Abstract
The blockade of platelet integrin glycoprotein (GP) IIb/IIIa is a promising new antiplatelet strategy. The binding of ligands or of the ligand-mimetic peptide RGD causes a conformational change of GP IIb/IIIa from the nonactivated to the activated state. Because several blocking agents/inhibitors are ligand-mimetics, the current study evaluates whether these agents have the intrinsic property to activate GP IIb/IIIa. Fibrinogen binding to GP IIb/IIIa on platelets or on CHO cells expressing recombinant GP IIb/IIIa was evaluated by flow cytometry or 125I-labeled fibrinogen. Incubation with the monoclonal antibody (MoAb) fragment c7E3 (abciximab) results in fibrinogen binding to GP IIb/IIIa and in the access of ligand-induced binding sites. At low concentrations (0.01 to 0.1 μg/mL), this intrinsic activating property of c7E3 can result in platelet aggregation. The disintegrin flavorodin and the RGD analogue fradafiban also induce fibrinogen binding, whereas the blocking MoAbs 2G12 and P2 and the activation-specific MoAb PAC-1 do not. Aspirin and indomethacin cannot block c7E3-induced fibrinogen binding to GP IIb/IIIa, but can inhibit c7E3-induced platelet aggregation. Thus, we conclude that GP IIb/IIIa inhibitors can demonstrate an intrinsic activating property, which can result in fibrinogen binding to GP IIb/IIIa and consequently in platelet aggregation. Cyclooxygenase inhibitors can inhibit platelet aggregation caused by GP IIb/IIIa inhibitors. Further studies will have to evaluate the clinical relevance of the potential intrinsic activating property of GP IIb/IIIa inhibitors and define consequences for the future drug development and evaluation of these potent antiplatelet agents.
© 1998 by The American Society of Hematology.
THERAPEUTIC STRATEGIES to inhibit platelet function, eg, by aspirin, are beneficial in various clinical settings.1 More recently, the platelet fibrinogen receptor, glycoprotein (GP) IIb/IIIa, which mediates platelet aggregation and partially platelet adhesion, has become a successful target for antiplatelet therapy.2-5 This glycoprotein belongs to the adhesion molecule family of integrins and is also termed αIIbβ3.2,6,7 Upon platelet activation, GP IIb/IIIa is functionally regulated by change in the anchorage of the receptor to the cytoskeleton8 and by a conformational change of GP IIb/IIIa that results in a high-affinity state for the ligand fibrinogen (activated GP IIb/IIIa).2,6,7 The amino acid sequence RGD, which is found in the α chain of fibrinogen but also in other ligands of GP IIb/IIIa, has been demonstrated to be directly involved in ligand binding to GP IIb/IIIa.2,7,9 For small peptides containing the RGD sequence, an effective inhibition of fibrinogen binding to GP IIb/IIIa and thus an effective inhibition of platelet aggregation has been demonstrated.9 Stimulated by the results with RGD peptides, several chemically synthesized RGD analogues have been developed.2,4 Furthermore, various snake venoms proved to be a rich source of a wide variety of GP IIb/IIIa blocking peptides, the so-called disintegrins.2 And finally, blocking monoclonal antibodies (MoAbs), such as c7E3 (abciximab), can be used for the blockade of GP IIb/IIIa.2-5
The binding of the ligand fibrinogen causes a conformational change of GP IIb/IIIa.10,11 RGD peptides mimic fibrinogen and thereby also induce a conformational change.10-14 In contrast to soluble fibrinogen, the small RGD peptides can bind to GP IIb/IIIa in the nonactivated, low-affinity state.13,14 If the RGD peptide is washed out from its receptor, GP IIb/IIIa is left in a high-affinity state with respect to the binding of fibrinogen.13 The present study evaluates whether GP IIb/IIIa inhibitors have the intrinsic property of inducing instead of blocking fibrinogen binding to GP IIb/IIIa. The major focus is on c7E3, because it is presently the most widely clinically used GP IIb/IIIa inhibitor.
MATERIALS AND METHODS
Blood preparation and cells.
Blood was collected by venipuncture with a 21-gauge butterfly needle from healthy volunteers and anticoagulated with citric acid. Platelet-rich plasma was obtained by centrifugation at 100g in plastic tubes at room temperature for 15 minutes in a laboratory centrifuge (Sorvall RT 6000; Du Pont, Bad Homburg, Germany).
CHO cells expressing either low-affinity (nonactivated) or high-affinity (activated) GP IIb/IIIa were produced as described elsewere in detail.15-17 For creation of the first, wild-type αIIb, for the second, a VGFFK-deleted αIIb, with an additional exchange of the αIIb cytoplasmic domain by the αLcytoplasmic sequence, were transfected together with wild-type β3 by electroporation.15 16 Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), 10% fetal calf serum, 1% MEM nonessential amino acids, 2 mmol/L L-glutamine, 700 μg/mL Geneticin, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from GIBCO, Eggenstein, Germany).
Antibodies and peptides.
The mouse/human chimeric Fab c7E3 (abciximab; ReoPro) was purchased from Beiersdorf-Lilly (Hamburg, Germany). IgG and F(ab′)2 of MoAb 7E3 were gifts of Centocor (Malvern, PA). The GP IIb/IIIa complex-specific MoAb 2G12 was provided by Dr Virgil Woods (University of California, San Diego, CA). The activation-dependent anti-GP IIb/IIIa MoAb PAC-118 was provided by Dr Sanford Shattil (Scripps Research Institute, La Jolla, CA). Anti-LIBS6 (anti-β3)19 was a gift from Dr Mark Ginsberg (Scripps Research Institute). The GP IIb/IIIa complex-specific MoAb P2 was purchased from Immunotech (Hamburg, Germany). The anti–P-selectin (anti-CD62P) MoAb (AK6) was purchased from Dianova (Hamburg, Germany). The fluorescein isothiocyanate (FITC)-labeled polyclonal chicken antifibrinogen antibody was purchased from Biopool (Umeå, Sweden), the peptide GRGDSP from Biomol (Hamburg, Germany), and flavorodin from Sigma (Deisenhofen, Germany). Fradafiban was a gift from the Dr Karl Thomae GmbH (Biberach, Germany).20 Human fibrinogen was purified from plasma as described.8 c7E3 binding was evaluated with biotinylated c7E3 and R-phycoerythrin–conjugated streptavidin (Dianova). Biotinylation was performed with BAC-SulfoNHS (Sigma) at a pH of 7.2, resulting in a biotin/protein ratio of 1.6.
Flow cytometry for the detection of P2, c7E3, and fibrinogen binding to GP IIb/IIIa as well as P-selectin expression on platelets.
Whole blood (1 μL) was diluted 1 to 50 in modified Tyrode’s buffer (150 mmol/L NaCl, 2.5 mmol/L KCl, 12 mmol/L NaHCO3, 2 mmol/L MgCl2, 2 mmol/L CaCl2, 1 mg/mL bovine serum albumin [BSA], 1 mg/mL dextrose, pH 7.4) and incubated with increasing concentrations of biotinylated c7E3 at room temperature for 20 minutes, either without or with the addition of 20 μmol/L ADP. A second incubation was performed with R-phycoerythrin–conjugated streptavidin (10 μg/mL; Dianova) at room temperature for 20 minutes. Fibrinogen binding and P-selectin expression was evaluated after dilution of whole blood as described above and one incubation step with FITC-labeled polyclonal chicken antifibrinogen Ab (10 μL) or FITC-labeled anti–P-selectin MoAb (5 μL), respectively. All samples were examined without centrifugation or fixation on FACScan with Lysis II (both Becton Dickinson, Mountain View, CA) software, gating platelets by forward/sideward scatter. Gates were established with an FITC–anti-GP Ib MoAb (Immunotech).
After detachment by Trypsin-EDTA (GIBCO), the GP IIb/IIIa-expressing CHO cells were washed twice in modified Tyrode’s buffer. Three hundred thousand cells per 50 μL modified Tyrode’s buffer were then incubated with biotinylated c7E3 (10 μg/mL) or FITC-conjugated MoAb P2 (20 μg/mL) for 30 minutes at room temperature and examined on FACScan.
Binding of fibrinogen to platelets and GP IIb/IIIa-expressing CHO cells pretreated with various GP IIb/IIIa inhibitors.
The percentage of platelets demonstrating binding of fibrinogen was measured by flow cytometry. The washout of GP IIb/IIIa inhibitors was performed similarly to the method described by Du et al.13Platelet-rich plasma (1 μL) was diluted 1 to 50 in modified Tyrode’s buffer and incubated for 30 minutes at room temperature with increasing concentrations of various GP IIb/IIIa inhibitors. Mild fixation was performed with 1% paraformaldehyde for 10 minutes at room temperature by the addition of CellFIX (Becton Dickinson) at one tenth of the sample volume. Then platelets were washed by dilution in 50 mL modified Tyrode’s buffer including fibrinogen at a final concentration of 3 g/L for 10 minutes and by a following centrifugation step. Pellets were resuspended in 50 μL modified Tyrode’s buffer with fibrinogen at a final concentration of 3 g/L, incubated for 30 minutes at room temperature together with 10 μL FITC-labeled polyclonal chicken antifibrinogen Ab, and analyzed on a FACScan. GP IIb/IIIa-expressing CHO cells were detached and washed as described above. Three hundred thousand cells per 50 μL modified Tyrode’s buffer were incubated with increasing concentrations of c7E3 or 2G12 for 30 minutes at room temperature. Without fixation, the CHO cells were washed and analyzed as described above for platelets.
Binding of fibrinogen to platelets induced by low concentrations of c7E3.
Whole blood (1 μL) was diluted 1 to 50 in modified Tyrode’s buffer and coincubated with various concentrations of c7E3 and 10 μL FITC-labeled polyclonal chicken antifibrinogen Ab for 30 minutes at room temperature. Without fixation or washing step, fibrinogen binding was evaluated as described above.
Purified human fibrinogen was radiolabeled with sodium [125I]-iodide using the chloramine-T method (Amersham International, Buckinghamshire, UK). Platelet-rich plasma was diluted with modified Tyrode’s buffer to a concentration of 150,000 platelets/μL. A total of 4.5 × 107 platelets per sample were incubated with 125I-fibrinogen (10 nmol/L) and various concentrations of c7E3 for 30 minutes at room temperature. Samples were either preincubated with the MoAb P2 (20 μg/mL) or without the addition. 125I-fibrinogen binding was evaluated without further fixation or washing by a Gamma counter LB 2103 (Berthold, Germany). Fifty-microliter aliquots were layered in quintuplets on 300 μL of 20% sucrose in modified Tyrode’s buffer and centrifuged for 3 minutes at 12,000 rpm. Counts associated with the cell pellet were determined.
Binding of MoAbs directed against a ligand-induced binding site (LIBS) on GP IIb/IIIa.
Whole blood (1 μL) was diluted l to 50 in modified Tyrode’s buffer and then incubated for 30 minutes at room temperature with 10 μg/mL of MoAb anti-LIBS6 and with saturating concentrations of c7E3, GRGDSP, or ADP (20 μmol/L) or without any addition. Cells were then fixed for 10 minutes at room temperature by the addition of CellFIX at one tenth of the sample volume. To specifically stain the anti-LIBS MoAb, but not c7E3, the pellet was resuspended after centrifugation in 50 μL modified Tyrode’s buffer containing 20 μg/mL of an FITC-labeled, polyclonal Fc-fragment-specific antimouse goat Ab (Dianova) that we proved did not bind to c7E3. After another incubation of 30 minutes at room temperature, cells were diluted in 300 μL modified Tyrode’s buffer and evaluated as described above. The fluorescence intensity of unspecific staining after incubation with an unspecific MoAb and with the secondary MoAb (mean fluorescence, 33 ± 5 arbitrary units) was subtracted from the evaluated specific mean fluorescence and the corrected value is given.
Aggregometry.
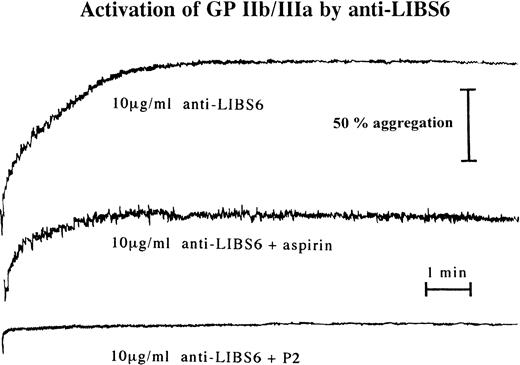
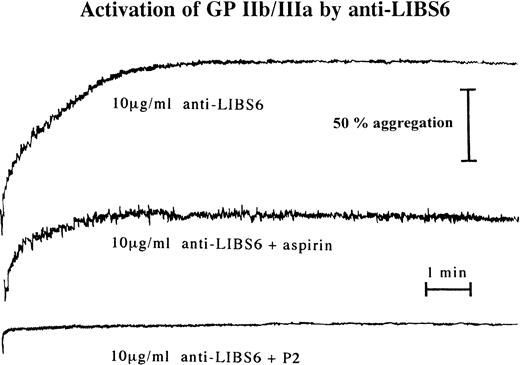
Aggregation of platelet-rich plasma was performed at a final volume of 220 μL after the addition of 20 μL of a 22 mmol/L CaCl2and MgCl2 solution on a Bio/Data PAP4 aggregometer (Moelab, Hilden, Germany) with a stirring rate of 1,000 rpm. As a positive control, aggregation was induced by 20 μmol/L ADP. Platelet-rich plasma was preincubated with either 100 μg/mL aspirin, 10 μmol/L indomethacin, 100 nmol/L prostaglandin I2 (PGI2), 20 μg/mL MoAb P2, or no addition for 15 minutes at room temperature. Incubation with 0.1 μg/mL c7E3, 10 μg/mL anti-LIBS6, or 0.1 μg/mL P2 was then performed for 30 minutes at room temperature. No stimulation (except for the positive control) and no fixation of platelets were performed.
RESULTS
c7E3 binds to the activated and nonactivated GP IIb/IIIa.
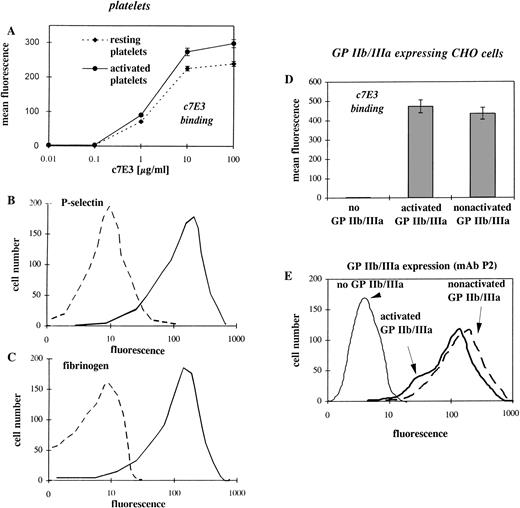
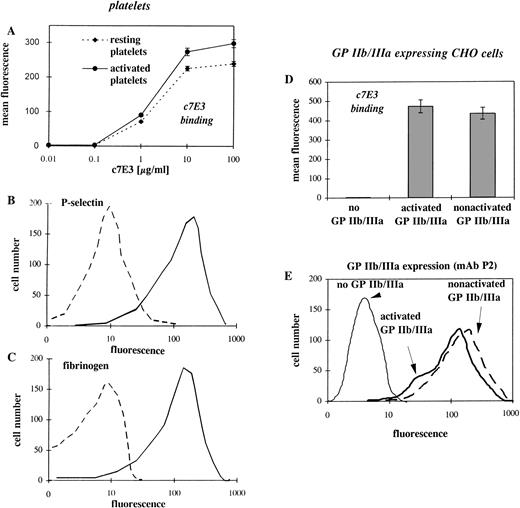
Because binding of the ligand fibrinogen and the ligand-mimetic peptide RGD can induce a conformational change of GP IIb/IIIa,10-14we hypothesized that the GP IIb/IIIa inhibitor, c7E3, by imitating a ligand, can also induce a conformational change. For this hypothesis, the binding of c7E3 at the low-affinity (nonactivated) GP IIb/IIIa is a precondition. Therefore, the binding of c7E3 to resting and activated platelets was evaluated by flow cytometry. In fact, steady-state binding of biotinylated c7E3 to activated platelets is only slightly higher than that to resting platelets (Fig1A). The activation status of platelets was affirmed by the expression of P-selectin and the binding of fibrinogen as evaluated in flow cytometry (Fig 1B and C). In those experiments with platelets, two variables make a cautious interpretation necessary. First, the number of the surface-exposed GP IIb/IIIa receptors can increase upon platelet activation. Second, platelets are potentially activated by the experimental procedure, and it cannot be excluded that the resting platelets are partially activated. To avoid these problems, two genetically engineered CHO cell lines expressing similar amounts of GP IIb/IIIa receptors (see Fig 1E), either in an activated or nonactivated state, were used as a model with clearly defined activation status of GP IIb/IIIa.15 16 In the comparison of the two cell lines, c7E3 binds similarly to activated and to nonactivated GP IIb/IIIa (Fig1D).
Flow cytometric comparison of c7E3 and fibrinogen binding as well as P-selectin expression on resting and activated platelets and evaluation of the expression level and c7E3 binding of CHO cells transfected with recombinant GP IIb/IIIa, either in the activated or nonactivated state. (A) Binding of various concentrations of biotinylated c7E3 to ADP-stimulated and resting platelets are expressed as the mean ± standard deviation of three determinations. (B) Histogram of FITC-conjugated anti–P-selectin MoAb binding to ADP-stimulated and resting platelets. (C) Histogram of FITC-conjugated polyclonal antifibrinogen chicken Ab binding to ADP-stimulated and resting platelets. (D) Binding of biotinylated c7E3 to CHO cells expressing no, activated, or nonactivated GP IIb/IIIa. Results are expressed as the mean ± standard deviation of three determinations. (E) Histograms of FITC-conjugated MoAb P2 (anticomplex GP IIb/IIIa) with GP IIb/IIIa (in the activated and nonactivated state) expressing CHO cell lines, together with CHO cells as a negative control. Typical histograms of several determinations are depicted.
Flow cytometric comparison of c7E3 and fibrinogen binding as well as P-selectin expression on resting and activated platelets and evaluation of the expression level and c7E3 binding of CHO cells transfected with recombinant GP IIb/IIIa, either in the activated or nonactivated state. (A) Binding of various concentrations of biotinylated c7E3 to ADP-stimulated and resting platelets are expressed as the mean ± standard deviation of three determinations. (B) Histogram of FITC-conjugated anti–P-selectin MoAb binding to ADP-stimulated and resting platelets. (C) Histogram of FITC-conjugated polyclonal antifibrinogen chicken Ab binding to ADP-stimulated and resting platelets. (D) Binding of biotinylated c7E3 to CHO cells expressing no, activated, or nonactivated GP IIb/IIIa. Results are expressed as the mean ± standard deviation of three determinations. (E) Histograms of FITC-conjugated MoAb P2 (anticomplex GP IIb/IIIa) with GP IIb/IIIa (in the activated and nonactivated state) expressing CHO cell lines, together with CHO cells as a negative control. Typical histograms of several determinations are depicted.
c7E3 induces high-affinity binding of fibrinogen to GP IIb/IIIa, in contrast to the MoAbs 2G12, P2, and PAC-1.
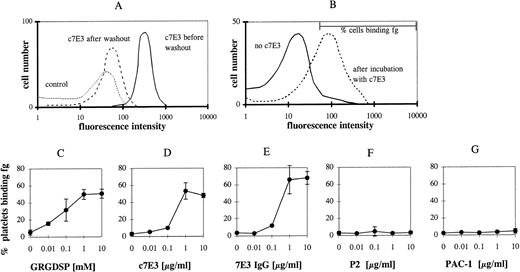
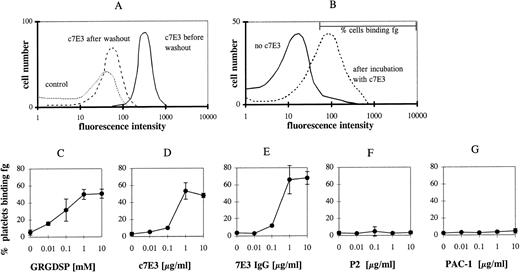
Receptor activating properties of inhibitors are primarily masked by the blockade of the receptor. Therefore, c7E3 was released by washing in modified Tyrode’s buffer. This washout of c7E3 was demonstrated in flow cytometry by evaluation of the remaining MoAb binding after the washout procedure (Fig 2A; shown for the incubation with 1 μg/mL c7E3). The specificity of c7E3 binding was evaluated by the competition in binding with the GP IIb/IIIa-blocking MoAb P2.
c7E3 binding before and after washout procedure and fibrinogen (fg) binding to unstimulated platelets after incubation with various GP IIb/IIIa inhibitors and following washout. (A) Initially bound biotinylated-c7E3 (solid line) and the remaining c7E3 binding after washout procedure (dashed line) as detected by R-phycoerythrin–conjugated streptavidin. Biotinylated unspecific Fab was used as negative control (pointed line). (B) Typical flow cytometric histogram of fg binding without c7E3 and after incubation with c7E3 and following washout. The marker that is used to determine the percentage of cells binding fg is shown. Platelets were incubated with increasing concentrations of GRGDSP (C ), c7E3 (D), 7E3 IgG (E), P2 (F), or PAC-1 (G) and then fixed and washed as described in Materials and Methods. After incubation with fibrinogen solution (3 g/L), fibrinogen binding was measured with an FITC-labeled polyclonal chicken antifibrinogen antibody in flow cytometry. The results are expressed as the percentage of platelets binding fibrinogen as the mean ± standard deviation of five determinations.
c7E3 binding before and after washout procedure and fibrinogen (fg) binding to unstimulated platelets after incubation with various GP IIb/IIIa inhibitors and following washout. (A) Initially bound biotinylated-c7E3 (solid line) and the remaining c7E3 binding after washout procedure (dashed line) as detected by R-phycoerythrin–conjugated streptavidin. Biotinylated unspecific Fab was used as negative control (pointed line). (B) Typical flow cytometric histogram of fg binding without c7E3 and after incubation with c7E3 and following washout. The marker that is used to determine the percentage of cells binding fg is shown. Platelets were incubated with increasing concentrations of GRGDSP (C ), c7E3 (D), 7E3 IgG (E), P2 (F), or PAC-1 (G) and then fixed and washed as described in Materials and Methods. After incubation with fibrinogen solution (3 g/L), fibrinogen binding was measured with an FITC-labeled polyclonal chicken antifibrinogen antibody in flow cytometry. The results are expressed as the percentage of platelets binding fibrinogen as the mean ± standard deviation of five determinations.
Fibrinogen binding was measured by an FITC-labeled chicken antifibrinogen MoAb in flow cytometry and expressed as percentage of cells binding fibrinogen. A marker region in the histograms depicting platelet fibrinogen binding was defined, allowing maximally 4% of nonstimulated platelets to be in the positive range (shown in Fig 2B). A similar increase in fibrinogen binding can be demonstrated by the measurement of mean fluorescence intensity (data not shown). Incubation of resting platelets with the peptide GRGDSP and a following washout resulted in a concentration-dependent binding of fibrinogen to GP IIb/IIIa (Fig 2C). Incubation with c7E3 and a following washout induced fibrinogen binding to GP IIb/IIIa (Fig 2D). Thus, c7E3 exhibits a similar intrinsic activating property as RGD peptides. Because the molecular size of ligands may determine potential intrinsic activating properties, the F(ab′)2 fragment and the whole IgG MoAb of 7E3 were used in the washout experiments described above. For both, the same activating property on GP IIb/IIIa could be demonstrated (depicted for IgG in Fig 2E). Thus, even large molecules with around 150 kD can induce a conformational change of GP IIb/IIIa. To prove whether the intrinsic activating effect is a general property of GP IIb/IIIa-blocking MoAbs, additional blocking MoAbs were evaluated. For the blocking MoAbs 2G12 and P2, no intrinsic activating property could be demonstrated (depicted for P2 in Fig 2F). Nevertheless, similar to c7E3, a washout of these MoAbs could be demonstrated in flow cytometry. After incubation with 1 μg/mL and the washout procedure, only 35% ± 3.8% of the originally occupied GP IIb/IIIa receptors for 2G12 and 29.2% ± 4.46% for P2 demonstrated remaining binding of these MoAbs. Differences in epitopes on GP IIb/IIIa of these two MoAbs compared with 7E3 may explain these contrasting findings.
The antibody PAC-1 is described as an activation-specific MoAb directed against GP IIb/IIIa that does not bind to the nonactivated GP IIb/IIIa.18,21 PAC-1 seems to bind in the fibrinogen-binding pocket of activated GP IIb/IIIa and thus to directly mimic soluble fibrinogen.21 There was no binding of PAC-1 to nonactivated GP IIb/IIIa on platelets or on CHO cells (data not shown) and no induction of fibrinogen binding by PAC-1 (Fig 2G). Thus, PAC-1 demonstrates no intrinsic activating property.
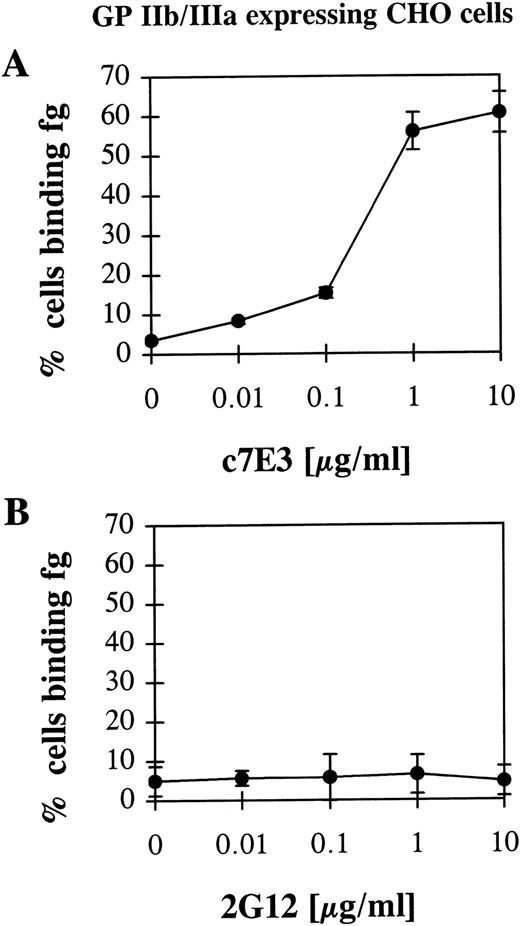
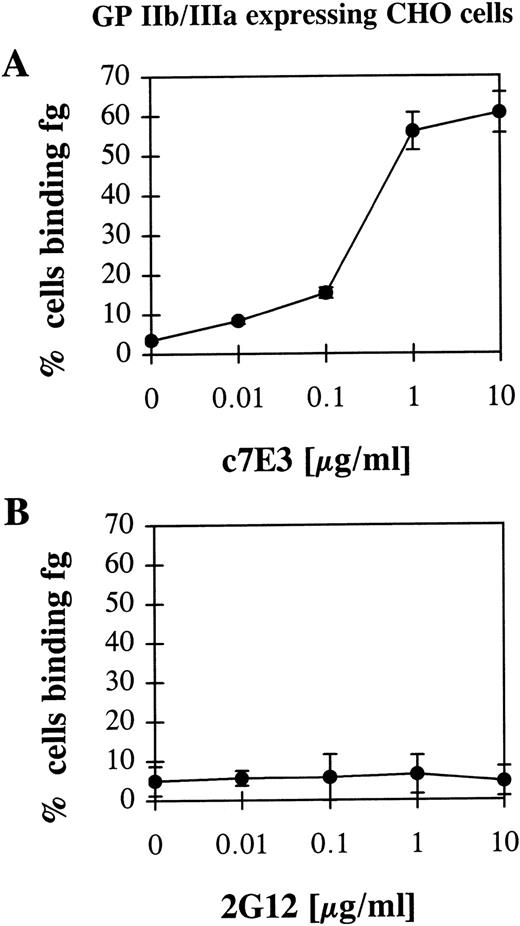
To exclude the possibility of a conformational change of GP IIb/IIIa by a general activation of platelets caused by c7E3 and thus to assure a direct effect of c7E3 on the GP IIb/IIIa receptor, a CHO cell line that expresses nonactivated GP IIb/IIIa was used as a model system. In these CHO cells, recombinant GP IIb/IIIa is not activatable by intracellular signals.17 Thus, an indirect effect that is not caused by a direct interaction between c7E3 and GP IIb/IIIa is not to be expected. Furthermore, in contrast to platelets, these cells can be centrifuged and washed without the risk of activating GP IIb/IIIa. Therefore, for the performed washout experiment, no fixation of these cells was necessary. Nevertheless, recombinant GP IIb/IIIa expressed by these cells is functional and can be activated to bind fibrinogen by activating anti-LIBS MoAbs.17 Thus, CHO cells expressing recombinant GP IIb/IIIa are an appropriate tool to check the hypothesis of whether GP IIb/IIIa inhibitors exert an activating effect on GP IIb/IIIa. After incubation with c7E3 and washout, fibrinogen binding on GP IIb/IIIa-expressing CHO cells could be detected by flow cytometry (Fig 3A). As control, 2G12 did not induce fibrinogen binding to GP IIb/IIIa (Fig 3B).
Fibrinogen (fg) binding to CHO cells expressing wild-type, nonactivated GP IIb/IIIa after incubation with c7E3 (A) or anti-GP IIb/IIIa MoAb 2G12 (B) and following washout. Experiments were performed as described in Fig 2 and in Materials and Methods.
Fibrinogen (fg) binding to CHO cells expressing wild-type, nonactivated GP IIb/IIIa after incubation with c7E3 (A) or anti-GP IIb/IIIa MoAb 2G12 (B) and following washout. Experiments were performed as described in Fig 2 and in Materials and Methods.
Detection of c7E3-induced conformational change of GP IIb/IIIa by MoAb anti-LIBS6.
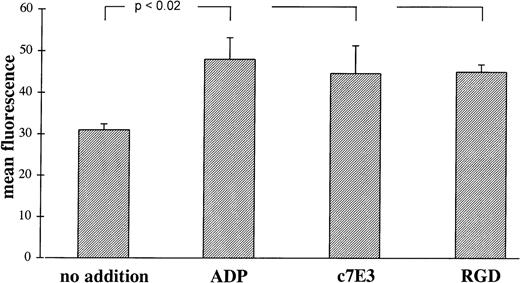
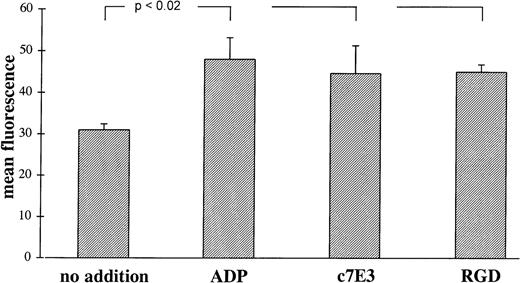
LIBS on GP IIb/IIIa are more accessible for anti-LIBS MoAbs after binding of the ligand fibrinogen or the ligand-mimetic peptide RGD.10,11 19 Incubation of platelets with c7E3 also induce an increase in anti-LIBS6 MoAb binding, which is small but statistically significant and which is similar in extent after incubation with ADP and RGD peptides (Fig4). This finding is consistent with a conformational change of GP IIb/IIIa induced by c7E3. For other anti-LIBS MoAbs, anti-LIBS1 and PMI-1, an increase in binding to GP IIb/IIIa induced by c7E3 has been demonstrated as well (Dr Meinrad Gawaz, Munich, Germany, personal communication, November 6, 1997).
Binding of MoAb anti-LIBS6 (10 μg/mL) induced by ADP (20 μmol/L), c7E3 (10 μg/mL), and GRGDSP (2 mmol/L) or no addition. Binding of MoAb anti-LIBS6 was detected by an FITC-labeled, polyclonal Fc-fragment-specific anti-IgG mouse Ab. Background binding with unspecific MoAb has been subtracted. Results are depicted as the mean of three determinations ± standard deviation. Using the unpaired Student’s t-test, P values less than .02 were obtained for comparisons between no addition and the addition of ADP, c7E3, and GRGDSP, respectively.
Binding of MoAb anti-LIBS6 (10 μg/mL) induced by ADP (20 μmol/L), c7E3 (10 μg/mL), and GRGDSP (2 mmol/L) or no addition. Binding of MoAb anti-LIBS6 was detected by an FITC-labeled, polyclonal Fc-fragment-specific anti-IgG mouse Ab. Background binding with unspecific MoAb has been subtracted. Results are depicted as the mean of three determinations ± standard deviation. Using the unpaired Student’s t-test, P values less than .02 were obtained for comparisons between no addition and the addition of ADP, c7E3, and GRGDSP, respectively.
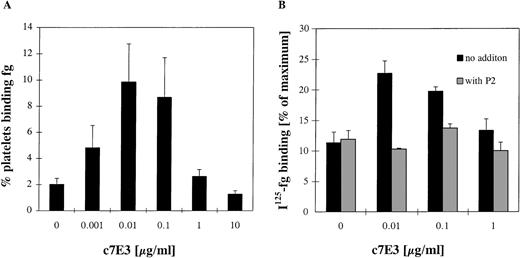
At low concentrations of c7E3, the intrinsic activating effect is strongest relative to the blocking effect.
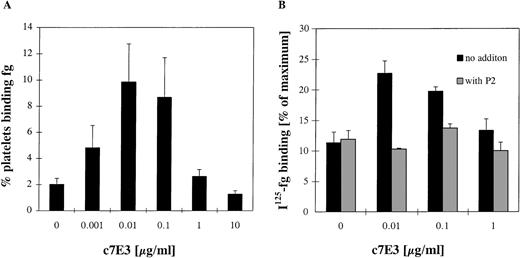
If c7E3 has an intrinsic activating property, the effect on fibrinogen binding is expected to be strongest, relative to the blocking effect, at low concentrations. Compared with the number of GP IIb/IIIa receptors, only a limited number of c7E3 is present. If c7E3 dissociates from a receptor, the probability that this receptor is not again occupied and thus blocked by another c7E3 molecule is higher at low concentrations of c7E3. Thus, the intrinsic activating effect of c7E3 may be unmasked at low concentrations. During incubation with increasing concentrations of c7E3 with no fixation of platelets and no washout of c7E3, fibrinogen binding of resting platelets was evaluated in flow cytometry and with 125I-labeled fibrinogen (Fig 5). At low concentrations (0.01 to 0.1 μg/mL) of c7E3, fibrinogen binding on platelets increased significantly. With higher concentrations of c7E3 (≥1 μg/mL), fibrinogen binding on resting platelets decreased. The effective blockade of the c7E3-induced fibrinogen binding by MoAb P2 demonstrates a specificity of this effect for GP IIb/IIIa. Overall, at low concentrations of c7E3, the activating property of this GP IIb/IIIa inhibitor is unmasked.
Evaluation of fibrinogen (fg) binding in flow cytometry (A) and with 125I-fibrinogen (B) at various concentrations of c7E3 without washout procedure. (A) The percentage of platelets binding fibrinogen was assessed by an FITC-labeled polyclonal chicken antifibrinogen antibody in flow cytometry. (B)125I-fibrinogen was determined as described in Materials and Methods in relation to the maximum binding by stimulation of platelets with 20 μmol/L ADP. The MoAb P2 (20 μg/mL) was preincubated for 10 minutes at room temperature. Results are depicted as the mean of five determinations ± standard deviation. Using the unpaired Student’s t-test, P values less than .01 were obtained for the comparisons between no addition of c7E3 and the additions of 0.01 and 0.1 μg/mL c7E3.
Evaluation of fibrinogen (fg) binding in flow cytometry (A) and with 125I-fibrinogen (B) at various concentrations of c7E3 without washout procedure. (A) The percentage of platelets binding fibrinogen was assessed by an FITC-labeled polyclonal chicken antifibrinogen antibody in flow cytometry. (B)125I-fibrinogen was determined as described in Materials and Methods in relation to the maximum binding by stimulation of platelets with 20 μmol/L ADP. The MoAb P2 (20 μg/mL) was preincubated for 10 minutes at room temperature. Results are depicted as the mean of five determinations ± standard deviation. Using the unpaired Student’s t-test, P values less than .01 were obtained for the comparisons between no addition of c7E3 and the additions of 0.01 and 0.1 μg/mL c7E3.
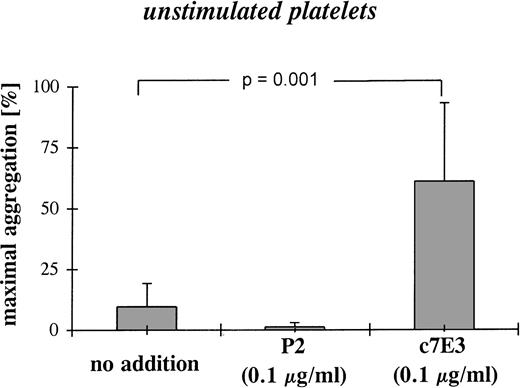
Low concentrations of c7E3 result in platelet aggregation.
To evaluate the functional consequence of fibrinogen binding at low c7E3 concentrations, aggregometry was performed with resting platelets. At a concentration of 0.1 μg/mL, c7E3 induces platelet aggregation (Fig 6). Whereas no addition or the incubation with MoAb P2 at 0.1 μg/mL did not induce platelet aggregation (Fig 6; representative examples of aggregation curves are shown in Fig 8). Aggregation induced by a low concentration of c7E3 could be blocked by the preincubation of platelets with 20 μg/mL of the GP IIb/IIIa-specific MoAb P2 (see Fig 8).
Platelet aggregation after incubation of unstimulated platelets with a low concentration (0.1 μg/mL) of c7E3 or P2 (anti-GP IIb/IIIa MoAb) or without addition. The bar graphs show mean maximal aggregation of 10 measurements ± standard deviations. Pvalue was evaluated with the unpaired Student’s t-test.
Platelet aggregation after incubation of unstimulated platelets with a low concentration (0.1 μg/mL) of c7E3 or P2 (anti-GP IIb/IIIa MoAb) or without addition. The bar graphs show mean maximal aggregation of 10 measurements ± standard deviations. Pvalue was evaluated with the unpaired Student’s t-test.
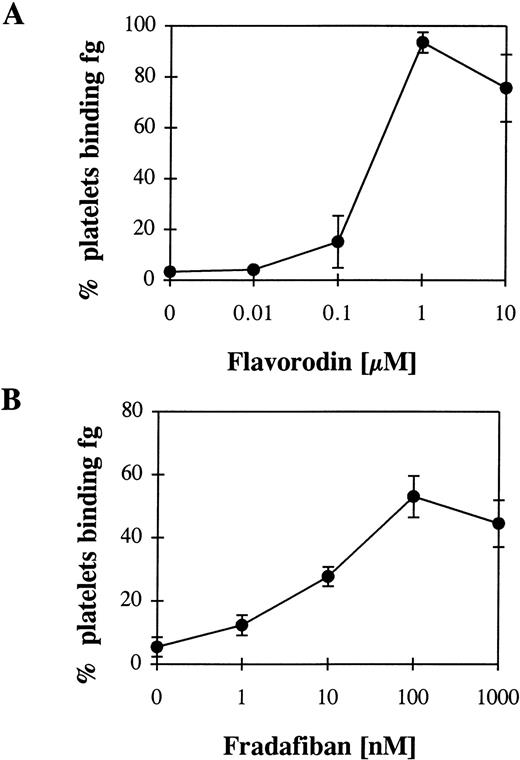
Flavoridin as a disintegrin, and fradafiban as a RGD-analogue demonstrate an intrinsic activating property.
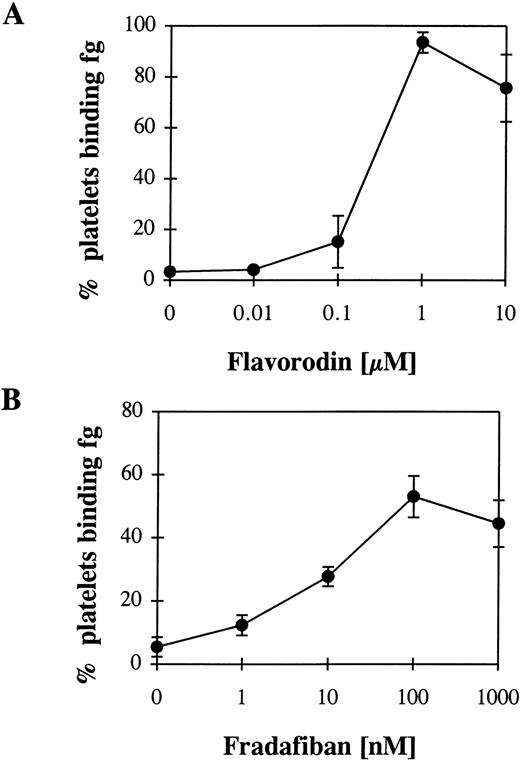
The snake venom flavorodin, as a member of the disintegrin group, inhibits GP IIb/ IIIa.22 Disintegrins contain a RGD sequence or a sequence similar to RGD and can bind to GP IIb/IIIa on unstimulated platelets.23 Thus, an intrinsic activating property of these proteins can be expected. Incubation with increasing concentrations of flavorodin and the following washout resulted in fibrinogen binding to platelets (Fig 7A). As an example for RGD analogues, fradafiban20 was evaluated in a similar experimental set-up. Again, a conformational change of the GP IIb/IIIa could be demonstrated (Fig 7B). Thus, both flavorodin and fradafiban demonstrate an intrinsic activating property.
Fibrinogen (fg) binding to unstimulated platelets after incubation with flavorodin as an example for a disintegrin (A) and fradafiban as an example for a RGD analogue (B) and a following washout. For the experimental procedure, see Fig 2 and Materials and Methods.
Fibrinogen (fg) binding to unstimulated platelets after incubation with flavorodin as an example for a disintegrin (A) and fradafiban as an example for a RGD analogue (B) and a following washout. For the experimental procedure, see Fig 2 and Materials and Methods.
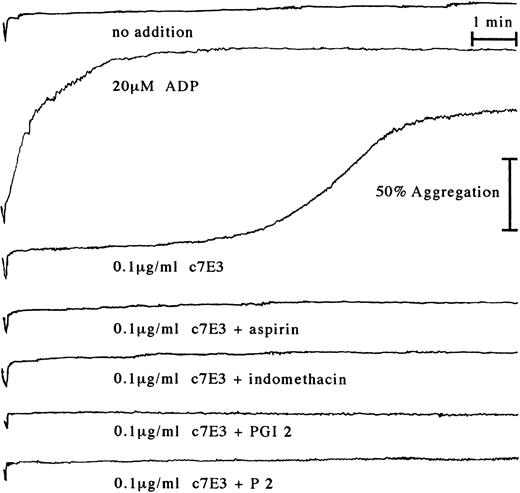
Aspirin does not inhibit c7E3-induced fibrinogen binding to GP IIb/IIIa, but does inhibit c7E3-induced platelet aggregation.
In clinical trials, c7E3 most often is combined with heparin and aspirin.2,4 5 Therefore, c7E3-induced fibrinogen binding and platelet aggregation were evaluated under coincubation with both drugs. Heparin does not influence c7E3-induced fibrinogen binding or platelet aggregation (data not shown). Incubation with c7E3 in the presence of aspirin (100 μg/mL) and the following washout of the MoAb fragment did not alter c7E3-induced fibrinogen binding to platelets (data not shown). In contrast, c7E3-induced platelet aggregation could be inhibited by coincubation with aspirin (Fig 8). Additionally to aspirin, another cyclooxygenase inhibitor, indomethacin (10 μmol/L), caused inhibition of c7E3-induced platelet aggregation (Fig 8). The same inhibiting effect could be demonstrated for PGI2 (100 nmol/L; Fig 8).
Platelet aggregation tracings starting with the addition of CaCl2 and MgCl2 to a final concentration of 2 mmol/L to platelet-rich plasma, either without addition, with incubation with 20 μmol/L ADP (as positive control), or with 0.1 μg/mL c7E3. c7E3 samples were additionally preincubated with either 100 μg/mL aspirin, 10 μmol/L indomethacin, 100 nmol/L PGI2, or 20 μg/mL MoAb P2. Representative tracings of several experiments as determined by increase of light transmission in a Bio/Data PAP4 aggregometer are depicted.
Platelet aggregation tracings starting with the addition of CaCl2 and MgCl2 to a final concentration of 2 mmol/L to platelet-rich plasma, either without addition, with incubation with 20 μmol/L ADP (as positive control), or with 0.1 μg/mL c7E3. c7E3 samples were additionally preincubated with either 100 μg/mL aspirin, 10 μmol/L indomethacin, 100 nmol/L PGI2, or 20 μg/mL MoAb P2. Representative tracings of several experiments as determined by increase of light transmission in a Bio/Data PAP4 aggregometer are depicted.
Aspirin inhibits platelet aggregation induced by an activating anti-LIBS MoAb.
Anti-LIBS MoAbs induce conformational changes of GP IIb/IIIa resulting in high-affinity binding of ligands. In their effect to cause a conformational change by interaction with the extracellular side of GP IIb/IIIa, anti-LIBS MoAbs can be compared with c7E3. Indeed, preincubation with aspirin inhibits anti-LIBS6–induced platelet aggregation (Fig 9). Thus, aspirin showed the same inhibitory effect on anti-LIBS–induced as on c7E3-induced platelet aggregation.
Anti-LIBS6–induced platelet aggregation is inhibited by aspirin. Platelet aggregation tracings start with the addition of CaCl2 and MgCl2 at a final concentration of 2 mmol/L to platelet-rich plasma. Aggregation is induced by addition of anti-LIBS6 to a final concentration of 10 μg/mL. Preincubation was performed with 100 μg/mL aspirin, 20 μg/mL MoAb P2, or no addition. Aggregation was determined by increase of light transmission in a Bio/Data PAP4 aggregometer. Representative tracings of several experiments are depicted.
Anti-LIBS6–induced platelet aggregation is inhibited by aspirin. Platelet aggregation tracings start with the addition of CaCl2 and MgCl2 at a final concentration of 2 mmol/L to platelet-rich plasma. Aggregation is induced by addition of anti-LIBS6 to a final concentration of 10 μg/mL. Preincubation was performed with 100 μg/mL aspirin, 20 μg/mL MoAb P2, or no addition. Aggregation was determined by increase of light transmission in a Bio/Data PAP4 aggregometer. Representative tracings of several experiments are depicted.
DISCUSSION
The major findings of this study are as follows. (1) The mouse/human chimeric Fab fragment c7E3 induces a conformational change of the platelet integrin GP IIb/IIIa. (2) c7E3 exhibits an intrinsic activating property that at low concentrations of c7E3 results in fibrinogen binding to GP IIb/IIIa. (3) As a functional consequence, c7E3 induces platelet aggregation at low concentrations and blocks platelet aggregation at high concentrations. (4) The GP IIb/IIIa-blocking MoAbs P2 and 2G12 and the activation-specific, anti-GP IIb/IIIa MoAb PAC-1 do not exhibit an intrinsic activating property. (5) The disintegrin flavorodin and the RGD analogue fradafiban also induce fibrinogen binding to GP IIb/IIIa. (6) Aspirin and indomethacin cannot inhibit c7E3-induced fibrinogen binding to GP IIb/IIIa, but can inhibit c7E3-induced platelet aggregation.
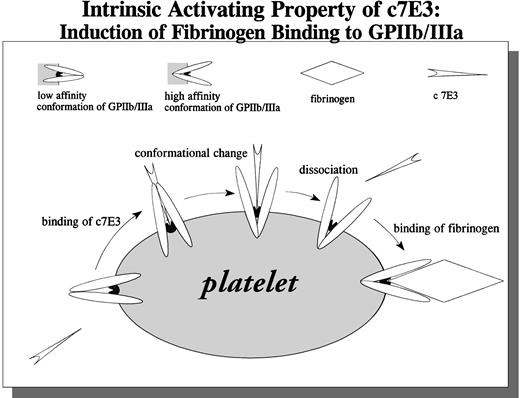
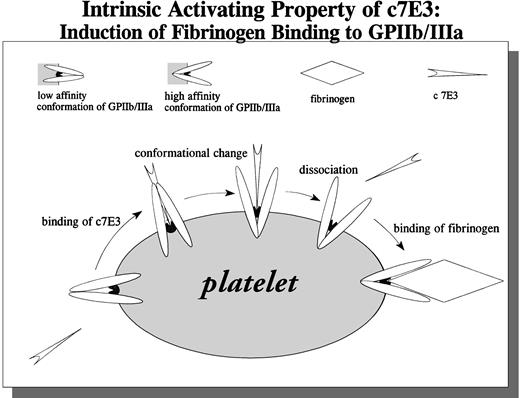
A potential explanation of our findings is shown in Fig 10. c7E3 binding to nonactivated GP IIb/IIIa results in a conformational change of GP IIb/IIIa. After dissociation of c7E3, GP IIb/IIIa is unblocked and left in an activated state, able to bind soluble fibrinogen. We could demonstrate that c7E3 binds to nonactivated GP IIb/IIIa on platelets and on CHO cells. Binding of the intact IgG, F(ab′)2, and Fab′ of 7E3 to resting platelets has been demonstrated.24,25 Furthermore, in several animal studies and clinical trials, a blockade of nonactivated GP IIb/IIIa by 7E3 is documented.2,4,5,26 Nevertheless, the combination of three binding characteristics of c7E3 implies a binding at or close to the fibrinogen binding pocket on GP IIb/IIIa. (1) The MoAb 7E3 exhibits an enhancend on-rate of binding to GP IIb/IIIa on activated compared with resting platelets.24,27 (2) 7E3 competes with fibrinogen for the binding to GP IIb/IIIa.3,5 (3) As evaluated in the present study, c7E3 has the intrinsic property to induce a conformational change of GP IIb/IIIa. This potential property of c7E3 has been hypothetically discussed5,36 but has not been demonstrated until now. For the MoAb OP-G2, similar binding characteristics as for c7E3 could be demonstrated; interestingly, this antibody can also induce a conformational change of GP IIb/IIIa as detected with an anti-LIBS MoAb (PMI-1).28Other MoAbs that block fibrinogen binding are expected to have epitopes outside the fibrinogen-binding pocket. For two of those blocking MoAbs, 2G12 and P2, we could demonstrate that they cannot induce binding of fibrinogen. MoAbs with these properties may provide an alternative basis for GP IIb/IIIa blocking drugs. An even more interesting alternative is MoAbs such as PAC-1. This MoAb is activation-dependent and does not bind to the nonactivated GP IIb/IIIa.18 21Therefore, as we could demonstrate, MoAb PAC-1 does not induce fibrinogen binding. Furthermore, because resting platelets are not blocked, GP IIb/IIIa-mediated adhesion of resting platelets on immobilized fibrinogen may still be possible. Thereby, if MoAbs with features such as PAC-1 could be used for clinical applications, no intrinsic activating effect and possibly less bleeding compared with c7E3 might be present.
Schematic drawing of the proposed intrinsic activating property of c7E3: Induction of fibrinogen binding to GPIIb/IIIa.
Schematic drawing of the proposed intrinsic activating property of c7E3: Induction of fibrinogen binding to GPIIb/IIIa.
In this study, we describe the use of two CHO cell lines expressing either a nonactivated or an activated GP IIb/IIIa. Because it has been proven that the expression of recombinant GP IIb/IIIa results in a funtional receptor,17 both cell lines have been used for various functional studies.15,16 Using the CHO cell line with nonactivated GP IIb/IIIa, affinity regulation29 and interaction with the cytoskeleton30 have been studied. Du et al13 used the same GP IIb/IIIa-expressing CHO cell line to demonstrate the activating effect of RGD peptides on GP IIb/IIIa. The CHO cell line with activated GP IIb/IIIa has been instrumental for the evaluation of the role of integrin affinity and cytoskeletal anchorage in αIIbβ3-mediated fibrinogen binding,16 cell adhesion,15 cell migration,31 and fibronectin matrix assembly.32Experiments with platelets are very often hampered by the fact that they are easily activated by the preparatory procedure. Furthermore, for many drugs it may be difficult to dissect general effects on platelets from isolated effects on GP IIb/IIIa. Therefore, our study may serve as an example for the direct evaluation of drug effects on the GP IIb/IIIa receptor, using recombinant GP IIb/IIIa.
Aspirin was not able to inhibit c7E3-induced fibrinogen binding to GP IIb/IIIa. Nevertheless, c7E3-induced platelet aggregation was inhibited by aspirin. This effect may be cyclooxygenase-dependent, because another cyclooxygenase inhibitor, indomethacin, also demonstrates similar inhibiton. Furthermore, PGI2 was able to inhibit c7E3-induced platelet aggregation. Anti-LIBS MoAbs-induced platelet aggregation is inhibited by indomethacin and aspirin, as shown by us (Fig 9) and others.19 For anti-LIBS MoAbs, as with c7E3, the conformational change of GP IIb/IIIa originates in the extracellular domain of the integrin. Cyclooxygenase inhibitors may modulate postreceptor effects such as changes in the cytoskeletal anchorage of GP IIb/IIIa or in the cytoskeletal organizaton itself.33 Further studies are needed to define the role of cyclooxygenase in GP IIb/IIIa inhibitor-induced platelet aggregation.
The data presented in this study are based on in vitro experiments and do not prove clinical relevance of the described intrinsic activating property of GP IIb/IIIa inhibitors. In vivo data from patients treated with c7E3 showed a transfer of c7E3 from initially occupied GP IIb/IIIa to GP IIb/IIIa of those platelets newly released into the circulation.34 This gradual recovery of c7E3 blockade for each platelet can be detected in flow cytometry in an unimodal decrease in c7E3 binding of the whole circulating platelet population.5,34 Therefore, GP IIb/IIIa receptors with a history of c7E3 occupancy and after dissociation are present5 and, thus, according to our findings, could demonstrate fibrinogen binding. Nevertheless, proaggregatory effects of GP IIb/IIIa inhibitors have not been seen or at least have not been reported yet. Several reasons may account for this. (1) Most patients treated with GP IIb/IIIa inhibitors did also receive aspirin. This comedication may have prevented a proaggregatory effect. (2) The c7E3-induced aggregation may not have the same properties as platelet aggregation induced in vivo by physiological platelet agonists. In the aggregation curves, the time at which maximal aggregation is reached and the maximal slope are different as compared for example with ADP-induced platelet aggregation. Similar differences in comparison to ADP-induced platelet aggregation are reported for anti-LIBS3–induced19 and RGDS-peptide–induced14 platelet aggregation. Thus, the stability of c7E3-induced aggregates may differ from regular platelet aggregates. (3) The number of GP IIb/IIIa receptors per platelet that are left in an activated state after dissociation of the GP IIb/IIIa inhibitors may be too low to result in stable aggregates. (4) The activation of GP IIb/IIIa may result in platelet adhesion to the endothelium and/or in sequestration within the spleen. A decrease in platelet count has been seen in many patients treated with GP IIb/IIIa inhibitors, even in those without bleeding complications.5,35,36 In two recent reviews,5,36 the induction of potentially allergenic neoepitopes (LIBS) on GP IIb/IIIa by a conformational change caused by the binding of GP IIb/IIIa antagonists are discussed as a potential reason for thrombocytopenia. Until now, there is no proven explanation for thrombocytopenia induced by GP IIb/IIIa inhibitors.5 36
Three groups of GP IIb/IIIa inhibitors are under investigation as therapeutics2-5: (1) GP IIb/IIIa blocking MoAbs, (2) short peptides originating from disintegrins of snake venoms that mostly contain RGD or KGD sequences, and (3) RGD/KGD analogues that are modeled according to the structure of RGD/KGD peptides. For members of all three groups, we could demonstrate an intrinsic activating property. Because disintegrins and also RGD/KGD analogues are ligand-mimetics, the same mechanism for change in conformation of GP IIb/IIIa, as shown in Fig 10, can be postulated. In fact, the disintegrins eristostatin and echistatin caused an increase in anti-LIBS MoAb binding37 and tetrafibricin, a nonpeptide GP IIb/IIIa inhibitor, transformed GP IIb/IIIa in a high-affinity state for fibrinogen and could induce platelet aggregation.38Nevertheless, there may also be members of the group of disintegrins (or short peptides derived from disintegrins) and RGD/KGD analogues (as there are also blocking MoAbs) that block GP IIb/IIIa but do not exhibit an intrinsic activating property. For example, anti-LIBS and washout experiments with pentamidine would suggest that this nonpeptide agent inhibits fibrinogen binding to GP IIb/IIIa without an intrinsic activating effect.39 Our description of an intrinsic activating property of various GP IIb/IIIa inhibitors may initiate a systematic evaluation of the large number of different GP IIb/IIIa inhibitors that are presently available or in development.
ACKNOWLEDGMENT
The authors thank Dr Timothy E. O’Toole and Dr Mark H. Ginsberg for their support and Dr Meinrad Gawaz for unpublished data. The expert technical assistance of Simone Bauer, Petra Jung, and Dr Siegfried Lang is gratefully acknowledged.
Supported by the German Research Foundation with a grant to K.P. and C.B. (SFB 320: C/3) and by a cooperation grant of the DAAD (Deutscher Akademischer Austauschdienst) and the Academy of Finland to K.P. and J.Y.
Presented in part at the American Heart Association Meeting 1997, Orlando, FL (Circulation 96:I-664, 1997).
Address reprint requests to Karlheinz Peter, MD, Innere Medizin III, Universität Heidelberg, Bergheimer Str. 58, 69115 Heidelberg, Germany; e-mail: kpeter@ukl.uni-heidelberg.de.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.