Abstract
Group I Burkitt lymphoma (BL) cell lines (L3055, Elijah, Louckes, BL2, and BL29) retaining the original biopsy phenotype were found to undergo prolonged phenotypic, functional, and molecular change on short-term exposure to soluble recombinant CD40L trimer. Sensitivity to, extent of, and duration of change appeared to reflect passage number in that the earliest passaged lines, L3055 and BL29, were generally the most susceptible. Culture of group I BL lines with CD40L resulted in significant growth arrest (without apoptosis) that, for L3055 cells, was sustained for 7 to 9 days after 72 hours of exposure. This was accompanied by the formation of large homotypic aggregates together with gross changes in individual cell morphology and a concomitant upregulation of CD54 (ICAM-1). Three of the five group I BL lines exhibited rapid downregulation of the hallmark CD77 surface antigen, which, for L3055 cells, was maintained for at least 12 days after 72 hours of incubation with CD40L. With the exception of BL2, all group I BL lines were induced to express CD40 homodimers on CD40-stimulation, whereas only monomers were detected in unstimulated cells. Experiments using CD40-transfected Rat-1 fibroblasts showed that the ability to signal for dimer formation required Thr234of CD40. For L3055 and BL29 cells, an initial 72 hours of exposure to CD40L resulted in the maintenance of homodimers for up to 14 and 10 days, respectively. There was a close correlation between the induction and duration of CD40 homodimers and the appearance of Bcl-2. For L3055 cells, which are sensitive to apoptosis-induction on BCR-engagement, exposure to CD40L for 72 hours was found to provide considerable protection from anti-IgM, which was still significant to 20 days. The implications of such sustained effects on relatively short-term exposure of tumor B cells to CD40L are discussed.
© 1998 by The American Society of Hematology.
BURKITT LYMPHOMA (BL) cell lines have been established from biopsy material of patients from both endemic and nonendemic regions. Epstein-Barr virus (EBV) is invariably associated with the disease in endemic areas but is harbored by the cells in only a minority of cases from nonendemic regions.1 Depending on the expression of specific latent EBV genes, established BL lines display a heterogeneous phenotype. Only EBNA1 protein can be detected in recently established, biopsy-like BL lines, whereas EBNAs 1 and 2 and LMP1 and 2 can often be detected in long-term EBV+ve BL lines that have undergone genotypic drift in culture.2,3Retention of the original biopsy-like phenotype in EBV−ve lines or in EBV+ve lines that have remained faithful to the starting genotype is identified from the following expression of cell surface markers: CD10+, CD77+, CD23−, CD39−, CD44−, LFA-1−, LFA-3−, and ICAM-1−. This profile is remarkably similar to that associated with normal B cells found in germinal centers (GC), and these lines are categorized as group I.2 Where genotypic drift occurs, there is a corresponding change in cell surface markers with the acquisition of a lymphoblastoid-like phenotype characterized as CD10−, CD77−, CD23+, CD39+, CD44+, LFA-1+, LFA-3+, and ICAM-1+. BL lines with fully blown lymphoblastoid characteristics are termed group III.2
In addition to expressing the full complement of EBV latent genes and the surface marker changes, group III lines, unlike group I lines (or normal GC B cells), express the antiapoptotic proto-oncogenebcl-2.3 Either by comparing group I with group III lines directly or by introducing bcl-2 into group I cells by gene transfection, it has been shown that the Bcl-2 protein can afford substantial protection of BL cells from a variety of otherwise apoptotic stimuli. Particularly for early passaged group I lines, the most physiological of these apoptotic triggers is provided by the cross-linking of sIgM with anti-IgM antibodies.3-5Stimulation through CD40 with either anti-CD40 monoclonal antibody (MoAb) or with CD40L protects, at least in the short-term, both group I BL cells and GC B cells from apoptosis.5 For both cell types, CD40-mediated protection from apoptosis can be independent of Bcl-2 induction as long as cells can be persuaded to remain in active cell-cycle. It has been argued that, once out-of-cycle, Bcl-2 expression becomes a prerequisite to continued survival.5
CD40, a member of the tumor necrosis factor receptor (TNF-R) superfamily, appears to play a central role in B-cell responses to TD antigens impinging on B-cell activation, growth, differentiation, isotype-switching, and survival at a number of key stages.6 It shares extracellular domain homology with other members of the TNF-R family, containing four homologous cysteine-rich extracellular domains. Engagement of CD40 by CD40L expressed on the surface of activated CD4+ T cells is believed to result in receptor trimerization and is thought to be a prerequisite event leading to signal transduction. Interestingly, preformed CD40 homodimers have been observed on a number of B-cell types, including BL Raji cells and normal B cells, although they appear absent on carcinoma lines or EBV-transformed cells.7 Although no function has as yet been assigned to the CD40 dimers, it could be envisaged that, given the presumed importance of receptor oligomerization, they may serve to modify signaling requirements or outcomes on cells where they are found.
Previous investigations into the consequences of engaging CD40 on BL cells in vitro have tended to concentrate solely on the maintenance of viability when the cells are confronted with apoptotic signals and, moreover, have usually looked only at effects operative in the short-term (<72 hours).8 Somewhat paradoxically, in contrast to the well-documented antiapoptotic actions observed in vitro, studies on human B-cell lymphomas developing in SCID mice have indicated that engagement of CD40 can provide protection from tumor development.9 In light of these results, we felt that a more detailed analysis of the response of group I BL cells to CD40L was warranted, with an examination of both short-term and long-term influences of CD40-ligation on morphology, phenotype, growth characteristics, and protection from apoptosis-induction. Corresponding analysis on the state of CD40 receptor dimerization together with the dynamics of Bcl-2 expression was also performed. Given the reported ability of the TH2-derived cytokine interleukin-4 (IL-4) to augment CD40-dependent change at several stages of normal B-cell development,10-13 comparisons were made in the responses of group I BL cells when confronted with CD40L both in the presence and absence of this cytokine.
MATERIALS AND METHODS
Cell lines, antibodies, and reagents.
BL cell lines L3055 (EBV−ve), Louckes (EBV−ve), BL2 (EBV−ve), Elijah (EBV+ve), and BL29 (EBV+ve) expressing a group I phenotype, as previously described by Rowe et al,14-16were used in this study. L3055 cells were from early passage, defined as cells that had not exceeded an in vitro passage number of 60 from initial establishment of the line from BL-biopsy tissue. Reference to late passage cells, defined as lines that had exceeded an in vitro passage number of 100, applies to the four other BL lines in this study. Late passage group I BL cells had different passage numbers, with the lowest being BL29 < BL2 < Elijah < Louckes. All BL cell lines were assessed by fluorescence-activated cell sorting (FACS) analysis before study with a panel of group I BL cell markers to ensure that cells retained the original phenotype.14-17 Cells were maintained in continuous culture with RPMI 1640 supplemented with 10% prescreened fetal calf serum (FCS; Bio-Whittacker, Wokingham, UK), 5,000 IU/mL penicillin, 5 mg/mL streptomycin (GIBCO/BRL, Paisley, UK), and 200 mmol/L glutamine (GIBCO/BRL). Rat-1 fibroblasts transfected with wild-type, full-length human CD40 were as previously described.18 In addition, Rat-I cells containing a Thr(234)-Ala mutation of CD40 were also generated. Briefly, this substitution was introduced by site-directed mutagenesis using the QuickChange site-directed mutagenesis kit of Stratagene (Cambridge, UK) and the mutated oligonucleotide primer 5′-CAC TGC TGC TCC AGT GCA GGA GGC TTT ACA TGG ATG-3′ and its complimentary oligo. The presence of the above mutation was verified by sequencing. Recombinant human IL-4 was purchased from R&D Systems Ltd (Oxford, UK). Recombinant soluble trimeric CD40 ligand was generated as described previously.8
The MoAbs used for phenotypic studies were obtained from the following sources: phycoerythrin (PE)- and fluorescein (FITC)-conjugated negative control isotype-matched Ab of irrelevant specificity, FITC-conjugated anti-CD10, anti-CD23, anti-IgM, and PE-conjugated anti-CD20 were all purchased from Dako Ltd (High Wycombe, UK). FITC-conjugated anti-CD40 EA5 MoAb and PE-conjugated anti-CD38 were purchased from Serotec and Becton Dickinson (Cowley, UK), respectively. Ascitic fluid containing rat IgM anti-CD77 (38.13) MoAb was a gift from Dr J. Weils (Institut Gustav-Roussy, Villejuif, France). Mouse anti-CD44 (BU52), anti-CD11a (BU17), anti-CD11b (OKM-1), anti-CD11c (BU15), and anti-CD54 (BU74) MoAb were purified by diethyl aminoethyl (DEAE) anion exchange from hybridomas grown in the Department of Immunology, University of Birmingham (Birmingham, UK); these MoAbs were conjugated to FITC by standard proceedures.
Immunodetection of CD40 and Bcl-2.
Cells (1 × 107) were lysed in 0.2 mL of 1% Triton X-100 lysis buffer (25 mmol/L Tris, pH 7.6, 150 mmol/L NaCl, 1 mmol/L EDTA, 50 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 1 μg/mL aprotinin, and 1 μg/mL leupeptin). Protein concentrations of each sample were determined. After adding 40 μL of 5× gel sample buffer (500 mmol/L Tris [pH 6.8], 12% sodium dodecyl sulfate [SDS], 25% glycerol, 5 μmol/L EDTA, 0.01% bromophenol blue) supplemented with 10% 2-mercaptoethanol for Bcl-2 lysates, the samples were boiled for 5 minutes. Samples were fractionated after loading equal protein concentrations onto 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) for CD40 blots and 12% SDS-PAGE for Bcl-2 blots. Proteins were transferred to Immobilon P membranes (Millipore, Watford, UK) using standard techniques, and specific proteins were detected using either mouse antihuman-Bcl-2 MoAb (100; Santa Cruz, Santa Cruz, CA) or mouse antihuman-CD40 MoAb (G28.5). Immunoblots were visualized using the ECL kit (Amersham International, Amersham, UK) according to the manufacturer’s instructions.
Measurement of apoptosis.
Cell samples (4 × 105 cells) were cytocentrifuged in duplicate onto glass slides at 250 rpm for 3 minutes. After air-drying and methanol-fixing, the slides were stained using the Romanoski method.19 Briefly, slides were stained in 1/3 dilution of Jenners (BDH/Merck, Lutterworth, UK) solution followed by 1/10 dilution of Giemsa (BDH/Merck) solution. Slides were air-dried and mounted using DPX mountant (BDH/Merck). Slides were counted blind, and cells were categorized according to apoptotic, viable, or lysed (late apoptotic/necrotic) morphology.
DNA synthesis.
DNA synthesis in 105 cells/200 μL was determined by the incorporation of [3H]thymidine ([3H]Thy; Amersham) at 1 μCi/50 μL of culture medium/well for 5 hours after 24 hours of culture. Samples were cultured and assayed in triplicate before harvesting using a Skatron cell harvester (Skatron, Newmarket, UK).
Homotypic adhesion.
Cells were cultured in 24-well plates under the conditions indicated in the text. Cell clumping was assessed under an inverted microscope (Olympus LK2; Olympus, London, UK), and photographs were taken using a mounted Nikon F302 camera (Nikon, Kingston-upon-Thames, UK) at the time points indicated in the text.
Analysis of B-cell surface phenotype.
Cells were harvested after culture under the conditions indicated in the text. Cells were washed once in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA) and 0.01% sodium azide (FACS buffer) before direct or indirect immunofluorescence analysis was performed. Briefly, cells were labeled by 30 minutes of incubation on ice with either MoAb specific for cell surface markers conjugated directly to fluorochromes or by an unconjugated MoAb that was detected by an additional second-stage incubation with fluorochrome-conjugated antimouse Ig Ab. Cells labeled either directly or indirectly were washed once and resuspended in FACS buffer. Flow cytometry data were obtained using a FACScan flow cytometer (Beckton Dickinson, Mountain View, CA). Viable cells were gated according to forward (FSC) and side (SSC) light scatter settings. Subsequent data were processed and analyzed using the Cell Quest program (Becton Dickinson).
RESULTS
Establishment of CD40L dose-response kinetics for phenotypic and functional change in group I BL cells.
First of all, the dose-response kinetics on the influence of soluble CD40L trimer on three of the basic parameters analyzed in this study was established for the EBV−ve L3055 group I BL cell line: (1) the induction of homotypic adhesions; (2) the induction of phenotypic change, assessed here by IL-4–dependent CD23 expression; and (3) the capacity to reverse anti-IgM (BU1)-mediated inhibition of [3H]-Thymidine incorporation, a measure of growth arrest that preludes surface IgM-driven apoptosis in these cells.8For the induction of all three parameters, a plateau effect was reached with 1 μg/mL of CD40L (Table 1) and this was the concentration chosen for all further experiments detailed in this study. It is worthy to note that the induction of homotypic adhesions was still being achieved, albeit suboptimally, with CD40L when present at levels as low as 0.008 μg/mL. By contrast, no significant change was observed in the other two parameters at levels less than 0.2 μg/mL of CD40L.
CD40L-mediated induction of homotypic adhesions in group I BL cells.
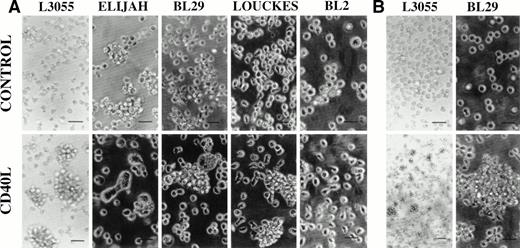
With the exception of BL2 and Elijah, large homotypic aggregates were observed in both early and late passage group I BL cells treated for 72 hours with CD40L (Fig 1A). CD40L-induced cell clustering was enhanced by the presence of IL-4 (20 ng/mL) in all three responding lines (data not detailed). Where, having washed out the stimuli at 72 hours, cells were maintained in culture for a total of 10 days, BL29 cells continued to display cell clusters comparable in size and frequency to those observed after the initial 72 hous of stimulation in the presence of CD40L, with or without IL-4 (Fig 1B). Although for L3055 cells the size of cell clusters was considerably reduced at 10 days, the cells were still forming large numbers of small loose cell aggregates that were absent in cultures that had not been precultured with CD40L (to effectively show small cell clusters, CD40L-treated L3055 cells are displayed at a lower magnification; Fig1B). By contrast, prestimulated Louckes cells at day 10 failed to show levels of cell clumping above those observed in untreated controls (data not detailed). It is worthy of note that, whereas the late passage group I BL cells Elijah, BL29, and Louckes displayed some background level of cell clustering, treatment with CD40L increased this level of clustering in all except the Elijah line (Fig 1A). Although Elijah cells failed to demonstrate cluster formation on culture with CD40L, they did respond to CD40-stimulation by forming large cells with pseudopodia-like extensions (Fig 1A). The formation of large cells could also be seen in BL29 cells, although these cells tended to extend fewer pseudopodia (Fig 1A). With the exception of Louckes, in which a small enhancement of aggregate formation was observed, culture with IL-4 alone failed to increase cell clustering in the group I BL lines (data not detailed).
Phase contrast photomicrographs of BL cell lines cultured at 37°C for 72 hours either alone (control) or with CD40L (1 μg/mL). Cell clumping was observed either after (A) the initial 72-hour treatment time or (B) after a total 10 days of culture, which included the initial 72-hour treatment. For all experiments in which cells were pretreated with reagents, cells at 5 × 105/mL received an initial culture with CD40L (with or without IL-4) for time stated and were then washed free of stimulants before replating at 5 × 105/mL in fresh culture medium in the absence of stimulants. The scale bar represents 50 μm.
Phase contrast photomicrographs of BL cell lines cultured at 37°C for 72 hours either alone (control) or with CD40L (1 μg/mL). Cell clumping was observed either after (A) the initial 72-hour treatment time or (B) after a total 10 days of culture, which included the initial 72-hour treatment. For all experiments in which cells were pretreated with reagents, cells at 5 × 105/mL received an initial culture with CD40L (with or without IL-4) for time stated and were then washed free of stimulants before replating at 5 × 105/mL in fresh culture medium in the absence of stimulants. The scale bar represents 50 μm.
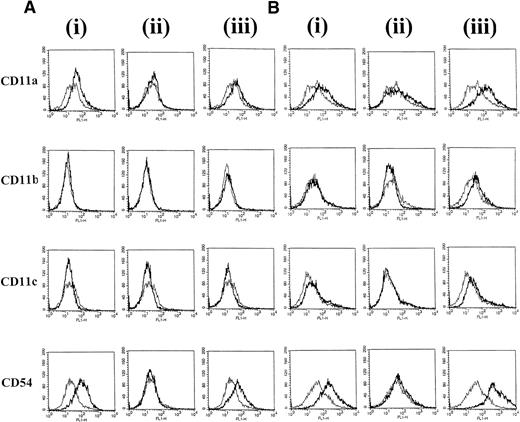
To identify surface molecules that may be involved in CD40-dependent clustering of group I BL cells, the expression of a panel of adhesion molecules was investigated on L3055 cells after the various treatments. At 24 hours, there was a slight increase in CD11a (LFA-1) and a substantial increase in CD54 (ICAM-1) expression on L3055 cells cultured with CD40L alone. The addition of IL-4 did not further enhance the CD40L-induced increase in expression of either of these molecules (Fig 2A). After 72 hours of stimulation with CD40L alone, no further large increases in CD11a or CD54 expression were observed above those seen at 24 hours (Fig 2B). L3055 cells stimulated with CD40L alone for either 24 or 72 hours failed to show increases in either CD11b (Mac-1) or CD11c (p150/95) expression compared with untreated control cells (Fig 2). However, after 72 hours of stimulation with CD40L in combination with IL-4, small but reproducible increases in CD11b and CD11c surface expression as well as large increases in CD11a and CD54 were observed (Fig 2B). Stimulation with IL-4 alone for either 24 or 72 hours did not induce any detectable changes in expression of all four adhesion molecules studied (Fig 2).
L3055 cells were stained with MoAb against adhesion molecules CD11a, b, c, and CD54 after (A) 24 hours or (B) 72 hours of treatment with (i) CD40L alone, (ii) IL-4 alone, or (iii) CD40L with IL-4. Untreated control cells are shown for comparison as a superimposed thin line on each histogram.
L3055 cells were stained with MoAb against adhesion molecules CD11a, b, c, and CD54 after (A) 24 hours or (B) 72 hours of treatment with (i) CD40L alone, (ii) IL-4 alone, or (iii) CD40L with IL-4. Untreated control cells are shown for comparison as a superimposed thin line on each histogram.
Phenotypic changes in group I BL cells stimulated with CD40L.
The capacity of CD40 to trigger phenotypic differentiation in early and late passage BL cell lines was investigated. Early and late passage group I BL cells were cultured for 72 hours with the stimulants indicated. Upon completion of the 72 hours of stimulation, cells were washed free of the factors and further maintained in culture for the periods indicated (which includes the initial 72-hour stimulation period) before removing aliquots for the analysis of cell surface phenotype. Preliminary experiments showed that, although CD40 stimulation induced a relatively early pattern of differentiation in group I BL cells, the effects were more pronounced after day 5 (including the 72-hour stimulation time); therefore, in this study, day 5 was chosen to show the immediate effects of CD40 on BL cell differentiation. Special attention is given to CD77, a hallmark group I surface marker that is lost when BL cells progress to a group III phenotype on long-term culture.2
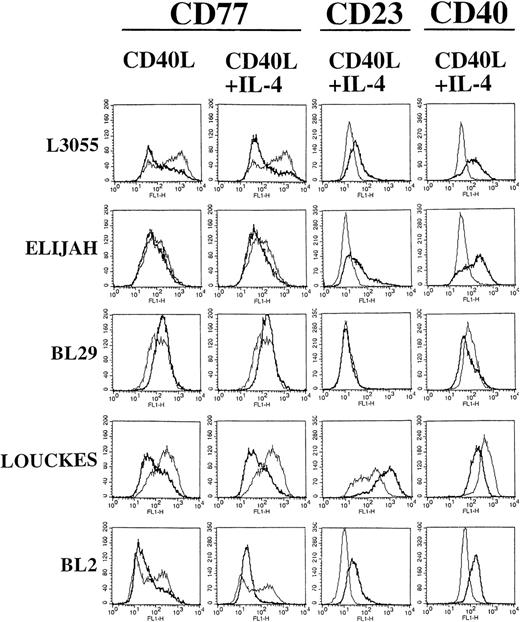
Figure 3 shows FACScan analysis of early passage L3055 cell surface phenotypes after 5 days (including the 72-hour preculture period). Every group I BL cell line tested in this study showed distinct populations of cells when observed on forward (FSC) and side (SSC) scatter dot plots (data not detailed). The large, nongranular cell populations represented viable cells, which were gated in every cell line before analysis of cell surface marker expression. Interestingly, group I BL cells stimulated with either CD40L alone or CD40L with IL-4, but not with IL-4 alone, showed increases in cell size and granularity that were detected by a shift in FSC and SSC dot plot profiles (data not detailed). In control cultures, a bimodal distribution of CD77 within the L3055 population with the presence of both CD77high and CD77low expressing cells was seen (Fig 3). Stimulation with CD40L induced a rapid downregulation in CD77 expression, resulting in the formation of a homogenous CD77low cell population (Fig 3). IL-4, although appearing to have no affect by itself (data not detailed), augmented the CD40-induced downregulation of CD77 expression (Fig 3). Two of the late passage BL lines, Louckes and BL2, also downregulated CD77 expression in response to stimulation through CD40 (Fig 3), with this effect again being enhanced by the presence of IL-4. Interestingly, for both Louckes and BL2 cells, culture with IL-4 alone could induce the downregulation of CD77 expression (data not detailed).
BL cells were stained with MoAb against CD77 after 72 hours of stimulation with CD40L alone. Additional staining with MoAb against CD77, CD23, and CD40 was performed after 72 hours of stimulation with CD40L in combination with IL-4. FACS analysis was performed after 5 days of culture (including the initial 72 hours of stimulation). Untreated control cells are shown for comparison as a superimposed thin line on each histogram.
BL cells were stained with MoAb against CD77 after 72 hours of stimulation with CD40L alone. Additional staining with MoAb against CD77, CD23, and CD40 was performed after 72 hours of stimulation with CD40L in combination with IL-4. FACS analysis was performed after 5 days of culture (including the initial 72 hours of stimulation). Untreated control cells are shown for comparison as a superimposed thin line on each histogram.
L3055 cells expressed a CD77low phenotype for at least 12 days after stimulation with CD40L either with or without IL-4 (data not detailed as day-12 FACScan histograms were essentially identical to those at day 5 as shown in Fig 3); indeed, CD77 expression remained low beyond the time scale of the experiment (>15 days). This finding is in contrast to BL2 and Louckes cells, which maintained low level CD77 expression for 5 days on a 72-hour prestimulation, but by day 12, CD77 expression had returned to normal or elevated levels, respectively (data not detailed). Changes in other cell surface markers were detected on L3055 cells by day 5, with a transient increase observed in the expression of CD40 itself after an initial 72 hours of culture with CD40L in combination with IL-4 (Fig 3). Small but reproducible increases in CD23 expression (a hallmark group III marker) were also observed on culture with CD40L, with or without IL-4 (Fig 3).
Elijah cells failed to display any changes in cell surface phenotype after culture with CD40L alone, at either 5 or 12 days with the initial 72 hours of stimulation (data not detailed). However, Elijah cells did display a transient 5-day increase in CD23 and CD40 expression when stimulated with CD40L in combination with IL-4 (Fig 3). These changes could not be detected at 12 days (data not detailed).
Louckes cells displayed a pattern of change in cell surface phenotype similar to that observed in L3055 cells (Fig 3), except that a more marked increase in CD23 expression was observed in cells treated with either CD40L alone (data not detailed) or CD40L in combination with IL-4 (Fig 3). Decreases (that returned to normal levels within 12 days) were also observed in the expression of CD40 when Louckes cells were treated with CD40L alone (data not detailed) or CD40L in combination with IL-4 (Fig 3). For BL2 cells, differences in surface CD40 expression were observed, depending on whether cells were previously stimulated with CD40L alone or CD40L in combination with IL-4. Culture with CD40L alone induced a large reduction in surface CD40 expression (data not detailed), whereas the combination of IL-4 and CD40L resulted in an increase in surface CD40 expression (Fig 3). Furthermore, stimulation with CD40L alone failed to induce any changes in CD23 expression, whereas culture with IL-4 and CD40L resulted in the increased surface expression of this molecule (Fig 3). The changes in BL2 cell phenotype mediated by either CD40L alone or CD40L in combination with IL-4 were also transient with levels returning to normal by day 12 (data not detailed).
Stimulation through CD40 induces growth arrest in group I BL cells.
Next, we assessed both the short-term and long-term influences of exposure to CD40L on DNA synthesis in group I BL lines. Culture with CD40L induced growth arrest in L3055 cells within 48 hours (day 2) when compared with untreated controls (Fig 4). The period for which L3055 cells then remained in growth arrest was dependent on the length of time the cells had been prestimulated with CD40L. Thus, L3055 cells stimulated through CD40 for 24 hours remained in growth arrest for a maximum of 5 days (Fig 4A); by extending the stimulation time to 72 hours, the duration of growth arrest was increased to 7 to 9 days (Fig 4B). Furthermore, cells stimulated with CD40L for 72 hours displayed a significant (P < .0001) decrease in DNA synthesis for up to 7 days compared with cells treated for just 24 hours (Fig 4). Reduction in cell proliferation was not accompanied by an increase in the rate of apoptosis occurring in these cultures as judged by examination of Romanowski-stained cytocentrifuge preparations (data not detailed).
L3055 cells were incubated with CD40L alone, IL-4 alone, or CD40L with IL-4 for either (A) 24 or (B) 72 hours. Aliquots were removed at the times indicated and cell growth was evaluated by incorporation of [3H]-Thy. Incorporation of [3H]-Thy for each treatment is expressed as a percentage of untreated control cultures. Data are presented as the mean with standard deviation from three separate experiments. The range of radioactivity incorporated in control (untreated) cultures for the three experiments was 7,698 to 11,218 cpm. Treatment with either CD40L alone or CD40L with IL-4 significantly (P < .0001) decreases proliferation of L3055 cells where indicated (*).
L3055 cells were incubated with CD40L alone, IL-4 alone, or CD40L with IL-4 for either (A) 24 or (B) 72 hours. Aliquots were removed at the times indicated and cell growth was evaluated by incorporation of [3H]-Thy. Incorporation of [3H]-Thy for each treatment is expressed as a percentage of untreated control cultures. Data are presented as the mean with standard deviation from three separate experiments. The range of radioactivity incorporated in control (untreated) cultures for the three experiments was 7,698 to 11,218 cpm. Treatment with either CD40L alone or CD40L with IL-4 significantly (P < .0001) decreases proliferation of L3055 cells where indicated (*).
For all four late passage BL lines, a significant (P < .0001) reduction in DNA synthesis was observed in response to stimulation through CD40 via CD40L (Fig 5). Cell proliferation was reduced within 24 hours (day 1) and with a 72-hour exposure remained lower than in untreated cells to 4 days; however, by day 7, proliferation had returned to normal levels (Fig 5). The addition of IL-4 to CD40L-stimulated cultures induced further significant (P < .0001) reductions in the DNA synthesis occurring in Elijah and Louckes cells at 24 hours, although this effect had disappeared in Louckes cells by day 4 (Fig 5). In contrast, for BL2 cells, the presence of IL-4 had a counteracting negative effect on growth arrest where, at day 4, DNA synthesis was significantly (P < .0001) higher in cells treated with both IL-4 and CD40L compared with cells treated with CD40L alone (Fig 5). Interestingly, BL2 cells also differed from both Elijah and Louckes in that CD40L-induced growth arrest was not at its maximal until 4 days into culture (Fig 5). The effects of CD40L on BL29 cells with regard to DNA synthesis were less pronounced, where a modest (∼30%) but still significant (P < .0001) reduction was observed at 24 hours that was maintained to day 4 (Fig 5). This reduction was not enhanced by the presence of IL-4 (Fig 5).
Four late passage BL cell lines were incubated with CD40L alone, IL-4 alone, or CD40L with IL-4 for 72 hours. Aliquots were removed at the times indicated and cell growth was evaluated by incorporation of [3H]-Thy, which is expressed as a percentage of untreated control cultures. Data are presented as the mean with standard deviation from three separate experiments. The range of radioactivity incorporated in control (untreated) cultures in these experiments for each cell line was: Elijah, 12,433 to 23,083 cpm; BL2, 14,053 to 22,767 cpm; BL29, 19,353 to 24,662 cpm; and Louckes, 13,471 to 23,622 cpm. Treatment with CD40L significantly (P < .0001) reduces proliferation of all late passage BL cell lines where indicated (*).
Four late passage BL cell lines were incubated with CD40L alone, IL-4 alone, or CD40L with IL-4 for 72 hours. Aliquots were removed at the times indicated and cell growth was evaluated by incorporation of [3H]-Thy, which is expressed as a percentage of untreated control cultures. Data are presented as the mean with standard deviation from three separate experiments. The range of radioactivity incorporated in control (untreated) cultures in these experiments for each cell line was: Elijah, 12,433 to 23,083 cpm; BL2, 14,053 to 22,767 cpm; BL29, 19,353 to 24,662 cpm; and Louckes, 13,471 to 23,622 cpm. Treatment with CD40L significantly (P < .0001) reduces proliferation of all late passage BL cell lines where indicated (*).
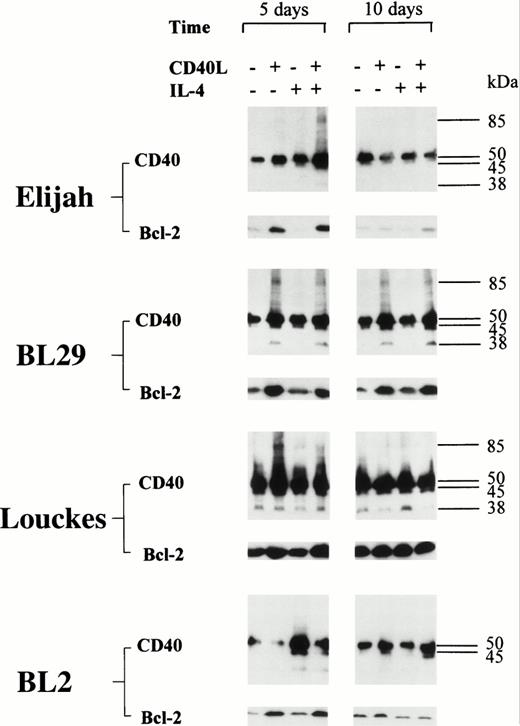
CD40 homodimer formation corresponds to an upregulation in the expression of Bcl-2 in group I BL cells.
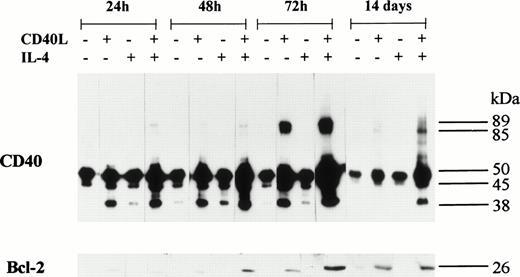
An upregulation in the expression of Bcl-2 was detected in all five BL B-cell lines after stimulation with CD40L; with one exception (BL2), this was accompanied by the formation of CD40 homodimers (Figs 6 and 7). Furthermore, in L3055 cells, CD40L induced low but detectable levels of Bcl-2 protein within 24 hours (Fig 7). Both the levels of Bcl-2 expression and the formation of CD40 homodimers were maximal after 72 hours, and the presence of IL-4 enhanced both CD40 dimer formation and Bcl-2 expression in L3055 cells (Fig 7). Stimulation with CD40L resulted in an increase in Bcl-2 expression, which was sustained for up to 14 days in L3055 cells (Fig 7) and up to 10 days in BL29 cells (Fig6). Interestingly, IL-4 did not enhance Bcl-2 protein expression when added with CD40L in all the late passage group I BL cells investigated (Fig 6). Furthermore, culture with IL-4 alone failed to upregulate Bcl-2 protein expression in both early and late passage BL cells (Figs6 and 7). Late passage group I BL B cells showed a diminished ability to maintain long-term increases in the levels of both Bcl-2 and CD40 homodimer expression. Indeed, with the exception of BL29, CD40 homodimers and Bcl-2 protein levels were either absent or had returned to normal levels within 5 days in all late passage group I BL cells (Fig 6).
BL cell lines were incubated either alone or with CD40L, IL-4, or CD40L with IL-4 for 72 hours. Cell lysates were prepared at either 5 or 10 days (including the initial 72-hour stimulation time), and equal amounts of total cellular protein was resolved on 10% SDS-PAGE. Western blotting was performed to determine the presence of CD40 homodimers using anti-CD40 MoAb G28.5. In addition, the same lysates were resolved on 12.5% SDS-PAGE, and Western blotting was performed to detect the presence of Bcl-2 using anti–Bcl-2 MoAb 100. The numbers to the right of each panel set indicate the positions of molecular weight markers.
BL cell lines were incubated either alone or with CD40L, IL-4, or CD40L with IL-4 for 72 hours. Cell lysates were prepared at either 5 or 10 days (including the initial 72-hour stimulation time), and equal amounts of total cellular protein was resolved on 10% SDS-PAGE. Western blotting was performed to determine the presence of CD40 homodimers using anti-CD40 MoAb G28.5. In addition, the same lysates were resolved on 12.5% SDS-PAGE, and Western blotting was performed to detect the presence of Bcl-2 using anti–Bcl-2 MoAb 100. The numbers to the right of each panel set indicate the positions of molecular weight markers.
L3055 cells were incubated either alone or with CD40L, IL-4, or CD40L with IL-4 for 72 hours. During the 72-hour stimulation time, the presence of CD40 homodimers and Bcl-2 was determined at 24, 48, and 72 hours. Immunoblots were also performed after cells were incubated with the same additives for 72 hours, followed by an extended 14-day culture (including the initial 72-hour stimulation time) in normal complete medium. Cell lysates were prepared and equal amounts of total cellular protein resolved on either 10% SDS-PAGE for CD40 homodimers or 12.5% SDS-PAGE for Bcl-2. Western blots were performed and CD40 homodimers were detected used anti-CD40 MoAb G28.5. Bcl-2 was detected using anti-Bcl-2 MoAb 100. The numbers to the right of each panel group indicate the positions of molecular weight markers.
L3055 cells were incubated either alone or with CD40L, IL-4, or CD40L with IL-4 for 72 hours. During the 72-hour stimulation time, the presence of CD40 homodimers and Bcl-2 was determined at 24, 48, and 72 hours. Immunoblots were also performed after cells were incubated with the same additives for 72 hours, followed by an extended 14-day culture (including the initial 72-hour stimulation time) in normal complete medium. Cell lysates were prepared and equal amounts of total cellular protein resolved on either 10% SDS-PAGE for CD40 homodimers or 12.5% SDS-PAGE for Bcl-2. Western blots were performed and CD40 homodimers were detected used anti-CD40 MoAb G28.5. Bcl-2 was detected using anti-Bcl-2 MoAb 100. The numbers to the right of each panel group indicate the positions of molecular weight markers.
In CD40-stimulated L3055 cells, the slow induction of Bcl-2 protein expression mirrored the gradual increase in CD40 homodimer formation. Both Bcl-2 protein and CD40 homodimers could be detected at low levels for up to 14 days in these cells (Fig 7). Late passage group I BL cells showed a similar pattern of Bcl-2 and CD40 homodimer expression (Fig6), although CD40 homodimers and Bcl-2 were detected in the individual cell lines for different lengths of time. Brief increases in CD40 homodimer expression were observed in Elijah and Louckes cells, each lasting no more than 5 days; again, this was also seen with Bcl-2, which also returned to normal levels after a transient 5-day increase in expression (Fig 6). Louckes cells displayed an apparent increase in Bcl-2 expression in untreated control cells after 10 days compared with 5 days, but this could be due to small variations in determining protein concentrations of cell lysates. It should be noted that observed differences in Bcl-2 expression are based on comparisons between cells cultured with the additions indicated and untreated control cells analyzed during the same time point. BL29 cells displayed a similar pattern in the upregulation of CD40 homodimer expression, as was seen in early passage L3055 cells. High levels of both homodimeric CD40 and Bcl-2 were detected in BL29 cells, and both CD40 homodimers and Bcl-2 expression levels remained elevated for a least 10 days (Fig6). Despite the consistent production of CD40 homodimers in all other CD40-stimulated group I BL cell lines, BL2 cells remained the exception, where the failure to detect CD40 homodimers was reflected by an upregulation in the expression of Bcl-2 protein, albeit a very modest one (Fig 6).
The inability to detect CD40 homodimers within 24 hours suggests that their formation is not simply a consequence of CD40L promoting direct aggregation of CD40 into dimeric complexes on the cell surface (Fig 7). This was further supported by the observation that Elijah cells expressed large amounts of homodimeric CD40 only when treated with both CD40L and IL-4 (Fig 6) and the fact that the BL2 cell line failed to produce CD40 homodimers under identical conditions of culture with CD40L, with or without IL-4 (Fig 6).
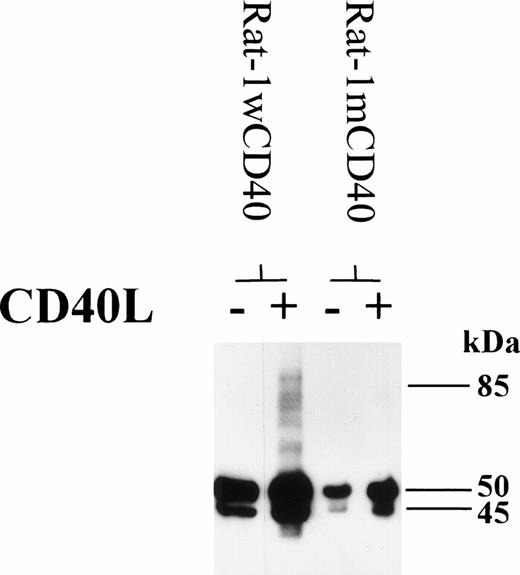
To gain some insights into the mechanism behind CD40-mediated dimer formation, the Rat-1 fibroblast cell line transfected with wild-type or mutated human CD40 was studied. Fibroblasts expressing wild-type CD40 could be induced to form homodimers after stimulation with CD40L, as seen with the group I BL lines (Fig 8). However, fibroblasts transfected with human CD40 mutated to substitute Ala at residue Thr234 were unable to signal through CD40 to produce high molecular weight CD40 immunoreactive material, including that corresponding to homodimers (Fig 8). This finding suggests that cells can only produce CD40 homodimers by transducing a signal through CD40 and that the essential signaling residue Thr234 is an important component of this pathway. These data are also consistent with the notion that CD40 homodimers were not produced merely as a result of nonspecific aggregation of the CD40 protein.
Rat-1 fibroblasts were transfected with either wild-type human CD40 (Rat-1wCD40) or mutant human CD40 (Rat-1mCD40). Stable transfectants were stimulated with CD40L for 72 hours. Cell lysates were prepared and equal amounts of total cellular protein was resolved on 10% SDS-PAGE. The presence of CD40 homodimers on immunoblots was determined using anti-CD40 MoAb G28.5. The numbers to the right of each panel set indicate the positions of molecular weight markers.
Rat-1 fibroblasts were transfected with either wild-type human CD40 (Rat-1wCD40) or mutant human CD40 (Rat-1mCD40). Stable transfectants were stimulated with CD40L for 72 hours. Cell lysates were prepared and equal amounts of total cellular protein was resolved on 10% SDS-PAGE. The presence of CD40 homodimers on immunoblots was determined using anti-CD40 MoAb G28.5. The numbers to the right of each panel set indicate the positions of molecular weight markers.
Exposure of L3055 cells to CD40L confers sustained protection from anti-IgM–induced apoptosis.
Early passage L3055 cells are sensitive to apoptosis driven by soluble anti-IgM, whereas later passage group I BL lines are relatively resistant. L3055 was thus chosen for a detailed analysis of the influence of short-term exposure to CD40L on the maintenance of protection from this apoptotic signal. L3055 cells were incubated after various times with anti-IgM MoAb (BU1) for 24 hours after an initial 72 hours of stimulation with either CD40L alone, IL-4 alone, or CD40L with IL-4. Apoptosis was assessed by examining Romanowski-stained cytocentrifuge preparations and enumerating the percentage of cells possessing classical morphological features of apoptosis compared with viable, intact cells. In addition, DNA synthesis was assessed by [3H]Thy incorporation in response to cross-linking sIgM. The validity of these techniques as measures of ant-IgM–induced apoptosis in L3055 cells and their detailed assessment has been fully documented elsewhere.8
Cells were again stimulated for 72 hours with the additives indicated, followed by maintenance in continuous culture. [3H]Thy incorporation was assessed at 14 and 20 days (which included the initial 72-hour stimulation time) after 24 hours of incubation with BU1. To determine the strength of survival signals delivered through CD40 by CD40L, BU1 was used at a range of concentrations (0.01 to 10 μg/mL) for both assessment of apoptosis and [3H]Thy incorporation in L3055 cells.
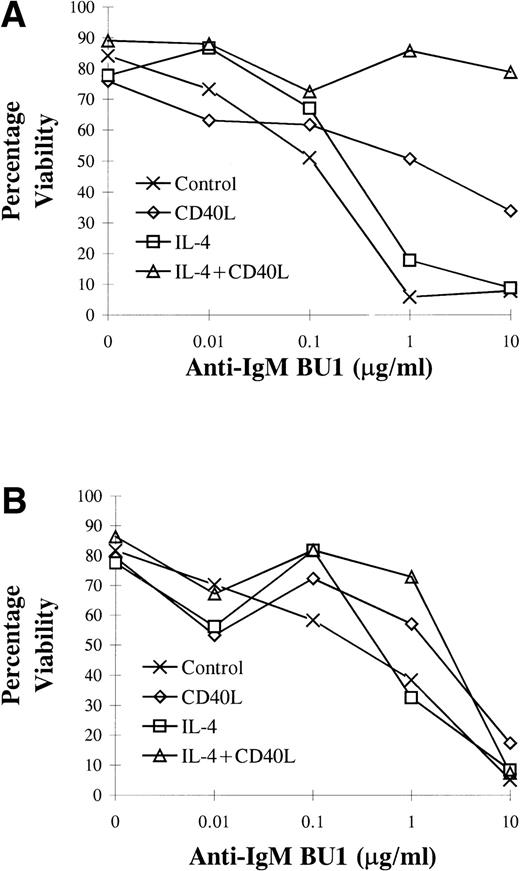
L3055 cells treated with CD40L displayed increased resistance to anti-IgM–induced apoptosis within 72 hours compared with untreated control cells (Fig 9A). Cells previously stimulated with either CD40L alone or CD40L in combination with IL-4 retained a degree of resistance to apoptosis induced by anti-IgM for at least 14 days (Fig 9B). The most potent antiapoptotic effects induced by CD40L were observed at 72 hours, where culture with CD40L conferred protection against anti-IgM–induced apoptosis even when added at concentrations of 10 μg/mL (Fig 9A). The addition of IL-4 in combination with CD40L to L3055 cells appeared to have a synergistic effect regarding protection, whereas L3055 cell viability in response to anti-IgM increased approximately 40% above that when only CD40L was providing protection (Fig 9A). IL-4 alone did not alter L3055 cell viability compared with untreated control cells on the addition of BU1 (Fig 9). At day 14, where cells had received an earlier 72 hours of exposure to either CD40L alone or CD40L in combination with IL-4, they still displayed some degree of resistance to the effects of BU1 when used at a concentration of 1 μg/mL (Fig 9B). Resistance to apoptosis induced by treatment with 1 μg/mL of BU1 after 14 days was enhanced in L3055 cells that had been stimulated previously with IL-4 in combination with CD40L compared with cells stimulated with CD40L alone. No enhanced resistance to BU1 at 10 μg/mL was evident at 14 days with any of the treatments (Fig 9B).
L3055 cells were incubated either alone or with CD40L, IL-4, or CD40L with IL-4 for 72 hours. Cells were then maintained in complete medium for up to 14 days (including the initial 72 hours of stimulation). During this time at (A) 3 and (B) 14 days of resistance to apoptosis induced by anti-IgM MoAb, BU1 was assessed by counting apoptotic cells from triplicate cultures on Romanoski-stained cytocentrifuge preparations. Data are representative of two separate experiments.
L3055 cells were incubated either alone or with CD40L, IL-4, or CD40L with IL-4 for 72 hours. Cells were then maintained in complete medium for up to 14 days (including the initial 72 hours of stimulation). During this time at (A) 3 and (B) 14 days of resistance to apoptosis induced by anti-IgM MoAb, BU1 was assessed by counting apoptotic cells from triplicate cultures on Romanoski-stained cytocentrifuge preparations. Data are representative of two separate experiments.
As mentioned above, it has been established that apoptosis promoted by anti-IgM in L3055 cells is preceded by growth arrest as registered by a decrease in the rate of DNA synthesis.8 In the present study, it was seen that culture of L3055 cells with CD40L alone or CD40L in combination with IL-4 engendered a high degree of resistance to BU1 in this regard when compared with untreated control cells (Fig 10). At day 14, where cells had been cultured with CD40L for the first 72 hours and then had the stimulants washed out, a significant (P < .0001) resistance to BU1-induced growth arrest was maintained even when they were confronted with high levels (10 μg/mL) of the antibody (Fig 10A). Whereas by day 20 the early 72-hour preculture with CD40L no longer appeared to be protecting from 10 μg/mL of BU1 with regard to its ability to reduce DNA synthesis, a significant degree (P < .0001) of protection was still being afforded against BU1 used at a lower dose of 1 μg/mL (Fig 10B). The addition of IL-4 was not seen to confer any significant additional increase in the resistance of L3055 cells to BU1 as measured by reductions in DNA synthesis over and above that observed in cells treated with CD40L alone at either 14 or 20 days (Fig 10).
L3055 cells were incubated either alone or with CD40L, IL-4, or CD40L with IL-4 for 72 hours. Stimulants were washed out and cells were maintained in complete medium for up to 20 days (including the initial 72 hours of stimulation). [3H]-Thy incorporation was assessed after a 24 hours of incubation with anti-IgM MoAb BU1 at (A) day 14 and (B) day 20. The percentage of incorporation [3H]-Thy for each treatment was determined from untreated control cultures, which ranged from 4,674 to 8,522 cpm in three experiments. Data are presented as the mean with standard deviation from these three separate experiments. Treatment with either CD40L alone or CD40L in combination with IL-4 significantly (P< .0001) increases L3055 cell proliferation after stimulation with anti-IgM MoAb BU1 where indicated (*).
L3055 cells were incubated either alone or with CD40L, IL-4, or CD40L with IL-4 for 72 hours. Stimulants were washed out and cells were maintained in complete medium for up to 20 days (including the initial 72 hours of stimulation). [3H]-Thy incorporation was assessed after a 24 hours of incubation with anti-IgM MoAb BU1 at (A) day 14 and (B) day 20. The percentage of incorporation [3H]-Thy for each treatment was determined from untreated control cultures, which ranged from 4,674 to 8,522 cpm in three experiments. Data are presented as the mean with standard deviation from these three separate experiments. Treatment with either CD40L alone or CD40L in combination with IL-4 significantly (P< .0001) increases L3055 cell proliferation after stimulation with anti-IgM MoAb BU1 where indicated (*).
DISCUSSION
This study investigated the induction and maintenance of a variety of phenotypic, functional, and molecular responses promoted in group I BL cells by their stimulation through CD40. Of particular note was the finding that exposure to CD40L not only protects group I BL cells from surface Ig-promoted apoptosis, but also directly induces growth arrest; furthermore, both outcomes could be relatively long-lived even after the CD40 signal was withdrawn. Another novel finding was that stimulation through CD40 induces the formation of CD40 homodimers in group I BL cells and that this was concomitant with the induction or increased expression of Bcl-2 protein, both of which could again be sustained even after removal of the CD40 signal. Of the phenotypic changes that were seen to accompany these events, which included the induction of homotypic adhesions, the most profound was the rapid and long-term downregulation of CD77, the hallmark group I BL marker. Simultaneous addition of IL-4 to CD40-stimulated cultures augmented many of these responses and, in some instances, IL-4 and CD40 signaling demonstrated marked synergy in determining both the intensity and duration of the responses.
As yet, no functional significance has been attributed to the formation of CD40 homodimers that has been detected on a number of B-cell types. Previous studies have shown that Raji cell lysates treated with alkylating agents such as N-ethylmaleimide or iodoacetimide fail to disrupt CD40 homodimers. This suggests that CD40 homodimers may be formed in vivo and are not produced through nonspecific sulphydryl interactions on CD40 monomers. This observation provides an important insight into the way CD40 is expressed, particularly given the requirement for CD40 to be cross-linked before receptor signaling can occur. It is therefore tempting to speculate that CD40 homodimers can enhance B-cell responses upon engagement with CD40L. This may be achieved by reducing the requirement for large amounts of CD40L, either in soluble or membrane-bound forms. It is possible that CD40 homodimers are actively produced either on the cell surface or synthesized intracellularly. From our present study, the slow induction of CD40 homodimers by CD40L leading to maximal homodimer expression at 72 hours supports the notion that CD40 homodimers are produced by active protein synthesis in vivo. Importantly, CD40L was unable to induce CD40 homodimer formation in Rat-1 fibroblasts transfected with CD40 expressing a point mutation at the essential signaling residue Thr234, whereas the same cells transfected with wild-type human CD40 could be induced to express homodimers in a manner analogous to that observed with the group I BL lines. These findings strongly suggest that CD40 homodimers can only be formed in cells that have the capacity to signal intracellularly through CD40. They also point to a role for TRAF adaptor molecules in this process, because the binding of some of them to the cytoplasmic domain of CD40 has been shown to involve residue Thr234 of the cytoplasmic domain.20 These findings potentially provide a mechanism for the rewiring of CD40 during B-cell differentiation as has been proposed previously.21 Further attempts to dissect the processes involved in CD40 homodimer production using chemical inhibitors to either protein synthesis or transport have been hindered by the toxic effects of these compounds on group I BL cells (our own unpublished data).
The expression of CD40 homodimers was mirrored closely by an increase in Bcl-2 protein levels in CD40L-stimulated group I BL cells. The levels of both CD40 homodimers and Bcl-2 protein increased at apparently similar rates over a 72-hour period. The ability of CD40 signals to induce a relatively late induction of Bcl-2 protein expression have been detailed previously5 and were confirmed in this study. It is difficult to speculate whether the formation of CD40 homodimers is directly or indirectly linked to induction of Bcl-2 expression or that the upregulation of these molecules is simply coincidental. However, the importance of this response may be reflected in the fact that (with the one exception of BL2) both early and late passage group I BL cell lines that were induced by CD40L, either alone or in combination with IL-4, to express CD40 homodimers, showed a corresponding increase in Bcl-2 expression.
One of the most surprising features of this study was that a short-term exposure to CD40L could lead to relatively long-term effects. In the example of CD40 homodimer formation and Bcl-2 induction, a 3-day culture with CD40L resulted in both these induced changes being maintained to day 10 or day 14 for BL29 and L3055 cells, respectively. Interestingly, these two lines showing the most durable changes were also the earliest in terms of their passage number.
It is also of note that low levels of dimeric CD40 could be detected in unstimulated Louckes cells, which had the highest in vitro passage number and the highest constitutive level of Bcl-2 protein among the cell lines studied. It is possible that the development of group I lines toward a more stable phenotype in culture may be accompanied by the constitutive expression of CD40 homodimers (and possibly also Bcl-2) together with a relative refractoriness to further induced long-term change. Indeed, as seen by us (unpublished data) and in previous studies,7 many well-established B-lymphoma cell lines, such as Raji, express CD40 as homodimers constitutively.
Given the duration of change in both CD40 homodimer formation and Bcl-2 protein expression, together with their previously reported sensitivity to surface IgM-mediated apoptosis, L3055 cells were selected for a study of possible long-term protection to apoptotic signals after short-term exposure to CD40L. L3055 cells stimulated through CD40 were resistant to apoptosis induced by high concentrations of anti-IgM from 72 hours and remained protected, albeit to a lesser extent, for up to 14 days even where CD40L had been removed from culture at day 3. Indeed, where stimulated via CD40 for 3 days, L3055 cells continued to proliferate optimally when treated with low, but still normally apoptosis-inducing, concentrations of anti-IgM at day 20. The maintenance of low-level, induced expression of Bcl-2 at day 14 may explain why L3055 cells were still afforded some degree of protection from apoptosis when treated with high concentrations of anti-IgM at this time. The lack of Bcl-2 expression at day 20 indicates that other long-term protective mechanisms induced by CD40L may confer partial protection from apoptosis. Earlier studies have shown that interferon-α protects Mutu BL cells from CaI-induced apoptosis through a pathway that does not appear to involve the upregulation of Bcl-2.22 It is possible that the long-term protective action of CD40L may be provided by separate mechanisms, such as those mediated by distinct members of the Bcl-2 family as has been noted in other studies of B-lymphoma cell lines.23-26
Not only did stimulation through CD40 provide protection from induced apoptosis, but it also promoted growth arrest directly in group I BL cells. Treatment with CD40L induced growth arrest in both early and late passage group I BL cell lines as measured by a reduction in [3H]Thy uptake compared with untreated control cells. Analysis of cell cycle profiles in previous studies on L3055 cells (and confirmed here; our own unpublished data) has shown relatively few cells arresting at any particular stage of the cell cycle when confronted with CD40L.8,27 However, the [3H]Thy incorporation experiments reported here show clearly that L3055 cells are in growth arrest after exposure to CD40L, due to their failure to synthesize DNA. Thus, it is likely that CD40-mediated signals may inhibit L3055 cell progression through the cell cycle by acting at multiple restriction points. Additional evidence obtained in a similar way from another study confirms that CD40L can induce growth arrest in B-lymphoma cell lines, including that of well-established BL lines such as Raji.9 Moreover, this type of growth inhibitory effect, without concommitant apoptosis, is not restricted to B cells, because it has also been observed in epithelial cells.18 Importantly, from our study, it is clear that growth arrest in group I BL cells does not inevitably lead to apoptosis. This may relate to the ability of CD40L to turn on Bcl-2 expression in group I BL cells. It has been suggested that only once cells have left the cell cycle does the potent protective action of Bcl-2 becomes mandatory, and in its absence the eventual fate is one of apoptotic death.5 The duration of the growth arrest promoted by CD40L and the additional effects of adding IL-4 could be quite variable among the five group I BL lines studied. Whereas IL-4 normally augmented CD40-dependent growth arrest, for BL2 cells it had a counteracting effect. The basis for this heterogeneity is unclear. Although for most of the cell lines growth arrest was relatively short-lived once the CD40L stimulus had been removed, for the early passage L3055 line, 24 hours of stimulation maintained growth arrest to day 5; with 72 hours of exposure to CD40L, growth arrest was extended to 7 to 9 days. This is considered remarkable for a cell line that is normally growing exponentially with a usual doubling time of approximately 24 hours (data not detailed). Again, there was no morphological evidence of enhanced apoptosis in these long-term arrested cultures, which, as discussed above, is consistent with the sustained expression of induced Bcl-2.
CD40L-promoted growth arrest, homodimer formation, induction of Bcl-2, and conferment of resistance to apoptosis were seen to be accompanied by changes in cell surface phenotype, cluster formation, and individual cell morphology, which, again, could be relatively long-lived. Most group I BL cell lines display little or no cell clustering, an observation supported by the absence of detectable adhesion molecule expression.28 However, it should be noted that late passage BL cell lines can drift phenotypically and display modest amounts of adhesion molecule expression, which can be accompanied by a small degree of cell clustering.28 This was seen in the present study for Elijah, BL29, and Louckes. As has been reported previously for normal resting B cells,13 three of the five lines were induced to form large homotypic aggregates when signaled through CD40, and this was enhanced by the addition of IL-4. For the early passage L3055 cells, this change in phenotype was once more relativley long-lived in that, with 72 hours of exposure to CD40L, there was a maintenance of small loose cell aggregates (which were absent in control cultures) even to day 10. Of the adhesion molecules studied, CD11a and CD54 were the ones that showed a corresponding upregulation on CD40-signaling. Untreated group I BL cells, particularly those recently established from biopsy tissue, display a resting cell morphology, where cells have a small cytoplasmic to nuclear ratio and the nuclear chromatin is relatively condense. Stimulation with CD40L resulted in a series of dramatic morphological changes in both early and late passage BL cell lines (full data not detailed). These changes were characterized by the production of large blastoid-like cells that often had pseudopodia-like extensions. These cells had a high cytoplasmic to nuclear ratio, and detailed morphological observations showed the presence of many cytoplasmic vesicles. The significance of these changes are unclear. However, the fact that nearly every cell line tested responded to stimulation with CD40L by this morphological criteria in an identical way highlights the importance of CD40 signaling in inducing a unique differentiation pathway in BL cells even when they are at somewhat differing stages of developmental arrest.
Whether CD40 signaling in BL cell lines accurately reflects events occurring in GC B cells, their presumed normal counterparts, is unclear. The importance of CD40 signals in GC differentiation is apparent in its requirement for the formation of either plasma or memory B cells.29 Studies involving in vitro culture of CD40-activated GC B cells have shown that withdrawal of CD40L stimulation leads to the differentiation of activated B cells into plasma cells, whereas prolonged CD40L stimulation results in the generation of memory B cells.29 Little work has been performed to investigate how CD40 signals influence the phenotype of lymphomas, such as the group I BL, which carry GC markers. This study has shown that stimulation through CD40 results in a heterogeneous phenotypic response in early and late passage group I BL cells. The addition of IL-4 to CD40L-treated cultures tended to enhance the existing effects of CD40 signals on a number of surface markers expressed by the BL cells. Interestingly, treatment of L3055 cells with both CD40L and IL-4, but not CD40L alone or IL-4 alone, resulted in a marked decrease in CD20 expression (unpublished data). A particularly striking observation was that both early passage L3055 cells and late passage Elijah, Louckes, and BL2 cells increased their expression of CD23 when treated with CD40L alone or CD40L in combination with IL-4. This indicates that the cells were moving towards a more lymphoblastoid, or group III, phenotype (see below). The fact that surface CD40 expression also increased on L3055, Elijah, and BL2 cells when treated with the same stimuli indicates that BL cell activation may involve an upregulation in the expression of various markers, including CD40. Importantly, the increase in surface CD40 expression observed by FACS analysis did not correspond directly with the ability of cells to form CD40 homodimers, in as much as BL29 cells that expressed homodimeric CD40 failed to show increases in surface CD40 expression, whereas BL2 cells that did not express CD40 homodimers did show increased surface CD40 expression on culture with CD40L.
The three EBV−ve group I BL lines all showed a reduction in surface CD77 expression in response to stimulation through CD40 that, for the early passage L3055 cells, was dramatic and prolonged. Unstimulated L3055 cells consisted of a mostly CD77high population. However, upon treatment with CD40L, the expression of CD77 skewed dramatically to produce an almost exclusively CD77low population. Furthermore, CD40L-treated L3055 cells failed to revert back to the normal phenotype by remaining CD77low over the duration of the experiment (>15 days), even though the stimulus had been removed at day 3. The importance of this observed reduction in CD77 expression remains unclear, although it should be noted that both the activation of CD40 and the downregulation of CD77 are effects observed during the in vitro progression of fresh or early passage EBV+ve BL cells (group I cells) into the differentiated and activated, lymphoblastoid-like cells, characterized by group III BL cells.3,16,30,31 Unlike the early group I counterparts, group III cells express the full repertoire of EBV latent antigens of which EBNA-4 is a member. Indeed, recent reports suggest that EBNA-4 is important in modulating CD40, vimentin, and CD77 on BL cells.32 It was observed in the same study that transfection of EBNA-4 into EBV−ve group I BL cells (dG75) resulted in an increase in CD40 and a decrease in CD77 expression, respectively. It is of interest in our study that CD77 expression remained unchanged on CD40-stimulation of both the EBV+ve BL lines. The expression of specific EBV-latent genes in the late passage EBV+ve BL cells used in this investigation has not been determined. However, it seems unlikely that EBNA-4 was expressed in these cells, because phenotypic analysis showed that all late passage BL cells expressed CD10 and CD77 but not CD23, typically observed in BL cells categorized as a group I phenotype. Taken together, these data suggest that stimulation through CD40 modulates CD77 expression in a manner similar to that observed in BL cells expressing EBNA-4. It has been proposed that EBNA-4 may modulate CD77 expression indirectly through intermediates of the glycolipid pathway.32 This may be a common point at which the effects of EBNA-4 in and EBV+ve BL cells and CD40 signals in EBV−ve BL cells converge to regulate CD77 expression. In common with CD40, CD77 may also be important in regulating apoptotic mechanisms during GC B-cell development.32 The fact that CD77 is expressed on GC B cells undergoing apoptosis could indicate a possible counterstructure for this molecule that may be involved in the induction of GC B-cell apoptosis. Therefore, CD40L-induced downregulation of CD77, along with the coordinate upregulation of CD40 seen on a number of BL cell lines tested in this study, could have important implications for cell survival. It should be noted that, despite the absence of EBV latent genes, CD40-stimulated L3055 and Louckes cells displayed a group III-like phenotype, in that CD77 expression was reduced, CD23 was upregulated, cells adopted a lymphoblastoid morphology, and large homotypic cell aggregates were produced. Some, or all, of these phenotypic responses were mirrored in the EBV-positive BL, BL29, and Elijah cells, suggesting that CD40 may have an important role in the progression of BL cells from a group I to a group III phenotype.
In summary, stimulating CD40 on both early and late passage and EBV−ve and EBV+ve BL cell lines results in multiple phenotypic, functional, and molecular changes. It is apparent that not all cell lines respond to CD40-meditated signals in an identical manner, despite initial similarities in cell surface phenotype and morphology. However, several of the cell lines follow similar trends in many or all of the ways they respond to CD40: eg, growth arrest, protection from apoptosis, coexpression of Bcl-2 and CD40 homodimers, and morphological and phenotypic changes. Importantly, the stimulation time during which cells are exposed to CD40L can dictate the strength and the duration of the response to CD40 signals. Most unexpectedly, and as highlighted by results obtained with the early passage L3055 cell line, some of the induced changes were maintained for 1 to 2 weeks after removal of the initial CD40L stimulus. These are not only important factors to consider when designing potential CD40L-based therapies for the treatment of B-cell lymphomas,9,33 34 but also raise the possibility that a transient exposure to CD40L, possibly via infiltrating T cells, may influence the course and nature of B-cell lymphomagenesis.
ACKNOWLEDGMENT
The authors thank Michelle Holder for expert technical assistance.
Supported by the Medical Research Council (U.K.) and the Cancer Research Campaign.
Address reprint requests to John Gordon, PhD, Department of Immunology, Medical School, University of Birmingham, Vincent Drive, Edgbaston, Birmingham, B15 2TT, UK; e-mail:j.gordon@bham.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 4. L3055 cells were incubated with CD40L alone, IL-4 alone, or CD40L with IL-4 for either (A) 24 or (B) 72 hours. Aliquots were removed at the times indicated and cell growth was evaluated by incorporation of [3H]-Thy. Incorporation of [3H]-Thy for each treatment is expressed as a percentage of untreated control cultures. Data are presented as the mean with standard deviation from three separate experiments. The range of radioactivity incorporated in control (untreated) cultures for the three experiments was 7,698 to 11,218 cpm. Treatment with either CD40L alone or CD40L with IL-4 significantly (P < .0001) decreases proliferation of L3055 cells where indicated (*).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/8/10.1182_blood.v92.8.2830/5/m_blod42010004x.jpeg?Expires=1769614702&Signature=jrVrL0dYR5wYaXa6E1k1TkDHWQvXvYapkoUYTbwuBSIQCaqYq72MGBIusqA2E4fBnZmsAHtLpOvkMHV-~vMlQN~I-dJGoudRAi2z3PcCYBMlGH8IXiv0n6usJ1BxlLwGT68IHzmHP01gVLHaVuf~cjwTU-dxPFQnB4rJGGm0q1l8EQntwVw4QDbwz152MmcTq1wSjrYwzAVtHt0DK0c1kZyDQHbAW2G3d79KlF6Zwg17GsFcYZuWhw1FqvBFfs7lO12s-7lhRJ3tsWJjTe1i3U6sGmjr4PyQtZnGVBWVFPCp3VKUK9RDZVD~geITMThGzCFlPnUZxepSHu55iNeZSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Four late passage BL cell lines were incubated with CD40L alone, IL-4 alone, or CD40L with IL-4 for 72 hours. Aliquots were removed at the times indicated and cell growth was evaluated by incorporation of [3H]-Thy, which is expressed as a percentage of untreated control cultures. Data are presented as the mean with standard deviation from three separate experiments. The range of radioactivity incorporated in control (untreated) cultures in these experiments for each cell line was: Elijah, 12,433 to 23,083 cpm; BL2, 14,053 to 22,767 cpm; BL29, 19,353 to 24,662 cpm; and Louckes, 13,471 to 23,622 cpm. Treatment with CD40L significantly (P < .0001) reduces proliferation of all late passage BL cell lines where indicated (*).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/8/10.1182_blood.v92.8.2830/5/m_blod42010005x.jpeg?Expires=1769614702&Signature=oPZv08f9wyWWQt6A0gk06p3HeIC9DQnFfxTAdBwgDqxUEAri71~P6eFEEfaaW39yRXHKxSfi5QbZzm8hV4zcE7CISKevrSDcsq87J78ta325Txx~AsBVNvA5dCaa0YX50EPtA18zGmsrJ0ARCnffb8MQ~bBl0PEGRL24PuuId5qKkzeZZkkpriHsf0oG0dwjGutmk~vRJkrbeDQeJkdzXAjN2ib7jW~WHb7mIVkShzm9QdBebvkRULLs-diNUyHj5lvjpCRWdAvdj375yLMI2sOg4hhdG-EBQ3uX00TcllprKR61BoVi8Y4M7k3EhX728J6Lq2SGadu0f0ZK13-2NQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 10. L3055 cells were incubated either alone or with CD40L, IL-4, or CD40L with IL-4 for 72 hours. Stimulants were washed out and cells were maintained in complete medium for up to 20 days (including the initial 72 hours of stimulation). [3H]-Thy incorporation was assessed after a 24 hours of incubation with anti-IgM MoAb BU1 at (A) day 14 and (B) day 20. The percentage of incorporation [3H]-Thy for each treatment was determined from untreated control cultures, which ranged from 4,674 to 8,522 cpm in three experiments. Data are presented as the mean with standard deviation from these three separate experiments. Treatment with either CD40L alone or CD40L in combination with IL-4 significantly (P< .0001) increases L3055 cell proliferation after stimulation with anti-IgM MoAb BU1 where indicated (*).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/8/10.1182_blood.v92.8.2830/5/m_blod42010010x.jpeg?Expires=1769614702&Signature=iMgZk6DnH4a96ivX~qettDKlh7dpj2GDj-6n-0AFJjYr0A-WFZQQJ2KwviRJS0se9B99OCuANqLweOJL4k4pX09HCVqub1vFH5-y3xodDaKs28hUtEdC4ZMfcpwLvCiwn7BZN23yUXosgHyT16dCr0tXWj~-kv0Qd7eAg4azwjJa8ZtkPGOkyN4cYwSX5BoC8ExdlvFpxfsMYL-hvY~~Smv2LEK3S0suXYNU~JjG0o79b0V4vrhG0Y~ZDTu5qpwbwDQvylyMNti6HcHaTskkH9AbvRZp0dkJZMfL3swwhrJUCTyN5M0Bwoi~Ra~OaKzv-AgUqPOORqf7rhqRYkpKeQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
