To the Editor:
p53 mutations have been detected in about 10% of all acute myeloid leukemia (AML) patients, mostly in patients with 17p monosomy.1-3 The scarcity of p53 mutations in AML could mean that, in the vast majority of AML patients, loss of p53 protein function is not required for the development of this disease. Alternatively, it is possible that inactivation of the p53 growth regulatory pathway is important and that this can occur either through disruption of downstream effector molecules or through epigenetic mechanisms that regulate p53 protein function. It has been suggested, for example, that inactivation of wild-type p53 protein in AML occurs through a mechanism involving conformational change of the protein4,5 or through binding to MDM2 protein.6-8 We have examined the functional status of the wild-type p53 protein expressed in cell lines derived from AML blasts on the basis of site-specific DNA binding activity, transactivation of p53-responsive genes, and ability to promote cell cycle arrest in G1 in response to γ-irradiation.9 The first two properties of p53 protein are strongly associated with its tumor suppressor function.10 11
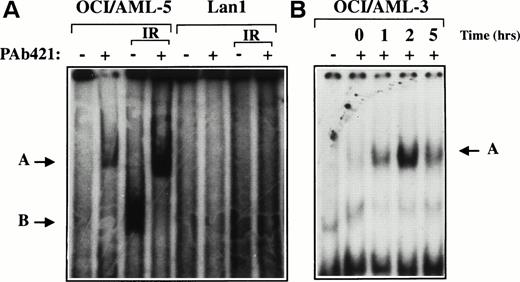
Nucleotide sequence analysis of the entire p53 coding region in four p53-expressing AML cell lines (OCI/AML-2, -3, -4, and -5)12 showed wild-type sequence. The site-specific DNA binding activity of p53 protein expressed in OCI/AML-3 and OCI/AML-5 cells was examined using an electrophoretic mobility shift assay (EMSA). Nuclear protein extracts were prepared from γ-irradiated or untreated cells and mixed with a 32P-labeled double-stranded oligonucleotide containing a p53 binding consensus sequence, p53CON.13 DNA damage increases the intracellular concentration of p53 protein and is also believed to activate the latent, sequence-specific DNA binding activity of p53. Whereas little, if any, DNA binding activity was detected in the nonirradiated extracts, the formation of a p53:DNA complex was evident when extracts were prepared from irradiated cells (Fig1). Inclusion of the p53-specific monoclonal antibody PAb421 in the binding reaction resulted in a supershifted p53:DNA complex and served to confirm the presence of p53 protein in the protein:DNA complex. DNA binding was not observed when an extract from the p53-negative cell line Lan1 was used in the EMSA.
DNA binding activity of p53 protein in AML cell lines. Nuclear extracts prepared from untreated or γ-irradiated OCI/AML-5 (A) and OCI/AML-3 (B) cells were incubated with a32P-labeled double-stranded oligonucleotide containing the p53 consensus sequence (p53CON) with (+) or without (−) the p53-specific monoclonal antibody PAb421 and analyzed by EMSA. Lan1 cells, which lack p53 protein, were used as a negative control. The OCI/AML-5 and Lan1 extracts were prepared 3 hours after γ-irradiation with a dose of 6 Gy. The OCI/AML-3 extracts were prepared at the times indicated after γ-irradiation with a dose of 2 Gy. The arrow labeled B points to the p53:DNA complex, and the arrow labeled A points to the supershifted antibody:p53:DNA complex.
DNA binding activity of p53 protein in AML cell lines. Nuclear extracts prepared from untreated or γ-irradiated OCI/AML-5 (A) and OCI/AML-3 (B) cells were incubated with a32P-labeled double-stranded oligonucleotide containing the p53 consensus sequence (p53CON) with (+) or without (−) the p53-specific monoclonal antibody PAb421 and analyzed by EMSA. Lan1 cells, which lack p53 protein, were used as a negative control. The OCI/AML-5 and Lan1 extracts were prepared 3 hours after γ-irradiation with a dose of 6 Gy. The OCI/AML-3 extracts were prepared at the times indicated after γ-irradiation with a dose of 2 Gy. The arrow labeled B points to the p53:DNA complex, and the arrow labeled A points to the supershifted antibody:p53:DNA complex.
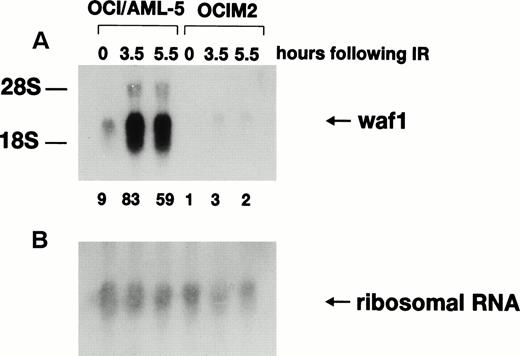
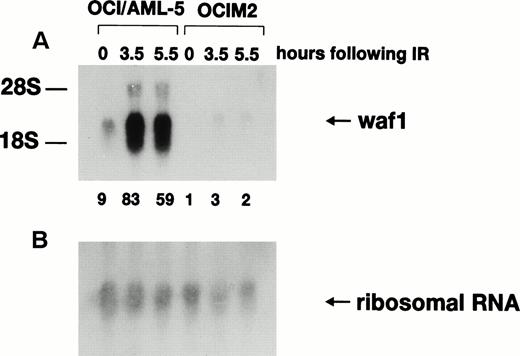
Activation of p21WAF1 gene transcription after γ-irradiation depends on wild-type p53 protein, and the p21WAF1 gene has been proposed to be a critical downstream effector in the p53-specific pathway of growth control in mammalian cells.14-17 Northern blot analysis (Fig2) indicated that the basal level of p21WAF1 mRNA was ninefold higher in OCI/AML-5 than in the mutant p53-expressing human erythroleukemia cell line OCIM2. Furthermore, 3.5 hours after irradiation with 6 Gy, p21WAF1mRNA levels increased ninefold in OCI/AML-5 and about threefold in OCIM2 cells. Irradiated OCI/AML-5 cells contained about 30-fold more p21WAF1 mRNA than did irradiated OCIM2 cells. No further increase in p21WAF1 mRNA levels was noted at later times after irradiation. p21WAF1 induction was also observed in irradiated OCI/AML-3 and OCI/AML-4 cell lines.18 The mRNA levels for GADD45 and MDM2, two other genes known to be transcriptionally regulated by p53 in response to DNA damage, also increased after γ-irradiation of AML cell lines (data not shown).
Expression of p21WAF1 mRNA in γ-irradiated OCI/AML-5 cells. Samples of total RNA (20 μg) prepared from cells at different times after exposure to 6 Gy of γ-irradiation were fractioned on an agarose-formaldehyde gel, transferred to a nylon membrane, and hybridized sequentially with 32P-labeled probes for human p21WAF1 cDNA (A) and 18S ribosomal RNA (B). OCIM2 cells, which express mutant p53 protein, were used as a control. Signal intensities were quantitated on a phosphorimager. The ratio of the p21WAF1 RNA signal to the 18S ribosomal RNA signal in the OCIM2 sample (0 hours) was arbitrarily set to 1.0 and the normalized values of p21WAF1 mRNA are shown at the bottom of (A).
Expression of p21WAF1 mRNA in γ-irradiated OCI/AML-5 cells. Samples of total RNA (20 μg) prepared from cells at different times after exposure to 6 Gy of γ-irradiation were fractioned on an agarose-formaldehyde gel, transferred to a nylon membrane, and hybridized sequentially with 32P-labeled probes for human p21WAF1 cDNA (A) and 18S ribosomal RNA (B). OCIM2 cells, which express mutant p53 protein, were used as a control. Signal intensities were quantitated on a phosphorimager. The ratio of the p21WAF1 RNA signal to the 18S ribosomal RNA signal in the OCIM2 sample (0 hours) was arbitrarily set to 1.0 and the normalized values of p21WAF1 mRNA are shown at the bottom of (A).
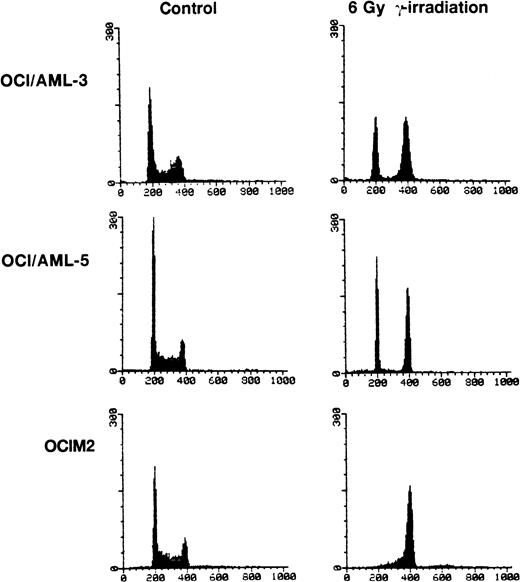
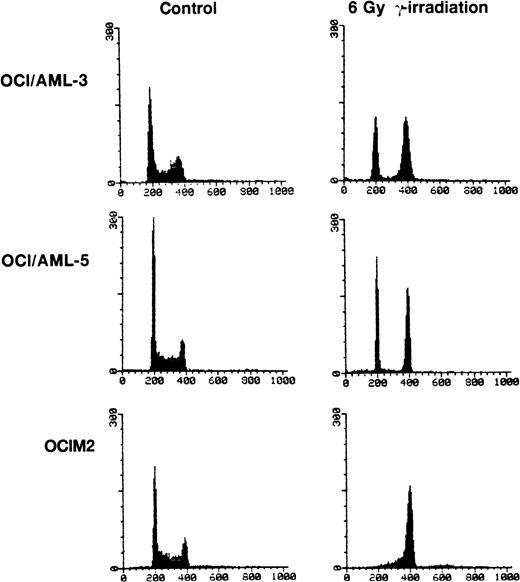
OCI/AML-3, OCI/AML-5, and OCIM2 cells were irradiated with a dose of 6 Gy and cell proliferation was assessed 16 hours later by propidium iodide staining and flow cytometry. Both OCI/AML-3 and OCI/AML-5 cells were blocked in the G1 and G2 phases of the cell cycle with little, if any, cells present in S phase. In contrast, the mutant p53-expressing OCIM2 cells accumulated in G2 and showed no evidence of a block in G1 (Fig 3). The failure of OCIM2 cells to arrest in G1 after γ-irradiation suggests that the G1 cell cycle block observed in irradiated OCI/AML-3 and OCI/AML-5 cells is likely to be dependent on functional p53 protein. Irradiation-induced G1 arrest was confirmed by dual-parameter flow cytometry after pulse labeling cells with BrdU and staining for DNA content with propidium iodide and for BrdU incorporation with a fluorescein isothiocyanate (FITC)-conjugated antibody for BrdU. OCI/AML-3 and OCI/AML-5 showed a ninefold and sixfold increase in the G1:S ratio 16 hours after γ-irradiation (6 Gy), respectively. An increase in the G1:S ratio provides a good indicator of G1 delay.
Cell cycle changes in AML cells after exposure to γ-irradiation (6 Gy). The DNA content was determined by staining the cells with propidium iodide and the resulting profiles resulting from propidium iodide fluorescence are shown. For the irradiated cells, the cell cycle analyses were performed 16 hours after irradiation. OCIM2 cells were used as a control.
Cell cycle changes in AML cells after exposure to γ-irradiation (6 Gy). The DNA content was determined by staining the cells with propidium iodide and the resulting profiles resulting from propidium iodide fluorescence are shown. For the irradiated cells, the cell cycle analyses were performed 16 hours after irradiation. OCIM2 cells were used as a control.
Our results indicate that p53 function (DNA binding, transactivation, and G1 checkpoint) is not lost during the development of AML or in the establishment of these AML cell lines. Functional p53 protein has also been demonstrated in human neuroblastoma,19 non-Hodgkin’s lymphoma,20 and even in certain HPV-positive cancer cell lines21 that contain wild-type p53 alleles. Hence, loss of p53 function or inactivation of the p53-dependent growth arrest pathway is not required for the development of certain malignancies, including AML.
ACKNOWLEDGMENT
Supported by grants from the Medical Research Council of Canada and the National Cancer Institute of Canada.