Abstract
The level of expression of the enzyme thiopurine methyltransferase (TPMT) is an important determinant of the metabolism of thiopurines used in the treatment of acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). Studies in red blood cells (RBC) have shown that TPMT expression displays genetic polymorphism with 11% of individuals having intermediate and one in 300 undetectable levels. The genetic basis for this polymorphism has now been elucidated and polymerase chain reaction (PCR)-based assays described for the most common mutations accounting for reduced activity. In previous studies, genotype has been correlated with red blood cell activity. In this report, we describe the relationship between genotype and TPMT activity measured directly in the target of drug action, the leukemic cell. We have demonstrated that the TPMT activity in lymphoblasts from 38 children and adults found by PCR to be homozygotes (*1/*1) was significantly higher than that in the five heterozygotes (*1/*3) detected (median, 0.25 v 0.08, P < .002, Mann-Whitney U). Similar results were obtained when results from children were analyzed separately. However, comparison of activity in blasts from AML and ALL showed a higher level in the former (0.35 v 0.22 nU/mg,P < .002, n = 17, 35), suggesting that factors other than genotype may also influence expression.
© 1998 by The American Society of Hematology.
SINCE THE INTRODUCTION of combination chemotherapy for the treatment of acute lymphoblastic leukemia (ALL) in children, the prognosis for this disease has improved steadily such that most trials now report a long-term survival rate of over 70%.1,2 However, current treatment protocols are complex and are associated with significant toxicity.3-6 There are therefore two major priorities in the treatment of childhood ALL; further improvement in the survival rate and a reduction in treatment-associated toxic side effects.7
One method, which may be used to achieve these goals, is the more effective use of currently available chemotherapy. At present, most patients are given cytotoxic drugs at a dosage determined by their surface area, despite the fact that, for several of the agents used, there is good evidence that this results in marked differences in the amount of drug which reaches its target.8 9 This is exemplified by the thiopurine, 6-mercaptopurine (6-MP), used in many regimes as part of the maintenance phase of therapy.
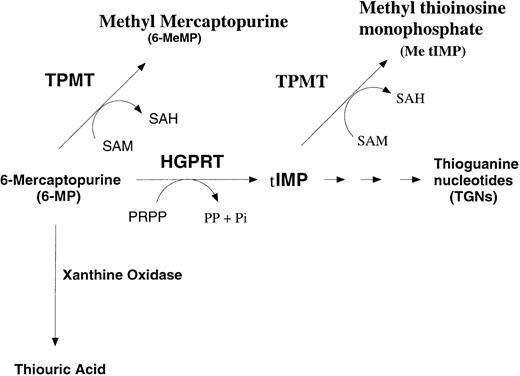
6-MP is a prodrug, which requires activation before it can exert its cytotoxic effects. As an analogue of hypoxanthine, it can act as a substrate for hypoxanthine-guanine phosphoribosyltransferase (HGPRT) leading to the formation of 6-thioinosine monophosphate (TIMP). Sequential reactions involving inosine monophosphate dehydrogenase and guanine monophosphate synthetase yields thioguanosine monophosphate (TGMP). Subsequent reactions by phosphokinases yields 6-thioguanosine diphosphate and triphosphate (TGDP and TGTP). TGMP, TGDP, and TGTP are collectively termed thioguanine nucleotides (TGNs, see Fig 1). Incorporation of 6-TGTP into DNA is believed to trigger cell death, probably by a process that involves the mismatch repair pathway.10,11 Two enzymes compete with HGPRT to reduce the intracellular levels of 6-TGNs. The first, xanthine oxidase, results in the formation of thiouric acid. Although this is an important route of inactivation of 6-MP, as demonstrated by the potentiation of 6-MP toxicity by the inhibitor allopurinol, xanthine oxidase levels do not appear to vary greatly between individuals.12 This is in contrast with the second enzyme, thiopurine methyltransferase (TPMT, EC 2.1.1.67), which inactivates 6-MP through the formation of methyl mercaptopurine. There is a clearly established variation in TPMT activity, which has been shown in several studies to follow a trimodal pattern of distribution within the normal population. In the original study reported by Weinshilboum,13 89% showed high, 11% intermediate, and one in 300 undetectable levels of TPMT activity in red blood cells (RBC). Similar patterns have subsequently been reported in several other populations (reviewed in Otterness et al14).
The importance of TPMT activity in the inactivation of 6-MP is demonstrated by the severe myelosuppression suffered by individuals with a congenital absence of the enzyme when given standard doses of 6-MP or the closely related drug, azathioprine.15-18 In addition, studies performed on children with ALL have shown an inverse relationship between TPMT expression and the level of red blood cell thioguanine nucleotides (RBC TGNs).19,20 The level of RBC TGNs have, in turn, been shown to predict outcome, with those children with levels below the population median having a significantly worse prognosis.21
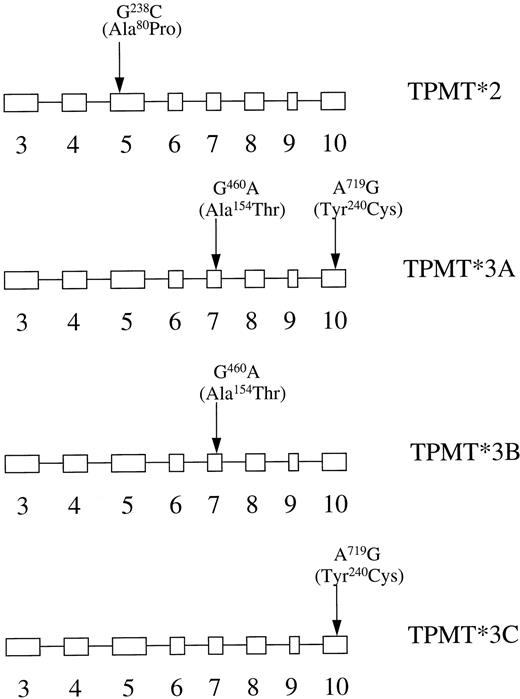
The genetic basis for 6-MP hypersensitivity and for the population distribution of TPMT activity has now been established following the cloning of the TPMT gene.22,23 A total of eight TPMT polymorphisms associated with reduced enzyme activity have now been identified.14 In describing gene mutations the convention has been adopted that the nonmutated gene is designated as TPMT*1 and mutated genes have been assigned as TPMT*2-*6 on the basis of the order in which they were first described. Recent results suggest that, in the Caucasian populations studied to date, the most common mutation genotype associated with low enzyme activity is TPMT*3, which accounts for more than 80% of the heterozygotes.14,24 In these cases, mutations are usually found at two sites, G460A and A719G (TPMT*3A, Fig 2). However, cases have been described in which the G460C or, more commonly, the A719G mutation occur in isolation (TPMT*3B and C, respectively). Studies of heterologous expression of mutated TPMT genes in yeast have shown that most mutations are associated with a decreased protein stability through the action of an adenosine triphosphate (ATP)-dependent proteasome-mediated pathway.25
Allelic variants of the TPMT gene. Boxes depict exons in the open reading frame of the human TPMT gene. The positions of the three point mutations detected by PCR-based assays are indicated.
Allelic variants of the TPMT gene. Boxes depict exons in the open reading frame of the human TPMT gene. The positions of the three point mutations detected by PCR-based assays are indicated.
Population studies have been performed on RBC, as these are a convenient source of material in which enzyme activity measurements can be made using small samples of venous blood. In patients with leukemia, such measurements may be rendered unreliable by prior red cell transfusion. It has been suggested that genotyping may be used as a reliable indicator of endogenous TPMT activity.24 The potential problem with this approach is that although most mutations occur at two loci (A719G or G460A), studies in which direct sequencing has been used across the entire open reading frame have shown that, in a significant minority of cases, especially in non-Caucasians, mutations may occur at other sites.14 Direct sequencing is, using current technology, not a practical approach for routine analysis. In this study, we have investigated the use of measurements of activity in leukemic blasts as an additional means of determining TPMT status and have correlated these measurements with genotype. We have shown that reliable measurements may be made on as few as 10 million blasts and that blast activity correlates with genotype. In addition we have compared the level of activity in blasts from patients with ALL with those from patients with acute myeloid leukemia (AML) and shown that expression is lineage-dependant.
MATERIALS AND METHODS
Patients and samples.
Samples were available for TPMT enzyme activity measurements from a total of 55 patients with ALL (35 children and 20 adults) and 17 patients with AML (all adults) who presented between March 1984 and September 1995 in the Northern Region of the United Kingdom. Of the children with ALL, 23 were boys and 12 were girls. The age ranged from 11 months to 15 years 6 months. Seven adults with ALL were men and 13 were women. The age range was 16 to 57 years. Of the adults with AML, 14 were men and three were women. The age range of this group was between 23 and 77 years.
Mononuclear cells were separated by centrifugation on a Ficoll-Hypaque density gradient from bone marrow or peripheral blood samples, which were surplus to diagnostic requirements, following guidelines established by the local ethics committee. Examination of cytocentrifuge preparations of these samples showed that they consisted of more than 85% malignant cells in each case studied, apart from two cases of AML with 61 and 69%. Leukemic blasts were stored as pellets of 10 to 20 million cells at −80°C before analysis.
TPMT activity in blasts.
For the measurement of TPMT activity in frozen pellets, cells were lysed by sonication (3 × 10 second bursts) on ice following the addition of 0.5 mL of 50 mmol/L Tris-HCl pH 7.8 containing 1 mmol/L EDTA and 1 mmol/L dithiothreitol (lysis buffer). Samples were centrifuged at 5,000 rpm in a microfuge and the supernatant removed for centrifugation at 100,000g for 1 hour using a benchtop ultracentrifuge (Beckman, Oxford, UK). Fifty-microliter aliquots were mixed with an equal volume of lysis buffer before measurement of TPMT activity according to the method described by McLeod.26 Total protein levels in the supernatant were measured using a commercially available kit (BioRad, Hemel Hempstead, UK). As variable red blood cell contamination was noted in several samples, the optical density (OD) of the supernatant after ultracentrifigation was measured at 430 nm and the amount of protein attributable to red blood cell contamination was assessed by reference to a standard curve of OD430 versus protein concentration calculated using normal RBC. This value was subtracted from the total protein measurement. The maximum contamination noted was equivalent to 0.2 g/L of haemoglobin. At this level, the effect on TPMT activity will be negligible, as this represents a 1:100 dilution of the concentration used in red blood cell assays.27 Results were expressed as units of enzyme activity per milligram of protein, where one unit represents the formation of 1 mmol of methyl mercaptopurine per hour of incubation at 37°C. Multiple assays of CCRF-CEM (a human T-lymphoblastoid cell line) cells gave a coefficient of variability of 8.4% (n = 10; mean activity, 0.24 nU/mg).
Genotype analysis.
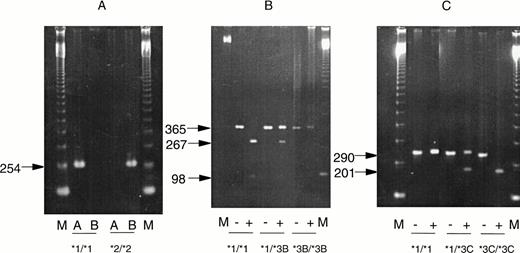
DNA was extracted from cell pellets obtained at the same time as those used for enzyme determination or, where not available, during remission. Detection of TPMT mutations using the polymerase chain reaction (PCR) was performed according to the method described by Yates et al24 using a Perkin Elmer thermal cycler (Perkin Elmer-Cetus, Foster City, CA). Briefly, detection of the G238C mutation relied on the use of wild-type and mutant specific primers (Fig 3A). Positive control DNA for this reaction was kindly provided by Professor W.E. Evans (St Jude Hospital, Memphis, TN). Detection of G460A used digestion of PCR products from the junction between intron 6 and exon 7 using theMwo I restriction enzyme. Wild-type DNA yields fragments of 267 and 98 bp, whereas mutated DNA fails to digest (Fig 3B). Detection of A719G used digestion of PCR products from the junction between intron 9 and exon 10 using Acc I. In this case, the presence of a mutation introduces a restriction site and leads to the production of fragments of 207 and 86 bp, whereas the wild-type DNA is undigested (Fig 3C).
Results of PCR assays for mutations at position G238C (A), G460A (B), and A719G (C). Photographs of ethidium bromide-stained gels are shown. “M” represents marker lanes. In (A) “A” indicates the result with wild-type and “B”, the result with mutation-specific primers. In (B and C), “−” indicates the result before digestion and “+” after digestion with restriction enzymes, as descibed in Materials and Methods. Homozygous mutated DNA was obtained from cases with absent TPMT activity not included in the present study.
Results of PCR assays for mutations at position G238C (A), G460A (B), and A719G (C). Photographs of ethidium bromide-stained gels are shown. “M” represents marker lanes. In (A) “A” indicates the result with wild-type and “B”, the result with mutation-specific primers. In (B and C), “−” indicates the result before digestion and “+” after digestion with restriction enzymes, as descibed in Materials and Methods. Homozygous mutated DNA was obtained from cases with absent TPMT activity not included in the present study.
Chemicals.
S-[methyl-14C]-adenosyl-L-methionine (specific activity, 57 mCi/mmol) was purchased from Amersham International (Bucks, UK). Optiphase Hisafe II scintillation cocktail was obtained from Amersham Pharmacia Biotech (Uppsala, Sweden), and Lymphoprep Ficoll-Hypaque solution from Nycomed (Oslo, Norway). Taq polymerase was from Promega (Southampton, UK), and the buffers used in the PCR reactions from Invitrogen (NV Leek, The Netherlands). All other chemicals were from Sigma Chemical Co (Pool, Dorset, UK).
Data analysis.
The Mann Whitney U test was used to compare enzyme activity between the groups of patients described below.
RESULTS
TPMT activity measurements.
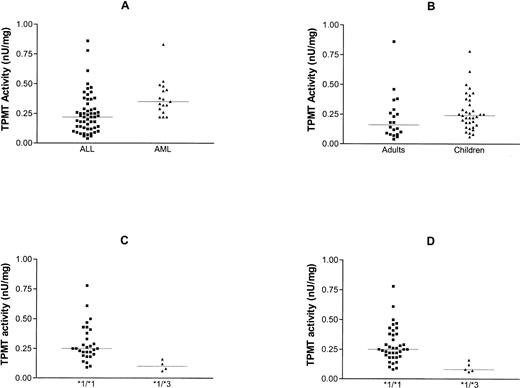
TPMT activity in lymphoblasts varied between 0.04 and 0.86 nU/mg protein, with a median of 0.22 nU/mg. This was significantly lower than the median level in myeloblasts (0.35 nU/mg, P < .002, Fig 4A) where the activity ranged from 0.22 to 0.83 nU/mg.
Comparison of TPMT activity in blasts from patients with ALL and AML (A), adults and children with ALL (B), and children with ALL who had the *1/*1 and *1/*3 genotype (C). (D) Shows the relationship between TPMT phenotype and genotype for all cases of ALL (adults and children). Lines indicate median values.
Comparison of TPMT activity in blasts from patients with ALL and AML (A), adults and children with ALL (B), and children with ALL who had the *1/*1 and *1/*3 genotype (C). (D) Shows the relationship between TPMT phenotype and genotype for all cases of ALL (adults and children). Lines indicate median values.
Comparison of the activity in lymphoblasts from children with measurements from cells from adults showed no significant difference (0.24 v 0.16 nU/mg, P = .08, Fig 4B).
TPMT genotyping.
Samples were available for genotyping for 33 of the 35 cases of childhood ALL, 10 of the 20 cases of adult ALL, and nine of the 17 cases of adult AML. Mutations at positions 230, 460, or 719 were detected in five cases in total (9%); four were in children with ALL and one in an adult with ALL. All were of the *3A type with mutations at both positions 460 and 719.
Correlation between genotype and phenotype in blasts.
Correlation of genotype with phenotype in children with ALL showed that the TPMT activity was significantly higher in those with no evidence of mutation at the three loci tested as compared with those with detectable mutations (0.25 v 0.1, P < .005, Fig 4C). The relationship between genotype and phenotype was similar when all cases of ALL were compared, ie, adults and children. The median activity in the nonmutated group was 0.25 and that in the heterozygotes 0.08 (P < .002, Fig 4D).
DISCUSSION
Although the empirical approach to the development of treatment for ALL has been highly successful, it has resulted in very complex regimes, which use multiple drugs, given either as short pulses (induction and intensification) or prolonged periods (continuing therapy). The way in which these trials have been performed has resulted in the titration of dosage and dose-intensity to a population determined level of toxicity. However, in recent years, there has been an increased awareness of interindividual variations in the metabolism of cytotoxic drugs due, in part, to pharmacogenetic factors and it has recently been demonstrated that improvements in long-term survival can be achieved by adjusting drug dosages in response to individual differences in pharmacokinetics.28 29
Although more than 95% of patients with ALL achieve complete morphologic remission using vincristine, steroids, and asparaginase, evidence from molecular studies suggests that many still have some residual disease at the end of intensification therapy.30This observation explains the marked impact that continuing therapy makes on treatment outcome. There is evidence to suggest that sustained treatment with the maximum tolerated doses of 6-MP and methotrexate is particularly important during this phase of treatment. For example, the introduction of continuous daily 6-MP and titration of the dosage to specified neutrophil and platelet counts during continuing therapy was associated with a 15% to 20% increase in the 4-year survival rate in a trial performed by the Medical Research Council in the United Kingdom (UKALL VIII). Although this trial also introduced intramuscular rather than intravenous asparaginase therapy, it was noted that the most striking clinical difference between UKALL VIII and earlier trials was sustained myelosuppression during continuing therapy.31Furthermore, the importance of continuing therapy in preventing relapse is supported by recent studies, which suggest that treatment with five drugs (vincristine, dexamethasone, asparaginase, methotrexate, and mercaptopurine) may be as effective as treatment with eight in low-risk patients.32 33
To increase the efficacy of 6-thiopurines in ALL, attempts have been made to maintain patients at the maximum tolerated dose at all times. However, in a study of the effect of individualized dose escalation of 6-MP during continuing therapy, Welch and Lilleyman34 found that the median cumulative dose was not increased above levels achieved in conventionally treated patients and that the chief consequence of this approach to therapy was to generate longer periods off therapy. The investigators noted that this could effectively decrease the antineoplastic activity of the drug. To achieve maximum tolerated dosage in individual patients, more information is required relating to variations in the clinical and molecular pharmacology of 6-MP.
6-Thiopurines are orally administered prodrugs, which are only effective once they have entered the lymphoblast and have been converted to thioguanine nucleotides before incorporation into DNA. It may be inferred that a number of pharmacokinetic and intracellular factors may influence the efficacy of these drugs. These include variations in compliance,35 hepatic metabolism, cellular uptake, catabolism and anabolism, DNA incorporation, and the development of tolerance to DNA incorporation of fraudulent bases.
6-MP undergoes extensive first-pass metabolism. At a dose of 75 mg/m2, the mean bioavailability is only 16% (range, 5% to 37%).36 One cause is the presence of xanthine oxidase in the intestinal mucosa and liver. This is demonstrated by the fact that allopurinol, a potent inhibitor of xanthine oxidase, increases bioavailability by a factor of 5. A second route of catabolism is via S-methylation through the action of TPMT. Unlike xanthine oxidase levels of TPMT expression vary considerably, as described above. Although studies of the role of TPMT in determining the response of patients to continuing therapy have concentrated on the effects of this enzyme on drug conversion within leukemic cells, it is possible that variations in expression in the liver and intestine will also influence response through an effect on systemic pharmacokinetics.
Family studies have shown that TPMT deficiency is inherited as an autosomal recessive trait.37 There are no apparent adverse effects unless and until treatment is instigated with 6-MP, 6-TG, or azathioprine (a widely used immunosuppresant that is metabolized to 6-MP). Administration of thiopurines to these patients is rapidly followed by life-threatening myelosuppression. Recently two groups have reported the cloning and characterization of cDNA from TPMT-deficient patients and showed a novel mutant allele (TPMT*3) containing two nucleotide transitions (G(460)→A and A(719)→G) producing amino acid changes at codons 154 (Ala→Thr) and 240 (Tyr→Cys).23 38 This differed from the rare mutant TPMT allele, ie, TPMT*2 with only G(238)→C, previously identified by the group from St Jude Hospital. Site-directed mutagenesis and heterologous expression established that either TPMT*3 mutation alone leads to a reduction in catalytic activity (G(460)→A, ninefold reduction; A(719)→G, 1.4-fold reduction), while the presence of both mutations leads to complete loss of activity. Using mutation-specific PCR-restriction fragment length polymorphism (RFLP) analysis, the TPMT*3 allele was detected in genomic DNA from approximately 75% of unrelated white subjects with heterozygous phenotypes, indicating that TPMT*3 is the most prevalent mutant allele associated with TPMT-deficiency in Caucasians. This is in agreement with our findings where all of the five mutations detected were of the TPMT*3A type.
Most population studies of TPMT activity have used RBC as a convenient source of samples. Levels in RBC have been shown to reflect levels in other tissues, including lymphoblasts.26 One major problem with the use of RBC is the fact that samples must be obtained before transfusion. This is often not practical in a disease that may present with life-threatening anemia. As an alternative, genotyping may be performed using DNA extracted from lymphoblasts or normal tissues. One potential problem with this approach is that although more than 80% of heterozygotes have the TMPT*3 mutation, a small, but significant, minority have more rare mutations. In the work reported in this publication, we assessed the value of measuring TPMT activity in leukemic blasts and, for the first time, have related this directly to genotype.
We found that reliable measurements could be made using 10 million leukemic cells and that the enzyme activity in cells with TPMT*3 mutations were significantly lower than those from cases in which mutations were not detectable. It was noticeable, however, that there were several cases in which low activity was not associated with detectable mutations, suggesting either the presence of mutations in regions of the gene, which were not examined, or that other factors may regulate TPMT expression.
The presence of factors that effect TPMT expression other than the number of nonmutated genes present is also suggested by the finding that the level in blasts from patients with AML is significantly higher than that in lymphoblasts (Fig 4A). Previous reports in which levels of TPMT activity in RBC increase during maintenance therapy also suggests that epigenetic factors may affect the rate of transcription.26 Interestingly, we could find no difference in the level of TPMT in blasts obtained from children and those from adults, suggesting that differences in the extent of 6-MP methylation does not contribute to the poor treatment outcome in older patients.
The finding of relatively high levels of TPMT in AML blasts is of interest in view of the fact that many treatment regimes for this disease use 6-thioguanine for remission induction. 6-Thioguanine is also a substrate for TPMT, although the fact that methyl thioinosine monophosphate (Me tIMP, Fig 1) is not formed with this drug means that the consequences of variations in TPMT expression are likely to differ, as me tIMP can act as an inhibitor of de novo purine synthesis.39
In summary, we have provided evidence that TPMT measurements in leukemic blasts reflect the genotype of the patient and may be used as an additional means of assessing the TPMT status. Such measurements may be of more direct relevance to the extent of drug inactivation by methylation than genotyping, as the effect of variations in the rate of transcription is also assessed. In addition the chance of false negative results due to the presence of rare mutations is eliminated. The general application of this method is limited by the need to obtain a relatively large number of cells at diagnosis. Furthermore, studies need to be performed on different ethnic groups to define accurate cut-off values of prognostic value in different populations. It is also important to note that genotyping and measurements in RBC may more accurately reflect systemic expression, which may in turn, be of importance in controling the amount of 6-MP that reaches the target tissues.
ACKNOWLEDGMENT
The assistance of Andrew Pearson, Herbie Newell, Mike Reid, Mathew Barnes, Gordon Taylor, Kay Maynard, Caroline Austin, Elizabeth Matkinson, and Celia Rabello is gratefully acknowleged.
Supported by grants from the Leukaemia Research Fund (London, UK) and the Kay Kendall Research Fund (London, UK).
Address reprint requests to Andrew G. Hall, MRCP, PhD, LRF Group, CRU, Medical School, Framlington Place, Newcastle Upon Tyne, NE2 4HH, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.