Abstract
Heparin-induced thrombocytopenia (HIT) is a potentially serious complication of heparin therapy. Antibodies to platelet factor 4 (PF4)/heparin complexes have been implicated in the pathogenesis of this disorder, but the antigenic epitope(s) on the protein have not been defined. To address this issue, we studied the binding of HIT antibodies to a series of recombinant proteins containing either point mutations in PF4 or chimeras containing various domains of PF4 and the related protein, neutrophil activating peptide-2 (NAP-2). Serum samples from 50 patients with a positive 14C-serotonin release assay (14C-SRA) and a clinical diagnosis of HIT and 20 normal controls were studied. HIT antibodies reacted strongly with wild-type (WT) PF4/heparin complexes, but reacted little, if at all, with NAP-2/heparin complexes (optical density [OD]405 = 2.5 and 0.2, respectively). Alanine substitutions at three of the four lysine residues implicated in heparin binding, K62, K65, and K66, had little effect on recognition by HIT antibodies (OD405 = 2.2, 2.8, and 2.0, respectively), whereas an alanine substitution at position K61 led to reduced, but still significant binding (OD405 = 1.0). Similar studies involving chimeras between PF4 and NAP-2 localized a major antigenic site to the region between the third and fourth cysteine residues for more than half of the sera tested. This site appears to involve a series of amino acids immediately after the third cysteine residue beginning with P37. Thus our studies suggest that whereas the C-terminal lysine residues of PF4 are important for heparin binding, they do not comprise a critical antigenic site for most HIT antibodies. Rather, we propose that maintaining a region near the third cysteine residue of PF4, distal from the proposed heparin-binding domain, is required to form the epitope recognized by many HIT antibodies.
© 1998 by The American Society of Hematology.
HEPARIN IS THE MOST common known cause of drug-induced immune thrombocytopenia,1,2 occurring in 1% to 3% of patients receiving unfractionated heparin and at a somewhat lower proportion of patients receiving low molecular weight heparin or heparinoids.3-5 Paradoxically, 10% to 20% of patients develop life- or limb-threatening thromboses if the exposure persists.6,7 It is currently believed that heparin-induced thrombocytopenia (HIT) is mediated by antibodies directed at complexes that form between heparin or other anionic mucopolysaccharides and platelet factor 4 (PF4) in plasma, on the surface of platelets, and/or on the endothelium.8-12 Immune complexes of HIT antibodies and PF4/heparin bind to the surface of platelets and induce their activation by cross-linking FcγIIA receptors4,13,14 and bind to the surface of the endothelium inducing procoagulant activity.4,15 A small proportion of HIT antibodies recognize other heparin-binding proteins such as neutrophil activating protein-2 (NAP-2) and interleukin-8.16 However, these HIT antibodies bind in the absence of heparin and some of these patients have had atypical presentations, making the biologic relevance of anti–NAP-2 antibodies uncertain.
The mechanism by which PF4/heparin complexes become antigenic and the portion(s) of PF4 recognized by HIT antibodies remain essentially unknown. PF4 is a 70-amino acid (AA) platelet-specific protein that belongs to the CXC (or beta) chemokine subfamily, in which the first two of the four conserved cysteine residues are separated by one AA residue.17 PF4 has been sequenced18 and cloned,19 and its x-ray crystallographic structure has been defined and compared with other chemokines (Fig1).20-22 PF4 exists as a tetramer with the three beta sheets of each subunit facing inwards and the N- and C-termini lying on the surface of the molecule. The C-termini are rich in lysines, which contribute to the tetramer’s high affinity for heparin. However, analysis of the crystallographic structure of PF4 points to additional residues that may contribute to the circumferential ring of positive charges that form the interface of the tetramer with heparin.23-25
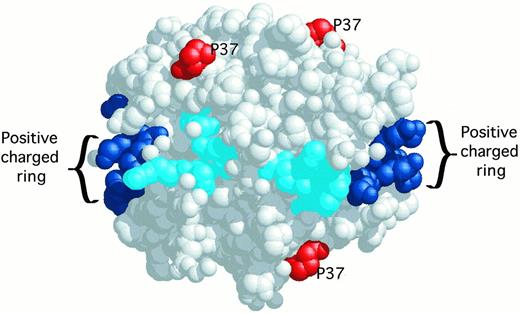
Molecular model of the PF4 tetramer derived from x-ray crystallography. The C-terminal lysines 61, 62, 65, 66 are shown in dark blue. Other lysines, arginines, and histidines contributing to the circumferential belt of positive charges around the PF4 tetramer are shown in cyan. Relevant to this report, the proline 37 amino acids are shown in red.
Molecular model of the PF4 tetramer derived from x-ray crystallography. The C-terminal lysines 61, 62, 65, 66 are shown in dark blue. Other lysines, arginines, and histidines contributing to the circumferential belt of positive charges around the PF4 tetramer are shown in cyan. Relevant to this report, the proline 37 amino acids are shown in red.
Superposition of the NAP-2 third domain (cyan) onto the homologous region of PF4 (yellow). The side-chains of P37 and the asparagine of NAP-2 are indicated. The superpositioning of the turn region indicates that the native structures are similar and that substitution of asparagine for proline in PF4 does not affect the turn conformation.
Superposition of the NAP-2 third domain (cyan) onto the homologous region of PF4 (yellow). The side-chains of P37 and the asparagine of NAP-2 are indicated. The superpositioning of the turn region indicates that the native structures are similar and that substitution of asparagine for proline in PF4 does not affect the turn conformation.
PF4 is expressed at high levels in the developing megakaryocyte and comprises 2% to 3% of the total protein in mature platelets.26 The tetramer is stored in the platelet α-granule and released in high concentrations at sites of platelet activation. Surprisingly, the biologic role(s) of PF4 has not been defined. Its most prominent characteristic is high affinity for heparin and heparin-like molecules,27 which may account for its binding to many cell surfaces including platelets themselves. Potential roles for PF4 in inflammation,28angiogenesis,29 megakaryocytopoiesis,30coagulation,27 and hemostasis31 have been suggested.
Platelet α-granules also store large amounts of the related platelet-specific CXC chemokine beta-thromboglobulin.26 On platelet activation, this protein is released into the circulation where it is cleaved by a number of proteases to NAP-2.32NAP-2 also consists of 70 AA and has ≈60% amino acid homology with PF4. Its crystal structure has also been defined.33 NAP-2 binds heparin with a somewhat lower affinity than does PF4, but this peptide is a potent chemotactic agent for neutrophils and monocytes.34 As mentioned above, a small number of patients with atypical HIT have antibodies to NAP-2 rather than to PF4/heparin complexes, but the biologic relevance of these antibodies remains uncertain.
The binding site for HIT antibodies in PF4/heparin is unknown, and more than one site may be involved when antibodies from different patients are examined.35 The results of two recent studies suggest that HIT antibodies do not recognize linear sequences of denatured PF4 and in the heparin-binding C-terminal portions of PF4.35,36Little more about the binding site(s) is known except that the antigenic site may reside on the PF4 tetramer itself.37 To begin to identify the structural determinants in PF4/heparin complexes recognized by HIT antibodies, we generated mutant proteins with specific substitutions in the lysine-rich C-terminus of PF4, and we created chimeric proteins comprising domains of PF4 and the related chemokine NAP-2. The result of our studies show that a significant proportion of HIT antibodies recognize an epitope that requires the conservation of sequence between the third and fourth cysteine residues of PF4.
MATERIALS AND METHODS
Source of antibodies.
Plasma samples were obtained from 50 patients who developed thrombocytopenia (with or without thrombosis) while receiving heparin which were referred to the Coagulation Laboratory at the Hospital of the University of Pennsylvania for the detection of heparin-dependent antiplatelet antibodies. All samples contained heparin-dependent antiplatelet antibodies determined by14C-SRA12,38 and anti-PF4/heparin antibodies detected by enzyme-linked immunosorbent assay (ELISA).8-12Research studies on discarded plasma from the clinically indicated studies were used with Institutional Ethics Committee approval. Twenty plasma samples obtained from patients with no known hematologic disturbances and negative 14C-SRA and PF4/heparin ELISA tests were used as normal controls. All plasma samples were stored at −70°C until use. Polyclonal rabbit antihuman PF4 antibody and polyclonal antihuman NAP-2 sera (Prepro Tech EC LTD, London, UK) were used to identify the purity and the quantity of the WT and variant PF4 proteins by immunoblotting and ELISA.
Recombinant PF4-like protein expression and purification.
A number of recombinant PF4-like proteins were generated (see Fig 2A). These included WT PF4 and NAP-2, a series of lysine to alanine substitutions in the lysine-rich C-terminus of PF4, and chimeric proteins containing various portions of PF4 and NAP-2. The WT PF4 and NAP-2 pT7-7 procaryote expression vectors have been described previously.34,39 Mutant and chimeric protein expression vectors were made using an overlapping polymerase chain reaction (PCR) approach beginning with these WT PF4 and NAP-2 expression vectors using VENT polymerase (New England Biolabs, Inc, Beverly, MA) to decrease mutation rate as previously described by us.40 All mutant constructs were sequenced using a Sequenase T7 DNA Polymerase Kit (Amersham Life Sciences, Inc, Cleveland, OH) to ensure that the desired construct was obtained.
The recombinant PF4 mutants and PF4/NAP-2 chimeric proteins were expressed in Escherichia coli BL21(DE3) pLysS (Novagen, Madison, WI) using the lacZ operon-driven pT7-7 expression vectors as previously described.39 Briefly, recombinant proteins were induced by the addition of 1 mmol/L isopropyl-thio-β-galactopyranoside (Fisher Biotech, Fairlawn, NJ) for 3 hours at 37°C after the bacterial growth had reached an optical density (600 nm) of 1.0 to 1.3 OD units. The bacterial pellet from 1 L of growth was lysed in 30 mL of TEN50 buffer (50 mmol/L Tris HCl, pH 8.0; 1 mmol/L EDTA; and 50 mmol/L NaCl) plus 100 μg/mL lysozyme (Sigma Chemical Co, St Louis, MO), 1 mmol/L phenylmethyl sulfonyl fluoride (PMSF) (Sigma), 0.5% Na deoxycholate (Sigma), and 10% Triton X-100 (Sigma). The sample was sonicated until it was no longer viscous and then centrifuged at 18,000g for 10 minutes to pellet the bacterial debris. Recombinant proteins were isolated from the supernatant of the bacterial lysate by adding it to a heparin agarose column (heparin immobilized on cross-linked 4% beaded agarose, cyanogen bromide activated type II-5 containing 500 mg heparin/mL packed gel, Sigma) and eluting the protein using a 0 to 2 mol/L NaCl gradient. The eluted proteins were further purified using FPLC chromatography using a μBindpak C18 column (Pharmacia Biotech Inc, Piscataway, NJ) and a gradient of acetonitrile and trifluoroacetic acid.
Protein purity was tested using 15% (wt/vol) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie blue R-250 staining (Sigma) and also by immunoblotting after electrotransferring the proteins to a PVDF (polyvinylidenedifluoride) transfer membrane (Millipore, Bedford, MA). The membrane was blocked with 5% (wt/vol) nonfat dry milk in Tris-buffered saline (TBS) (50 mmol/L Tris, pH 8.0; 150 mmol/L NaCl) containing 0.01% (vol/vol) Tween-20 (PBS-T, (BioRad, Richmond, VA) (TBST) for 30 minutes at room temperature (RT) and then incubated for 1 hour at RT with commercial rabbit anti-PF4 and anti–NAP-2 polyclonal antibodies in TBST. After washing twice with TBST, the PVDF membrane was incubated with affinity-purified horseradish peroxidase-conjugated swine antirabbit IgG (Dako Als, Glostrup, Denmark) for 1 hour at RT, and washed twice with TBST. The membrane was treated with enhanced chemiluminescence (ECL) reagent (Amersham Life Sciences, Inc, Arlington Heights, IL) according to the manufacturer’s instructions and developed on XAR film (Eastman Kodak Co, Rochester, NY). Protein concentrations were determined using bicinchoninic acid (BCA) protein assay (Pierce Co, Rockford, IL) with bovine serum albumin as a standard, according to manufacturer’s instructions.
Direct ELISA.
Binding of HIT antibodies to complexes between heparin and WT and variant PF4 was determined by ELISA as described.8 12 For each recombinant protein, sera from 50 HIT patients and 20 controls were analyzed. To do this, the optimal molar ratio of each protein to heparin required for recognition by each HIT plasma sample was first determined using a grid in which the final protein concentration was varied from 0 to 15 μg/mL at 5 μg/mL increments, and the heparin concentration was varied from 0 to 1 U/mL at 0.2 U/mL increments. Specifically, these protein/heparin complexes were formed by mixing WT PF4, mutant PF4, or chimeric proteins with unfractionated heparin (Heplock; Elkins-Sinn Inc, Cherry Hill, NJ) in phosphate-buffered saline (PBS) (0.01 mol/L sodium phosphate, 0.138 mol/L NaCl, 0.0027 mol/L KCl, pH 7.4). Microtiter 96-well plates (NUNC Maxisorp, Roskilde, Denmark) were coated overnight at RT with 100 μL of the protein/heparin complexes preformed at a predetermined optimal molar ratios. The wells were then washed three times with PBS containing 0.1% Tween-20 to remove unbound antigen. Unreactive sites were blocked by adding 10% (wt/vol) fetal calf serum (FCS; GIBCO-BRL, Grand Island, NY) in PBS for 2 hours at RT and the wells were washed three times with PBS-T. Patient or control serum diluted 1:200 in PBS/10%FCS (100 μL per well) was added to duplicate wells at RT for 1 hour. After washings with PBS-T, alkaline phosphatase-conjugated goat antihuman IgG, IgA, IgM (Cappel, Organon Teknika, Westchester, PA) diluted 1:2,000 was added for 1 hour at RT. After final washings with PBS-T, p-nitrophenylphosphate (Sigma) in diethanolamine, pH 9.8 was added for 1 hour, and the OD at 405 nmol/L (OD450) was read.
Binding of WT PF4, NAP-2, and mutant proteins to microtiter wells and to 3H-heparin.
To validate the ELISA assays conducted to measure the binding of HIT antibodies to variant proteins, we first measured the capacity of these variant proteins to form complexes with heparin and the capacity of the resultant complexes to bind to the 96-well microtiter plates (NUNC Maxisorp) used in the ELISA. In the first step, each recombinant protein (2 μmol/L) was incubated with 0.1 to 0.6 μmol/L3H-heparin (0.29 mCi/mmol, 6 to 20 kD, NEN, Boston, MA) in 200 μL PBS in microtiter wells. The plates were rocked gently overnight at RT, washed five times with 200 μL PBS/0.1% Tween-20, and the liquid contents were drained. The wells were then incubated overnight with 200 μL of UltimaGold (Packard, Meriden, CT) with gentle agitation and a 100-μL aliquot was counted for radioactivity (2,000 CA TRI-CARB Liquid Scintillation Analyzer, Packard). In the second step, the amount of the recombinant protein/heparin complex that bound to microtiter plates was measured as described for the HIT ELISA above. Wells were coated with a 2-μmol/L final concentration of each recombinant protein in PBS. Bound protein was detected using either polyclonal anti-PF4 or anti-NAP–2 sera in PBS/10% bovine serum albumin (BSA) (Sigma) and a 1:2,000 dilution of alkaline phosphatase-conjugated goat antirabbit IgG in PBS/10% BSA. Binding of antibody to each protein variant was compared with that of WT PF4 or NAP-2.
Role of the C-terminal peptide (48-70) of PF4 in HIT antibody binding.
To examine the role of the C-terminal portion of PF4, we generated a rabbit polyclonal antibody against a 24-AA peptide corresponding to amino acids 48-70 of human PF434 (generously provided by Dr Stefan Niewiarowski, Temple University Medical School, Philadelphia, PA). To do this, the previously-described synthetic peptide34 was coupled to rabbit serum albumin carrier using 20 mmol/L glutaraldehyde (Sigma).41 The peptide was injected alone or after preincubation with heparin at an equimolar ratio immediately before immunization. Rabbit polyclonal antibodies against the peptide/carrier conjugate/heparin complex were generated by the Pocono Rabbit Farm & Laboratory, Inc (Canadensis, PA).
The capacity of rabbit anti-C terminal antibody to compete with human HIT antibodies was then determined. Heparin/PF4-coated plates were preincubated with excess of rabbit anti-PF4 C-terminus antisera (1:50 dilution) for 2 hours at RT, and the unbound protein was removed by extensive washing with PBS-T. HIT antibodies were then added for 1 hour at RT at a concentration that generated 50% of their maximal binding. Binding of HIT antibodies was then measured by the addition of alkaline phosphatase-conjugated antihuman IgG. The percent binding of HIT antibody in the presence of rabbit anti-PF4 was then calculated. Cross-reactivity with rabbit IgG was tested by measuring the binding of the alkaline phosphatase antihuman IgG to the previously added rabbit serum, in the absence of human IgG.
Inhibition ELISA for HIT antibody binding to PF4/heparin by different chimeras.
The IgG fraction from selected HIT serum was isolated using Protein G Sepharose 4 Fast Flow Media Column, Pharmacia, according to manufacturer’s instructions. HIT plasma samples or IgG fraction was diluted to give 50% of its maximal binding to WT PF4/heparin. The capacity of WT or the various chimeric PF4 proteins to inhibit antibody binding was then determined. To do so, this concentration of HIT plasma samples or IgG was preincubated for 2 hours at RT with WT PF4, NAP-2, or PF4/NAP-2 chimeras or BSA (0.5 to 50 μg/mL in PBS). Residual binding was measured by adding the antibody/inhibitor mixture to microtiter wells precoated with WT PF4/heparin and blocked with 10% FCS/PBS for 1 hour at RT. Antibody binding was then measured as described above. The percent inhibition of HIT antibody by WT or variant PF4 protein was calculated as follows: % binding= (OD405 binding with inhibitor × 100)/OD405 binding without inhibitor.
Neutrophil activation.
To test the functionality of the various recombinant proteins, the capacity of the WT and mutant proteins to activate neutrophils was measured by their ability to induce intracytoplasmic Ca2+flux as described.34 Briefly, neutrophils (isolated by Polymorph Prep, NYCOMED, Oslo, Norway, according to manufacturer’s instructions) were loaded with Fura-2-AM pentacetoxymethylester (Cal Biochem Inc, San Diego, CA) at RT for 40 minutes, allowing the cell permeant Fura-2 ester to enter the cells and to be hydrolyzed to the impermeant species, free Fura-2, by intracellular esterases. Two milliliters of cells resuspended in 0.133 g/L CaCl2·2H2O, and 0.1 g/L MgCl2·6H2O was placed in a four-sided warmed curvette with a magnetic stirbar. An excitation wavelength of 340 nmol/L and an emission wavelength of 510 nmol/L were used. Fluorescence (F) of an unstimulated control and cells stimulated with various concentrations of recombinant proteins was recorded with a spectrofluorometer (Perkin-Elmer LS-5; Perkin-Elmer, Oakbrook, IL). After this, 10 μL of 10% (vol/vol) Triton X-100 was added to lyse the cells and release Fura-2 to chelate with Ca2+ (excess) in the suspending solution. Cytosolic free calcium was calculated as previously described.34
Modeling constructs/model building and energy refinement.
Studies were performed to examine potential localized changes associated with the third cysteine in PF4 induced by the various mutations, which affected HIT antibody binding. The crystal structures for PF4 and NAP-2 were obtained from the Brookhaven Crystallographic Databank.42 These structures were used as templates to construct models of the chimeric proteins. Least Squares superpositioning of the respective domains was used as a measure of the similarity in the respective structures despite differences in amino acid sequence. The templates were mutated to those of the respective sequence using the model building program Insight II (Biosym Technologies/MSI San Diego, CA). Side chain angles of the substituted residues were set according to angles identified in a database of side chains. The program Discover (version 2.95 Biosym Technologies) was used for conformational calculations with the supplied consistent valence force field (CVFF) parameters, as discussed previously.43 44 After model building, the respective structures were energy optimized to convergence. Molecular dynamics (MD) at 300°K was used to further alleviate any close contacts within the chimeric proteins. Initially, an MD simulation over 100 picoseconds using the program Discover was performed. The structure was then energy minimized using conjugate gradients to convergence. After this initial equilibration, the calculation was resumed for another 100 picoseconds at 300°K at constant pressure. The resulting structure for the respective proteins was energy minimized using conjugate gradients to convergence.
Statistical analysis.
Results of ELISA assays were analyzed using one-way analysis of variance (ANOVA) test for comparison between groups or T-test for comparison between two groups. A P value <0.05 was considered statistically significant.
RESULTS
Characterization of the recombinant proteins.
The goal of our study was to begin to identify the determinants in PF4/heparin complexes recognized by HIT antibodies. To do so, we generated recombinant PF4 variants, assessed their purity, heparin affinity, and biologic activity. We then measured their capacity to form complexes with heparin capable of binding to plastic wells to compare their binding of HIT antibodies relative to WT PF4/heparin.
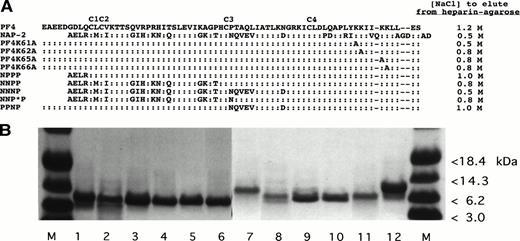
We have previously shown that recombinant PF4 and plasma-derived PF4 express essentially identical biological properties39 and binding of HIT antibodies when complexed with heparin in an ELISA,12 despite retention of the N-terminal methionine in the recombinant molecule. This permitted us to use this recombinant WT PF4 as the template for subsequent mutations. The PF4 variants and control proteins we studied and their heparin-binding affinity, determined by the NaCl concentration needed to elute the proteins from a heparin-agarose column, are shown in Fig2A. These data show that each recombinant protein retained high affinity for heparin, requiring ≥0.5 mol/L NaCl to be eluted from heparin-agarose. Figure 2B shows that the eluted proteins were pure as assessed by SDS-PAGE. Further, WT NAP-2 and chimera proteins NPPP, NNPP, and NNNP (see Fig 2A) induced neutrophil activation as measured by changes in cytosolic Ca2+ concentrations but PF4 did not (not shown), consistent with previous studies described by us and others.34 45
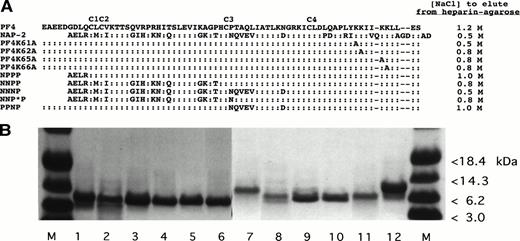
Recombinant WT and mutant PF4 and NAP-2 proteins studied. (A) Schematic presentation of the proteins tested and the concentration (molarity) of NaCl required to elute each protein from a heparin agarose bead column. (B) Coomassie blue staining of 10 μg of various recombinant proteins separated on an SDS-PAGE gel. M, protein markers. Lane 1, WT PF4; lane 2, PF4K61A; lane 3, PF4K62A; lane 4, PF4K65A; lane 5, PF4K66A; lane 6, PPNP; lane 7, WT NAP-2; lane 8, NPPP; lane 9, NNPP; lane 10, NNNP; lane 11, NNP*P; and lane 12, WT PF4.
Recombinant WT and mutant PF4 and NAP-2 proteins studied. (A) Schematic presentation of the proteins tested and the concentration (molarity) of NaCl required to elute each protein from a heparin agarose bead column. (B) Coomassie blue staining of 10 μg of various recombinant proteins separated on an SDS-PAGE gel. M, protein markers. Lane 1, WT PF4; lane 2, PF4K61A; lane 3, PF4K62A; lane 4, PF4K65A; lane 5, PF4K66A; lane 6, PPNP; lane 7, WT NAP-2; lane 8, NPPP; lane 9, NNPP; lane 10, NNNP; lane 11, NNP*P; and lane 12, WT PF4.
We then examined whether the recombinant proteins would bind equally well to the ELISA plates. We added an equal amount of each protein and measured their binding to the wells using polyclonal antibodies to PF4 and NAP-2. The sum of binding to these two antibodies was essentially equivalent for all of the recombinant proteins, suggesting that each bound equally well to the ELISA plate (Fig3A). We then asked whether the bound proteins would bind equivalent amounts of heparin (Fig 3B). Over the range of heparin used in subsequent experiments (0.20 to 0.35 μmol/L), all of the tested proteins bounded, within a twofold range, approximately equal amounts of 3H-heparin. Thus, by each of these criteria, the variant PF4 molecules could be used to identify the binding site(s) of HIT antibodies.
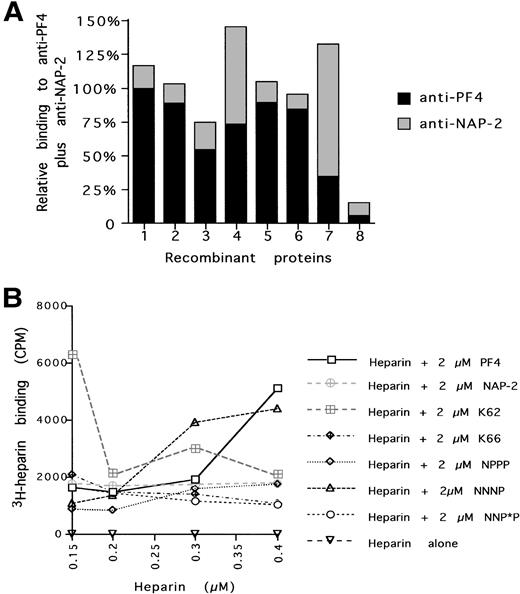
Characterization of the recombinant proteins binding to ELISA plates and to heparin. (A) Amount of protein bound to individual wells as indicated by the sum total of relative binding to a PF4 polyclonal antibody (with PF4 given a value of 1) plus relative binding to a NAP-2 polyclonal antibody (with NAP-2 given a value of 1). (B) Amount of 3H-heparin bound to equal amounts of various recombinant proteins in an ELISA well. Protein 1 = WT PF4; 2 = NPPP; 3 = NNP*P; 4 = NNNP; 5 = PF4, K62; 6 = PF4, K66; 7 = WT NAP-2; and 8 = no recombinant protein.
Characterization of the recombinant proteins binding to ELISA plates and to heparin. (A) Amount of protein bound to individual wells as indicated by the sum total of relative binding to a PF4 polyclonal antibody (with PF4 given a value of 1) plus relative binding to a NAP-2 polyclonal antibody (with NAP-2 given a value of 1). (B) Amount of 3H-heparin bound to equal amounts of various recombinant proteins in an ELISA well. Protein 1 = WT PF4; 2 = NPPP; 3 = NNP*P; 4 = NNNP; 5 = PF4, K62; 6 = PF4, K66; 7 = WT NAP-2; and 8 = no recombinant protein.
Lysine to alanine substitutions in the C-terminus of PF4.
We first addressed the role of the C-terminal lysine-rich region of PF4, which contributes to its heparin affinity, inhibition of angiogenesis,29 and megakaryocytopoiesis30 in the binding of HIT antibodies. Mutation of any of the four C-terminal lysine residues to alanine individually decreases, but does not abolish heparin binding (Fig 2A). This result is consistent with a previous study in which variants with individual alanine substitutions at K62 and K66 in recombinant PF4 retained high affinity for heparin.23 Similar substitutions at K61 and K65 were not evaluated in that study.
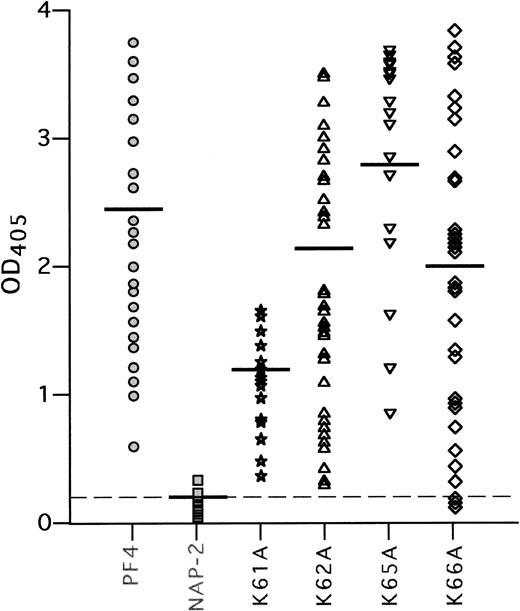
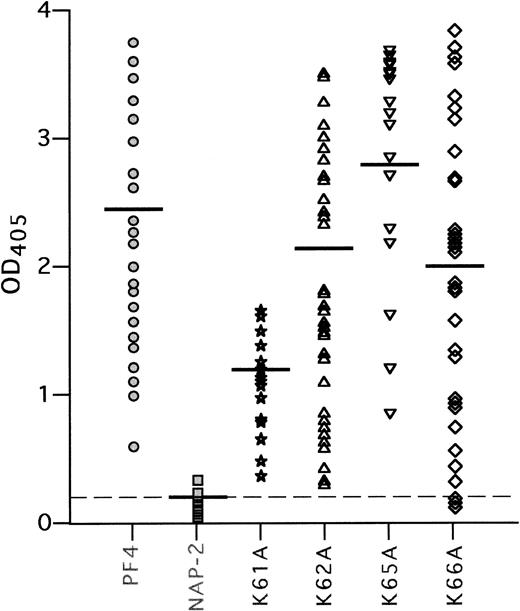
We then tested the binding of 50 HIT and 20 control plasma samples to complexes of these mutant PF4 proteins with heparin,8 12using WT PF4/heparin as the positive control and WT NAP-2/heparin as the negative control (Fig 4). None of the 50 HIT plasma samples contained antibodies to NAP-2/heparin, nor did sera from the 20 controls react with either the PF4/heparin or NAP-2/heparin (mean OD405 = 0.2 ± 0.1 for each). In contrast, HIT antibodies bound strongly to complexes of heparin and PF4 with alanine substitutions at K62, K65, and K66 (OD405 = 2.2 ± 0.9, 2.8 ± 1.0, 2.0 ± 1.1, respectively, compared with WT-PF4/heparin [OD405 = 2.5 ± 0.7] and compared with WT-NAP-2/heparin, P < .001 for each). Somewhat different results were obtained with PF4/heparin with an alanine substitution at K61. This variant eluted from heparin-agarose at the same NaCl concentration as did NAP-2. Yet, in contrast to NAP-2, each HIT plasma tested reacted with the PF4 mutant complexed to heparin (OD405 = 1.0 ± 0.3; P < .001 compared with WT NAP-2/heparin), albeit slightly less well than with WT PF4/heparin (P < .001). These data suggest that the C-terminal lysine residues may contribute to heparin binding, but are not in themselves required for the binding of HIT antibodies in the presence of heparin.
HIT antibody binding to the K to A mutant PF4 proteins. Binding of 50 antibodies from HIT patients to WT PF4, WT NAP-2, and K to A mutant proteins complexed with heparin was tested in ELISA, as described in Materials and Methods. Each point represents binding activity of an individual plasma sample. Results are shown as light absorbency at 405 nmol/L. The mean OD405 readings are indicated by a horizontal line for each protein tested. The mean OD405 for the 20 normal sera tested with PF4/heparin complexes is shown as a dashed line.
HIT antibody binding to the K to A mutant PF4 proteins. Binding of 50 antibodies from HIT patients to WT PF4, WT NAP-2, and K to A mutant proteins complexed with heparin was tested in ELISA, as described in Materials and Methods. Each point represents binding activity of an individual plasma sample. Results are shown as light absorbency at 405 nmol/L. The mean OD405 readings are indicated by a horizontal line for each protein tested. The mean OD405 for the 20 normal sera tested with PF4/heparin complexes is shown as a dashed line.
Competition between HIT antibody and polyclonal antibodies against C-terminal PF4 peptide/heparin for binding to PF4/heparin.
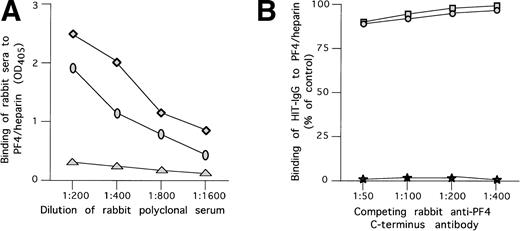
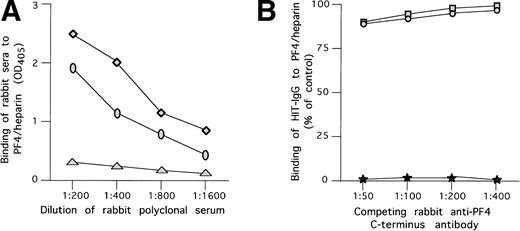
We tested the capacity of polyclonal antibodies to the C-terminal portion of PF4 to compete with the binding of HIT antibodies to WT PF4/heparin complexes. As depicted in Fig5A, antisera raised in rabbits against the C-terminal peptide of PF4 (PF448-70) specifically recognized PF4/heparin complex in a direct ELISA. However, even when preincubated in saturating dilution with PF4/heparin complex, these antibodies had only a minimal effect on the binding of HIT antibodies to PF4/heparin complex (Fig 5B).
Antibodies to the PF4 c-terminus do not compete with HIT antibodies. (A) Binding of rabbit polyclonal anti-C-terminal antibodies to PF4/heparin complex. (⧫) Binding of rabbit antibody developed to C-terminal PF4 peptide complexed to heparin at various concentration to microtiter wells coated with PF4/heparin complex. (○) Same as above, but for antibody raised against the C-terminus only. (▴) Binding of rabbit antibody developed to C-terminal PF4 peptide complexed to heparin at various concentration to uncoated wells. (B) Competition of binding to PF4/heparin in ELISA by polyclonal anti-PF4 C-terminus and HIT antibodies. Polyclonal anti-PF4 C-terminus antibodies raised in rabbits were used at different concentrations and preincubated with immobilized WT PF4/heparin or NAP-2/heparin complexes attached to microtiter plates. HIT antibodies in a predetermined concentration giving 50% binding were added to the plates and residual binding was measured. As shown, no significant competition with HIT antibody binding was demonstrated by either rabbit antibody. (□) Polyclonal antibody raised against PF4/heparin and tested with PF4/heparin complexes on microtiter wells. (•) Polyclonal antibody raised against PF4 and tested with PF4/heparin complexes on microtiter wells. (★) Binding of the alkaline phosphatase antihuman IgG to the previously added rabbit serum in the absence of HIT antibody.
Antibodies to the PF4 c-terminus do not compete with HIT antibodies. (A) Binding of rabbit polyclonal anti-C-terminal antibodies to PF4/heparin complex. (⧫) Binding of rabbit antibody developed to C-terminal PF4 peptide complexed to heparin at various concentration to microtiter wells coated with PF4/heparin complex. (○) Same as above, but for antibody raised against the C-terminus only. (▴) Binding of rabbit antibody developed to C-terminal PF4 peptide complexed to heparin at various concentration to uncoated wells. (B) Competition of binding to PF4/heparin in ELISA by polyclonal anti-PF4 C-terminus and HIT antibodies. Polyclonal anti-PF4 C-terminus antibodies raised in rabbits were used at different concentrations and preincubated with immobilized WT PF4/heparin or NAP-2/heparin complexes attached to microtiter plates. HIT antibodies in a predetermined concentration giving 50% binding were added to the plates and residual binding was measured. As shown, no significant competition with HIT antibody binding was demonstrated by either rabbit antibody. (□) Polyclonal antibody raised against PF4/heparin and tested with PF4/heparin complexes on microtiter wells. (•) Polyclonal antibody raised against PF4 and tested with PF4/heparin complexes on microtiter wells. (★) Binding of the alkaline phosphatase antihuman IgG to the previously added rabbit serum in the absence of HIT antibody.
PF4/NAP-2 chimeric protein.
As these results suggest that the C-terminal lysines of PF4 do not comprise the major antigenic site for HIT antibodies, we adopted an alternative strategy for assessing the contribution of the other domains by making chimeras between PF4 and NAP-2, as outlined in Fig2A. This approach is predicated on the observation that although NAP-2 shares ≈60% AA homology with PF4 and demonstrates significant affinity for heparin, few HIT sera recognize NAP-2 or NAP-2/heparin complexes (Amiral et al16). The chimeras were constructed by considering the PF4 and NAP-2 proteins as being composed of four domains: (1) an N-terminal domain that terminates at the first cysteine residue, (2) a domain that lies between the second and third cysteines, (3) a domain that lies between the third and fourth cysteines, and (4) a C-terminal domain that begins after the fourth cysteine residue. The first and fourth domains are located on the outer surface of the tetramer, whereas the second and third domains form beta sheets, part of which are on the surface and part of which lie between the monomeric subunits. In the studies described below, the chimeric proteins are considered to be composed of these four domains derived either from NAP-2, designated by an “N,” or from PF4, designated by a “P” (see also Fig 2A).
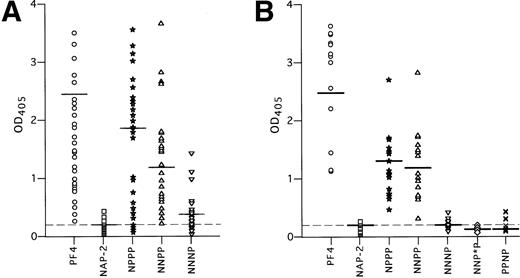
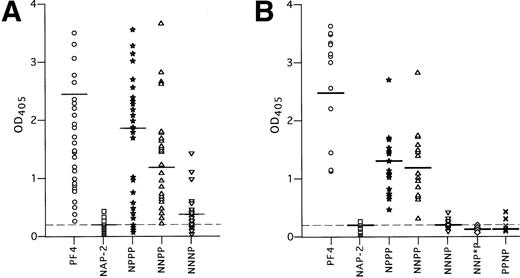
HIT antibodies bound to complexes between heparin and the chimeras NPPP and NNPP (Fig 6A). Binding was intermediate between that seen with WT-PF4 and NAP-2 (OD405 = 1.5 ± 1.0 and 1.0 ± 0.7, respectively; P < 0.001 for each protein compared with NAP-2/heparin). In contrast, HIT antibodies were unable to bind to complexes between heparin and the chimeric protein NNNP above the level seen with NAP-2/heparin (OD405 = 0.25 ± 0.03, compared with 0.20 ± 0.01, P > 0.05).
HIT antibody binding in ELISA to the chimeric PF4-NAP-2 proteins complexed to heparin. (A) Binding of 50 HIT sera samples to WT PF4, WT NAP-2, and the PF4/NAP-2 chimeric proteins complexed to heparin. (B) Similar to (A), but only the 18 HIT sera with greatest reactivity to NNPP in (A) were studied. Each point represents binding activity of an individual serum sample. IgG binding is measured by OD405. The mean OD405 readings are indicated by a horizontal line for each protein. The mean OD405 for the 20 normal sera tested with PF4/heparin complexes is shown as a dashed line.
HIT antibody binding in ELISA to the chimeric PF4-NAP-2 proteins complexed to heparin. (A) Binding of 50 HIT sera samples to WT PF4, WT NAP-2, and the PF4/NAP-2 chimeric proteins complexed to heparin. (B) Similar to (A), but only the 18 HIT sera with greatest reactivity to NNPP in (A) were studied. Each point represents binding activity of an individual serum sample. IgG binding is measured by OD405. The mean OD405 readings are indicated by a horizontal line for each protein. The mean OD405 for the 20 normal sera tested with PF4/heparin complexes is shown as a dashed line.
However, we found considerable variation in the extent to which the 50 HIT sera bound to these chimeric proteins. For example, some HIT sera reacted with heparin complexed to NPPP and NNPP as strongly as with PF4, whereas others showed little or no reactivity. These data are compatible with the existence of a variable polyclonal response to an antigen comprised of more than one binding site.35 36 Thus, to define an individual site more precisely, we reasoned that it would be most appropriate to study in more detail those sera that react most strongly with that determinant. Therefore, in a separate experiment, we restudied the 18 plasma that reacted most strongly with the NNPP/heparin complex (Fig 6B). Each of these 18 plasma reacted strongly with both NPPP/heparin and NNPP/heparin (1.0 ± 0.4, 1.0 ± 0.5, respectively, in this study) whereas none reacted with NNNP/heparin (0.2 ± 0.1 v NAP-2/heparin, 0.1 ± 0.1, P < .05). From this we conclude that one or more epitopes seen by these sera is lost when the third domain of NAP-2 is substituted for the analogous portion of PF4.
NNPP and NNNP differ by six amino acids, including a stretch of five amino acids immediately after the third cysteine residue (Fig 2A). Therefore, we made an additional chimera, NNP*P, in which the most N-terminal amino acid of this group, P37, was switched to the asparagine present in the homologous position in NAP-2. The change of this one amino acid led to a near total loss of antibody binding (OD405 = 0.12 ± 0.03, compared with 1.0 ± 0.5 for NNPP/heparin, P < 0.001) (Fig 6B).
It remains possible that the effect seen with NNP*P was due to an accumulative effect of the changes in the first two domains of the chimeric proteins plus the P37 → N change in the third domain. To further demonstrate the importance of the third PF4 domain in HIT antigenicity, an additional chimeric protein PPNP was constructed, which differs from WT PF4 only in the third domain (Fig 2A). This protein has high heparin affinity, but also demonstrated virtually no antibody binding when bound to heparin (OD405 = 0.19 ± 0.05, compared with 2.4 ± 0.5 for WT PF4/heparin, P < 0.0001) (Fig 6B).
Inhibition ELISA assays.
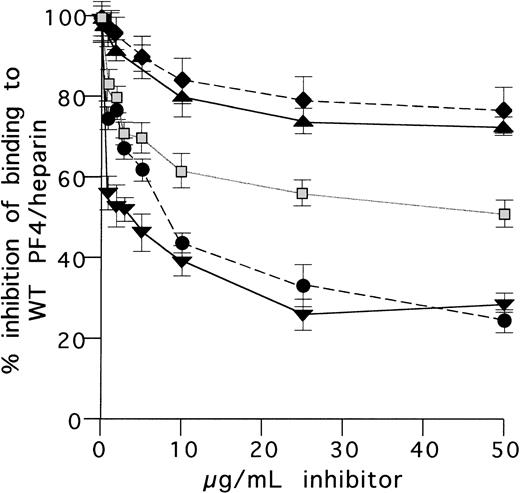
Competition-inhibition studies were then performed to investigate the potential importance of the region between the third and fourth cysteines of PF4 through an independent approach. We compared the capacity of WT and various chimeric PF4 proteins to inhibit the binding of HIT antibodies known to bind to NNPP/heparin by solid phase ELISA. Representative results from one such assay are shown in Fig 7. NNPP/heparin in fluid phase inhibited HIT antibody binding to immobilized WT PF4/heparin to almost the same extent as did WT PF4/heparin. NNPP and PF4 at a concentration of 12 μg/mL, when complexed with heparin, inhibited the binding by 61% ± 2% and 57% ± 2%, respectively, whereas NNNP and NAP-2, when complexed with heparin, did not inhibit antibody binding to this extent even at a fivefold higher concentration. NAP-2/heparin inhibited HIT antibody binding to the same extent as did BSA/heparin, representing a nonspecific inhibition.
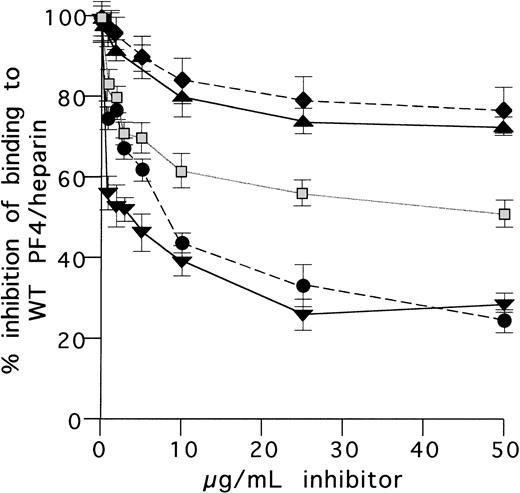
Inhibition of HIT antibody binding to WT-PF4/heparin complex by chimeric proteins and by anti–C-terminal PF4 antibodies. HIT antibody sample in a predetermined concentration giving 50% binding to WT PF4/heparin was preincubated with various WT or chimeric proteins at different concentrations. Residual antibody binding to WT PF4/heparin was then measured in ELISA and expressed as HIT antibody binding: % of control (100% binding without inhibitor). Note the similar maximal inhibition obtained in the presence of WT PF4/heparin and the chimera NNPP/heparin. (⧫) BSA; (▴) NAP-2; (▧) NNNP; (▾) PF4; (•) NNPP.
Inhibition of HIT antibody binding to WT-PF4/heparin complex by chimeric proteins and by anti–C-terminal PF4 antibodies. HIT antibody sample in a predetermined concentration giving 50% binding to WT PF4/heparin was preincubated with various WT or chimeric proteins at different concentrations. Residual antibody binding to WT PF4/heparin was then measured in ELISA and expressed as HIT antibody binding: % of control (100% binding without inhibitor). Note the similar maximal inhibition obtained in the presence of WT PF4/heparin and the chimera NNPP/heparin. (⧫) BSA; (▴) NAP-2; (▧) NNNP; (▾) PF4; (•) NNPP.
Effect of mutations on antibody binding versus PF4 structure.
We then asked how the structure of PF4 was likely to have been altered by mutations that disrupted HIT antibody binding. The Root Mean Square Deviation (RMS) of spatial positions of the backbone structure of two proteins provides a measure of their similarity in structure. Superpositioning of the individual subunits of PF4 and NAP-2 result in an RMS deviation of 1.5Å between subunits. Superpositioning of the structures between their third and fourth cysteine (the third domain) results in an RMS of 0.6Å, indicating that this region is highly conserved structurally notwithstanding differences in their amino acid sequences (see Fig 8, page 3251). This observation suggests further that the four domains of PF4 and NAP-2 have the same general conformations and should be relatively interchangeable, particularly the domain that lies between the third and fourth cysteines (Fig 2A). Substitution of the six AA differences between the two chemokines into the PF4 starting geometry or the six amino acids of NAP-2 into the PF4 starting geometry does not change the structures of these domains (0.6Å RMS). Nor was there evidence of dramatic structural changes when the proline was replaced by the asparagine notwithstanding the differences in antibody binding.
DISCUSSION
The goal of this study was to begin to identify those portions of PF4 that contribute to its recognition by HIT antibodies in the presence of heparin. Previous approaches using PF4-based peptides or denatured protein have been interpreted to suggest that multiple sites on PF4 might be involved and that HIT antibodies do not recognize linear amino acid sequences.35 36 Therefore, our approach was to introduce mutations into native PF4 so that the molecule was more likely to maintain conformational constraints and retain the relevant interactions with heparin to permit antibody binding. In accord with this, the mutants we studied formed complex with heparin, and these complexes bound to ELISA wells in a way that permitted comparisons with WT PF4 to be made.
Binding of HIT antibodies was disrupted to only a limited extent by mutations made in the lysine residues in the C-terminal α helical portion of the molecule, which are involved in heparin binding.24 Indeed, only the K61 → A substitution showed a significant impact in HIT antibody binding (Fig 4). The same conclusion was drawn from studies in which we found that binding of HIT antibodies was not inhibited by a polyclonal antibody to the C-terminus of PF4 (Fig 5B). These findings are in accord with previous studies showing that C-terminal-based peptides are not recognized by HIT-Ig,35 36 and suggest that dominant epitopes lie outside the circumferential band of positive charge residues.
Similarly, changes in the N-terminus of PF4 were tolerated. The N-terminus of PF4 could be substituted by the ELR-containing sequence of NAP-2 sequence in the NPPP chimera with little reduction in antibody binding (Fig 6A). Most HIT antibodies also bound to the NNPP chimera in which the two N-terminal domains of NAP-2 were introduced into PF4 (Fig6A).
In contrast, antibody binding was lost in most cases when NNNP was complexed with heparin. These data are consistent with the hypothesis that a major antigenic site for HIT antibodies requires the integrity of the region between the third and fourth cysteine residues. This was especially evident when plasma samples from the subgroup of patients with high titers of antibodies to NNPP were examined, none of which showed significant binding to NNNP/heparin. Further support for this concept comes from the finding that NNPP/heparin inhibited binding of HIT antibodies with WT PF4/heparin almost as well as WT PF4/heparin itself, whereas NNNP/heparin was much less effective (Fig 7). Further, substitution of a single amino acid in PF4 that lies at the interface of the third and fourth domains to that found in NAP-2 (P37 → N) in NNP*P virtually abolished antibody binding (Fig 6B). Finally, the chimera PPNP was tested in which the first two and the last domains of PF4 were conserved and only the differences between PF4 and NAP-2 in the third domain remained. This construct also demonstrated little HIT antigenicity and supported the importance of the domain between the third and fourth cysteine residues for antibody binding.
The P37 residue is located near the surface of the tetramer away from the heparin binding ring (Fig 1). Computerized modeling indicates that the P37 → N substitution is not expected to introduce dramatic structural changes that would disrupt its conformation or folding. Thus, the P37 → N substitution in NNP*P most likely impairs antibody binding by directly perturbing the antigen recognition site, although an untoward effect such as disruption of the multimer composition of the protein/heparin complex cannot be formally excluded.
P37 is located near the surface of PF4 tetramer away from the positive ring of amino acids that bind heparin. Binding of heparin may alter the conformation of the PF4 tetramer, exposing a cryptic epitope that involves the P37 residue. The four amino acids adjacent to the C-terminus of P37 in PF4 and the homologous residues in NAP-2 differ as well. Each are buried within a hydrophobic pocket created by the beta sheet strands. It is possible that the binding of heparin exposes one or more of these residues as well. Heparin is able to cause profound conformational changes in antithrombin III, which exposes its P-P′ site and dramatically accelerates its serpin activity.46 The results of our studies are also reminiscent of the conformational changes induced in the positively-charged β-2-glycoprotein by anionic phospholipids, which may generate neo-epitopes involved in the pathogenesis of the antiphospholipid syndrome.47 48
Significant heterogeneity was seen in the binding of HIT antibodies from different patients to the various PF4 mutants. A likely explanation for this finding is that serum antibodies are polyclonal and that patients differ in their response to various epitopes. Some plasma contained antibodies that bound poorly to the NNPP and NPPP chimeras, suggesting that some epitopes require structural features imposed by the first two “domains” of the molecule. In others, antibody binding to NNNP/heparin and the K61A was impaired, consistent with an epitope proximal to the α helix in the C-terminus of PF4. Alternatively, these sites may transmit conformational changes induced by heparin to other parts of the molecule that are contacted by the HIT antibodies directly. Additional studies will be required to localize and characterize epitopes on other parts of the PF4 molecule, to understand how heparin induces “neo-epitope formation” and to determine whether antibody specificity correlates with the risk of thrombosis.
ACKNOWLEDGMENT
We thank Dr M. Anna Kowalska for assistance in performing the neutrophil activation studies and Stefan Niewiarowski for sharing the PF4 C-terminal synthetic peptide with us.
Supported in part by Grant No. RO1-HL54749 from the National Institutes of Health, Bethesda, MD.
Address reprint requests to M. Poncz, MD, The Children’s Hospital of Philadelphia, 34th St & Civic Center Blvd, ARC, Room 316H, Philadelphia, PA 19104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.