Abstract
RP105 was originally discovered as a mouse B-cell surface molecule that transmits an activation signal. The signal leads to resistance against irradiation-induced apoptosis and massive B-cell proliferation. Recently, we found that mouse RP105 is associated with another molecule, MD-1. We have isolated here the human MD-1 cDNA. We show that human MD-1 is also associated with human RP105 and has an important role in cell surface expression of RP105. We also describe a monoclonal antibody (MoAb) that recognizes human RP105. Expression of RP105 is restricted to CD19+ B cells. Histological studies showed that RP105 is expressed mainly on mature B cells in mantle zones. Germinal center cells are either dull or negative. RP105 is thus a novel human B-cell marker that is preferentially expressed on mature B cells. Moreover, the anti-RP105 MoAb activates B cells, leading to increases in cell size, expression of a costimulatory molecule CD80, and DNA synthesis. The B-cell activation pathway using RP105 is conserved in humans.
© 1998 by The American Society of Hematology.
THE LEUCINE-RICH REPEAT (LRR) is a protein motif that has been implicated in protein-protein interaction. It is present in a number of proteins with diverse functions and cellular locations.1 Some members have a role in defense against pathogens. Tomato disease resistance (R) genes cf-2 and cf-9 are transmembrane proteins that possess LRRs in extracellular domains.2,3 They recognize specific pathogen molecules with LRRs and activate plant’s defense responses.4 Similarly, the Toll receptor, another LRR molecule of Drosophila, elicits defense responses against the fungal pathogens.5 A human homologue of Toll was recently cloned.6 Human Toll is expressed on lymphocytes and monocytes and transmits an activation signal. Thus, LRR molecules seem to have a basic role in the immune system among diverse species.
RP105 is another LRR molecule that is expressed on mouse B lymphocytes. It was first identified by establishing the RadioProtective (RP) monoclonal antibody (MoAb) that protected spleen B cells from irradiation-induced apoptosis.7,8 The RP105 molecule, when cross-linked by the MoAb, transmits the activation signal that leads to massive B-cell proliferation as well as resistance against apoptosis. Interestingly, these activated and proliferating B cells arrest their growth and undergo apoptosis upon a signal from the antigen receptor.9 RP105 may be involved in regulation of B-cell growth and an antigen-induced death. We have recently identified MD-1 as a molecule that is physically associated with RP105.10 MD-1 was originally reported as av-myb–regulated gene.11 It is a secretory molecule but tethered to the cell surface by RP105. MD-1 seems to regulate cell surface expression of RP105.10
A human homologue of RP105 was previously identified.12 The amino acid sequence shows 75% identity to that of mouse RP105, and the deduced molecular structure, including LRRs, is well conserved. Despite such structural similarities, cell surface expression, transduction of an activation signal, and association with human MD-1 are not clear yet. To address these issues, we conducted molecular cloning of human MD-1 and established an MoAb against human RP105.
MATERIALS AND METHODS
Cells and antibodies.
A mouse B-cell lymphoma M12 was transfected with an expression vector pEFBOS (a gift from Dr Shigekazu Nagata, Osaka University Medical School, Osaka, Japan) that codes for human RP105 with the flag epitope at the carboxy-terminus. Expression of human RP105 was confirmed with immunoblotting with an anti-flag MoAb M2 (Eastman Kodak Co, New Haven, CT) and with cell surface biotinylation and subsequent immunoprecipitation by the anti-flag MoAb. The cell line was referred to as M12HRP. The BaHRP cell line was prepared by transfecting the same vector as above into an mouse interleukin-3 (IL-3)–dependent line Ba/F3. Ba/F3 cells do not express either MD-1 or RP105. Expression of human RP105 was detected with immunoprecipitation and immunoblotting with the anti-flag MoAb. The BaHRPMD cell line was established by further transfection of BaHRP cells with an expression vector encoding human MD-1 of which the carboxy-terminus was tagged with the flag epitope. Cells bearing MD-1 on a cell surface were sought by staining with the anti-flag MoAb. Although already transfected human RP105 also bears the flag epitope, it is located inside the cell. Therefore, the flag epitope on human RP105 is not detectable with cell surface staining.
Human cell lines, including Daudi, Ramos, Nalm-6, U937, K562, JY, RPMI8866, CEM, and Molt4, were obtained from Japan Cancer Resources Banks (JCRB, Osaka, Japan). Peripheral blood leukocytes were isolated from heparinized blood by Ficoll-Hypaque density gradient centrifugation (Pharmacia, Uppsala, Sweden). Normal tonsil tissues were obtained from tonsillectomies at the Saga Medical School Hospital (Saga, Japan). In the cell proliferation assay (see Table2), tonsillar B cells were enriched by negative selection using anti-CD2 MoAb-coupled Dynabeads (Dynal Inc, Oslo, Norway).
Anti-CD19 MoAb SG/16 was established in this laboratory. Another anti-CD19 MoAb conjugated with fluorescein isothiocyanate (FITC) was obtained from Becton Dickinson Immunocytometry Systems (San Jose, CA). The biotinylated anti-CD80 MoAb was purchased from PharMingen (San Diego, CA). The MHR73 MoAb (IgG1/κ) was established from BALB/c mice immunized with the M12HRP cell line (see Results). The MoAb was purified from ascitic fluid with the ABx column chromatography (J.T. Baker Inc, Phillipsburg, NJ).
An Est cDNA clone and sequencing.
An Est cDNA clone encoding human MD-1 (Genbank accession no. T84854) was obtained from Genome Systems Inc (St Louis, MO). Sequencing was conducted with ALFexpress DNA sequencer and Thermo Sequenase cycle sequencing kit (Pharmacia Biotech Japan, Tokyo, Japan).
Cell surface biotinylation, immunoprecipitation, and Western blotting.
Cell surface biotinylation and immunoprecipitation was conducted as described previously.13 Briefly, cells were washed in Hanks’ balanced salt solution (HBSS) and adjusted to 5 × 107/mL in saline containing 100 mmol/L HEPES (pH 8.0). Sulfosuccinimidobiotin (Pierce, Rockford, IL) was added to cell suspension at 0.5 mg/mL. After 30 minutes of incubation at room temperature with occasional shaking, cells were washed in HBSS and lysed in a buffer consisting of 50 mmol/L Tris/HCl (pH 7.5), 150 mmol/L NaCl, 1% Triton X-100, 50 mmol/L iodoacetamide, 1 mmol/L phenylmethyl sulfonyl fluoride (PMSF), 10 μg/mL soybean trypsin inhibitor, 5 mmol/L EDTA, and 0.1% sodium azide. After 30 minutes of incubation on ice, lysate was centrifuged and supernatant was recovered. Anti-RP105–coupled Hi-Trap beads (Pharmacia Biotech Japan) were added to cell lysate and rotated for 2 hours at 4°C. Beads were washed in the lysis buffer and bound proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Biotinylated proteins were detected with streptavidin-peroxidase (Amersham Japan, Tokyo, Japan) and Supersignal chemiluminescent substrate (Pierce). The flag epitope was demonstrated with the anti-flag MoAb followed by goat antimouse IgG-horseradish peroxidase (BioSource International, Camarillo, CA).
Northern hybridization.
Total RNA was extracted with Isogen (Nippon Gene, Toyama, Japan) and subjected to agarose electrophoresis (10 μg/lane). After transfer to a nylon membrane (Hybond N+; Amersham Japan, Tokyo, Japan), RNA was hybridized to a probe that had been labeled by random priming of the cDNA clone encoding human MD-1. Hybridization buffer consisted of 10% dextran sulfate (Pharmacia), 1 mol/L NaCl, 1% SDS, 50 mmol/L Tris/HCl (pH 7.5). Hybridization was conducted at 65°C for 20 hours. Washing was performed in 2× SSC with 0.1% SDS at up to 65°C. Radioactive signals were visualized with an image-analyzer BAS2000 (Fuji Film Co Ltd, Tokyo, Japan). The same membrane was reprobed for Glyceraldehyde-3-phosphate dehydrogenase.14
Flow cytometry and cell permeabilization.
Cells were incubated with the MHR73 MoAb or the anti-flag MoAb for 20 minutes on ice. After washes with a staining buffer (phosphate-buffered saline [PBS] containing 2% fetal calf serum [FCS] and 0.1% azide), goat antimouse IgG-FITC was added. Propidium iodide was included in the second incubation to exclude dead cells. For dual-staining, the biotinylated MHR73 followed by avidin-phycoerythrin and FITC-labeled anti-CD19 MoAb were used. Cells were analyzed on a FACScan (Becton Dickinson, Mountain View, CA). For cell permeabilization, 0.1% saponin detergent was added to the staining buffer as described.15
Cell proliferation assay.
B cells enriched from a normal human tonsil were cultured in a 96-well plate for 4 days with an indicated MoAb. Cells were pulsed with 1 μCi of [3H] TdR (ICN Radiochemicals, Irvine, CA) for the last 6 hours. They were then harvested onto glass fiber filters and the incorporated radioactivity was determined on a Beta plate flat-bed liquid scintillation counter (Pharmacia-Wallac, Gaithersburg, MD). The results are presented as a mean ± SD of triplicate wells.
Immunohistochemistry.
Normal tonsil tissues were embedded in Tissue-Tek II OCT compound (Miles Inc, Elkhart, IN), frozen in liquid nitrogen, and stored at −80°C. Acetone-fixed cryostat sections of 4 μm were incubated with the anti-RP105 MoAb MHR73. After washes, ENVISION+/HRP (DAKO Japan, Inc, Tokyo, Japan) was added. RP105 was finally visualized with 3,3′-diaminobenzidine.
RESULTS
Molecular cloning of human MD-1.
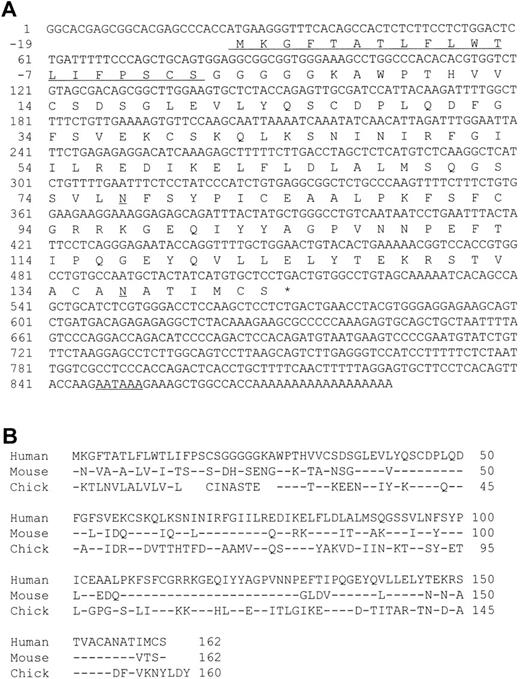
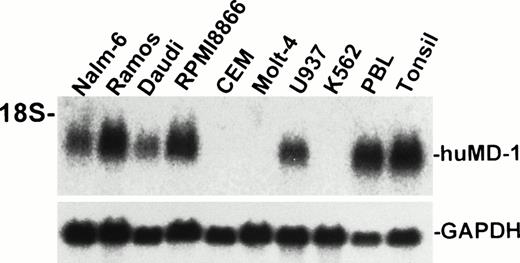
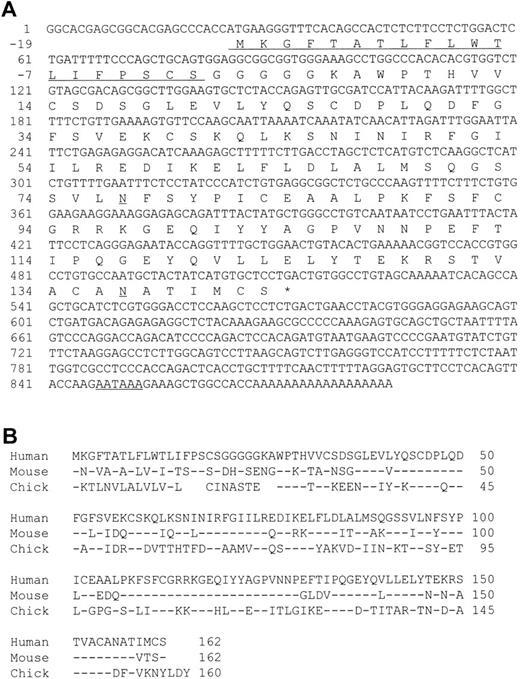
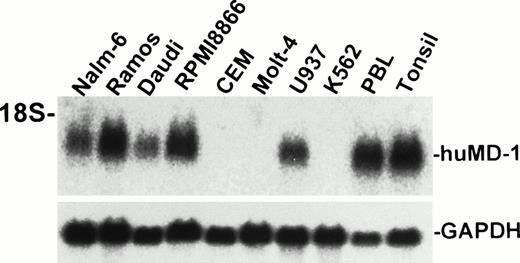
A cDNA encoding human MD-1 was isolated. We used the expressed-sequence tag (EST) database at the National Center for Biotechnology Information (NCBI, Bethesda, MD) to obtain the human MD-1 cDNA. A search with the mouse MD-1 nucleotide sequence identified a cDNA clone from human fetal liver and spleen (Genbank accession no. T84854). A whole sequence of the clone was determined. It contained the full coding region as well as a poly(A) signal (residue 847-852, underlined in Fig 1A). The cDNA clone encodes 162 amino acids. There is a hydrophobic stretch from the 1st to the 19th amino acid residue. This portion is likely to be a signal peptide (underlined in Fig 1A). The mature peptide consists of 143 amino acids, and does not have any additional stretches of hydrophobic amino acids. Therefore, human MD-1 seems to be a secretory molecule. Two canonical N-glycosylation sites are contained (underlined in Fig 1A). The amino acid sequence of human MD-1 has 66% and 38% identity to mouse and chicken MD-1, respectively (Fig 1B). Six of seven cysteine residues in the mature human MD-1 peptide are conserved in all three species. Northern hybridization showed that the MD-1 transcript is about 1 kb and demonstrable in all four B-cell lines (Nalm-6, Ramos, Daudi, and RPMI8866) and a monocyte line U937, but not in two T-cell lines (CEM and Molt-4) or an erythroleukemia line K562 (Fig 2).
A nucleotide sequence of human MD-1 and alignment of its amino acid sequence with mouse and chicken MD-1. (A) The nucleotide sequence of human MD-1 is shown. A poly(A) signal is underlined. An encoded amino acid sequence is also shown. A signal sequence and two canonical N-glycosylation sites are underlined. (B) Amino acid alignment of human, mouse, and chicken MD-1. Identical residues in mouse and chicken MD-1 are denoted by (−). Five gaps are introduced in chicken to optimize alignment and denoted by blanks.
A nucleotide sequence of human MD-1 and alignment of its amino acid sequence with mouse and chicken MD-1. (A) The nucleotide sequence of human MD-1 is shown. A poly(A) signal is underlined. An encoded amino acid sequence is also shown. A signal sequence and two canonical N-glycosylation sites are underlined. (B) Amino acid alignment of human, mouse, and chicken MD-1. Identical residues in mouse and chicken MD-1 are denoted by (−). Five gaps are introduced in chicken to optimize alignment and denoted by blanks.
Northern hybridization of human MD-1. Total RNA from human cell lines were electrophoresed, blotted, and hybridized with the human MD-1 probe. An approximately 1-kb signal is apparent and indicated as huMD-1. The same blot was reprobed for GAPDH and is shown below.
Northern hybridization of human MD-1. Total RNA from human cell lines were electrophoresed, blotted, and hybridized with the human MD-1 probe. An approximately 1-kb signal is apparent and indicated as huMD-1. The same blot was reprobed for GAPDH and is shown below.
Establishment of an antihuman RP105 MoAb, MHR73.
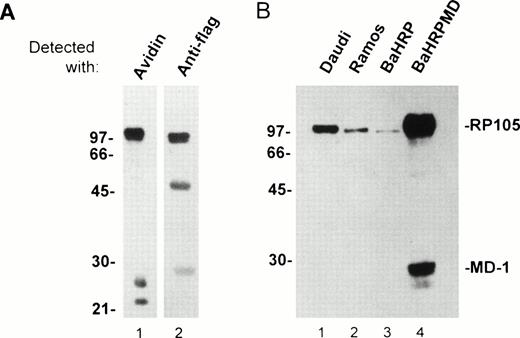
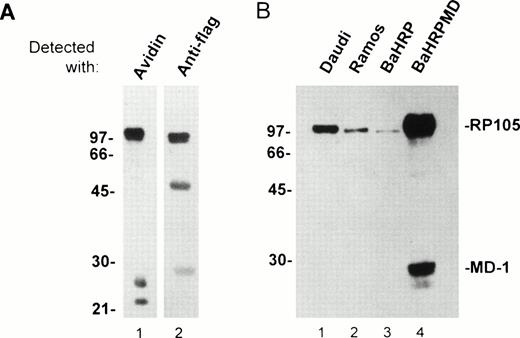
An anti-RP105 MoAb is requisite to study expression/function of human RP105 and its association with MD-1. We immunized BALB/c mice with a mouse B-cell line M12HRP that expresses human RP105 complexed with mouse MD-1 (Fig 3A and see below). Immunized spleen cells were fused with the SP2/0 myeloma. Supernatant from resultant hybridoma cells were used for staining of 293T cells that had been transiently transfected with vectors encoding human RP105 and mouse MD-1. Positive Abs were further characterized by staining human B-cell lymphomas. One MoAb was finally chosen and referred to as MHR73. The MHR73 MoAb reacted with M12HRP cells but not with the original line M12. M12HRP was used for cell surface biotinylation and immunoprecipitation. MHR73 precipitated three distinct antigens of 97, 25, and 22 kD (Fig 3A1). Introduced human RP105 had been tagged with the flag epitope. Human RP105 corresponds to the 97-kD species, because it bore the flag epitope (Fig 3A2). The smaller species are likely to be mouse MD-1, because similar signals were coprecipitated with endogenous mouse RP105 and identified as mouse MD-1.10 The MoAb bound to two human B-cell lines, Ramos and Daudi. The size of the target antigen on these cell lines was similar to ectopically expressed human RP105 on M12 cells (Fig 3B1 and 3B2). Thus, MHR73 is an MoAb against human RP105.
The MHR73 MoAb recognizes RP105. (A) Cell surface proteins on M12HRP cells were biotinylated. After extraction with a lysis buffer, immunoprecipitation was conducted with the MHR73 MoAb. Precipitated proteins were subjected to SDS-PAGE (12% acrylamide, reduced condition) and blotted onto a nitrocellulose membrane. Blotted proteins were detected with either avidin horseradish peroxides (for cell surface molecules: lane 1) or an anti-flag MoAb followed by goat antimouse IgG-horseradish peroxidase (for human RP105 that bears the flag epitope at the carboxy-terminus: lane 2). Signals of about 50 and 28 kD are observed in lane 2. They correspond to the heavy chain and the light chain of the MHR73 MoAb, because they were detected with goat antimouse IgG-horseradish peroxidase alone (data not shown). (B) Indicated cell lines were subject to cell surface biotinylation. Cell surface molecules were then precipitated with either the MHR73 MoAb (lanes 1, 2, and 4) or the anti-flag MoAb (lane 3). Precipitated molecules were resolved on SDS-PAGE, blotted on an nitrocellulose membrane, and detected with avidin-horseradish peroxidase. The MD-1 signal was very faint in Daudi or Ramos cells in this experiment. However, we confirmed additional signals of 25 and 22 kD in other experiments (data not shown).
The MHR73 MoAb recognizes RP105. (A) Cell surface proteins on M12HRP cells were biotinylated. After extraction with a lysis buffer, immunoprecipitation was conducted with the MHR73 MoAb. Precipitated proteins were subjected to SDS-PAGE (12% acrylamide, reduced condition) and blotted onto a nitrocellulose membrane. Blotted proteins were detected with either avidin horseradish peroxides (for cell surface molecules: lane 1) or an anti-flag MoAb followed by goat antimouse IgG-horseradish peroxidase (for human RP105 that bears the flag epitope at the carboxy-terminus: lane 2). Signals of about 50 and 28 kD are observed in lane 2. They correspond to the heavy chain and the light chain of the MHR73 MoAb, because they were detected with goat antimouse IgG-horseradish peroxidase alone (data not shown). (B) Indicated cell lines were subject to cell surface biotinylation. Cell surface molecules were then precipitated with either the MHR73 MoAb (lanes 1, 2, and 4) or the anti-flag MoAb (lane 3). Precipitated molecules were resolved on SDS-PAGE, blotted on an nitrocellulose membrane, and detected with avidin-horseradish peroxidase. The MD-1 signal was very faint in Daudi or Ramos cells in this experiment. However, we confirmed additional signals of 25 and 22 kD in other experiments (data not shown).
Human MD-1 positively regulates cell surface expression of human RP105.
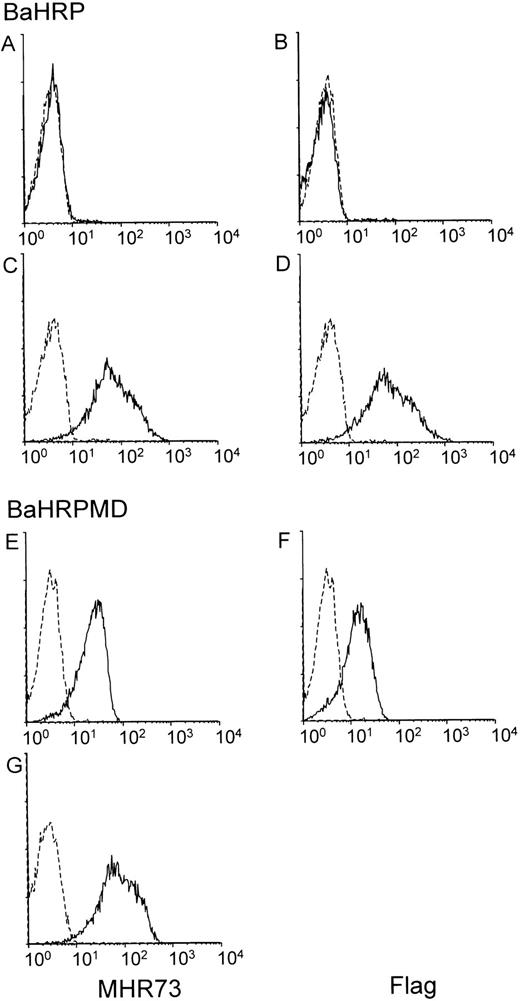
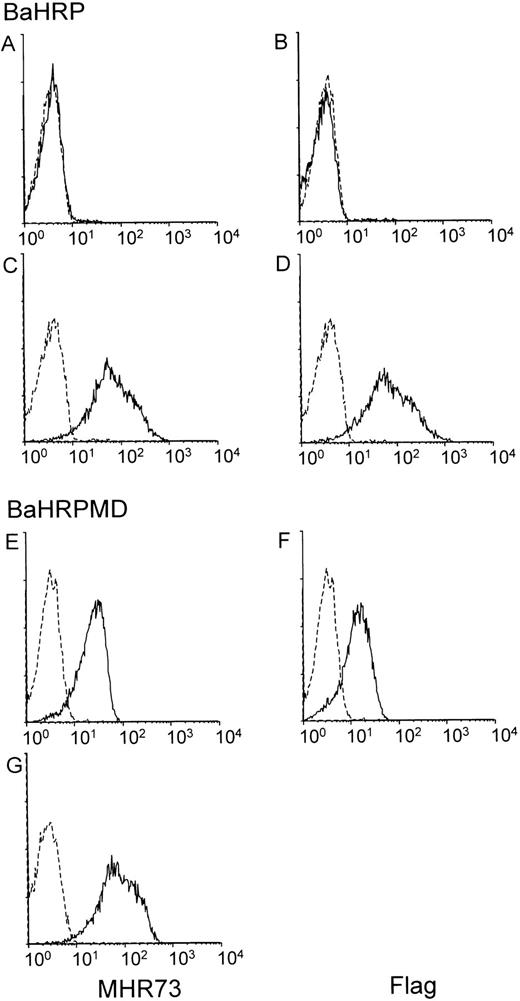
The BaHRP line was established by transfection of the mouse IL-3–dependent line Ba/F3 with a vector encoding human RP105. Ba/F3 cells do not express either mouse RP105 or MD-1 (data not shown). Introduced RP105 had been tagged with the flag epitope at the carboxyl terminus. Expression of human RP105 in BaHRP cells was therefore confirmed by immunoprecipitation and subsequent probing with the anti-flag MoAb (data not shown). However, MHR73 MoAb did not react with BaHRP cells (Fig 4A), and only a faint signal was obtained with cell surface biotinylation and immunoprecipitation with the anti-flag MoAb (Fig 3B3). We assumed that the majority of human RP105 proteins did not come out but stayed inside the cell. To see intracellular proteins, BaHRP cells were permeabilized with saponin detergent and stained with MHR73 or the anti-flag MoAb. Either antibody was able to demonstrate human RP105 inside the BaHRP line (Fig 4C and D). Therefore, the majority of human RP105 is located inside the cell. Another BaHRP line also had most RP105 molecules inside the cell (data not shown). The human RP105 protein expressed alone seemed to have difficulty in reaching the cell surface (see Discussion).
MD-1 has an important role in cell surface expression of human RP105. Two IL-3–dependent lines, BaHRP (A through D) and BaHRPMD (E through G), were stained with either the anti-RP105 MoAb MHR73 (the left column) or the anti-flag MoAb M2 (the right column). Cell permeabilization was conducted in (C), (D), and (G). RP105 and MD-1 were tagged with the flag epitope at carboxy-terminus. Carboxy-temini of RP105 and MD-1 are located inside or outside cells, respectively. Therefore, the anti-flag MoAb detects MD-1 but not RP105 in cell surface staining of BaHRPMD cells (F).
MD-1 has an important role in cell surface expression of human RP105. Two IL-3–dependent lines, BaHRP (A through D) and BaHRPMD (E through G), were stained with either the anti-RP105 MoAb MHR73 (the left column) or the anti-flag MoAb M2 (the right column). Cell permeabilization was conducted in (C), (D), and (G). RP105 and MD-1 were tagged with the flag epitope at carboxy-terminus. Carboxy-temini of RP105 and MD-1 are located inside or outside cells, respectively. Therefore, the anti-flag MoAb detects MD-1 but not RP105 in cell surface staining of BaHRPMD cells (F).
The BaHRP line is the mouse IL-3–dependent line that ectopically expresses human RP105 (see Materials and Methods). The BaHRPMD line was then established by further transfection of BaHRP cells with an expression vector encoding the human MD-1 cDNA. The MD-1 cDNA was tagged with the flag epitope. The anti-flag MoAb is therefore able to detect MD-1. Human MD-1 does not have a transmembrane portion (Fig 1). Nevertheless, it was expressed on a cell surface (Fig 4F). RP105 also appeared on the cell surface (Fig 4E). Cell surface biotinylation and subsequent immunoprecipitation with MHR73 showed a signal of about 30 kD as well as human RP105 (Fig 3B4). Immunoprobing with the anti-flag MoAb showed that the 30-kD species bore the flag epitope (data not shown). Human MD-1 is therefore associated with human RP105. Ectopically expressed MD-1 was bigger than endogenous MD-1 that was about 22 or 25 kD. A similar change in size between ectopic and endogenous MD-1 also occurred to the mouse homologue.10This may stem from a difference in posttranslational modification such as glycosylation.
Introduction of MD-1 complemented cell surface expression of RP105 (Fig4A and E) in the BaHRPMD line. Moreover, we conducted transient transfection into a human kidney line 293T cells with vectors encoding human RP105 or human MD-1. Cell surface staining with MHR73 showed that human RP105 did not appear on a cell surface when human RP105 was transfected alone, but did when human MD-1 was coexpressed (data not shown). Thus, MD-1 has an important role in cell surface expression of RP105.
Expression of human RP105.
Expression of RP105 was studied with flow cytometry staining. Results are summarized in Table 1. Among cell lines studied, only two B-cell lines (Ramos and Daudi) were positive. Negative cell lines include two B-cell lines (Nalm-6 and RPMI8866), a monocyte line (U937), an erythroleukemia line (K562), and two T-cell lines (CEM and Molt-4). Results with Ramos and Daudi were consistent with that of northern hybridization (Fig 2 and Table 1). However, those with Nalm-6 and U937 were not. They express the transcripts of RP105 and MD-1, but RP105 was not expressed on cell surfaces. RPMI8866 had only MD-1 mRNA.
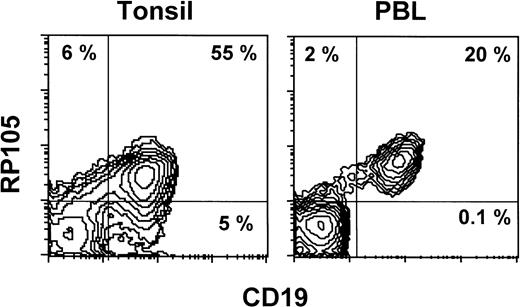
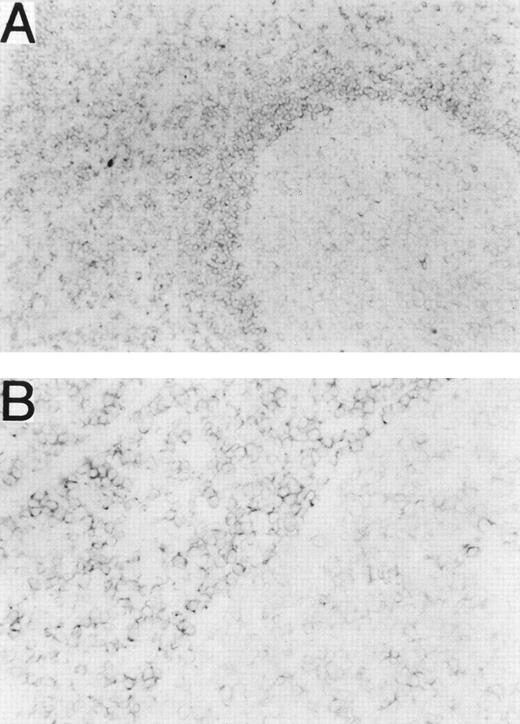
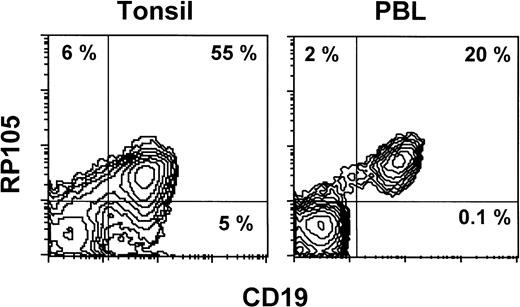
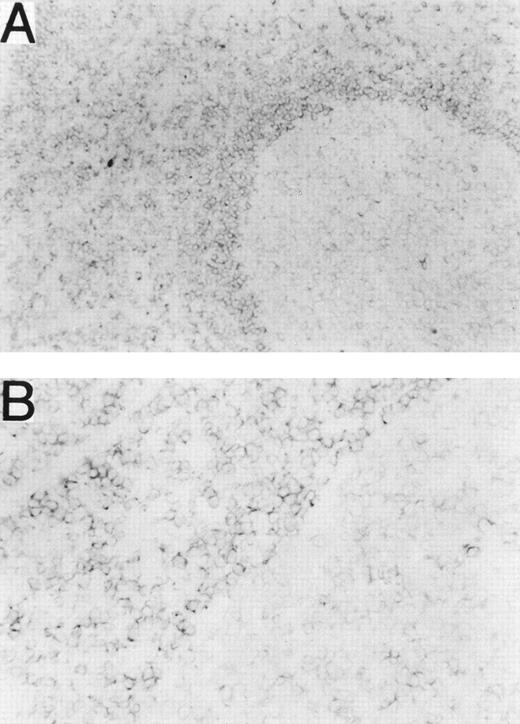
Normal leukocytes from peripheral blood and tonsils were also stained with MHR73. RP105 was restricted to CD19+ B cells (Fig 5). Interestingly, all B cells in peripheral blood are positive, whereas some tonsillar B cells were negative. Percentages of RP105− B cells in whole B cells were variable from 10% to 30%. To see RP105−B cells in more detail, sections of a normal tonsil were stained with MHR73 (Fig 6). RP105 was mainly expressed on B cells in mantle zones. Cells in germinal centers were either dull or negative. Considering that recirculating B cells reside in follicles and mantle zones, the result of section staining is consistent with that of flow cytometry. B cells in follicles and mantle zones correspond to mature B cells. RP105 is thus a B-cell marker that is preferentially expressed on mature B cells.
RP105 expression is restricted to CD19+ B cells. Leukocytes from peripheral blood or a tonsil were stained with the FITC-labeled antibody against CD19 and biotinylated MHR73 followed by avidin-phycoerythrin. Stained cells were analyzed on a FACScan.
RP105 expression is restricted to CD19+ B cells. Leukocytes from peripheral blood or a tonsil were stained with the FITC-labeled antibody against CD19 and biotinylated MHR73 followed by avidin-phycoerythrin. Stained cells were analyzed on a FACScan.
RP105 is mainly expressed on B cells in mantle zones. Frozen sections from a human tonsil were stained with the MHR73 MoAb. (A) RP105+ B cells are mainly located in a mantle zone that surrounds a germinal center where expression of RP105 is dull or negative (original magnification × 50). (B) Higher magnification (original magnification × 200) of a boundary between the mantle zone and the germinal center of (A). No significant signal was observed by staining with the second reagent alone.
RP105 is mainly expressed on B cells in mantle zones. Frozen sections from a human tonsil were stained with the MHR73 MoAb. (A) RP105+ B cells are mainly located in a mantle zone that surrounds a germinal center where expression of RP105 is dull or negative (original magnification × 50). (B) Higher magnification (original magnification × 200) of a boundary between the mantle zone and the germinal center of (A). No significant signal was observed by staining with the second reagent alone.
Human RP105 transmits an activation signal.
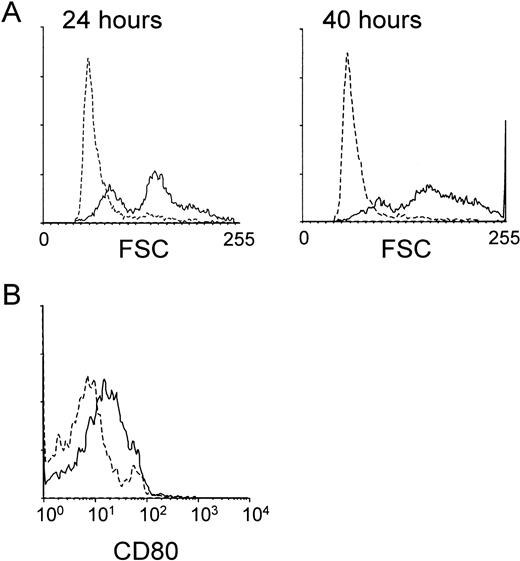
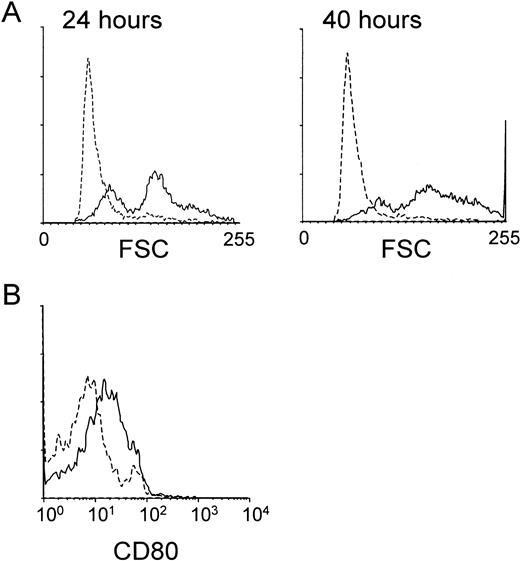
We next studied whether the MHR73 MoAb was able to induce B-cell activation. Peripheral blood mononuclear leukocytes were cultured in the presence or absence of MHR73. CD19+ B cells were examined about changes in cell size and expression of a costimulatory molecule CD80. An increase in cell size, measured by forward scatter with flow cytometry, was apparent as early as 24 hours after incubation had started (Fig 7A). After 40 hours, the change in cell size became more remarkable, and a costimulatory molecule CD80 was significantly induced (Fig 7). B cells were enriched from tonsils and cultured with MHR73 for 3 to 4 days. An increase in DNA synthesis was apparent (Table 2). With peripheral blood leukocytes, a similar increase in DNA synthesis was observed after 3 to 4 days of culture with MHR73 (data not shown). Thus, B cells were activated in response to cross-linking of human RP105.
The anti-RP105 MoAb induces B cells to increase in cell size and expression of a costimulatory molecule CD80. Peripheral blood mononuclear cells were collected by Ficoll-Hypaque density gradient centrifugation and cultured in a 24-well plate at 2 × 106cells/well with or without the anti-MHR73 MoAb (20 μg/mL). After 24 hours (the left panel of A) or 40 hours (the right panel of A and B), cells were harvested and stained with FITC-labeled anti-CD19 MoAb and biotinylated anti-CD80 MoAb followed by avidin-phycoerythrin. Stained cells were analyzed on a FACScan. Only CD19+ B cells were examined, and profiles of forward scatter (A) or CD80 expression (B) are shown. Solid lines and hatched lines depict histograms of cells cultured with or without the MHR73 MoAb, respectively.
The anti-RP105 MoAb induces B cells to increase in cell size and expression of a costimulatory molecule CD80. Peripheral blood mononuclear cells were collected by Ficoll-Hypaque density gradient centrifugation and cultured in a 24-well plate at 2 × 106cells/well with or without the anti-MHR73 MoAb (20 μg/mL). After 24 hours (the left panel of A) or 40 hours (the right panel of A and B), cells were harvested and stained with FITC-labeled anti-CD19 MoAb and biotinylated anti-CD80 MoAb followed by avidin-phycoerythrin. Stained cells were analyzed on a FACScan. Only CD19+ B cells were examined, and profiles of forward scatter (A) or CD80 expression (B) are shown. Solid lines and hatched lines depict histograms of cells cultured with or without the MHR73 MoAb, respectively.
DISCUSSION
Human MD-1, a molecule interacting with RP105, was isolated in the present study. Amino acid sequence showed 66% and 38% identity to mouse and chicken MD-1, respectively. Mouse MD-1 binds to human RP105 ectopically expressed on mouse M12HRP cells. On the other hand, human MD-1 was associated with mouse RP105 transiently expressed on 293 T cells (Miyake et al, unpublished observation). Interaction of RP105 and MD-1 is therefore well conserved between humans and mice and works easily beyond the species barrier.
MD-1 plays an important role in cell surface expression of RP105. Without MD-1, the majority of human RP105 was held inside the cell (Fig4A and C). RP105 alone may have difficulty in intracellular maturation or traffic to cell surfaces. In this regard, it has to be noted that RP105 without MD-1 seemed to be smaller and less heterogeneous than RP105 with MD-1. We compared RP105 molecules in BaHPRMD or BaHRP cells by immunoprecipitation and subsequent probing with the anti-flag MoAb (Miyake et al, unpublished observation). Two closely located signals were obtained from BaHRPMD cells that expressed RP105 and MD-1. The bigger one appeared to be cell surface RP105, because it was similar in size to the RP105 signal obtained by cell surface biotinylation (Fig 3B4). The other, smaller and less heterogeneous signal is likely to be intracellular precursor of RP105. Smaller size and lesser heterogeneity of intracellular RP105 would stem from immature glycosylation. Only the smaller signal was apparent from BaHRP cells that expressed RP105 but not MD-1. In the absence of MD-1, the RP105 protein seems to be held before or during maturation steps.
It remains to be clarified how MD-1 contributes to posttranslational modification and subsequent cell surface expression of RP105. One possibility is that RP105 and MD-1 is associated before maturation and the association itself is important for maturation and subsequent cell surface expression. Association of MD-1 might confer more stable conformation on the RP105 molecule. RP105 alone could be unstable and easily degraded. Further studies are under way to seeking for differences between RP105 alone and RP105 associated with MD-1.
The MHR73 was able to recognize intracellular and immature RP105 in the absence of MD-1 (Fig 4C). The epitope of MHR73 is therefore not dependent on either glycosylation or association of MD-1, but on the protein backbone.
It is important to know expression of MD-1 as well as RP105, because cell surface expression of RP105 is dependent on MD-1 (Fig 4). Distribution of MD-1 mRNA seems to be broader than that of RP105. The RP105 transcript was previously observed in four lines: Nalm-6, Ramos, Daudi, and U937.12 All of them express the MD-1 transcript as well (Fig 2). A B-cell line RPMI8866 expresses MD-1 but not RP105 mRNA (Fig 2 and Table 1). Moreover, we found two additional EST clones similar to MD-1 from human fetal brain or lung, where RP105 would not be present. Also, in mouse MD-1, the transcript was observed in liver and brain as well as in lymphoid organs.10 In the absence of RP105, eg, in liver or brain, MD-1 can be secreted or might be associated with another LRR molecule. MD-1 may have additional roles outside the immune system. The anti–MD-1 MoAb is needed to see localization of MD-1 and association with other LRR molecules.
Recirculating B cells have a bigger chance of encountering a pathogen and contributing to an inductive phase of B-cell responses than sessile B cells. It is reasonable that recirculating B cells express molecules that receive a signal from the innate immune system, a system that has an important role in the development of an antibody response.16 For example, CD21 is preferentially expressed on mature B cells. It is a receptor for the C3d fragment of the complement system that belongs to innate immunity. In concert with CD19, CD21 augments B-cell responses against an antigen to which the C3d fragment attaches.17 Thus, CD21 assists communication of B cells with the innate immune system and helps B cells to mount antibody production. In the present study, we showed that RP105 is expressed on mature and recirculating B cells and that RP105 does transmit an activation signal leading to increases in cell size and DNA synthesis and to induction of CD80 expression. Induced CD80 would help B cells to interact with T cells and accomplish further activation. RP105 may also contribute to the inductive phase of B-cell activation in response to innate immunity. This hypothesis is supported by other LRR molecules in the immune system. CD14 is an lipopolysaccharide (LPS) receptor and activates macrophages.18Activated macrophages then activate lymphocytes by secreting cytokines or expressing costimulatory molecules. The Drosophila Toll receptor and tomato disease resistance gene cf-2 and cf-9 are requisite for eliciting defense responses against fungal pathogens.2-5 A human homologue of the Toll receptor is expressed on macrophages or dendritic cells and is expected to activate them in responses against pathogens.6 These LRR molecules are thought to augment innate immunity and to mount adaptive immune responses. RP105 may belong to these LRR molecules in terms of a role in the immune system as well as of molecular structure. A ligand for RP105 might be derived from a pathogen or a molecule produced in cells working in innate immunity.
Another expectation of a role of RP105 comes from our previous study in mice.9 B cells activated with anti-RP105 MoAb showed growth arrest and apoptosis in response to a signal through the antigen receptor. This result suggests that RP105 might be involved in regulation of an antigen-induced B-cell death. The antigen-induced death of mature lymphocytes is thought to contribute to establishment or maintenance of peripheral B-cell tolerance and to termination of B-cell responses by downsizing activated B-cell clones.19Loss of function or malfunction of RP105 could lead to augmented B-cell responses and autoimmune diseases. The MHR73 MoAb opens a human study of RP105. It is interesting to study B cells from patients suffering from recurrent infections or autoimmune diseases. Abnormalities of B cells may be shown with staining or stimulation with MHR73. These studies would complement results from mouse studies.
Y.M. and R.S. contributed equally to this study.
Supported by grants from the Ministry of Education, Science, and Culture of Japan; the Osaka Cancer Research Foundation; the Ichiro Kanehara Foundation; the Ryoichi Naito Foundation for Medical Research; and Ciba-Geygy Foundation (Japan) for the Promotion of Science.
Sequence data from this article have been deposited with the DDBJ, EMBL, and GenBank Data Libraries under Accession No. AF05718.
Address reprint requests to Kensuke Miyake, MD, PhD, Department of Immunology, Saga Medical School, Nabeshima, Saga 849-8501, Japan; email: miyake@post.saga-med.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.