes on the surface of different HRS cells in fresh lymph node biopsies (Fig 2). The use of paraffin-embedded sections that facilitate the testing of large numbers of cases was not feasible for two main reasons. First, as mentioned earlier, the CerCLIP.1 MoAb recognizes a conformation-dependent epitope on MHC class II αβ–CLIP complexes, which is lost during PFA fixation (data not shown). Second, isolated labeling of plasma-membrane–localized MHC class II molecules requires intact living cells. Although the use of fresh lymph node biopsies to study protein expression in HRS cells has many advantages, the loss of cytoarchitecture requires a novel strategy to identify HRS tumor cells in suspension. To overcome this difficulty, we made use of a nuclear labeling protocol in which the fluorescent DNA-binding dye, Hoechst 33258, is taken up by viable cells and accumulated in the nucleus. Visualization of nuclear size and shape combined with cell-surface fluorescent labeling allowed us to examine individual HRS cells for expression of MHC class II αβ–CLIP complexes. Interestingly, the data obtained from viable lymph node cell suspensions showed a marked difference when compared with the data obtained from HD-cell cultures. A subset of HRS cells expressed markedly high levels of plasma-membrane–localized MHC class II αβ–CLIP complexes, whereas the levels of expression of MHC class II αβ dimers on the surface of individual HRS cells were similar. Because in the absence of HLA-DM molecules, which catalyze the exchange of CLIP for antigenic peptides,28 MHC class II αβ–CLIP complexes are trafficked to the cell membrane, one would predict that some HRS tumor cells express lower levels of HLA-DM. Future experiments are needed to determine, in HRS tumor cells, the levels of HLA-DM or HLA-DO, another HLA-linked gene product that blocks HLA-DM function.29
We attempted to determine the stability of MHC class II molecules in the presence of SDS to obtain direct evidence of antigenic peptide loading in HRS cells. Although fluorescence-activated cell sorting–based enrichments of cells expressing high levels of the tumor necrosis factor receptor protein, CD30,20 from lymph node cell suspensions contained over 80% bona fide HRS cells as judged by Hoechst 33258 fluorescent staining (data not shown), the cell numbers obtained in our experiments were too small for analysis of SDS-stable MHC class II αβ dimers by Western blotting. However, the morphological observation that a significant number of MHC class II αβ–CLIP complexes are routed to the surface in some HRS cells suggests a loss of APC function. To determine the functionality of MHC class II and costimulatory molecules expressed by HRS cells, it will be necessary to determine whether these cells are potent stimulators of primary mixed lymphocyte cultures.
It has been shown that in a proportion of cases HRS cells are latently infected with EBV, expressing high levels of the viral gene product latent integral membrane protein 1 (LMP 1).30 Cells presenting LMP 1–derived peptides in an MHC class I–restricted fashion are capable of activating CD8+ cytotoxic T cells.31 Intriguingly, HRS cells fail to activate CD8+ cytotoxic T cells irrespective of infection with EBV.32 Recent studies addressing a possible downregulation of MHC class I molecules by HRS cells as a mechanism of immune escape produced conflicting results.33,34 Using a conformation-dependent anti-MHC class I antibody, we found abundant expression of MHC class I molecules on the surface of HRS cells (data not shown). The results presented in this study indicate that, in Hodgkin disease, effective MHC class II antigen presentation by a fraction of HRS cells is compromised. The predicted lack of CD4+ T-cell activation offers an attractive explanation how HRS cells could evade killing by CD8+ cytotoxic T cells. Moreover, the high levels of MHC class II αβ–CLIP complexes on the surface of HRS cells could provide a rational basis for autologous bone marrow transplantation in patients who have failed chemotherapy regimens and who cannot be cured with conventional therapies. Recently, it has been shown that the anti-tumor activity of the cyclosporine-induced graft-versus-host disease after autologous bone marrow transplantation depends on the induction of promiscuous CD8+ cytotoxic T cells that recognize MHC class II αβ–CLIP complexes.35 Further studies are needed to determine whether disease progression correlates with the selective outgrowth of an HRS cell population presenting high levels of surface MHC class II αβ–CLIP peptides.
ACKNOWLEDGMENT
We thank Lesley Shield for expert technical assistance; Bob Jackson, Nick Rooney, and Robin Reid for providing patient material; Peter Cresswell, David Jones, Hans Drexler, and Michael Steel for their gift of antibodies and cell lines; and Alexander Rudensky for advice. We thank the members of the staff at the Beatson Laboratories (Glasgow, UK) for advice and assistance with the densitometric analysis of data.
Supported by Grant No. 9520 from the Leukemia Research Fund. H.B. is a Leukemia Research Fund Clinical Research Fellow.
Address correspondence to Herbert Bosshart, MD, Leukemia Research Fund Virus Centre, Bearsden Road, Glasgow G61 1QH, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.