Abstract
The interaction between p145c-KIT and p210bcr-abl in transduced cell lines, and the selective outgrowth of normal progenitors during long-term culture of chronic myeloid leukemia (CML) cells on stroma deficient in stem-cell factor (SCF) suggests that the response of CML cells to SCF may be abnormal. We examined the proliferative effect of SCF(100 ng/mL), provided as the sole stimulus, on individual CD34+ cells from five normal donors and five chronic-phase CML patients. Forty-eight percent of isolated single CML CD34+ cells proliferated after 6 days of culture to a mean of 18 cells, whereas only 8% of normal CD34+ cells proliferated (mean number of cells generated was 4). SCF, as a single agent, supported the survival and expansion of colony-forming unit–granulocyte-macrophage (CFU-GM) from CML CD34+CD38+ cells and the more primitive CML CD34+CD38− cells. These CFU-GM colonies were all bcr-abl positive, showing the specificity of SCF stimulation for the leukemic cell population. Coculture of CML and normal CD34+ cells showed exclusive growth of Ph+cells, suggesting that growth in SCF alone is not dependent on secretion of cytokines by CML cells. SCF augmentation of β1-integrin–mediated adhesion of CML CD34+cells to fibronectin was not increased when compared with the effect on normal CD34+ cells, suggesting that the proliferative and adhesive responses resulting from SCF stimulation are uncoupled. The increased proliferation may contribute to the accumulation of leukemic progenitors, which is a feature of CML.
CHRONIC MYELOID leukemia (CML) is characterized cytogenetically by the Philadelphia chromosome (Ph), t(9;22)(q34;q1),1 and at the molecular level, by expression of the bcr-abl fusion gene.2 The cell targeted by the Ph translocation is usually a pluripotent stem cell,3 but excessive proliferation is confined to the myeloid and megakaryocytic lineages. Leukemic progenitors are capable of differentiation to functional, mature hematopoietic cells.
In the early chronic phase of CML, the coexistence of normal (Ph−) and leukemic (Ph+) hematopoiesis is demonstrable in the bone marrow (BM) and blood of CML patients.4-8 The underlying mechanisms for the accumulation of Ph+ progenitors in CML have been only partially elucidated, but they include increased survival due to an antiapoptotic effect of p210bcr-abl9 and reduced effect on proliferation by inhibitors such as macrophage inflammatory protein 1-α.10
Normal hematopoiesis is a tightly regulated process involving a balance between signals that stimulate and those that inhibit the proliferation and differentiation of pluripotent progenitors. Many of these regulatory signals are provided by interaction with the BM stromal elements.11-19 The close association with stroma provides hematopoietic cells with an array of cytokines that directly influence cell survival, differentiation, and proliferation. Stromal cells produce stem cell factor (SCF), which is an early acting cytokine of 248 amino acids that promotes the growth of all hematopoietic cell lineages. SCF acts synergistically with other cytokines to promote proliferation of normal progenitor cells in a variety of culture systems. It does not increase the numbers of committed colony-forming units (CFUs) but enhances granulocyte colony-stimulating factor– (G-CSF), granulocyte-macrophage colony-stimulating factor– (GM-CSF), interleukin-3– (IL-3), and IL-6–stimulated myelopoiesis in both short- and long-term CD34+ cell cultures. SCF on its own cannot recruit quiescent hematopoietic progenitor cells into cycle, but it can prevent their apoptosis and is able to maintain the active cell-cycle characteristics of isolated CD34+ cells in 2-day cultures.20
SCF stimulates the growth of progenitor cells from most patients with myelodysplastic syndromes21 and acute myeloid leukemia (AML),22,23 but reports on the effect of SCF on CML cell proliferation have been conflicting. The addition of SCF to CML cultures stimulated by G-CSF and/or GM-CSF has been reported to induce little, or no, effect on proliferation.24Conversely, erythroid CFU can be grown from CML cells in the absence of erythropoietin, but only if SCF is present.25 Recently, Hallek et al26 showed that the SCF receptors p145c-KIT and p210bcr-abl form an intracellular complex in bcr-abl positive, c-KIT positive cell lines. Fetal calf serum (FCS)–supplemented colony-forming unit–granulocyte-macrophage (CFU-GM) assays of CML bone marrow (BM) revealed a lack of response of CML cells to SCF, and the authors concluded that activation of p145c-KIT by p210bcr-abl usurped the role of SCF and so rendered CML cells insensitive to this cytokine. Further evidence of an abnormal response of CML cells to SCF is suggested by the loss of CML cells, and a concomitant increase in normal progenitors, during long-term culture on SCF-deficient stroma.27
Cytokines also promote the attachment of normal hematopoietic progenitors to stromal elements via activation of β1-integrins. The interaction of SCF with p145c-KIT increases the avidity of α4β1- and α5β1-integrins for their ligands in cell lines28 and in normal hematopoietic progenitors.29 In vitro adhesion assays of normal hematopoietic progenitors to immobilized fibronectin (FN) has shown that SCF causes a transient increase in the affinity of the β1-integrins for their ligand. The cells subsequently become unresponsive to further stimulation by SCF, and adhesion to FN returns to basal levels.
The attachment of progenitors to FN in vitro has a negative effect on proliferation that is independent of differentiation, necrosis, and apoptosis.30,31 CML progenitors have an adhesive defect that reduces their interaction with stroma in vitro.32 They also show impaired attachment to FN.33 This reduced attachment is brought about by a reduction in the steady-state affinity of β1-integrins for FN rather than by altered β1-integrin expression. Modulation of β1-integrin affinity of CML progenitors by the activating antibody 8A2 results in attachment of these cells to FN and a subsequent decrease in their proliferation in vitro.31These studies raise the possibility that lack of β1-integrin–mediated attachment of CML progenitors to stroma and stromal elements frees CML cells from some of the negative regulatory influences to which normal cells are subject.
To investigate the effect of SCF on primary CML cells, we have examined the proliferation of single leukemic CD34+ cells grown in serum-deprived medium (SDM) with and without the addition of cytokines. This assay system removes the potentially confounding influence of FCS and possible paracrine growth factor production by accessory cells and reveals the true response of leukemic hematopoietic progenitors to individual cytokines or cytokine combinations.
We have further investigated the role of SCF in serum-deprived conditions in augmentation of attachment of CML cells to immobilized FN because proliferation and adhesion are correlated events in normal CD34+ cells.34
Though p210bcr-abl may indeed activate p145c-KIT in CML cells, 26 our findings indicate that this activation is not complete because SCF, through p145c-KIT, can provide additional adhesive and proliferative signals. However, stimulation of CML cells with SCF alone results in a potent proliferative signal that is not observed in cells lacking bcr-abl gene expression.
MATERIALS AND METHODS
BM Cells
BM aspirates were collected after informed consent from normal adult donors under a program approved by the Human Ethics Committee of the Royal Adelaide Hospital, and after informed consent from CML patients undergoing routine hematologic investigation at presentation. BM mononuclear cells (BMMNC) were recovered from a Lymphoprep (Nycomed Pharma As, Oslo, Norway) density gradient and were resuspended in Hanks' balanced salt solution (HBSS) supplemented with 20 mmol/L HEPES (Life Technologies, Glen Waverley, Australia), pH 7.3, and 5% FCS (Trace Biosciences, Castle Hill, NSW, Australia). The cells were washed twice with HBSS/5% FCS and resuspended in SDM before analysis. SDM consists of Iscove's modified Dulbecco's medium (IMDM) supplemented with 1% bovine serum albumin (BSA; No. A2153; Sigma Chemical Co, St Louis, MO), 200 μg/mL iron-saturated transferrin (No. T2158; Sigma Chemical Co), 10 μg/mL insulin, 20 μg/mL low-density lipoprotein (Sigma Chemical Co), and 10−5 mol/L 2-mercaptoethanol.
Antibody Labeling and Cell Sorting
BMMNC were incubated on ice with a fluorescein isothiocyanate (FITC)-conjugated anti-CD34 antibody (HPCA-2-FITC) or with a mixture of HPCA-2-FITC and a phycoerythrin (PE)-conjugated anti-CD38 antibody (Leu 17) for 45 minutes. Cells were washed twice in HBSS/5% FCS and resuspended in SDM. Cells incubated with nonbinding isotype-matched antibodies conjugated to FITC or PE (mouse IgG1 FITC and mouse IgG1 PE; Dako, Glostrup, Denmark) were used as controls.
BMMNC were sorted by a FACStarPLUS (Becton Dickinson, Mountain View, CA) equipped with an argon laser emitting 488 nm light at 200 mW. CD34+ cells in the lymphocyte/blast region were collected into SDM. Cells were pelleted by centrifugation then resuspended in SDM. Cells used for adhesion assays were starved overnight at 37°C to eliminate the residual effects of FCS, whereas cells for pre-CFU culture were plated directly into culture wells with the appropriate cytokines. For single-cell deposition assays the automated cell deposition unit deposited individual normal or CML CD34+ cells into Terasaki plate wells that contained 10 μL of SDM with or without cytokines.
After 12 to 24 hours, wells were checked microscopically for the presence of a single cell. Wells not containing one cell at this stage were excluded from future analyses. The number of cells per well was determined microscopically at defined time intervals.
Antibody Labeling for Immunofluorescence Analysis
Cells were resuspended in blocking buffer (HBSS/5% FCS, 1% BSA, 5% heat-inactivated normal human serum) and incubated on ice for up to 1 hour to block Fc-mediated antibody binding. For single-layer staining, cells were then incubated with fluorochrome-conjugated antibodies recognizing CD34, CD14, CD15, CD61, glycophorin A, CD19, or CD3 on ice for 45 minutes and then washed twice in ice cold immunofluorescence (IF) buffer (HBSS/5% FCS, 0.2 g/500 mL sodium azide) and fixed in 1% formamide in PBS. For two-layer staining, the cells were incubated with the anti-p145c-KIT antibody YB5.8 on ice for 45 minutes. After two washes in IF buffer, a secondary antibody was added (PE-conjugated sheep F(ab)2 fragments directed against mouse IgG) and incubated on ice for 30 minutes. The cells were washed twice in IF buffer and fixed in 1% formamide in PBS. Antibodies used in this study are shown in Table 1.
Cytokines and Chemicals
Recombinant human SCF, IL-3, IL-6, G-CSF, and GM-CSF were generously provided by Amgen Inc (Thousand Oaks, CA). FN was purchased from Boehringer Mannheim (Mannheim, Germany). Culture media were purchased from Life Technologies.
Pre-CFU Culture
This is a stroma-free, cytokine-dependent, suspension culture system based on the method of Iscove et al.35 It measures the de novo generation of CFU-GM colonies as an index of preprogenitors. One thousand sorted CD34+ cells were resuspended in 1 mL of SDM with appropriate growth factors in 24-well plates. Cells were incubated at 37°C in a humidified atmosphere and, at 7-day intervals, were given fresh medium and growth factors. Before medium changes, an aliquot of cells was removed and plated in the CFU-GM assay.
CFU-GM Assay
Cells were plated in triplicate in 1-mL cultures containing 0.9% methylcellulose in IMDM supplemented with 30% FCS, 3 mmol/l L-glutamine, IL-3 (10 ng/mL), GM-CSF (10 ng/mL), IL-6 (20 ng/mL), G-CSF (100 ng/mL), and SCF (100 ng/mL). After 14 days incubation at 37°C in a humidified atmosphere with 5% CO2, CFU-GM colonies were scored as aggregates of greater than 50 cells.
Reverse-Transcription Polymerase Chain Reaction (RT-PCR) of CFU-GM Colonies
RNA extraction.
Individual colonies were plucked with 5 μL of semisolid culture medium and vigorously mixed in 100 μL RNAZOL B (Biotecx Laboratories, Houston, TX). RNA extraction was continued according to the manufacturer's recommendations with the addition of 20 μg Glycogen (Boehringer Mannheim).
cDNA synthesis.
RNA was dissolved in 8 μL of diethyl pyrocarbonate–treated water and heated at 65°C for 10 minutes. After cooling the RNA rapidly on ice, 12.5 μL of RT mix was added and cDNA synthesis was performed at 37°C for 2 hours. RT mix consists of 80 mmol/L Tris pH 8.3; 120 mmol/L KCl; 4.8 mmol/L MgCl2; 16 mmol/L DTT; 0.1 mmol/L each of dATP, dCTP, dGTP, and dTTP; 0.01 μg/μL pdN6 (Pharmacia, Victoria, Australia); 1.4 U/μL RNAguard (Pharmacia); and 16 U/μL M-MLV RT (GIBCO-BRL, Gaithersburg, MD). RT was inactivated by heating at 65°C for 10 minutes.
Nested PCR.
The presence of abl gene transcripts (cDNA control) and bcr-abl transcripts was determined by two rounds of PCR using nested primers as previously described.36
Fluorescence in Situ Hybridization (FISH) Analysis
FISH using bcr-abl probes (Vysis pty ltd, Downers Grove, IL) was performed as previously described.37 Cells were placed on a poly-L-lysine–coated microscope slide (Polysine; Biolab Scientific, Auckland, New Zealand) and swollen in situ in hypotonic (0.075 mol/L) KCl for 20 minutes. The cytoplasm was removed by three changes of ice cold 3:1 methanol:acetic acid, and then the slides were allowed to air dry. Cells thus treated were stored at 4°C for a maximum of 2 weeks before hybridization. Cells were treated with RNaseA (0.1 mg/mL 37°C) for 1 hour and were then heat denatured in 70% formamide/2× SSC. The denatured probe mixture was added to the slides, which had been dehydrated through graded ethanol and air dried. Hybridization continued overnight at 37°C. Posthybridization washes in 50% formamide/2× SSC were performed, and slides were mounted in antifade solution before examination by fluorescence microscope. On examination of interphase nuclei, the normal abl gene is seen as a red dot, the normal bcr gene is seen as a green dot, and the bcr-abl fusion gene appears either as a yellow dot or as adjacent red and green signals.
Sex determination by FISH was performed using X and Y chromosome-specific repetitive sequence probes. The digoxygenin-labeled X chromosome probe (DXZ1) was obtained from Oncor (Gaithersburg, MD), and the Y probe (HRY) was a gift from Ken Reed. Cell preparation, hybridization, and washing were performed as described. The X chromosome signal was detected with anti-DIG FITC, and the biotin-labeled Y chromosome probe was detected with avidin-Texas Red. Male cells contained one red and one green signal, whereas female cells contained two green signals.
Functional Assays Of Adhesion
Estimation of the percent of the total cell population that is adherent.
Ninety-six–well tissue culture–treated plates (Nunc, Roskilde, Denmark) were incubated overnight at 4°C with 50 μg/mL FN in PBS. This solution was removed by aspiration, and nonspecific binding to the wells was minimized by incubation at 37°C for 2 hours in 100 μL 2% BSA in RPMI 1640. Plates were washed three times with 0.2% BSA in RPMI 1640 (adhesion medium), transferred onto ice, and used immediately. Cytokines were added to protein-coated wells as specified. Cells, which had been deprived of growth factors overnight, were washed twice and resuspended in approximately 500 μL of RPMI 1640 containing 5 μmol/L Calcein-AM (Molecular Probes, Eugene, OR). The cells were incubated at 37°C for one hour, then washed three times in IMDM with 0.2% BSA, resuspended at 2 to 4 × 105cells/mL in adhesion medium, and incubated on ice alone or with appropriate antibodies for 30 minutes. One hundred microliters of the cell suspension was then added, in triplicate, to FN- or BSA-coated microtitre plates on ice. The plates were centrifuged at 1,000 rpm for 5 minutes at 4°C to ensure direct and even contact with treated surfaces. After warming the plates to 37°C for 2 minutes on an incublock, they were transferred to a humidified 37°C incubator for 30 minutes. Nonadherent cells were removed by aspiration, and the wells were washed three times (or until the BSA-coated wells were clear) by the addition of 150 μL of adhesion medium. One hundred and fifty microliters of 1% sodium dodecyl sulfate (SDS), 1% NaOH solution was added to lyse the cells, and the fluorescence was measured using a Fluorimager (Molecular Dynamics, Sunnyvale, CA). Analysis of data was performed with ImageQuant software (Molecular Dynamics). The percentage of adherent cells was determined by dividing the fluorescence of the adherent fraction lysate by the fluorescence of the initial cell loading.
Estimation of the number of CFU-GM that are adherent.
The assays were performed as above in 6-well clusters (Nunc), except that cells were not prestained with calcein-AM. After removal of nonadherent cells, methylcellulose mixture and cytokines (as described in the CFU-GM assay) were added to the adherent cells and incubated at 37°C for 14 days. CFU-GM assays were also established for nonadherent cells. The number of colonies grown at day 14 was expressed as a percent of the total number of colonies that were obtained from 1,000 CD34+ cells that were plated directly in the CFU-GM assay and that had not undergone the adhesion assay.
RESULTS
CML CD34+ Cells Proliferate With SCF as the Sole Stimulus
BM CD34+ cells from five CML patients and from five normal donors were cultured at a concentration of 103/mL in SDM + SCF (100 ng/mL), SDM alone, or SDM + 4 hematopoietic growth factor (HGF; IL-3 10 ng/mL, IL-6 20 ng/mL, G-CSF 100 ng/mL, and SCF 100 ng/mL). Growth of normal and leukemic CD34+ cells was below the limits of detection in the cytokine-free cultures. Normal CD34+ cells and CML CD34+ cells showed a 3,000-fold expansion in the presence of 4HGF after 21 days in culture. In contrast, only the CML cells proliferated in response to SCF alone (2,400-fold expansion ), whereas normal CD34 cells did not (Fig 1A).
Proliferation of 1,000 sorted cells in the serum-free pre-CFU assay. (A) CD34+ cells from five normal donors and five CML patients were cultured in SCF alone or in 4HGF. Cell counts for normal CD34+ cultured in SCF alone remain below the limit of detection and so are represented by a dotted line. (B) CML CD34+ from three patients were subfractionated into CD38+/−and cultured in SCF alone or in 4HGF. CD34+CD38+/− cells from three normal donors showed no discernible proliferation in this assay and are not plotted. (A) □, CML in SCF; ○, CML in 4HGF; •, normal in SCF; ▪, normal in 4HGF. (B) ▪, CD34+CD38+ in SCF; •, CD34+CD38− in SCF; □, CD34+ in 4HGF; ○, CD34+CD38− in 4HGF.
Proliferation of 1,000 sorted cells in the serum-free pre-CFU assay. (A) CD34+ cells from five normal donors and five CML patients were cultured in SCF alone or in 4HGF. Cell counts for normal CD34+ cultured in SCF alone remain below the limit of detection and so are represented by a dotted line. (B) CML CD34+ from three patients were subfractionated into CD38+/−and cultured in SCF alone or in 4HGF. CD34+CD38+/− cells from three normal donors showed no discernible proliferation in this assay and are not plotted. (A) □, CML in SCF; ○, CML in 4HGF; •, normal in SCF; ▪, normal in 4HGF. (B) ▪, CD34+CD38+ in SCF; •, CD34+CD38− in SCF; □, CD34+ in 4HGF; ○, CD34+CD38− in 4HGF.
To investigate the response of the CD34+CD38+and the more primitive CD34+CD38−fractions of the CML and normal CD34+ cell populations to SCF, cells were sorted on the basis of CD34 and CD38 expression and cultured as described above. Normal CD34+CD38+and CD34+CD38− cells proliferated in 4HGF, but neither fraction showed proliferation in SCF alone. The CML CD34+CD38+ cells proliferated in a quantitatively similar manner in the presence of either 4HGF or SCF alone, reaching a plateau at day 14 (Fig 1B). In SCF alone, the CML CD34+CD38− cell proliferation was lower than was seen in 4HGF (P < .01). Growth of this cell population did not plateau at day 14 but continued for several weeks whether the stimulus was SCF alone (P < .05) or 4HGF (P < .01).
CML CD34+ Cells Proliferate in a Single-Cell Assay With SCF as the Sole Stimulus
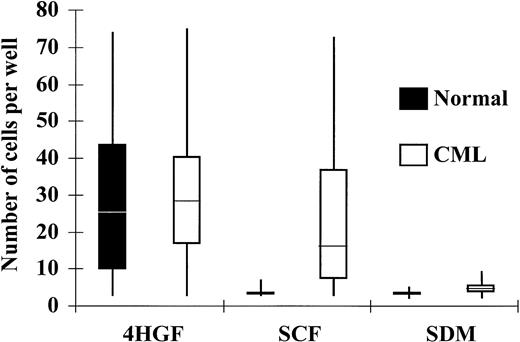
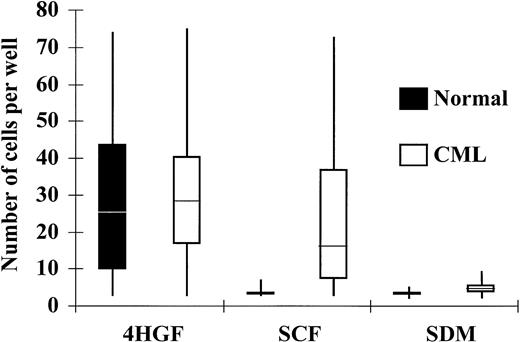
Because SCF induces marked proliferation of normal CD34+cells only when it acts in synergy with other cytokines, we postulated that the survival and subsequent growth of CML cells in SCF alone may have resulted from paracrine production of additional cytokines. To investigate whether individual CD34+ cells will survive in the absence of other cells, and to examine the number of cells that respond to SCF and the extent of their proliferation, single CD34+ cells were deposited by the ACDU of the FACStarplus into SDM alone, SDM supplemented with 100 ng/mL SCF, or SDM + 4HGF. CD34+ cells from normal donors failed to proliferate to a significant extent in SDM without cytokines and showed only minimal expansion in SCF (100 ng/mL) alone. At day 6 in SCF, 8% of normal CD34+ cells had proliferated to a mean of 4 cells/well (range 3 to 8, n = 48). Expansion in response to 4HGF was seen in 52% of normal CD34+ cells at day 6, and the mean number of cells grown from a single cell at this time point was 32 (range 3 to 65, n = 123). CML CD34+ cells showed only 8% proliferation in SDM without cytokines (mean number of cells generated was 5, range 3 to 9, n = 67). CML CD34+ cells showed significantly greater proliferation in SCF compared with normal cells (P < .001); 48% of CML cells showed proliferation in the presence of SCF alone. The mean number of cells grown from a single cell at this time point was 18, (range 3 to 72, n = 403). In 4HGF, a higher percentage (88%) of CML CD34+ cells proliferated compared with normal cells (P < .001), and the mean number of cells grown from a single cell was 49 (range 3 to 71, n = 152; Fig 2, Table 2)
Response of CD34+ cells to cytokines in single-cell culture at day 6. Individual CD34+ cells were deposited into Terasaki plate wells containing 10 μL of SDM alone, SDM supplemented with SCF (100 ng/mL), or SDM supplemented with 4HGF. The number of cells in each well was enumerated after 6 days and plotted in quartiles for CML and normal CD34+ cells.
Response of CD34+ cells to cytokines in single-cell culture at day 6. Individual CD34+ cells were deposited into Terasaki plate wells containing 10 μL of SDM alone, SDM supplemented with SCF (100 ng/mL), or SDM supplemented with 4HGF. The number of cells in each well was enumerated after 6 days and plotted in quartiles for CML and normal CD34+ cells.
Single-cell deposition assays using CD34+CD38+and CD34+CD38− subsets were performed to investigate the response of these subsets to SCF alone. At the single-cell level, 42% of the CML CD34+CD38+cells were proliferating at day 6, and the mean number of cells grown from a single cell was 31.5 (range 3 to 72, n = 117); 19% of the CML CD34+CD38− cells were proliferating at day 6, and the mean number of cells grown from a single cell was 10 (range 3 to 32, n = 118). After 13 days in culture, the CML CD34+CD38− population continued to proliferate and showed a mean number of 52.6 cells (range 3 to 150, n = 17). Normal CD34+CD38+ cells showed minimal proliferation at day 6 (data not shown), and normal CD34+CD38− cells did not proliferate.
CML CD34+ Cells Show Dose-Dependent Proliferation in SCF Whereas Cells Derived From Normal Donors Do Not
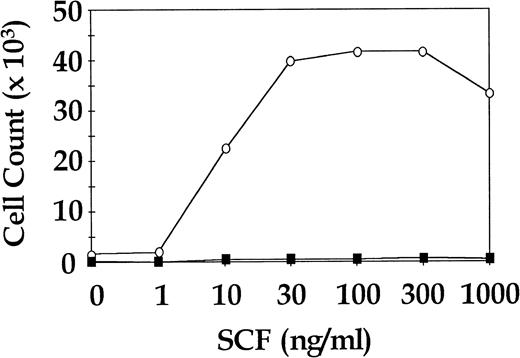
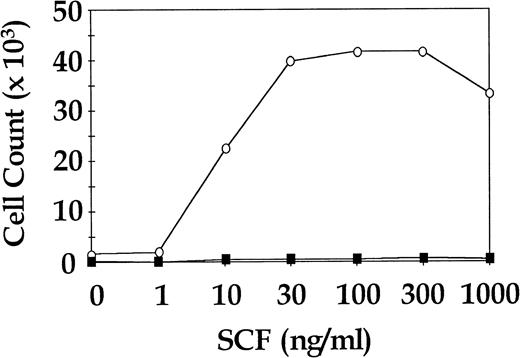
Total CD34+ cells from three CML patients and three normal donors were cultured for 7 days in SDM with SCF in doses ranging from 0 ng/mL to 1,000 ng/mL in approximately half log increments. Cells were also cultured in 4HGF to assess viability. The CML cells showed a dose-dependent increase in proliferation to SCF, whereas the normal cells were not observed to proliferate at any concentration of SCF. All CML samples and normal samples proliferated in 4HGF (data not shown). In two of the three normal cell cultures, enumeration of cells at day 7 was not possible because the cell numbers were below the level of detection. The third normal cell culture was seeded at a concentration of 13.5 × 103 cells/mL and yielded enumerable cells after 7 days in SDM + SCF. A representative CML dose response curve to SCF and the single assay of normal cells for which results were obtained are shown in Fig 3.
Proliferative response of sorted CML and normal CD34+ cells to increasing concentrations of SCF. Representative results for a single CML sample and a single normal control are shown. ○, CML CD34+; ▪, normal CD34+.
Proliferative response of sorted CML and normal CD34+ cells to increasing concentrations of SCF. Representative results for a single CML sample and a single normal control are shown. ○, CML CD34+; ▪, normal CD34+.
Cells That Proliferate in Response to SCF Alone Are bcr-abl Positive
Single CML CD34+ cells from two CML patients were cultured for 14 days in SCF alone or in 4HGF. RNA was extracted from expanded clones, and RT-PCR for the bcr-abl fusion gene was performed to assess the origin of the proliferating cells. All 18 clones expanded in SCF alone were positive for abl and bcr-abl gene transcripts by RT-PCR. This finding indicates that only the leukemic cells were SCF responsive. Furthermore, fusion of bcr and abl was shown in an additional five SCF responsive clones from one patient by FISH. Twenty-four clones expanded in 4HGF for 14 days were subjected to RT-PCR. Only 16 were bcr-abl positive, but all 24 were positive for expression of the normal abl allele (Table3).
The Progeny of Cells Stimulated With SCF Alone Is Morphologically and Immunophenotypically Distinct From That Cultured in 4HGF
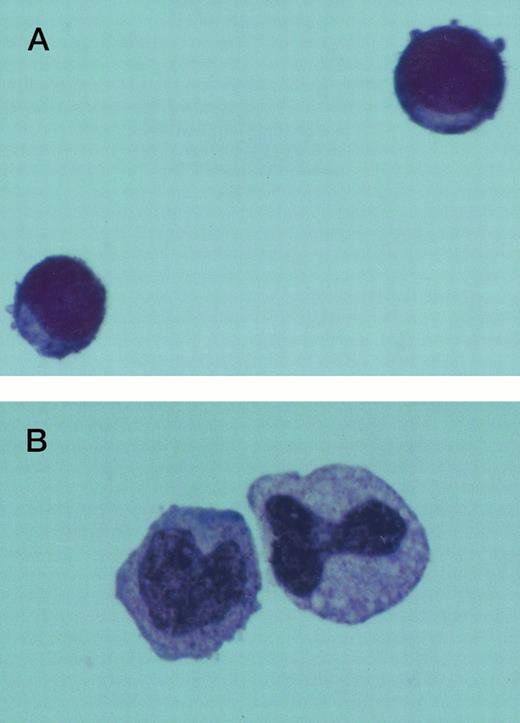
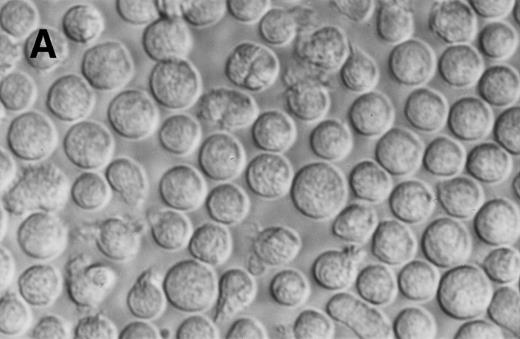
The morphology of Jenner Giemsa–stained preparations of cultured normal and CML BM cells was examined. Six days after culture in SCF alone, the expanded CML cells showed an increase in size and resembled large blast cells morphologically. They had a high nuclear to cytoplasmic ratio and densely stained chromatin. Following an additional 7 days in SCF, some of these cells retained their primitive appearance, whereas others assumed a monocytoid appearance. Normal and CML cells grown in 4HGF differentiated into granulocytes and monocytoid cells by day 14. Typical cell morphology is shown in Fig 4. After 24 days of culture, the difference in morphology between CML cells grown in SCF and those grown in 4HGF was even more pronounced. SCF-derived cells appeared smaller and more homogeneous in size and shape than the 4HGF-derived cells, which varied in size, granularity, and nuclear shape (Fig 5).
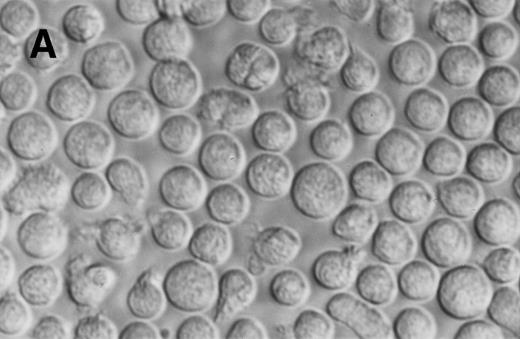
Jenner Giemsa stain showing the morphology of CD34+CD38− CML cells cultured in the pre-CFU assay for 14 days with SCF alone, or with 4HGF (original magnification 200×). (A) SCF-stimulated cells; (B) 4HGF-stimulated cells.
Jenner Giemsa stain showing the morphology of CD34+CD38− CML cells cultured in the pre-CFU assay for 14 days with SCF alone, or with 4HGF (original magnification 200×). (A) SCF-stimulated cells; (B) 4HGF-stimulated cells.
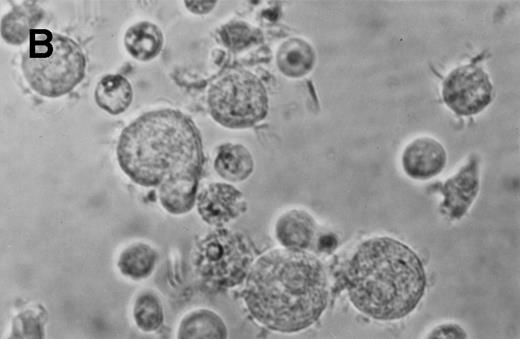
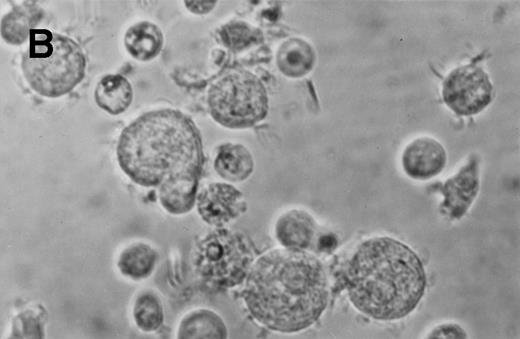
Phase contrast photomicrograph showing the morphology of CD34+CD38− CML cells cultured in the pre-CFU assay for 24 days with SCF alone or with 4HGF (original magnification 200×). (A) SCF-stimulated cells; (B) 4HGF-stimulated cells.
Phase contrast photomicrograph showing the morphology of CD34+CD38− CML cells cultured in the pre-CFU assay for 24 days with SCF alone or with 4HGF (original magnification 200×). (A) SCF-stimulated cells; (B) 4HGF-stimulated cells.
The immunophenotype of cells from 3 CML patients grown in either 4HGF or in SCF alone was examined to determine whether cells produced in culture with SCF as a single agent showed lineage restriction. Cultures were initiated with 1,000 CD34+CD38−cells and were immunophenotyped after 14 days. The results of the semiquantitative flow cytometric analysis are shown in Table 4.
Tryptase enzyme expression for mast cell identification was determined by the alkaline phosphatase anti-alkaline phosphatase technique, and occasional cells showed positivity both in CML cells cultured in 4HGF and in SCF alone for 14 days. These findings show that myeloid and monocytic lineage markers are less frequently expressed on cells generated by culture in SCF alone compared with cells generated in 4HGF, but the expression of differentiation markers from myeloid, monocytic, erythroid, and megaryocytic lineages indicates that the response of CML cells to the single agent SCF is not lineage restricted.
Culture in SCF Alone Supports Survival of Leukemic Preprogenitor Cells
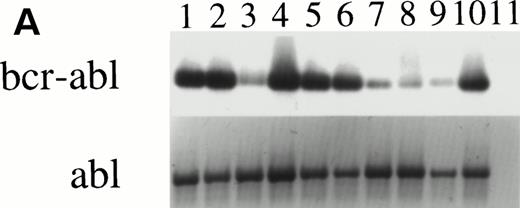
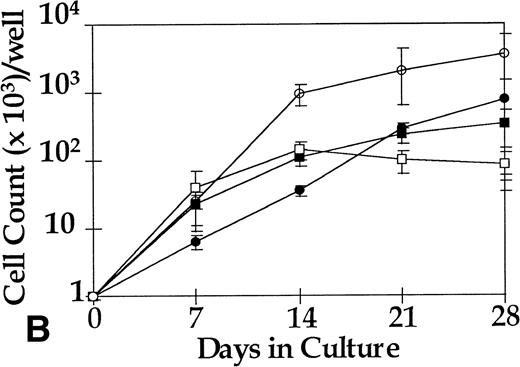
CFU-GM were assayed at days 0, 14, and 28 from 4HGF- or SCF-stimulated serum-deprived cultures (pre-CFU assay) of CD34+ cells from three normal donors and from three CML patients. The temporal pattern of generation of CFU-GM colonies was similar for normal and leukemic cells cultured in 4HGF. At day 0, CFU-GM were generated mainly from CD34+CD38+ cell cultures, and at days 14 and 28, colonies were derived mainly from the CD34+CD38− fraction. The kinetics of generation of CFU-GM colonies from CML cells cultured in SCF alone mirrored that observed for cells cultured in 4HGF (Fig 6). The ability of CFU-GM to be grown from CML cells cultured for up to 28 days in SCF alone shows the ability of this cytokine to recruit preprogenitors into cycle sequentially. Insufficient normal cells were available after culture in SCF alone to be tested for CFU-GM generation. The leukemic origin of the pre-CFU from the three CML samples was determined by RT-PCR. CFU-GM colonies obtained from cells plated after 14 days of culture stimulated by either 4HGF or SCF alone were assessed for expression of bcr-abl. All 28 colonies examined from SCF-stimulated cultures were positive for bcr-abl by RT-PCR, whereas only 10 of 18 colonies from 4HGF-stimulated cultures were positive for bcr-abl by RT-PCR (Fig 7).
Generation of CFU-GM from 1,000 sorted cells from three CML patients in the pre-CFU assay over 28 days. Aliquots of cells for the CFU-GM assay were removed from the pre-CFU cultures at intervals. The total number of CFU generated (from 1,000 original cells) was calculated from the number of colonies derived and the proliferation of the remaining cells, ie (Number of Colonies/1,000 Cells) × (Number of Cells Generated [× 1,000]). ○, CD34+CD38− in 4HGF; •, CD34+CD38− in SCF; ▪, CD34+CD38+ in SCF; □, CD34+CD38+ in 4HGF.
Generation of CFU-GM from 1,000 sorted cells from three CML patients in the pre-CFU assay over 28 days. Aliquots of cells for the CFU-GM assay were removed from the pre-CFU cultures at intervals. The total number of CFU generated (from 1,000 original cells) was calculated from the number of colonies derived and the proliferation of the remaining cells, ie (Number of Colonies/1,000 Cells) × (Number of Cells Generated [× 1,000]). ○, CD34+CD38− in 4HGF; •, CD34+CD38− in SCF; ▪, CD34+CD38+ in SCF; □, CD34+CD38+ in 4HGF.
RT-PCR of individual granulocyte-macrophage colonies derived from CML cells after culture in the pre-CFU assay. (A) Lanes 1 to 10: PCR of ten individual colonies derived from cells cultured in SCF alone for 14 days, then transferred into the CFU-GM assay for 14 days. Lane 11 is a CFU-GM culture medium negative control. (B) Lanes 1 to 8: PCR of eight individual colonies derived from cells cultured in 4HGF for 14 days, then transferred into the CFU-GM assay for 14 days. Lane 9 is a CFU-GM culture medium negative control.
RT-PCR of individual granulocyte-macrophage colonies derived from CML cells after culture in the pre-CFU assay. (A) Lanes 1 to 10: PCR of ten individual colonies derived from cells cultured in SCF alone for 14 days, then transferred into the CFU-GM assay for 14 days. Lane 11 is a CFU-GM culture medium negative control. (B) Lanes 1 to 8: PCR of eight individual colonies derived from cells cultured in 4HGF for 14 days, then transferred into the CFU-GM assay for 14 days. Lane 9 is a CFU-GM culture medium negative control.
Expression of P145c-kit Is Not Elevated in CML CD34+ Cells
To investigate whether the unique response of CML CD34+cells to SCF was due to overexpression of p145c-KIT, we assessed the level of expression of p145c-KIT on CD34+ cells in six normal and six CML BM samples by semiquantitative flow cytometric analysis. The peak fluorescence of the anti-p145c-KIT antibody on the gated CD34+population of normal BM cells was 11.7 ± 2.4, and on CML BM cells it was 10.2 ± 1.1. The difference in p145c-KITexpression between normal CD34+ cells and CML CD34+ cells was not statistically significant when analyzed by one-way analysis of variance (ANOVA); these data indicate that the marked proliferation of CML CD34+ cells that resulted from stimulation by SCF alone was not due to an alteration in the level of receptor expression.
Autocrine Secretion of HGF by CML CD34+ Cells Is Not Sufficient to Stimulate Proliferation of Normal Cells in the Presence of SCF Alone
Synergy between exogenous SCF and cytokines secreted by CML cells may explain why these cells respond to SCF in the absence of other exogenous cytokines. We explored this possibility by culturing mixtures of 1,000 male CML CD34+ cells and 1,000 female normal CD34+ cells in SCF or in 4HGF. The ratio of male (CML) cells to female (normal) cells was determined by FISH at day 14 using X and Y chromosome–specific probes. Two separate experiments were performed and contained cells from two male CML patients admixed with cells from two individual normal donors. The expansion of female cells in the SCF-stimulated cultures would suggest that their proliferation was enhanced by coculture with CML cells. Results of the FISH analyses are shown in Table 5. Proliferation of CML cells in 4HGF was quantitatively similar to proliferation of cells from a normal donor in 4HGF, but CML cells stimulated with only SCF had a marked growth advantage over normal donor cells grown in these conditions. Inability to detect female cells in 100 cells examined by FISH after 14 days in culture with SCF alone shows that these cells comprised fewer than 3% of the total cell number.38 These results indicate that autocrine cytokine secretion by CML cells is not sufficient to support the proliferation of other cytokine-dependent cells.
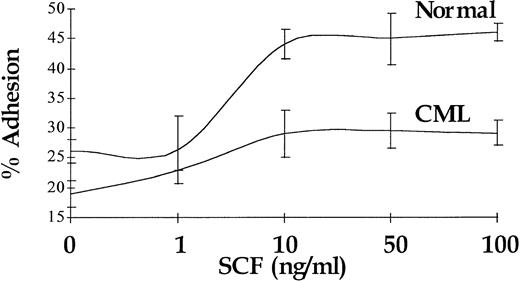
Enhancement of Adhesion to FN in Response to SCF Is Reduced in CML CD34+ Cells and Is Not Coupled to the Proliferative Response
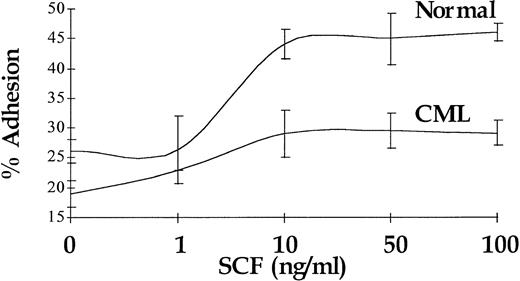
In normal CD34+ cells, increased proliferation in response to SCF stimulation is coupled with enhanced adhesion to FN.34 In accord with previously published data,29 a 30-minute stimulation with SCF resulted in a 2.4-fold increase (P < .01) in the number of normal CD34+ cells adhering to immobilized FN (n = 4), and the augmentation of adhesion of CML CD34+ cells in response to SCF stimulation was also significant (P < .05), but it was limited to 1.4-fold (n = 4). Maximal response was seen at 10 ng/mL for CML and normal CD34+ cells. A representative experiment (performed in triplicate) is shown in Fig 8.
Augmentation of CD34+ cell adhesion to immobilized FN in response to increasing doses of SCF. Representative results for a single CML sample and a single normal control are shown.
Augmentation of CD34+ cell adhesion to immobilized FN in response to increasing doses of SCF. Representative results for a single CML sample and a single normal control are shown.
We also investigated whether adhesion of CFU-GM to FN was increased in response to SCF. One thousand CD34+ normal or CML cells were allowed to adhere to immobilized FN either with or without SCF (10 ng/mL) treatment. After 30 minutes the nonadherent cells were removed by washing and were plated in a CFU-GM assay. The adherent cells were obtained and plated. Colonies were enumerated after a further 14-day incubation, and the result for each fraction was expressed as a percentage of the total number of colonies arising from the assay (ie, as a percentage of the number of colonies derived from 1,000 CD34+ cells). Twenty-one colonies from both fractions of the SCF-treated CML cells and 20 colonies from both fractions of untreated CML cells were subjected to RT-PCR analysis for expression of the bcr-abl fusion gene. Following SCF stimulation, there was an increase in the number of colonies that were adherent to FN in the normal controls, but no such increase was evident in the CML samples. SCF treatment did not result in an increase in the percentage of colonies that were bcr-abl positive compared with the unstimulated cells (Table 6).
DISCUSSION
In the present study, we have examined the role of SCF in the proliferation and adhesion of CML Ph+ progenitors in vitro. Our results show that SCF alone is sufficient to provide a strong proliferative signal for CML CD34+ cells. This is in contrast to the response of normal CD34+ cells, which show substantial proliferation in SCF only when other growth factors are present. In CML both the CD34+CD38+ cells and the more primitive CD34+CD38− subsets proliferate in response to SCF alone. The growth curve is initially slower for CML CD34+CD38− cells stimulated with SCF alone when compared with their proliferation in 4HGF. This difference may reflect the greater speed of recruitment of cells into cycle by 4HGF and probably results also from the larger number of cells (normal and leukemic) that respond to this cytokine combination. Responsiveness of the leukemic progenitors and preprogenitors to SCF is shown by the survival and expansion of pre-CFU. Comparison of the morphology of cells grown in 4HGF for 24 days and those grown in SCF for 24 days in serum-deprived conditions shows that the 4HGF-derived cells are heterogeneous in appearance with features typical of maturation, such as lobed nuclei and cytoplasmic granulation. The cells derived from culture in SCF as the sole stimulus are more homogeneous in appearance, and there is less morphologic evidence of maturation. At day 14 the immunophenotype of SCF-derived cells indicates that myeloid, megakaryocytic, erythroid, and monocytic lineage development occurs in response to SCF. This demonstration of SCF-dependent proliferation in multiple lineages extends the finding of Isaad et al,25 who showed that SCF alone could promote CML erythroid colony formation in semisolid media. CD34 expression is lost both from SCF and 4HGF stimulated cells, but maintenance of a degree of immaturity can be surmised from the ability of these cells to form colonies in semisolid media after up to 28 days in pre-CFU culture. The increase in the number of colony forming cells during CML cell culture in SCF alone provides additional evidence of expansion of leukemic granulocyte and macrophage precursors.
The progenitors that form CFU-GM colonies from day 14 of culture in SCF are bcr-abl positive by RT-PCR, showing the specificity of the proliferative effects of SCF alone for the leukemic cell population within the CML sample. Of interest are other studies39 40that have shown an apparent decline in the number of leukemic preprogenitor cells in liquid culture systems using multiple growth factors. It is uncertain whether this reduction in leukemic CFU in culture results from massive expansion of normal progenitors that mask the residual leukemic population. The present study has confirmed that CML preprogenitors reside in the CD34+CD38− subpopulation and that they may outgrow the normal cells that are present if SCF is provided as the sole cytokine. The proliferative response of CML CD34+cells to SCF is dose dependent up to approximately 100 ng/mL, whereas normal cells do not proliferate at any concentration of SCF in the absence of other cytokines.
Although SCF as a single agent induces selective proliferation of Ph+ CML cells, we cannot exclude the possibility that SCF selectively supports viability of Ph+ cells and that proliferation is permitted rather than directly induced. However, the different morphology and immunophenotype of cells cultured in SCF alone compared with those cultured in 4HGF suggests to us a more instructive rather than permissive role for SCF in these cultures. The increased proliferative response of CML CD34+ cells to SCF is not due to overexpression of the receptor because p145c-KIT is expressed at normal levels on these cells.
Autocrine cytokine production has been shown in normal CD34+ cells,41,42 and more recently the presence of IL-3 and G-CSF gene transcripts in CML cells have been shown by RT-PCR.43 Cytokines thus produced may synergize with SCF to enhance the proliferative signal generated by this cytokine in CML cells. Although coculture of normal and CML BM with SCF as the only stimulus shows that cytokine secretion in CML cells is insufficient to sustain the proliferation of normal cells, it is possible that the cytokines exert an autocrine-proliferative effect by binding to intracellular receptors. In this case, synergy between SCF and the endogenously produced cytokines may be responsible for providing CML cells with their unique response to SCF. Indeed, in the presence of IL-3 at 10 ng/mL, the maximum proliferative response of CML and normal cells occurs at equivalent SCF concentrations and the extent of proliferation is similar (data not shown). However, two other possible mechanisms may render CML cells responsive to SCF as a single agent. Firstly, tyrosine kinase oncogenes such as v-src, v-fms, and trk, and including bcr-abl, have been shown to abrogate IL-3 dependence of various hematopoietic cell lines44-48 by a mechanism that does not involve autocrine IL-3 production. This finding suggests that IL-3 and p210bcr-abl may share a final common pathway in signal transduction.49 In the absence of IL-3, p210bcr-abl in CML cells may activate IL-3 signaling pathways, and so provide synergy with signaling through p145c-KIT to result in proliferation in response to SCF alone. We have previously shown that synergy between IL-3 and SCF greatly enhances the proliferative response of normal CD34+cells.50 Secondly, cytoplasmic interaction of p210bcr-abl with p145c-KIT may partially activate the p145c-KIT signaling pathways that drive proliferation. The interaction between p145c-KIT and its ligand may then provide an increased strength of signal that enhances proliferation. CML cells do not have a global increase in sensitivity to all cytokines, because although single CML CD34+ cells from some patients stimulated with IL-3 or GM-CSF alone yielded increased growth, the effect was not seen in all patient samples (unpublished data, April 1997). In all cases studied, IL-6 and G-CSF yielded growth comparable to that seen in normal controls.
The positive effect of SCF on CML cell proliferation led us to ask whether the effect of SCF on β1-integrin–mediated adhesion was also increased. Augmentation of adhesion of the CD34+ cell population by SCF in CML was not as great as the increase seen in normal CD34+ cells, and indeed, SCF did not augment adhesion of leukemic CFU-GM to FN. This finding is at odds with the profound effect of SCF as a single agent on CML cell proliferation and shows that adhesive and proliferative responses to SCF are uncoupled in CML. This decrease in the adhesive response to SCF is consistent with increased expression of the adhesion inhibitory β1B-integrin isoform in CML cells.51
Our data suggest that increased proliferation in the presence of SCF alone, without a concomitant increase in the antiproliferative signals that are provided by β1-integrin activation, may contribute to the massive accumulation of leukemic progenitors that is a constant feature of this disease. This hypothesis is supported by our previous demonstration that SCF promotes preferential engraftment of the leukemic cell population in sublethally irradiated NOD/SCID mice that are injected with unselected CML blood cells.52
The selective activity of SCF alone on Ph+ primitive progenitors provides a unique culture assay that can be used to examine the number and clonogenic potential of these cells under different conditions. The potential use of SCF to induce differentiation of Ph+ progenitors to extinction, while having an antiapoptotic effect on Ph− progenitors, may raise the prospect of a new in vitro strategy to purge long-term repopulating CML cells from autograft material.
ACKNOWLEDGMENT
The authors thank Dr J. Marty of Amgen Inc for gifts of recombinant growth factors, Mario Nicola and Jeff Suttle for assistance with diagnostic cytogenetic and RT-PCR analysis of CML samples, and Alan Bishop and Sandy McIntyre for their expertise in flow cytometry.
Supported by a grant from the National Health and Medical Research Council of Australia to T.P.H.
Address reprint requests to Sarah Moore, Division of Haematology, Hanson Centre for Cancer Research, IMVS, Frome Rd, Adelaide, SA, Australia; e-mail: sarah.moore@imvs.sa.gov.au.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




![Fig. 6. Generation of CFU-GM from 1,000 sorted cells from three CML patients in the pre-CFU assay over 28 days. Aliquots of cells for the CFU-GM assay were removed from the pre-CFU cultures at intervals. The total number of CFU generated (from 1,000 original cells) was calculated from the number of colonies derived and the proliferation of the remaining cells, ie (Number of Colonies/1,000 Cells) × (Number of Cells Generated [× 1,000]). ○, CD34+CD38− in 4HGF; •, CD34+CD38− in SCF; ▪, CD34+CD38+ in SCF; □, CD34+CD38+ in 4HGF.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2461/3/m_blod41936006x.jpeg?Expires=1768876570&Signature=F3BBlaEkTZ7~6vkpUdsXQ1ujm-ruWXDFo8FQlov~4yznUCxbaFQLYW-Nb4SY63GbJ-GokH8EMZWo3hRKKFLIdYNjx2VitN59YvTPHyXnH88zQF0-cSzyjzjxpLiVJAN~ZcdkgwyMV7Ep39ruCCWmhBb3y9KWtykY6rgu8W1yROkePofYJ72HmraCZOFPK1GcWvpZeVUYTbydXEWMhUXGAoDjtJGaUx1rrPIPap3c1Ecj8L~obmy0SG1Yf5mMiSUlQHrD0x19tSTTLr3WNTHbuWH4Ff~6fxD57LABzdiT751YKo-OQmwrFSobbRXYdlw6P5R7aDGPCLV0pIlv2gxPMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 6. Generation of CFU-GM from 1,000 sorted cells from three CML patients in the pre-CFU assay over 28 days. Aliquots of cells for the CFU-GM assay were removed from the pre-CFU cultures at intervals. The total number of CFU generated (from 1,000 original cells) was calculated from the number of colonies derived and the proliferation of the remaining cells, ie (Number of Colonies/1,000 Cells) × (Number of Cells Generated [× 1,000]). ○, CD34+CD38− in 4HGF; •, CD34+CD38− in SCF; ▪, CD34+CD38+ in SCF; □, CD34+CD38+ in 4HGF.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2461/3/m_blod41936006x.jpeg?Expires=1769054172&Signature=27l82IeGtJNuKvhi3UiXAFtGctVrUjGFXUPDy425wopz~NJFpneE05GiFyFvCA9fD91VFomg0ZIknX0RAbIUGYvb98413P7V~Az6HbOV3OIyO7K-1ikWaADlhHcEk6RXtj89OVPk1gOfMi~6huYudcX7vscc-TNM5oWPLOLOK1p6B96PDibg7MeGCt8HCCf2-ZinDTL-JIK9plYDHpSn5yqEyNfbMbnXi7bhTGEB2DKsmfMQHUY7QizrZzFo~bUFbkTypHGZ8ehJZmzxEnRtgJNlHMD0N1M-3iBaEGk-Lra1GaMZiReeKd0BVVBxY5OKRPZCnbOZTSwrtIcO7aYSQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)