Abstract
Noninfectious lung injury is common after allogeneic bone marrow transplantation (BMT), but its association with acute graft-versus-host disease (GVHD) is unclear. Using a murine BMT system where donor and host differ by multiple minor histocompatibility (H) antigens, we investigated the nature of lung injury and its relationship both to systemic GVHD and host-reactive donor T cells. Lethally irradiated CBA hosts received syngeneic BMT or allogeneic (B10.BR) T-cell–depleted (TCD) bone marrow (BM) with and without the addition of T cells. Six weeks after BMT, significant pulmonary histopathology was observed in animals receiving allogeneic BMT compared with syngeneic controls. Lung damage was greater in mice that received allogeneic T cells and developed GVHD, but it was also detectable after TCD BMT when signs of clinical and histologic acute GVHD were absent. In each setting, lung injury was associated with significant alterations in pulmonary function. Mature, donor (Vβ6+and Vβ3+) T cells were significantly increased in the broncho-alveolar lavage (BAL) fluid of all allogeneic BMT recipients compared with syngeneic controls, and these cells proliferated and produced interferon-γ (IFN-γ) to host antigens in vitro. These in vitro responses correlated with increased IFN-γ and tumor necrosis factor-α (TNF-α) in the BAL fluid. We conclude that alloreactive donor lymphocytes are associated with lung injury in this allogeneic BMT model. The expansion of these cells in the BAL fluid and their ability to respond to host antigens even when systemic tolerance has been established (ie, the absence of clinical GVHD) suggest that the lung may serve as a sanctuary site for these host reactive donor T cells. These findings may have important implications with regard to the evaluation and treatment of pulmonary dysfunction after allogeneic BMT even when clinical GVHD is absent.
LUNG INJURY IS a frequent and severe complication following allogeneic bone marrow transplantation (BMT). Pulmonary insults in various forms occur in 25% to 55% of transplanted patients and account for approximately 40% of transplant-related mortality.1-6 Idiopathic pneumonia syndrome (IPS) refers to diffuse noninfectious pneumonia that occurs in this setting.1 A recent study showed a lower incidence and earlier onset of IPS than previously reported, but the typical clinical course involving the rapid onset of respiratory failure leading to death remained unchanged, underscoring the critical nature of this transplant-related problem.6 Although IPS has been associated with the development of clinical and experimental acute graft-versus-host disease (GVHD),1,5-8 it has also been reported after allogeneic T-cell–depleted (TCD) BMT when signs and symptoms of GVHD are absent,9,10 making a causal relationship between the two entities difficult to establish. We have developed a murine model of IPS where GVHD and lung injury are induced by minor histocompatibility (H) antigenic differences between donor and host (B10.BR → CBA) and have previously described the inflammatory aspects of the pulmonary damage.7 11 Using this BMT system, we have now examined the relationship of lung injury to alloreactive donor T cells and to acute GVHD. Our data show that noninfectious lung injury that occurs after allogeneic BMT is associated with an expansion of host-reactive donor T cells in the broncho-alveolar lavage (BAL) fluid and results in significant alterations in pulmonary function. Depletion of donor T cells in the bone marrow inoculum at the time of transplant significantly reduces but does not eliminate this injury, even though it prevents the development of acute GVHD. Host-reactive donor T cells were identified in the BAL fluid but not the spleens of TCD BMT recipients, which helps to explain the dichotomous nature of injury to host tissues and which suggests that the lung may be a particularly reactive site for donor T lymphocytes after allogeneic BMT.
MATERIALS AND METHODS
Mice, BMT, and assessment of GVHD.
The protocol for BMT and GVHD induction has been described previously.12,13 Female CBA/J (H-2k) and B10.BR (H-2k) mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and were transplanted between the ages of 10 and 20 weeks. Bone marrow (BM) was obtained from the femurs and tibias of donor CBA or B10.BR mice and depleted of T cells using an anti-Thy 1.2 monoclonal antibody (MoAb; American Type Culture Collection, Rockville, MD) and Low-Tox-M rabbit complement C′ (Accurate Corp, Westbury, NY). We have previously shown that TCD with two rounds of anti-Thy 1.2 MoAb and complement leaves fewer than 1 in 104 mitogen responsive T cells in the BM by limiting dilution assay.14Cell mixtures of 5 × 106 BM cells supplemented with 1 × 106 nylon wool nonadherent splenic T cells from either syngeneic (CBA) or allogeneic (B10.BR) donors were resuspended in Leibovitz's L-15 medium (Life Technologies, Grand Island, NY) and transplanted into CBA recipients via tail vein infusion (0.25 mL total volume). Other CBA animals received allogeneic TCD BM only. Before transplant, host mice received 11 Gy of total body irradiation (137Cs source) delivered in two fractions, separated by 3 hours to reduce gastrointestinal toxicity. This dose of irradiation does not cause histologically detectable pulmonary injury in normal CBA mice.14 Mice were subsequently housed in sterilized microisolator cages and received normal chow and autoclaved hyperchlorinated water for the first 2 weeks post-BMT and filtered water thereafter.
The severity of GVHD was assessed by percent weight change (recipient mice were ear punched and individual weights were obtained and recorded on day +1 and weekly thereafter until the time of analysis) and a clinical scoring system previously described that incorporates five clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity.7 At the time of analysis, mice from coded cages were evaluated and graded from 0 to 2 for each criterion. A clinical index was subsequently generated by summation of the five criteria scores (maximum index = 10).
Tissue procurement and histopathologic analysis of GVHD.
The presence of acute GVHD was also assessed by detailed histopathologic analysis of classic target organs. Samples of oral mucosa and a portion of the right lobe of the liver were obtained from animals 6 weeks after BMT and placed in buffered formalin. Formalin-preserved specimens were then embedded in paraffin, cut into 5 μm thick sections, and stained with hematoxylin and eosin for histological examination. Slides were coded without reference to mouse type or prior treatment status and systematically examined by pathologists (oral mucosa, R.S.; liver, J.M.C.) to establish an index of injury. Samples of oral mucosa were analyzed by counting the total number of lymphocytes present per high-powered field as previously described, and averages were taken from four to five fields per section.13 Analysis of liver tissue was performed by scoring 14 pathologic features, including inflammatory infiltrates in bile ducts and portal tracts, vascular endothelialitis, and hepatocellular damage as previously reported.11 13 A severity scale from one to four was used where 0 = normal, 0.5 = rare scattered, 1= minimal or focal, 2 = mild and more diffuse, 3 = moderate damage, and 4 = severe damage. Scores for each individual feature were added to yield a composite score of liver pathology.
Examination of lung histopathology and measurement of pulmonary function.
The presence of pulmonary toxicity after BMT was determined by examination of lung histopathology and pulmonary function in transplanted animals 6 weeks after BMT. Lungs from each mouse were inflated with 1 mL of Tissue Tek OCT compound (Miles, Elkhart, IN) and removed from the thoracic cavity. The right lower lobe and left lung were immersed in 10% buffered formalin. Formalin-preserved specimens were then embedded in paraffin, cut into 5 μm thick sections, and stained with hematoxylin and eosin for histological examination. Slides were coded without reference to mouse type or prior treatment status and systematically examined by L.K. to establish an index of injury. Lung tissue was evaluated for the presence of periluminal infiltrates (around airways and vessels) or parenchymal pneumonitis (involving the alveoli or interstitium) using a semiquantitative scoring system as previously described that incorporates both the severity and extent of histopathology.7
Dynamic pulmonary compliance (Cdyn) and airway conductance (GL) were measured in live mice using a plethysmographic technique as previously described.15,16 A state of general anesthesia was induced using 70 to 90 mg/kg of pentobarbital injected intraperitoneally, and 1% lidocaine was used as an additional local anesthetic in the region of the anterior neck. A 19-gauge tubing adapter (used as a tracheostomy tube) was then surgically inserted into the trachea and secured with silk suture. Mechanical ventilation was instituted with a rodent ventilator (Harvard Apparatus, Natick, MA) set to deliver a tidal volume of 0.07 mL per 10 g of body mass at a frequency of 150 breaths per minute and a positive end-expiratory pressure of 2 to 3 cm H20. A small portion of the thoracic wall was excised to eliminate any effect of the chest wall on pulmonary function. The mice were placed in a plexiglas chamber, which was subsequently sealed to a constant volume plethysmograph system. Pressures in the chamber and in the tracheostomy tube were detected by separate transducers (Celesco, Canoga Park, CA), amplified, and converted from analog to digital data to be processed by a computer programmed to calculate Cdynand GL.15 16 Thirty seconds before each determination, a deep inspiration (3 to 4 × tidal volume) was delivered to provide a constant volume history and to prevent atelectasis. Measurements of Cdyn and GL were obtained at 1-minute intervals for 5 minutes for each mouse and the results averaged. The coefficient of variation for values of Cdyn and GL from individual mice was less than 5%. Standard curves for both measures of pulmonary function were generated for CBA mice by measuring Cdyn and GLin naive animals from age 10 to 32 weeks at 2- to 4-week intervals (n = 4 to 5 animals per age group). Measurements of Cdyn and GL obtained from transplanted animals were therefore compared with mean values for age-matched naive controls, and the results are expressed as the percent predicted score.
Broncho-alveolar lavage (BAL), cytokine determination, and cellular differential.
After the determination of pulmonary function, mice were killed by exsanguination and BAL was performed. A 0.8 mL aliquot of 1× phosphate buffered saline (PBS) containing 0.6 mmol/L EDTA was instilled into the lungs through the secured tracheostomy tube, of which 0.7 mL was removed and placed into a sterile tube on ice. This procedure was repeated nine additional times with subsequent aliquots combined in a second tube. The tubes were centrifuged at 1,500 rpm for 5 minutes, and supernatant from the first tube was frozen for subsequent analysis. Cell pellets from both tubes were combined, washed twice, and counted. Aliquots of cell suspensions (2 × 106 cells per mL) were placed on glass cover slips, air-dried, stained with Wright-Giemsa, and mounted on microscope slides. Coded slides were then evaluated visually for morphologic differentials. Concentrations of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) were measured in BAL fluid supernatant (obtained from the first laved aliquot) by sandwich enzyme-linked immunosorbent assay (ELISA) using specific anti-murine MoAbs for capture and detection and the appropriate standards purchased from Pharmingen (IFN-γ; San Diego, CA) and Genzyme (TNF-α; Cambridge, MA). Assays were performed according to the manufacturer's protocol. Lower limits of detection of these assays were 15 pg/mL (TNF-α) and 0.25 U/mL (IFN-γ). For determination of endotoxin concentration in BAL fluid, the limulus amebocyte lysate (LAL) assay QLC-1000 test kit was used (Bio Whittaker, Walkersville, MD). Assays were performed according to the manufacturer's protocol as previously described.7 In all cytokine and endotoxin determinations, samples, and standards were run in duplicate.
Cell culture, analysis of proliferative response, and IFN-γ production.
All culture media reagents were purchased from GIBCO-BRL (Gaithersburg, MD). For analysis of proliferative response and IFN-γ production, BAL cells and splenocytes were obtained from individual recipients 6 weeks post-BMT and pooled within treatment groups. Splenocytes (ranging in concentration from 0.5 to 2.0 × 105) were cultured in flat-bottomed 96-well Falcon plates (Lincoln Park, NJ) for 72 hours in the presence of irradiated and TCD (anti-Thy 1.2 MoAb and C′) stimulator cells from CBA/J mice (2 × 105/well). Cell culture was performed in 5% fetal calf serum (FCS)/RPMI supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 0.1 mmol/L nonessential amino acid, 0.02 mmol/L β-mercaptoethanol, and 10 mmol/L HEPES, pH 7.75 at 37°C in a humidified incubator supplemented with 7% CO2. BAL cells were preincubated for 90 minutes at 37°C to remove adherent macrophages and then cultured in a similar manner. Supernatants were collected at 48 hours for IFN-γ analysis by ELISA, and proliferative response to host antigen was measured after 72 hours by incorporation of [3H] thymidine (1μCi) for the last 24 hours of incubation.
Statistical considerations.
All values are expressed as the mean ± SEM. Statistical comparisons between groups were completed using the nonparametric, unpaired, Mann-Whitney test.
RESULTS
Significant GVHD and lung injury develops after allogeneic BMT.
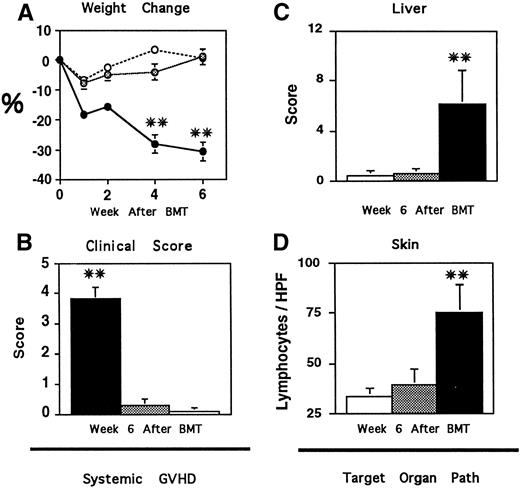
CBA mice were first transplanted with syngeneic (CBA) or allogeneic (B10.BR) TCD bone marrow and 1 × 106 donor T cells. Animals were assessed weekly for systemic GVHD using percent weight change and the clinical scoring system described in Materials and Methods. Recipients of allogeneic BM and T cells developed significant weight loss within the first week after BMT and, despite a transient recovery, continued to lose weight throughout the observation period consistent with the development of acute GVHD (Fig 1A, P < .01). Approximately 40% of these animals died of GVHD by 6 weeks after BMT (data not shown). By contrast, mice receiving syngeneic BMT all survived and were at or above their pretransplant weight 6 weeks after BMT. Recipients of allogeneic BMT also developed significant signs of GVHD compared with syngeneic controls as assessed by alterations in fur texture, skin integrity, posture, mobility, and weight (Fig 1B, P < .01). The extent of acute GVHD was further evaluated 6 weeks after BMT by histopathologic evaluation of target organ tissue as described in Materials and Methods. As shown in Fig 1C and D, semiquantitative analysis showed significant tissue injury in the liver and oral mucosa of animals receiving allogeneic BM and 1 × 106 donor T cells compared with recipients of syngeneic BMT (P < .01), consistent with the presence of clinical GVHD in these animals.
Analysis of systemic (A and B) and target organ (C and D) GVHD after BMT. CBA mice received syngeneic TCD BM and T cells □, TCD allogeneic (B10.BR) BM alone ▩, or with the addition of 1 × 106 T cells ▪, as described in Materials and Methods. Recipients of allogeneic BM and T cells developed significant GVHD as determined by weight loss (A) and clinical score (B), compared with recipients of syngeneic BMT (**P < .01). The extent of acute GVHD was also assessed 6 weeks after BMT by histopathologic evaluation of target organ tissue as described in Materials and Methods. Six weeks after transplant, recipients of allogeneic BM and T cells developed significant tissue injury in the liver (C) and oral mucosa (D) compared with syngeneic BMT controls (**P < .01). Transplantation of allogeneic TCD BM alone completely prevented the development of GVHD; weights, clinical scores, and target organ pathology scores of these animals were indistinguishable from syngeneic controls. Data are expressed as mean ± SEM. (A and B, n = 12 to 15 per group; C and D, n = 6 to 9 per group)
Analysis of systemic (A and B) and target organ (C and D) GVHD after BMT. CBA mice received syngeneic TCD BM and T cells □, TCD allogeneic (B10.BR) BM alone ▩, or with the addition of 1 × 106 T cells ▪, as described in Materials and Methods. Recipients of allogeneic BM and T cells developed significant GVHD as determined by weight loss (A) and clinical score (B), compared with recipients of syngeneic BMT (**P < .01). The extent of acute GVHD was also assessed 6 weeks after BMT by histopathologic evaluation of target organ tissue as described in Materials and Methods. Six weeks after transplant, recipients of allogeneic BM and T cells developed significant tissue injury in the liver (C) and oral mucosa (D) compared with syngeneic BMT controls (**P < .01). Transplantation of allogeneic TCD BM alone completely prevented the development of GVHD; weights, clinical scores, and target organ pathology scores of these animals were indistinguishable from syngeneic controls. Data are expressed as mean ± SEM. (A and B, n = 12 to 15 per group; C and D, n = 6 to 9 per group)
To evaluate the extent of pulmonary toxicity that developed 6 weeks after BMT, lung tissue was obtained, examined microscopically, and scored semiquantitatively as described in Materials and Methods. Consistent with previous studies, lungs of mice receiving syngeneic transplants maintained virtually normal histology, whereas two major abnormalities were apparent in the group receiving allogeneic BM and T cells.7 First, a dense mononuclear cell infiltrate was found around both pulmonary vessels and bronchioles, and second, an acute pneumonitis was observed involving both the interstitium and alveolar spaces. The alveolar infiltrate was composed of macrophages, lymphocytes, epithelial cells, and scattered polymorphonuclear cells within a fibrin matrix (data not shown). Semiquantitative evaluation of lung sections showed that significant pulmonary damage was present after allogeneic BMT compared with syngeneic controls (Fig 2A, P < .01). Potential infectious causes of pulmonary injury were ruled out by screening sentinel mice from each transplant group for a panel of both viral and bacterial organisms as previously described.7 No pathogenic organisms were identified in any mice, and lung tissue was negative for Pneumocystis carinii by silver staining.
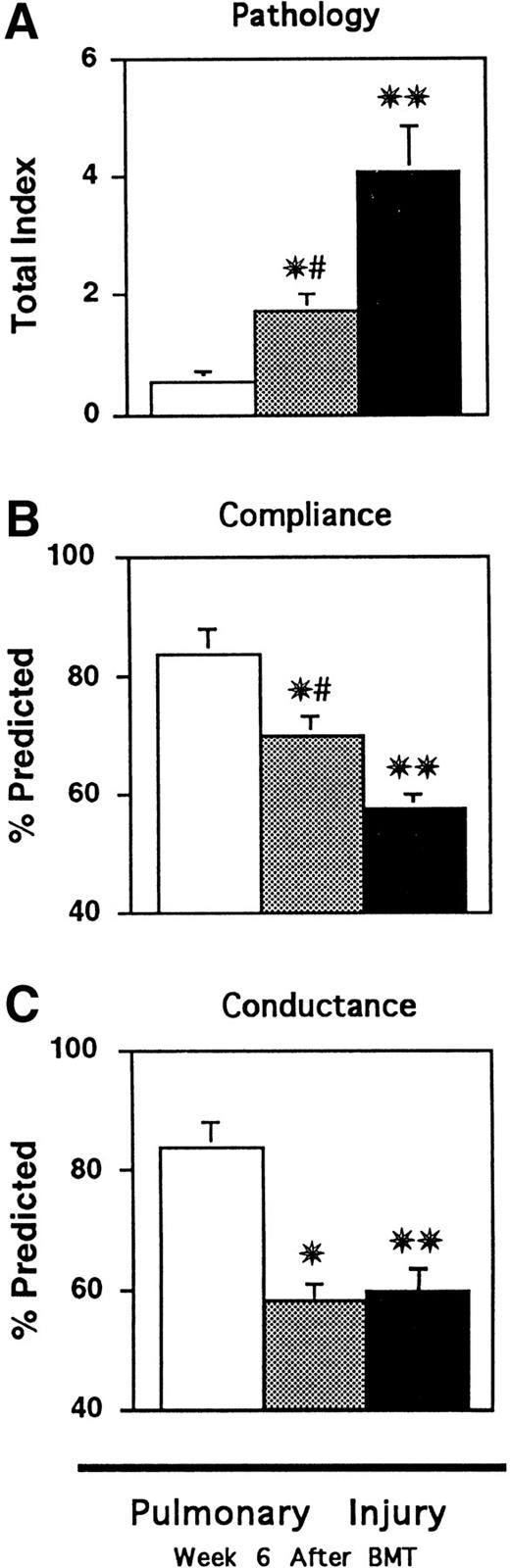
Semiquantitative analysis of lung histopathology (A) and pulmonary function (B and C) 6 weeks after BMT. CBA mice received BMT as in Fig 1 (syngeneic □, allo TCD BM alone ▩, allo TCD BM and T cells ▪). Lungs were obtained after BMT and analyzed as described in Materials and Methods. Significant lung injury was present in animals receiving allogeneic T cells compared with syngeneic BMT recipients (**P < .01). TCD significantly reduced (#P < .01), but did not eliminate, lung injury and pathology scores after TCD BMT remained significantly increased compared with syngeneic controls (*P < .01). Pulmonary dynamic compliance (Cdyn) and airway conductance (GL) were measured in live transplanted animals as described in Materials and Methods. Lung injury that developed in mice with GVHD was associated with significant decreases in Cdynand GL compared with syngeneic controls (**P < .01). Mice receiving TCD BMT showed significant improvements in Cdyn (B) but not GL (C) compared with animals with GVHD (#P = .01), but both measurements remained significantly reduced compared with syngeneic controls (*P < .01). Data are expressed as mean ± SEM. (n = 12 to 15 per group).
Semiquantitative analysis of lung histopathology (A) and pulmonary function (B and C) 6 weeks after BMT. CBA mice received BMT as in Fig 1 (syngeneic □, allo TCD BM alone ▩, allo TCD BM and T cells ▪). Lungs were obtained after BMT and analyzed as described in Materials and Methods. Significant lung injury was present in animals receiving allogeneic T cells compared with syngeneic BMT recipients (**P < .01). TCD significantly reduced (#P < .01), but did not eliminate, lung injury and pathology scores after TCD BMT remained significantly increased compared with syngeneic controls (*P < .01). Pulmonary dynamic compliance (Cdyn) and airway conductance (GL) were measured in live transplanted animals as described in Materials and Methods. Lung injury that developed in mice with GVHD was associated with significant decreases in Cdynand GL compared with syngeneic controls (**P < .01). Mice receiving TCD BMT showed significant improvements in Cdyn (B) but not GL (C) compared with animals with GVHD (#P = .01), but both measurements remained significantly reduced compared with syngeneic controls (*P < .01). Data are expressed as mean ± SEM. (n = 12 to 15 per group).
We next evaluated the physiologic consequences of the lung pathology present after BMT by measuring pulmonary dynamic compliance (Cdyn) and airway conductance (GL) in live transplanted mice using the well-established plethysmographic technique described in Materials and Methods. Abnormalities in Cdyn, defined as the change in lung volume resulting from a given increase in distending transpulmonary pressure, would be expected with pulmonary parenchymal alterations, including consolidation, atelectasis, and interstitial inflammation or fibrosis. GL, measured as flow per unit pressure and which represents the reciprocal of resistance, would be influenced by conditions that result in alteration of bronchial diameter. Six weeks after transplant, mice receiving allogeneic BMT showed significant reductions in both dynamic compliance and airway conductance compared with syngeneic controls consistent with both the interstitial and peribronchial infiltrates seen microscopically (Fig 2B and C, P < .01).
TCD prevents GVHD and significantly reduces, but does not eliminate, lung injury after BMT.
We analyzed the contribution of donor T cells to GVHD and lung injury that developed after allogeneic BMT by using the method of TCD described in Materials and Methods. CBA recipients of allogeneic TCD BM alone (TCD BMT) at no time showed evidence of active GVHD, and their weight curves and clinical scores at the time of analysis were indistinguishable from syngeneic controls (Fig 1A and B). Similarly, target organs from recipients of TCD BMT had equivalent pathology scores compared with syngeneic controls (Fig 1C and D), confirming the clinical analysis and consistent with multiple studies that TCD BMT prevents GVHD.13,17 18 As shown in Fig 2A, evaluation of lung sections showed that TCD significantly reduced, but did not eliminate, the development of lung injury after BMT (P<0.01). This damage was manifest primarily as mononuclear cell infiltration around pulmonary vessels and bronchi, while a mild pneumonitis was seen in the alveoli and interstitium of approximately 50% of animals (data not shown). As shown in Fig 2B and C, analysis of pulmonary function confirmed these surprising results; recipients of TCD BMT showed significant improvements in Cdyn (P= .01) but not in GL compared with mice with GVHD, and both of these measurements remained significantly reduced compared with syngeneic controls (P < .01). These pulmonary function test findings correlated well with the extent and nature of histologic lung damage present after TCD BMT and confirmed that these animals develop significant lung toxicity in the absence of systemic and histopathologic GVHD as measured in usual target organs (Fig 1C and D).
Lung injury in recipients of allogeneic BMT is associated with increases of TNF-α, IFN-γ, and host-reactive T cells in the BAL fluid.
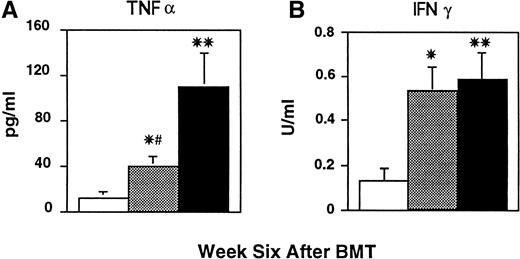
In an attempt to delineate the mechanisms responsible for lung injury occurring after allogeneic BMT, BAL fluid was collected from all transplanted mice before fixation of lung tissue as described in Materials and Methods. We have previously shown that inflammatory changes in BAL fluid correlate with this injury,7 and therefore we evaluated cellularity, cytokine content, and LPS levels in the BAL fluid of all transplanted mice. As shown in Table 1, lung injury in recipients of allogeneic BM and T cells that developed GVHD was associated with a significant increase in the number of total BAL cells compared with syngeneic controls (P < .01). Also noted in this group was a fivefold increase in lymphocytes, and significant increases in macrophages and neutrophils, consistent with the mixed inflammatory alveolar infiltrates observed on histopathology. BAL fluid cellularity remained significantly elevated in recipients of TCD BMT compared with syngenic controls (P < .01) and included increased numbers of both neutrophils and lymphocytes (P < .05). Nearly identical changes were noted with respect to BAL TNF-α, which was reduced after TCD BMT by more than 50% (Fig 3A,P = .01) but which remained significantly elevated compared with syngeneic controls (Fig 3A, P < .05). Despite the increased levels of both neutrophils and TNF-α, LPS levels in the BAL fluid from mice receiving TCD BMT were not elevated compared with syngeneic controls (data not shown). We hypothesized that the increase in TNF-α in the absence of LPS in BAL fluid after TCD BMT might be derived from T cells or activated “primed” pulmonary macrophages. We therefore analyzed BAL fluid IFN-γ levels to evaluate this hypothesis. As shown in Fig 3B, IFN-γ levels in the TCD BMT group were significantly increased compared with syngeneic recipients (P < .01), and somewhat surprisingly, were comparable with those measured in animals with GVHD. We next investigated whether T cells in BAL fluid might represent a source of this IFN-γ. Responses of BAL lymphocytes to alloantigens in vitro have been used as a reliable indicator of both acute and chronic rejection after lung transplantation,19 and we therefore evaluated the responses of BAL lymphocytes to host antigens in this BMT model. BAL cells obtained from transplant recipients at week 6 were cultured in vitro with host stimulator cells, and as shown in Fig 4A, BAL cells from mice with GVHD responded vigorously. The proliferative response of these cells was sixfold higher than syngeneic controls, and their production of IFN-γ was markedly increased (20.3 ± 1.2 v ND). TCD reduced but did not eliminate these responses, as proliferation and IFN-γ production to host antigens by BAL cells from recipients of TCD BMT remained significantly increased compared with syngeneic controls. Thus, host-reactive donor T cells appeared to contribute to the BAL fluid changes and pulmonary damage observed after allogeneic BMT.
BAL fluid TNF-α and IFN-γ concentrations after BMT. CBA mice received BMT as in Fig 1 (syngeneic □, allo TCD BM alone ▩, allo TCD BM and T cells ▪). BAL fluid was obtained 6 weeks after BMT and analyzed for TNF-α and IFN-γ concentrations as described in Materials and Methods. Both TNF-α and IFN-γ were significantly increased in the BAL fluid from mice receiving allogeneic BM and T compared with syngeneic controls (**P < .01). BMT with TCD BM only lead to a 50% reduction in TNF-α (#P = .01) but not IFN-γ, and levels of both cytokines remained significantly elevated compared with syngeneic controls (*P < .05). Data are expressed as mean ± SEM. (n = 8 to 12 per group).
BAL fluid TNF-α and IFN-γ concentrations after BMT. CBA mice received BMT as in Fig 1 (syngeneic □, allo TCD BM alone ▩, allo TCD BM and T cells ▪). BAL fluid was obtained 6 weeks after BMT and analyzed for TNF-α and IFN-γ concentrations as described in Materials and Methods. Both TNF-α and IFN-γ were significantly increased in the BAL fluid from mice receiving allogeneic BM and T compared with syngeneic controls (**P < .01). BMT with TCD BM only lead to a 50% reduction in TNF-α (#P = .01) but not IFN-γ, and levels of both cytokines remained significantly elevated compared with syngeneic controls (*P < .05). Data are expressed as mean ± SEM. (n = 8 to 12 per group).
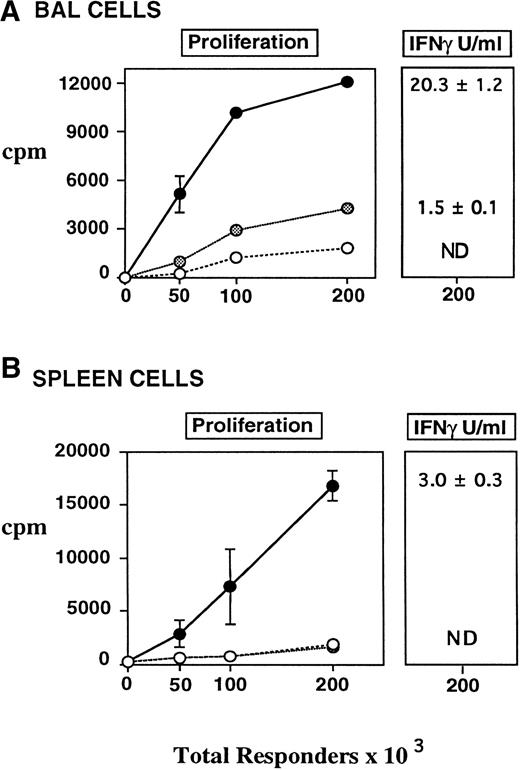
Proliferation and IFN-γ production of BAL fluid (A) and Splenic (B) T cells to host (CBA) antigens in vitro. CBA mice received BMT as in Fig 1 (syngeneic, allo TCD BM alone •, allo TCD BM and T cells •). BAL cells and splenocytes were obtained from transplanted animals 6 weeks after BMT and cultured with irradiated host stimulator cells as described in Materials and Methods. BAL and splenic T-cell populations from recipients of allogeneic BM and T cells responded to host antigens in vitro. TCD reduced, but did not eliminate, proliferation and IFN-γ production of pulmonary T cells to host antigens, whereas similar responses by splenocytes were absent, consistent with the lack of GVHD in these animals. Values for proliferation were normalized for the percent T cells present in each group (CD4+ and CD8+). IFN-γ levels expressed in U/mL were measured at maximum proliferative response (200 × 103 responders per well). Data are expressed as mean ± SEM of triplicate wells and represent one of two similar experiments.
Proliferation and IFN-γ production of BAL fluid (A) and Splenic (B) T cells to host (CBA) antigens in vitro. CBA mice received BMT as in Fig 1 (syngeneic, allo TCD BM alone •, allo TCD BM and T cells •). BAL cells and splenocytes were obtained from transplanted animals 6 weeks after BMT and cultured with irradiated host stimulator cells as described in Materials and Methods. BAL and splenic T-cell populations from recipients of allogeneic BM and T cells responded to host antigens in vitro. TCD reduced, but did not eliminate, proliferation and IFN-γ production of pulmonary T cells to host antigens, whereas similar responses by splenocytes were absent, consistent with the lack of GVHD in these animals. Values for proliferation were normalized for the percent T cells present in each group (CD4+ and CD8+). IFN-γ levels expressed in U/mL were measured at maximum proliferative response (200 × 103 responders per well). Data are expressed as mean ± SEM of triplicate wells and represent one of two similar experiments.
The response to host antigens by BAL fluid T cells after TCD BMT was somewhat surprising because very few T cells remained in the BM inoculum after the TCD procedure, and the effectiveness of that protocol was confirmed by the absence of clinical or histologic GVHD in these animals (Fig 1). To determine whether T-cell reactivity to host antigens was confined to the lungs of these animals, similar in vitro cultures were performed using splenocytes from transplanted animals as responders to host antigens in a mixed lymphocyte reaction (MLR). As shown in Fig 4B, the proliferative response of splenocytes from TCD BMT recipients to host antigens was equivalent to that of syngeneic controls. IFN-γ production was also undetectable in supernatants of these splenocyte cultures, consistent with the absence of systemic GVHD. By contrast, splenocytes from animals with GVHD both proliferated and produced IFN-γ when stimulated with host antigens. Interestingly, BAL cells from mice with GVHD produced approximately sevenfold more IFN-γ than did splenocytes from the same animals. Taken together, these findings show that T cells that are responsive to host antigens are present in the lung after allogeneic BMT and that T-cell alloreactivity may be enhanced there even when such responses are low or undetectable in the periphery.
Donor Vβ6+/αβTCR+ T cells expand in the lungs after allogeneic BMT.
The above responses to host antigens in vitro strongly suggested the presence of donor T cells in the BAL fluid of mice after allogeneic BMT. To confirm the origin of these cells, we used differences in the T cell Vβ repertoire between donor and host. Mature Vβ6+and Vβ3+ T cells are normally deleted by negative selection in the thymuses of host (CBA) but not donor (B10.BR) animals due to differences in Mls expression.20 However, both Vβ6+ and Vβ3+ T-cell populations, which recognize CBA antigens in vitro, have been found in the peripheral circulation of CBA recipients of allogeneic BMT as a result of thymic dysfunction and damage associated with the development of GVHD.21 We therefore analyzed BAL fluid and splenic T-cell populations by flow cytometry for the expression of Vβ6, Vβ3, and TCR αβ antigens. As shown in Table 2, the percentage of splenic Vβ6+ T lymphocytes was significantly increased in mice with GVHD compared with both syngeneic and TCD BMT recipients (P < .01). Dramatic increases in the percentage of donor T cells were also seen in the BAL fluid of these mice; nearly 40% of αβTCR+ T cells were Vβ6+, a level fourfold higher than in naive B10.BR mice. Consistent with their reactivity to host antigens in vitro, approximately 21% of BAL fluid αβTCR+ lymphocytes from TCD BMT recipients also expressed Vβ6, confirming the donor origin of those T cells (P < 0.01, compared to syn). A smaller albeit significant increase in Vβ6+ cells was apparent in the splenic T-cell population of these animals (5.5 ± 0.8% v0.7 ± 0.1%), where donor tolerance of host antigens was demonstrable in vitro (Fig 4B) and confirmed in vivo by the absence of systemic or histologic GVHD (Fig 1A through D). Thus, Vβ6+ donor T cells homed to the lungs after allogeneic BMT, persistently responded to host antigens, and were associated with clinically and histologically significant tissue injury. Depletion of donor T cells at the time of BMT reduced, but did not abrogate, donor T-cells response in the lungs even though the number of T cells in the donor BM inoculum was insufficient to cause clinical or histologic GVHD and the small percentage of donor T cells identified in the spleens of these animals did not recognize host antigens in vitro.
DISCUSSION
Using a well-characterized murine BMT model, we have examined the nature of lung histopathology that develops after allogeneic BMT and its relationship to both acute GVHD and host-reactive donor T cells. Our data show that significant noninfectious lung damage occurs in animals with GVHD (Fig 2). This injury is associated with an expansion of host-reactive donor (Vβ6+) T cells (Fig 4, Table 2) and increases in TNF-α and IFN-γ in the BAL fluid of affected animals (Fig 3). The physiologic significance of this injury is underscored by abnormalities in pulmonary function, where both dynamic compliance and airway conductance were decreased after BMT (Fig 2). Depletion of donor T cells at the time of BMT significantly reduced but did not eliminate this injury, even though it effectively prevented the development of systemic GVHD (Figs 1 and 2). The residual lung injury after TCD BMT resulted in alteration in pulmonary function, particularly with respect to airway conductance, which failed to improve compared with mice with GVHD (Fig 2). Interestingly, an expansion of host-reactive donor Vβ6+ T cells was identified in the BAL fluid but not in the spleens of TCD BMT recipients, consistent with the lack of clinical and histologic GVHD seen in these animals. These results support the hypothesis that host-reactive donor T cells can significantly contribute to noninfectious lung injury that occurs after BMT. They also show that the lung is susceptible to immune-mediated damage from small numbers of donor T cells present following allogeneic TCD BMT and suggest the lung may represent a sanctuary site for donor lymphocytes even when systemic tolerance between donor and host is established.
The role of GVHD and specifically of alloreactive donor T cells in the pathogenesis of IPS remains a topic of considerable debate. Although a variety of pulmonary complications and histopathologic findings have been associated with the development of GVHD, a mechanistic relationship between noninfectious lung injury and immunologically active donor T cells has not been clearly established. The principal objection to the identification of the lung as a target organ of acute GVHD is that epithelial cell apoptosis, a finding classically attributed to selective T-cell–mediated injury and considered pathognomonic for acute GVHD in the gut, liver, and skin of patients after BMT, has not been consistently identified among the myriad of histologic findings noted in the lungs of patients with IPS.22-25 In 1978, Beschorner et al22 noted an association between the severity of clinical GVHD and a histologic pattern consistent with lymphocytic bronchitis found on postmortem exams. This finding was not seen in patients who received autologous BMT or in untransplanted controls.22 Although initially considered a potential histopathologic correlate for GVHD of the lung, the association between lymphocytic bronchitis and the development of systemic GVHD was not consistently identified in subsequent reports.23-25
However, the heterogeneity of pulmonary histopathology in clinical BMT is complicated by both the nonspecific changes that occur after mechanical ventilation and the suboptimal quality and quantity of pathology specimens due to the significant risks associated with lung biopsy procedures. Despite the lack of classic GVHD histopathology, it is not unreasonable to suggest that pulmonary parenchymal and endothelial cells can be potential targets for activated donor T cells after allogeneic BMT. Firstly, the lung is a rich source of major and minor HC antigens and professional antigen-presenting cells,26,27 and it is the site of complex immunologic networks, the proper balance of which allows for infectious surveillance and maintenance of structural integrity, whereas dysregulation of such networks can result in tissue injury and scarring.28 The role of T lymphocytes to immune-mediated inflammatory reactions in the lung has recently been confirmed by several groups and is thought to involve dendritic cells, macrophages, and the secretion of cytokines that can influence an emerging T-cell response.29,30 Secondly, the association of chronic GVHD with obstructive lung disease after BMT is well accepted.31-36 Although a causal link between these two entities has yet to be definitively established, the striking similarities between the consistent histopathologic features of obstructive bronchiolitis after BMT and the bronchiolitis obliterans associated with lung transplant rejection along with reports of improvement in lung function with immunosuppressive agents strongly suggests an immunological component to this pulmonary process.31,35,36 It would be inconsistent for the lung to be a target of a chronic but not an acute immune attack. Thirdly, epithelial cell apoptosis is not a requirement of GVHD pathology. The thymus is a known target of GVHD and displays extensive cytolytic damage early in the course of this process, but epithelial cell apoptosis is not a prominent histologic feature.37 Finally, recent studies have shown that GVHD target organs vary with respect to their susceptibility to injury by inflammatory effectors such as CTLs, TNF-α, and FasL.38 39 If the mechanisms of GVHD-related tissue injury can differ between individual target organs, it is possible that the histopathologic manifestation of this injury may also vary.
Recently, several studies using established rodent BMT models to explore the potential causal relationship between GVHD and IPS have shown the development of pulmonary injury in animals with systemic GVHD.7,40-42 Advantages of these systems include the unlimited availability of tissue for pathologic analysis and the ability to analyze the development of tissue injury without the confounding influences of immunosuppressive chemoprophylaxis, underlying disease, or prior treatment. Surprisingly, even under controlled experimental conditions, several patterns of lung injury have been identified. For example, using a B10 → (CBA × B10) F1 murine BMT model, Piguet and coworkers40 observed both an acute hemorhagic alveolitis and a late onset interstitial pneumonitis (IP) after infusion of B10 parental lymphocytes, whereas induction of GVHD with T cells from CBA donors led to IP only. Subsequently, the development of interstitial pneumonitis and lymphocytic bronchiolitis/bronchitis similar to the histopathology seen in lung allograft rejection was noted in an unirradiated rat GVHD model.41 We have observed similar pulmonary pathology in the murine BMT system studied here and have consistently identified parenchymal pneumonitis and mononuclear cell infiltration around both bronchial and vascular structures as the two major microscopic patterns of injury.7
Experimental models have also provided insight into the possible pathophysiologic mechanisms responsible for noninfectious lung injury occurring after BMT. TNF-α has been detected in the lungs of animals with GVHD,40,43 and administration of TNF antiserum blocked the development of alveolar hemorrhage but did not reduce the accompanying cellular infiltrate.40 We have previously shown that lung injury that occurred during active GVHD was associated with increased BAL fluid levels of neutrophils, TNF-α, and LPS.7 Elevations of proinflammatory cytokines in the lungs of patients with pneumonitis has been confirmed in one report44 but not in another where lung toxicity after BMT was associated with a Th-2 cytokine response.45 The importance of lymphocytes to lung injury after BMT has also been suggested by several groups.42,46,47 Panoskaitsis-Mortari and coworkers42 showed that donor T cells were important mediators of lung toxicity that developed within the first week of BMT across MHC antigens. Additionally, donor T-cell clones that recognize CD45 polymorphisms contributed to a rapidly progressive pulmonary vasculitis within the first 3 days after their injection into nonirradiated recipients.47 These findings support the clinical observation of Leblond and colleagues,48 who suggested that the alveolar lymphocytosis that accompanied interstitial pneumonitis after allogeneic BMT could represent a pulmonary manifestation of chronic GVHD.
The current study further dissects the pathogenesis of IPS by showing a role for donor T-cell reactivity in mediating lung damage that occurs after allogeneic BMT, even when systemic GVHD is absent. Our observation that host-reactive donor lymphocytes are present in the BAL fluid but not the spleens of animals after TCD BMT is intriguing and suggests that the lung may be particularly sensitive to the effects of these cells even when systemic tolerance has been established. It remains unresolved, however, as to whether the GVHD induced alterations in BAL fluid cytokine levels and in pulmonary histology after TCD BMT are mediated by mature donor T cells remaining in the BM innoculum, by effector cells that have differentiated from an engrafted BM within an allogeneic thymic environment, or by a combination of these cells. With respect to the latter, previous work using this strain combination has shown that acute GVHD is accompanied by loss of normal thymic repertoire selection.21 In the current study, thymic cellularity was reduced in mice receiving TCD BMT compared with syngeneic BMT controls (26.3 ± 2.5 × 106v 42.9 ± 3.9 × 106, P < .01), whereas the number of thymocytes in both groups remained significantly higher than those in mice with clinically evident GVHD (6.4 ± 2.0 × 106, P < .01). Thus, mild thymic dysfunction and a loss of negative selection could account for the persistence of host-reactive donor T cells after TCD BMT. Data supporting the second hypothesis can be found in an study by Korngold et al,17 where as few as 3 × 104 B10.BR T cells (equivalent to 0.3% T-cell contamination of the marrow) resulted in significant systemic disease and mortality from GVHD in CBA recipients. It is possible, therefore, that transplantation of a very small number of mature, host-responsive T cells could result in small but significant changes in cytokine production and cause damage in a sensitive target organ, such as the thymus or the lung, without producing significant systemic or histologic GVHD in other target organs. Further studies using genetically marked bone marrow and thymectomized recipients will be needed to address the relevant contributions of these two mechanisms to lung damage after allogeneic BMT.
Enhanced lymphocyte activation in the lungs after BMT has been reported by others.9,49 Using an experimental BMT model, Gartner and colleagues49 showed that pulmonary natural killer (NK) cell activity remained increased over an extended period of time during GVHD in contrast to the transient and mild increase in splenic NK activity that occurred during the same interval. Clinically, BAL fluid lymphocytosis has been described after TCD BMT during pneumonitis that resulted from a local immune response; pulmonary T cells appeared to be activated despite systemic immune suppression.9Furthermore, chronic noninfectious lung injury, as diagnosed by clinical symptoms, radiographic abnormalities, and alterations in pulmonary function, has been reported in patients without evidence of systemic GVHD.31-34 50
Although we have provided data to support a role for alloreactive donor T cells in the evolution of lung damage after BMT, the precise mechanisms by which these cells traffic to the lung, interact with host antigens, and cause injury remain unresolved. This process is likely to be complex and to ultimately involve the interaction of donor lymphocytes with pulmonary antigen presenting cells (APC). It is conceivable that pulmonary dendritic cells, which are potent stimulators of primary T-cell responses, are intimately involved with this process.51,52 These cells are thought to play a critical role in the initiation and regulation of immune responses in the lung, and recent data suggest that they are important to both acute and chronic rejection after lung transplantation.53-56Furthermore, the Th-1 cytokines interleukin-2 (IL-2) and IFN-γ, which are critical to the development of GVHD, are felt to be involved in the activation and recruitment of dendritic cells to sites of inflammation.57,58 The specific requirement of host APCs for the generation of acute GVHD was recently reported in a CD8+ T-cell driven GVHD model where chimeric animals that did not express MHC class I on their APCs but did express MHC class I antigens on target tissue were used as BMT recipients.59 It is possible that radioresistant, pulmonary APCs in the host persist longer than those in other organs, thus allowing sustained presentation of host antigens in the lung but not in other visceral sites to small numbers of donor T cells that are trapped within the pulmonary microvascular circulation. Studies are in progress to determine whether the kinetics of dendritic cell turnover after BMT can account for the apparent “sanctuary” status of the lung with respect to donor T cells, a concept that may have important implications with regard to the evaluation and treatment of pulmonary dysfunction after BMT even when clinical GVHD is absent.
Supported by National Institutes of Health Grant Nos. HL55162, CA 39542, and DK39512; J.L.M.F. is a scholar of the Leukemia Society of America. M.R.M.V. is the recipient of a Howard Hughes Physician Postdoctoral Fellowship.
Address reprint requests to Kenneth R. Cooke, MD, Dana-Farber Cancer Institute, Department of Pediatric Oncology, D1420, 44 Binney St, Boston, MA 02115;e-mail kenneth_cooke@dfci.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.