Abstract
Diffuse large B-cell lymphoma (DLCL) is characterized by a marked degree of morphologic and clinical heterogeneity. We studied 156 patients with de novo DLCL for rearrangements of the BCL2, BCL6, and MYC oncogenes by Southern blot analysis and BCL2 protein expression. We related these data to the primary site of presentation, disease stage, and other clinical risk factors. Structural alterations of BCL2, BCL6, and MYC were detected in 25 of 156, 36 of 116, and 10 of 151 patients, respectively. Three cases showed a combination of BCL2 and BCL6 rearrangements, and two cases had a combination of BCL6 and MYC rearrangements. BCL2 rearrangement was found more often in extensive (39%) and primary nodal (17%) lymphomas than in extranodal cases (4%) (P = .003). BCL2 rearrangement was present in none of 40 patients with stage I disease, but in 22% of patients with stage II to IV (P = .006). The presence of BCL2 rearrangements did not significantly affect overall survival (OS) or disease-free survival (DFS). In contrast, high BCL2 protein expression adversely affected both OS (P = .008) and DFS (P = .01). BCL2 protein expression was poorly correlated with BCL2 rearrangement: only 52% of BCL2-rearranged lymphomas and 37% of BCL2-unrearranged cases had high BCL2 protein expression. Rearrangement of BCL6 was found more often in patients with extranodal (36%) and extensive (39%) presentation versus primary nodal disease (28%). No significant correlation was found with disease stage, lymphadenopathy, or bone marrow involvement. DFS and OS were not influenced by BCL6 rearrangements. MYC rearrangements were found in 16% of primary extranodal lymphomas, versus 2% of primary nodal cases (P = .02). In particular, gastrointestinal (GI) lymphomas (5 of 18 cases, 28%) were affected by MYC rearrangements. The distinct biologic behavior of these extranodal lymphomas was reflected by a high complete remission (CR) rate: 7 of 10 patients with MYC rearrangement attained complete remission and 6 responders remained alive for more than 4 years, resulting in a trend for better DFS (P = .07). These data show the complex nature of molecular events in DLCL, which is a reflection of the morphologic and clinical heterogeneity of these lymphomas. However, thus far, these genetic rearrangements fail as prognostic markers.
© 1998 by The American Society of Hematology.
CLONAL CHROMOSOMAL abnormalities are associated with but not always specific for distinct types of non-Hodgkin’s lymphoma (NHL). These include t(8;14) and its variants t(2;8) and t(8;22) in Burkitt’s lymphoma, t(14;18) in follicular lymphoma, and t(11;14) in mantle cell lymphoma, involving MYC, BCL2, and BCL1 loci, respectively.1
By karyotyping, t(14;18)(q21;q34) has been identified in up to 85% to 90% of all cases of follicular lymphoma1; most translocations can be detected by Southern blot analysis. The reported incidence of t(14;18) translocations in diffuse large B-cell lymphoma (DLCL) is lower, with a range of 12% to 30%.2-7 The BCL2 gene was originally discovered by virtue of its involvement in this translocation. This leads to constitutive activation and increased expression of BCL2, which has been shown to inhibit apoptosis8 and, more generally, may block chemotherapy-induced cell death. Multiple BCL2 homologs that inhibit or promote cell death, as well as homologs that modulate BCL2 and BCL-XL activity, have been described.9 However, little is known about their possible involvement in cancer, especially as to whether these genes can be mutated. Recently, BAX mutations have been described in some B-cell lymphoma cell lines.10
The BCL6 gene was discovered due to its engagement in chromosomal translocations involving band 3q27 in NHL.11 BCL6 rearrangements are found in DLCL in up to 35% of cases.12-17 These rearrangements juxtapose heterologous sequences derived from other chromosomes to the BCL6 coding domain, causing its deregulated expression.18 The 5′ noncoding region of the BCL6 gene can also be altered by somatic point mutations that are detectable, independent of rearrangement, in 70% of DLCL cases.19 BCL6 encodes for a POZ/zinc-finger protein and acts as a transcriptional repressor.20 BCL6 is normally expressed in both B cells and CD4+ T cells within germinal centers, controlling germinal-center formation and Th2-type inflammation,21 but its precise function is unknown.
Translocation t(8;14)(q24;q32) was the first recurring chromosomal translocation shown to be associated with a lymphoproliferative disease.22 On the molecular level, the translocation juxtaposes the MYC gene in chromosome region 8q24 next to the IgH locus in chromosome region 14q32, leading to deregulation and overexpression of the transcription factor MYC. Translocation t(8;14) can be observed in most, if not all, cases of Burkitt’s lymphoma/leukemia, and in up to 15% of large-cell lymphoma cases.23-26 In addition, in Burkitt’s lymphoma27 and less frequently in DLCL,28 mutations affect both the coding and intron 1 sequences of MYC harboring MYC regulatory elements.
From both a clinical and histopathologic point of view, DLCL is highly heterogeneous. Survival can be predicted on the basis of pretreatment clinical characteristics as established by the International NHL Prognostic Factors Project.29 The genetic and molecular profile of DLCL in relation to biologic behavior and clinical outcome has been elucidated to a certain extent.21 There is controversy about the clinical significance of oncogene rearrangements in nodal and extranodal DLCL. DLCL is relatively frequent at extranodal sites. Studies of gastrointestinal (GI) and primary cutaneous large-cell lymphomas30 demonstrated a very low frequency of BCL2 rearrangements, whereas a higher frequency of MYC rearrangements was found in DLCL of the GI tract.31-33 Furthermore, a relatively high frequency of BCL6 rearrangements in extranodal DLCL was found.14 In the latter study, BCL6 rearrangements correlated with a favorable clinical outcome, but this could not be confirmed by others.13 BCL2 rearrangements are often confined to nodal DLCL, and here also, conflicting results are reported about the clinical impact.2-7
The population-based NHL Registry of the Comprehensive Cancer Center West (CCCW) in The Netherlands provided the opportunity to perform a molecular study on frozen tumor tissue from 156 patients with de novo DLCL to investigate the value of BCL2, BCL6, and MYC rearrangements in relation to clinical presentation, response to therapy, and survival. We correlated these results with BCL2 protein expression, which is a strong independent clinical risk factor for DLCL as reported by our group34 and others.6,7 35
SUBJECTS AND METHODS
Patients and materials.
In the population-based NHL Registry of the CCCW in The Netherlands, 1,168 patients with a histologically proven diagnosis of NHL were included between 1981 and 1989.34 The region of the CCCW contains 1.6 million inhabitants and 15 hospitals. Initial diagnoses were made by the local pathologists. All patients had disease staging according to the Ann Arbor classification.36 For adequate staging, computed tomographic scans of the thorax and abdomen and bone marrow histology were required. Patients were treated according to the recommendation of the local internist, and this treatment was considered “adequate” when doxorubicin-containing polychemotherapy with or without radiotherapy or radiotherapy alone was administered for patients with fully documented stage I disease. All tumors including immunohistochemistry were reviewed by a regional panel of pathologists. Primary cutaneous T-cell lymphoma, plasmacytoma, acute lymphoblastic leukemia, and chronic lymphocytic leukemia were excluded from the registry. Clinical parameters and treatment modality and outcome were recorded. The follow-up evaluation was updated annually for patients who were still alive.
In the CCCW records, 508 B-cell lymphomas were recognized with a histopathologic diagnosis of diffuse centroblastic-centrocytic (diff CbCc), diffuse centroblastic (diff Cb), immunoblastic (Ib), and mucosa-associated lymphoid tissue of high-grade malignancy (MALT high), compatible with a diagnosis of DLCL as recently defined by the Revised European-American Classification of Lymphoid Neoplasms (REAL) classification.37 For 122 patients, no or only bad-quality tissue blocks were available. Representative paraffin-embedded tumor tissue from the remaining 386 patients included material from all 15 hospitals of the CCCW. All cases were reviewed by three hematopathologists and reclassified according to the updated Kiel and REAL classification.37 38 In 14 of 386 patients, the initial diagnosis was revised: seven cases of diff CbCc were reclassified as follicular CbCc (n = 2) and mantle cell lymphoma (n = 5), and seven cases of diff Cb were reclassified as follicular Cb (n = 6) and mantle cell lymphoma (n = 1). This resulted in a final cohort of 372 patients with DLCL.
From 156 of these 372 cases, frozen tissue could be collected from the frozen tissue bank of the Department of Pathology, Leiden University Medical Center, for Southern blot analysis. The series includes 40 cases published previously; for these cases, only BCL2 and MYC rearrangements were investigated.39 The remaining 116 patients were tested for BCL2, BCL6, and MYC rearrangements.
For 84 patients, treatment was adequate with doxorubicin-containing polychemotherapy (with or without radiotherapy) or radiotherapy alone for patients with fully documented stage I disease.
Three patterns of initial disease presentation were defined: (1) primary nodal NHL with presentation in the lymph node, Waldeyer’s ring, spleen, or bone marrow, (2) primary extranodal NHL with presentation in other sites with or without local lymph node involvement, and (3) extensive NHL with presentation in both extranodal and distant nodal sites (often the other side of the diaphragm).
Southern blot analysis.
High–molecular-weight DNA was isolated from frozen tissue blocks kept at −80°C. Five to 10 tissue slides (20 μm) were treated by standard proteinase K and phenol-chloroform extraction as described previously.39 One additional slide was used for estimation of the presence of lymphoma cells in each particular DNA sample. Ten micrograms of DNA were digested with each restriction enzyme using buffers recommended by the supplier (Boehringer, Mannheim, Germany), size-fractionated by gel electrophoresis on 0.8% agarose gels, denatured, and transferred with 0.4 mol/L NaOH/1 mol/L NaCl onto nylon membranes (Hybond N+; Amersham, Amersham, UK). After transfer, the membranes were neutralized in 0.2 mol/L Trishydrochloride, pH 7.5/2× SSC (1× SSC is 0.15 mol/L NaCl plus 0.015 mol/L sodium citrate), dried, and baked at 80°C for 2 hours.
The membranes were prehybridized for 2 hours at 65°C in 6× SSC, 5× Denhardt solution (0.1% Ficoll, 0.1% polyvinylpyrrolidone, and 0.1% bovine serum albumin), 0.5% sodium dodecyl sulfate (SDS), 10% PEG 6000, and 50 μg/mL salmon sperm DNA. Purified DNA probes were radiolabeled with 20 μCi [α32P]dCTP (> 3,000 Ci/mmol; Amersham) using the random-primed DNA labeling kit (Pharmacia, Uppsala, Sweden) with a specific activity of approximately 10−8 to 10−9 cpm/μg DNA. After 16 hours of hybridization, the membranes were washed for 30 minutes in 1× SSC/0.1% SDS and 30 minutes in 0.1× SSC/0.1% SDS at 65°C and then exposed to Kodak X-Omat AR films (Eastman Kodak, Rochester, NY) with DuPont Cronex Lightning-Plus screens (DuPont de Nemours, Wilmington, DE) at −70°C.
The following probes were used: pCμ51/Jh, a 2.5-kbEcoRI-BgIII fragment of the Ig heavy chain–joining genes (kindly provided by P. Leder, Boston, MA); pSP64/18-4RH, a 2.8-kbEcoRI-HindIII fragment from the major breakpoint region (MBR) of the BCL2 gene (kindly provided by Tsujimoto, Osaka, Japan); pFL-2, a 4-kb Eco-HindIII fragment containing the minor cluster region (mcr) of the BCL2 gene (kindly provided by M. Cleary, Stanford, CA); pMYC/exIII/3138, a 1.4-kb ClaI-EcoRI fragment representing exon 3 of MYC derived from the 10-kbEcoRI-EcoRI genomic clone pHM-1 (kindly provided by I. Laird, Leiden, The Netherlands); pGSc4.0, a 4-kb SacI-Sac I fragment representing the 3′ region of exon 1a of the BCL6 gene (kindly provided by R. Dalla-Favera, New York, NY); p486a (probe F372) and p486 (probe F370), a 2.6-kb BamHI-XhoI and 3.4-kb Xba I-BamHI fragment, respectively, covering the breakpoint region between exon 1a and 1b of the BCL6/LAZ3 gene (kindly provided by C. Bastard, Lille, France).
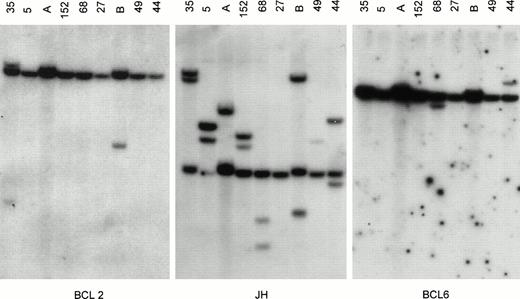
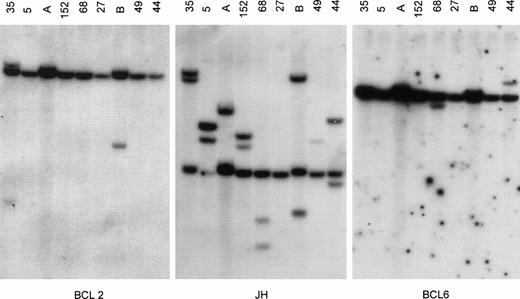
All DNAs digested with BgIII, EcoRI, andHindIII were successively hybridized with all probes for Jh, BCL2, BCL6, and MYC. Only DNA samples that carried one or more Jh signals in addition to the germline signal (ratio extra/germline > 10%) were included in the DNA analysis. The presence of breakpoints within BCL2, BCL6, or MYC was defined when two or more rearrangements were observed in three different digests. In the BCL6 breakpoint region, breakpoints are scattered over a region of 10 kb and small mutations affecting the restriction enzyme recognition sites, as well as restriction enzyme polymorphisms, are observed.17 Similarly, point mutations affect the first intron of MYC. For both genes, the clinical significance of the mutations is unknown. Therefore, when a rearrangement was observed in only one of the three digests, these DNA samples were additionally digested with Xba I and BamHI and hybridized with BCL6 probes. A representative Southern blot with hybridized tumor DNA is shown in Fig 1.
Southern blot analysis of IgH, BCL2, and BCL6 gene rearrangement. Tumor DNA samples from cases 35, 5, 152, 68, 27, 49, 44 (see Table 1) and 2 other lymphomas (lanes A and B) not included in the study were digested with BgIII and hybridized with probes for BCL2 (pSP64/18-4RH for MBR), JH (pCm51/JH), and BCL6 (pGSc4.0 for the 3′ region of exon 1a of BCL6). All tumors except case 27 showed 1 or more rearranged JH bands, indicating sufficient tumor DNA. Case 27 showed 2 strong rearranged JH bands in both theHindIII and EcoRI digests (not shown), indicating that the BgIII digest was false-negative. Case 35 showed rearrangement for BCL2, and cases 68 and 44 showed rearrangements for BCL6. Comigration of 1 rearranged BCL2 and 1 rearranged JH band in case 35 indicates t(14;18). Case 152 did not show BCL6 rearrangement but had BCL6 rearrangement upon analysis with the pGSc4.0 probe in theHindIII digest, as well as F370 and F372 BCL6 probes in theBgIII digest and the F370 probe in the EcoRI digest. This indicates that the BCL6 breakpoint is present in the MTC of BCL6 (not shown).
Southern blot analysis of IgH, BCL2, and BCL6 gene rearrangement. Tumor DNA samples from cases 35, 5, 152, 68, 27, 49, 44 (see Table 1) and 2 other lymphomas (lanes A and B) not included in the study were digested with BgIII and hybridized with probes for BCL2 (pSP64/18-4RH for MBR), JH (pCm51/JH), and BCL6 (pGSc4.0 for the 3′ region of exon 1a of BCL6). All tumors except case 27 showed 1 or more rearranged JH bands, indicating sufficient tumor DNA. Case 27 showed 2 strong rearranged JH bands in both theHindIII and EcoRI digests (not shown), indicating that the BgIII digest was false-negative. Case 35 showed rearrangement for BCL2, and cases 68 and 44 showed rearrangements for BCL6. Comigration of 1 rearranged BCL2 and 1 rearranged JH band in case 35 indicates t(14;18). Case 152 did not show BCL6 rearrangement but had BCL6 rearrangement upon analysis with the pGSc4.0 probe in theHindIII digest, as well as F370 and F372 BCL6 probes in theBgIII digest and the F370 probe in the EcoRI digest. This indicates that the BCL6 breakpoint is present in the MTC of BCL6 (not shown).
Immunohistology for BCL2 protein expression.
Paraffin-embedded sections were cut and fixed on 3′ aminopropyl triethoxysilan (APES)-coated glass slides. The sections were dried overnight at 37°C and deparaffinized in three changes of xylene for 5 minutes. Slides were incubated for 5 minutes in methanol and 20 minutes in methanol plus peroxide 1%, followed by a brief wash in deionized water. The slides were heated to 100°C in sodium citrate buffer (0.06 mol/L) for 20 minutes and slowly cooled for at least 2 hours. They were washed in phosphate-buffered saline (PBS) and preincubated with 10% normal goat serum for 15 minutes, followed by overnight immunostaining with the anti-BCL2 monoclonal antibody BCL2 124 (kindly provided by D. Mason, Oxford, UK) and by incubation with biotinylated rabbit anti-mouse Igs (1:200) and streptavidin-biotin peroxidase complex (1:100) (Dako, Glostrup, Denmark). The slides were stained with diaminobenzidine (Sigma, St Louis, MO). In addition, immunostaining with the L26 monoclonal antibody specific for B cells (CD20) and UCHL1 specific for T cells (CD45RO) (Dako) was performed to verify the B-cell nature of the neoplastic cells.
The presence of BCL2 protein expression was investigated, and three categories were defined: absent, none or less than 5% of tumor cells with cytoplasmic staining; low, variable and weak cytoplasmic staining of tumor cells; and high, strong cytoplasmic staining of almost all tumor cells. In all cases, reactive T lymphocytes served as an internal control.
Statistical analysis.
Survival curves were calculated according to the Kaplan-Meier method; survival analysis was performed using the log-rank test. Overall survival (OS) was calculated from the date of diagnosis until death or last follow-up evaluation. For patients with a complete remission (CR), disease-free survival (DFS) was estimated from the date of CR until first relapse or last contact, if disease-free.
RESULTS
Pathologic and clinical characteristics.
Clinical characteristics of 156 patients are shown in Table1, including clinically and/or pathologically documented tumor sites and staging data according to the Ann Arbor classification. Patients were grouped according to site of presentation: primary nodal, extranodal, or extensive.
In 93 patients, lymphoma presented with lymphadenopathy, and these were classified as primary nodal. Staging revealed additional extranodal tumor sites in seven patients. In 18 (21%) of 85 patients with a bone marrow biopsy, the marrow contained tumor. According to the Ann Arbor system, 16 of 85 adequately staged patients had stage I disease (19%), 27 stage II (32%), 21 stage III (25%), and 21 stage IV (25%). Sixteen additional patients presented with tonsillar localization, and of these, 14 also had cervical lymphadenopathy.
Forty-five patients presented with extranodal disease: stomach (n = 10), intestine (n = 8), breast (n = 4), soft tissue/muscle (n = 4), bone (n = 3), salivary gland (n = 2), lacrimal gland (n = 1), testis (n = 2), thyroid gland (n = 2), skin (n = 2), central nervous system ([CNS] n = 2), lung (n = 1), kidney (n = 1), adrenal gland (n = 1), and paranasal cavity (n = 1). Subsequent staging revealed that eight patients had multiple extranodal tumor sites. It also disclosed additional lymph node involvement in 17 cases. In 6 (15%) of 39 assessable patients, the bone marrow contained tumor. Nineteen (46%) of 41 adequately staged patients had stage I disease, 10 stage II (24%), and 12 stage IV (29%).
Eighteen patients presented with both extensive nodal and extranodal disease (GI tract, lung, pleura, breast, liver, bone, soft tissue/muscle, parotic gland, kidney, testis, and CNS). All patients had lymphadenopathy, 13 at both sides of the diaphragm (72%). In 7 of 16 adequately staged patients, the bone marrow showed infiltration of tumor cells (44%).
Bone marrow involvement was found in 31 patients: in 18, it was mainly concordant large cells; in five, it was discordant small cells; and in five, it could not be determined. There was no relation between discordant bone marrow involvement and any oncogene rearrangement tested.
Oncogene rearrangements in relation to pathologic and clinical characteristics.
The incidence of BCL2, BCL6, and MYC rearrangements in 156 patients is displayed in Tables 1 and 2 in relation to clinically and/or pathologically documented tumor sites and staging data according to the Ann Arbor classification. The series contained 40 cases published previously39: for these cases, only MYC and BCL2 rearrangements (36 cases with the MBR and mcr probe and four cases with the MBR probe only) were investigated. The remaining 114 patients were tested for BCL2, BCL6, and MYC rearrangements. Representative cases are shown in Fig 1. Survival curves in association with oncogene rearrangements are shown in Figs 2-4. Survival curves in association with BCL2 protein expression are shown in Fig5.
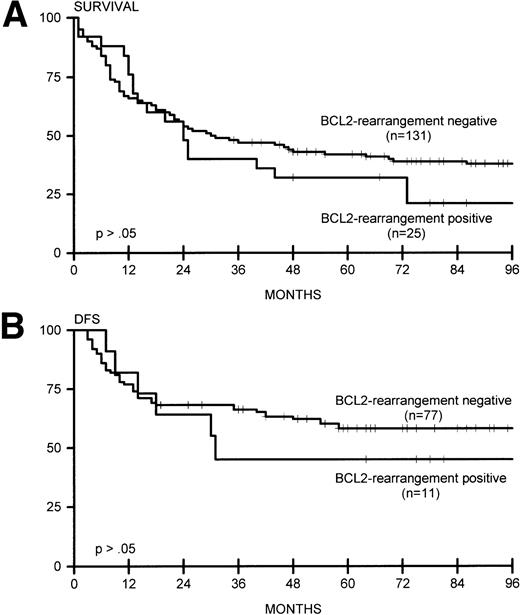
(A) Overall survival for BCL2-rearrangement positive (n = 25) and BCL2-rearrangement negative (n = 131) lymphomas. (B) DFS for BCL2-rearrangement positive (n = 11) and BCL2-rearrangement negative (n = 77) lymphomas.
(A) Overall survival for BCL2-rearrangement positive (n = 25) and BCL2-rearrangement negative (n = 131) lymphomas. (B) DFS for BCL2-rearrangement positive (n = 11) and BCL2-rearrangement negative (n = 77) lymphomas.
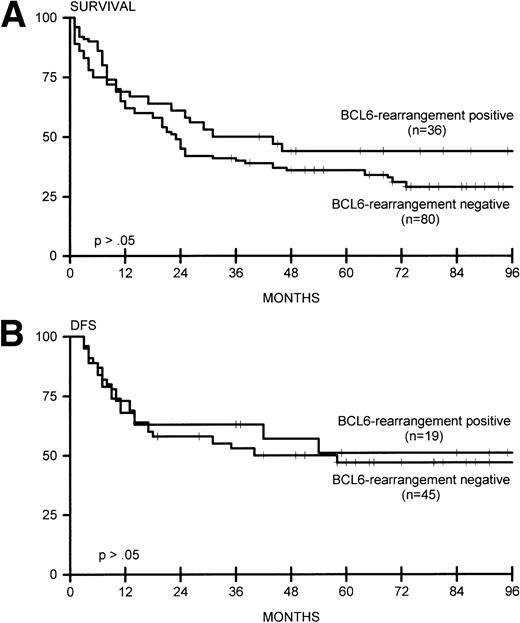
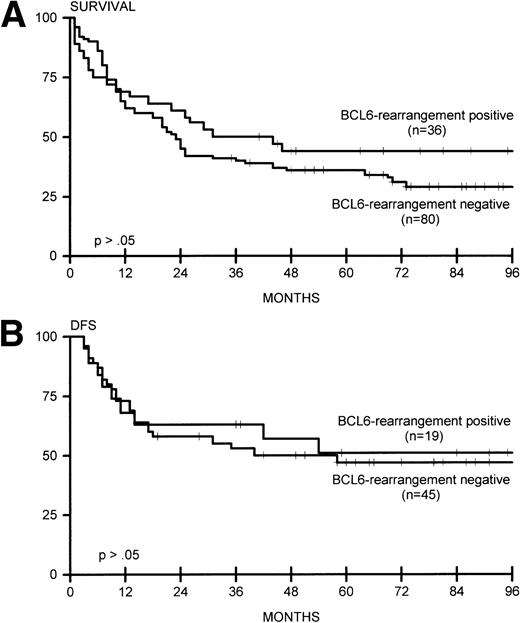
(A) Overall survival for BCL6-rearrangement positive (n = 36) and BCL6-rearrangement negative (n = 80) lymphomas. (B) DFS for BCL6-rearrangement positive (n = 19) and BCL6-rearrangement negative (n = 45) lymphomas.
(A) Overall survival for BCL6-rearrangement positive (n = 36) and BCL6-rearrangement negative (n = 80) lymphomas. (B) DFS for BCL6-rearrangement positive (n = 19) and BCL6-rearrangement negative (n = 45) lymphomas.
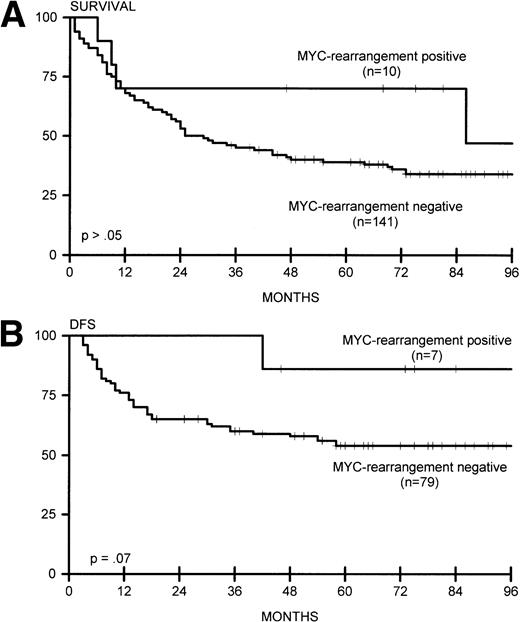
(A) Overall survival for MYC-rearrangement positive (n = 10) and MYC-rearrangement negative (n = 141) lymphomas. (B) DFS for MYC-rearrangement positive (n = 7) and MYC-rearrangement negative (n = 79) lymphomas.
(A) Overall survival for MYC-rearrangement positive (n = 10) and MYC-rearrangement negative (n = 141) lymphomas. (B) DFS for MYC-rearrangement positive (n = 7) and MYC-rearrangement negative (n = 79) lymphomas.
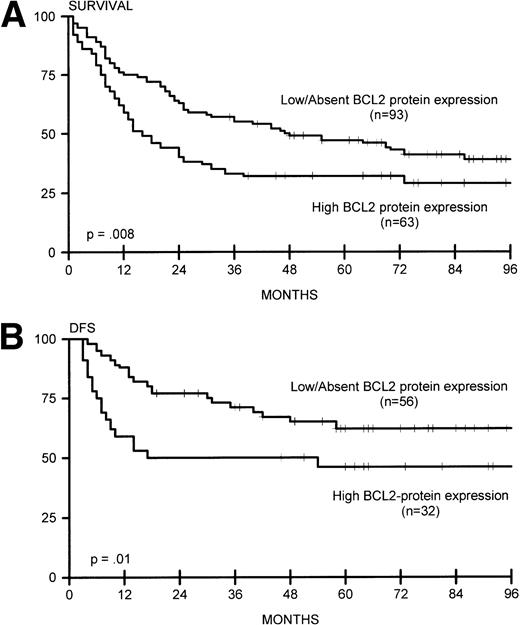
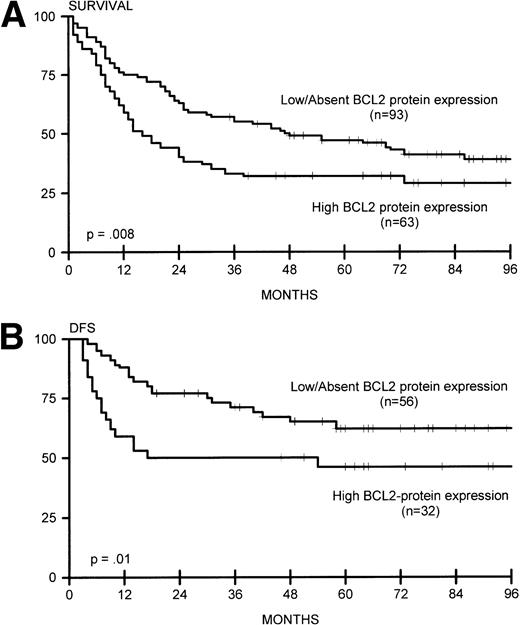
(A) Overall survival for lymphomas with high BCL2 protein expression (n = 63) and low/absent BCL2 protein expression (n = 93). (B) DFS for lymphomas with high BCL2 protein expression (n = 32) and low/absent BCL2 protein expression (n = 56).
(A) Overall survival for lymphomas with high BCL2 protein expression (n = 63) and low/absent BCL2 protein expression (n = 93). (B) DFS for lymphomas with high BCL2 protein expression (n = 32) and low/absent BCL2 protein expression (n = 56).
BCL2.
Sixteen of 93 nodal lymphomas (17%) versus 2 of 45 extranodal cases (4%) showed rearrangement of BCL2. In extranodal cases, the tumor presented in the thyroid gland and colon (with simultaneous rearrangement of BCL6). BCL2 rearrangement was found in none of 40 patients with stage I disease and in 25 (22%) of 116 patients with stage II to IV disease (P = .006), including 7 (39%) of 18 patients with extensive disease (P = .003). BCL2 rearrangement was significantly correlated with the presence of lymphadenopathy (24 of 123 patients with lymphadenopathy v 1 of 33 patients without, P = .03). Bone marrow involvement was not correlated with the presence of BCL2 rearrangements. In all cases with BCL2 rearrangement, comigration with a rearranged IgH gene sequence was detected with the JH probe, indicating t(14;18). BCL2 rearrangements involved the MBR in 23 and the mcr in two cases. We also analyzed BCL2 protein expression in these lymphomas. High expression was not exclusively confined to tumors with BCL2 rearrangements: 49 of 131 cases without BCL2 rearrangements were strongly BCL2 protein-positive (37%). Of note, 12 (48%) of 25 cases with BCL2 rearrangements had low or absent expression of BCL2 protein (Table3). BCL2 rearrangement was not associated with induction of complete remission or with OS or DFS (Fig 2A and B), in contrast to high BCL2 protein expression, which correlated with OS and DFS (Fig 5A and B). These data were also found for 84 patients who were adequately treated with doxorubicin-containing polychemotherapy with or without radiotherapy, or radiotherapy alone for patients with fully documented stage I disease (data not shown).
BCL6.
BCL6 rearrangements were studied in 116 patients, with 36 positive cases (31%). Twenty-one of 74 nodal cases (28%), 10 of 29 extranodal cases (34%), and 5 of 13 extensive cases (38%) showed rearrangement for BCL6. No significant correlation was found with the disease stage and the presence of lymphadenopathy. Bone marrow involvement was found in 4 of 36 patients with BCL6 rearrangement, versus 21 of 95 patients without rearrangement. Three of four patients with DLCL of the CNS showed rearrangement of BCL6. In two patients with nodal DLCL and one patient with extranodal DLCL, rearrangements of both BCL2 and BCL6 were found. Combined rearrangement of BCL6 and MYC was found in one nodal and one extensive case. Complete remission and OS and DFS was not significantly different between BCL6 rearrangement-positive and -negative cases (Fig 3A and B). These data were also observed for 65 patients who were adequately treated with doxorubicin-containing polychemotherapy with or without radiotherapy, or radiotherapy alone for patients with fully documented stage I disease (data not shown).
MYC.
MYC rearrangements were predominantly found in primary extranodal large-cell lymphomas (7 of 45 cases, P = .02), particularly GI lymphomas (5 of 18 v 4 of 116 cases, P = .001); the other sites were lung/pleura, soft tissue, and testis. Two nodal lymphomas showed MYC rearrangement, one together with rearrangement of BCL6. Additionally, one extensive case contained both MYC and BCL6 rearrangement. Comigration of rearranged MYC and JH gene sequences was found in 4 of 10 cases. Seven of 10 patients with MYC rearrangement attained complete remission, and 6 responders remained alive for more than 4 years (Fig 4A and B), resulting in a trend for better DFS (P = .07).
DISCUSSION
According to all available studies, including the present one, BCL2 rearrangements are detectable in 12% to 30% of DLCL.2-7In almost all cases, the rearrangement is caused by translocation t(14;18). We expanded our previous cohort of de novo DLCL39with even stronger proof that BCL2 rearrangements are found more often in primary nodal versus primary extranodal cases. Our data corroborate the study by Offit et al3 in that their stage I to III DLCLs with BCL2 rearrangement also presented with nodal involvement (no data were presented for stage IV cases). Similarly, in a cytogenetic analysis by Ott et al,33 no translocation t(14;18) was reported in high-grade MALT lymphomas of the stomach and parotid gland. Our results essentially differ from two other studies4,5showing BCL2 rearrangements in some cases with primary extranodal involvement. However, the latter cohorts used different definitions for nodal and extranodal disease. Interestingly, in the present study, BCL2 rearrangements were strongly associated with disseminated disease, since none of 40 patients with stage I disease showed a rearrangement. This is not reflected by a significantly higher percentage of bone marrow involvement or by discordant morphology. It may be proposed that a higher rate of bone marrow infiltration in BCL2 rearrangement-positive DLCL can be expected, since translocation t(14;18) is thought to arise from an error in the recombination process of precursor B cells in bone marrow,40 and some believe that t(14;18)-positive DLCL may represent progressed follicle center cell lymphoma, with bone marrow involvement in a high percentage of cases.41 Nevertheless, the finding that DLCL with BCL2 rearrangement is restricted to disseminated disease and nodal presentation fits with the hypothesis that these lymphomas have a distinct origin.
Interestingly, and in accordance with the results of Gascoyne et al,7 there is almost no correlation between BCL2 rearrangement and BCL2 expression in DLCL: almost half of DLCL cases with BCL2 rearrangement have no or weak BCL2 protein expression, and in reverse, approximately 40% of DLCL cases with high BCL2 protein expression lack detectable rearrangement. The first observation is difficult to justify, although this discrepancy can be explained by mutations in the open reading frame of the translocated BCL2 gene.42 Alternatively, t(14;18) and deregulation of BCL2 may have played an essential role in the initial lymphomagenesis, but may have lost their significance during further tumor progression. A similar transient role has been attributed to Epstein-Barr virus in African Burkitt’s lymphoma.43 One explanation for the fact that so many DLCL cases without detectable BCL2 rearrangement show overexpression of the protein is DNA amplification instead of translocation. Indeed, amplification of the BCL2 gene has been recently reported in a small series of DLCL cases with BCL2 overexpression.44 45 In our series, no significant amplification of BCL2 could be detected with Southern blotting analysis, with the possible exception of one case (data not shown).
In line with previous reports by our group34 and others,3-7,35 we found a different prognostic impact for BCL2 rearrangement and BCL2 protein expression in DLCL. No significant difference in BCL2 rearrangements in relation to CR, DFS, and OS was observed in the present study, in accordance with most reports,4-7 whereas other studies found a difference for DFS and one study also for OS.2,3,18 In contrast, in our cohort of patients, BCL2 protein expression was associated with poor DFS and OS. In a multivariate analysis, BCL2 protein expression was an independent risk factor in relation to well-defined clinical risk parameters.34 In a separate study on primary cutaneous DLCL, a similar relation between BCL2 expression and prognosis was found: primary DLCL of the head and trunk without BCL2 expression has a favorable clinical course compared with primary DLCL of the leg with concomitant high expression of BCL2 protein; both types of cutaneous DLCL lacked a detectable t(14;18) as tested by PCR.46
Previous studies have indicated that MYC rearrangements are detectable in 5% to 15%23-26 of patients with DLCL, especially in the GI tract.31,32 In the present study, rearrangement of MYC was found in 7% of cases with a majority of extranodal lymphomas originating from the GI tract. These results corroborate recent data on high-grade MALT lymphomas reported by Ott et al.33 They found t(8;14) in tumor tissue of the stomach in three of 21 high-grade MALT lymphomas. In our cohort, the distinct biologic behavior of these lymphomas with MYC rearrangements was reflected by a high CR rate and better DFS, with six of seven complete responders alive for more than 4 years. Offit et al14 reported nine cases with t(8;14); five of these presented at extranodal sites, but no correlation was found with GI lymphoma. Of note, we did not find any case with a combined t(14;18) and t(8;14) in the present series of de novo DLCL. Such cases often represent progressed follicular lymphoma or de novo leukemia/lymphoma with Burkitt-like morphology.47 48
The role of BCL6 rearrangements in DLCL is less distinct. BCL6 rearrangement is found in 23% to 37% of cases with Southern blotting,12-14 but only a minority are visible on the cytogenetic level.49 Cytogenetic detection is impeded by localization of the 3q27 breakpoint at the tip of chromosome 3q and by the great diversity in chromosomal partners involved in the breakpoint. On the other hand, with Southern blot analysis, the frequent point mutations in BCL6 can be easily interpreted as chromosomal breakpoints, especially if too few restriction enzymes and/or probes are used. The frequency of BCL6 rearrangements in the present series of DLCL (31%) is similar to that found by other groups.12-14In the present study, no significant correlation was found between BCL6 rearrangement and the site of presentation, although there was a slight preponderance of extranodal cases and cases with extensive disease. An association with extranodal disease was also found by Offit et al.14 We observed involvement of many different extranodal sites (CNS, testis, and bone), including MALT of the stomach, intestine, and breast. This relative unspecificity of BCL6 rearrangements is also encountered on the molecular level, since they can coexist with BCL2 and MYC rearrangements as shown by our group and others.14 Interestingly and in contrast to the present results and those of Bastard et al,13 in the study by Offit et al,14 patients with BCL6 rearrangements had a significantly better clinical outcome. In a supplementary analysis, Offit et al50 showed a correlation between prognosis and occurrence of chromosomal aberrations additional to BCL6 rearrangements. Therefore, it cannot be excluded that these different results for prognosis depend on a different occurrence of these additional genetic events.
Our observations, in part, support the hypothesis that the natural history of DLCL may vary depending on the pathogenesis of the tumor.41 According to a model proposed by Dalla-Favera et al,41 at least two distinct genetic pathways may lead to DLCL development: the first pathway consorts with DLCL transformed from a clinically undetectable follicular phase with BCL2 rearrangement; the second pathway involves BCL6. Our finding that BCL2 rearrangements are mainly present in a subset of patients with nodal and disseminated disease supports a distinct BCL2 pathway. However, assuming that these DLCLs arise from clinically occult follicular lymphoma, the events secondary to t(14;18) seem to differ from the events involved in tumor progression from overt follicular lymphoma, ie, P53 mutations51-53 and MYC activation by translocation,54 especially since no such events were encountered in a series of de novo DLCL with t(14;18)21 and since we did not find any MYC rearrangements in the presently analyzed t(14;18)-carrying DLCL. The occurrence of a distinct BCL6 pathway in de novo DLCL is not strongly supported by the present data or by prior data showing the coexistence of these rearrangements in (untransformed or transformed) t(14;18)-carrying follicular lymphomas.17 19
In conclusion, this report addresses the relevance of BCL2, BCL6, and MYC rearrangements in the biologic and clinical behavior of DLCL. Although these markers might, to some degree, delineate different oncogenetic pathways in DLCL, they fail as prognostic markers, especially when compared with the well-defined clinical variables of the international prognostic index.29
Supported by the Institute of Radioprotection/J.A. Cohen Institute (6.2.3), the Dutch Cancer Society (IKW 91-06), and the Ank van Vlissingen Fund.
Address reprint requests to P.M. Kluin, MD, PhD, Department of Pathology, Leiden University Medical Center, L1Q, PO Box 9600, 2300 RC Leiden, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.