Abstract
Congenital deficiency of factor XI is a rare condition associated with a mild to moderate bleeding diathesis that is most commonly found in persons of Jewish ancestry. The disorder has been reported sporadically in a number of other ethnic groups, but rarely in the black population. We report on the genetic analysis of the factor XI genes of two African American patients: a 9-year-old boy (the propositus) with mild factor XI deficiency and his mother. Both individuals have lifelong histories of excessive bleeding. Dideoxyfingerprinting, a technique combining components of single-strand conformational polymorphism analysis and dideoxy-chain termination sequencing, was used in the analysis. Both patients were found to be heterozygous for a mutation changing serine 248 to glutamine, whereas the propositus was heterozygous for an additional mutation on the paternal allele changing glutamine 226 to arginine. Both mutations reside in the third apple domain of the factor XI heavy chain, an area that has been shown to contain binding sites for factor IX, platelets, and glycosaminoglycans. Previously reported mutations in the factor XI gene seem to cause deficiency primarily by reducing protein expression. Because both alleles in the propositus contain amino acid substitutions, the significant amount of circulating factor XI in his plasma must be comprised entirely of abnormal molecules. Factor XI circulates as a homodimer, and the presence of mutations in both alleles of the factor XI gene suggests that his bleeding disorder is caused in part by the effect of the two abnormal gene products forming dimers in different combinations. Three neutral (not associated with amino acid changes) DNA polymorphisms were also identified in the two subjects: a C to T change at nucleotide 472 in exon 5, A to G at nucleotide 844 in exon 8, and T to C at nucleotide 1234 in exon 11. Analysis of a random sample of normal volunteers showed that these polymorphisms are relatively common, with allele frequencies of 7.4%, 19%, and 18%, respectively. This suggests that there is considerable genetic heterogeneity in the factor XI gene.
© 1998 by The American Society of Hematology.
COAGULATION FACTOR XI is the zymogen of a plasma serine protease that contributes to hemostasis by activating factor IX through limited proteolysis.1 Congenital deficiency of factor XI is a rare condition with a particularly high prevalence in individuals of Ashkenazi Jewish descent.2,3The disorder causes a mild to moderate bleeding diathesis characterized primarily by postoperative and traumatic hemorrhage.2,4-6Curiously, bleeding seems to correlate poorly with the level of plasma factor XI activity as determined by assays based on contact activation–initiated coagulation, such as the activated partial thromboplastin time (aPTT).7-9 Although it is generally agreed that the risk of hemorrhage is greatest for those with severe decreases in factor XI activity (<15% of the activity of normal plasma),2,10,11 the bleeding tendency can be highly variable, and persons with severe deficiency may have no abnormal bleeding.2,7-9 More controversial is the issue of abnormal hemostasis in mild factor XI deficiency (>25% of the activity of normal plasma), where the patients are presumed to have one normal and one abnormal copy of the factor XI gene. Some studies have reported similar bleeding problems for those with mild and severe deficiency,8,9 and there are families in which bleeding symptoms seem to be inherited in an autosomal-dominant manner.12-14 This suggests that some forms of factor XI deficiency may differ from those of other components of the coagulation cascade which are typically symptomatic only in the homozygous or compound heterozygous condition.
Factor XI is unique among the proteases of the coagulation cascade in that it circulates as a disulfide-linked dimer of identical polypeptide chains.15 Therefore, hypothetically, mild factor XI deficiency could behave in some instances as an autosomal-dominant disorder in the same manner as is observed in certain forms of von Willebrand disease.16 The dimeric protein may exist as a mixture of normal and abnormal subunits with unpredictable effects on hemostasis in vivo. However, data to support this premise are lacking because virtually all mutations in the factor XI gene identified to date seem to either disrupt or greatly decrease expression of protein.17-20 We used dideoxyfingerprinting (ddF),21 22 a powerful technique combining features of single-strand conformational polymorphism (SSCP) analysis and dideoxy-chain termination DNA sequencing, to analyze the factor XI genes in two members of an African American family with histories of excessive bleeding. The propositus, a 9-year-old boy with mild factor XI deficiency, is a compound heterozygote for mutations in the factor XI third apple domain, indicating that the factor XI protein circulating in his plasma is entirely comprised of abnormal molecules. In addition to the amino acid changes, several neutral polymorphisms that indicate that there may be significant heterogeneity in the factor XI gene were identified in these individuals.
MATERIALS AND METHODS
Materials and Reagents
Molecular biology.
The complementary DNA (cDNA) for human factor XI was a gift from Dr Dominic Chung (University of Washington, Seattle, WA). TaKaRa long-range DNA polymerase for polymerase chain reaction (PCR) was from Pan Vera Corp (Madison, WI). NuSieve agarose and MDE gel solution for preparing polyacrylamide gels for ddF were from FMC BioProducts (Rockland, ME). QIAamp blood kits and QIAEX II gel extraction kits were purchased from Qiagen (Chatsworth, CA). A Thermo-sequenase radiolabeled terminator cycle sequencing system for DNA sequencing and γ-32P-dATP were from Amersham Life Sciences (Arlington Heights, IL). The Chameleon double-stranded site-directed mutagenesis kit, XL-1 blue strain of Escherichia coli, and plasmid pBluescript were from Stratagene (La Jolla, CA). T4 polynucleotide kinase and restriction endonucleases SmaI andAatII were purchased from New England Biolabs, Inc (Beverly, MA). Taq DNA polymerase was from GIBCO-BRL (Gaithersburg, MD).
Tissue culture.
The 293 human embryonal kidney fibroblast cell line was purchased from American Tissue Type Collection (ATCC CRL 1573; Rockville, MD). Cell-gro Complete serum-free medium was from Mediatech, Inc (Herdon, VA).
Measurement of factor XI.
Goat anti-human factor XI polyclonal antibodies, with and without conjugated horseradish peroxidase (HRP), for enzyme-linked immunosorbent assay (ELISA) were purchased from Enzyme Research Laboratories (South Bend, IN). Rabbit anti-goat IgG polyclonal antibody conjugated to alkaline phosphatase was from Sigma Chemical (St Louis, MO). Factor XI–deficient and pooled normal human plasma were from George King Bio-Medical (Overland Park, KS). Thrombosil aPTT reagent was from Ortho Diagnostic (Raritan, NJ).
Patients and Controls
Patient one is a 9-year-old African-American boy with a history of excessive bleeding after dental procedures and frequent epistaxis that his physicians believe has contributed to the development of iron deficiency. He has required plasma transfusions for several of these episodes. In addition, he had prolonged bleeding after circumcision at birth. Laboratory evaluation has revealed a normal prothrombin time (PT), a mild prolongation (a few seconds) of the aPTT, and a factor XI activity level that has ranged from 40% to 55% of normal plasma levels. No other coagulation factor was found to be below normal range, and a bleeding time test and evaluations for von Willebrand disease have been negative. His mother, patient two, has a lifelong history of abnormal hemostasis including bleeding with dental work, frequent epistaxis, and heavy bleeding after childbirth. Her PT, aPTT, and bleeding time are normal. At the age of 8 she was given the diagnosis of von Willebrand disease; however, multiple evaluations since that time have not verified this. Evaluation of her von Willebrand factor levels during the course of this study showed antigen and activity levels of 65% to 70% of normal plasma levels, which are within normal range. Patient three is a 67-year-old man of European Jewish ancestry with severe factor XI deficiency (plasma activity 2% to 3% of normal plasma levels) and a history of postoperative and posttraumatic bleeding requiring multiple transfusions of fresh frozen plasma. He was included in this study as an abnormal control for the genetic analysis. Twenty-seven healthy volunteers with normal PTs and aPTTs served as a normal control population. Platelet-poor plasma was prepared on all individuals from peripheral venous blood collected into a one-tenth volume of 3.8% sodium citrate. Blood samples were also collected into EDTA and frozen to serve as a source of DNA. Protocols for obtaining samples were approved by the institutional review boards of both of the involved institutions.
Factor XI activity and antigen levels in platelet-poor plasma were determined for the three patients and compared with values for a group of 10 normal controls. Activity levels were measured by modified aPTT comparing the capacity of patient and pooled normal plasma to correct the abnormal clotting time of factor XI–deficient plasma.23 Measurements were performed in duplicate on an STA coagulation analyzer (Diagnostica Stago, Asnieres-sur-Seine, France) and were verified on a Dataclot 2 fibrometer (Helena Lab, Beaumont, TX). Factor XI antigen levels were determined by ELISA using goat anti-human factor XI polyclonal capture and HRP-conjugated detection antibodies according to the supplier’s (Enzyme Research Lab) instructions. Serial dilutions of pooled normal plasma were used to construct a control curve for the ELISA to which the results for patient samples were compared. Results are shown in Table 1.
Isolation and Polymerase Chain Reaction Amplification of DNA
Genomic DNA was isolated from peripheral blood anticoagulated with EDTA using a QIAamp blood kit. For PCR amplification of DNA, pairs of oligonucleotide primers were generated for each exon encoding the mature factor XI molecule (exons 3-15, Table 2) using Oligo Selection Program (OSP) software24 and the published sequence of the factor XI gene.25 Exons 8 and 9, and 9 and 10 were amplified together because of their close proximity to each other. PCR was performed on a Perkin Elmer model 460 DNA Thermal Cycler (Foster City, CA) and was performed in a total volume of 50 μL containing 10× TaKaRa reaction buffer, 1.5 mmol/L magnesium chloride, 100 μmol/L of each deoxyribonucleotide triphosphate (dNTP), 10 pmol of each primer, 2.5 U of TaKaRa LA Taq polymerase, and 100 ng of genomic DNA. The reaction mixture was initially denatured at 95°C for 3 minutes, followed by annealing at 60°C for 40 seconds and extension at 72°C for 1 minute. Subsequently, the following cycling parameters were used: 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute for 39 cycles. PCR products were size fractionated by electrophoresis on 2% NuSieve agarose gels and the DNA was cut from the gels and purified using a QIAEX II gel extraction kit according to the manufacturers’ recommendations.
ddF and DNA Sequencing
ddF was performed as recently described.22 The oligonucleotide primers were the same as those used for PCR amplification of the exons, and ddF was performed in both 5′ and 3′ directions for each exon. The primers were end labeled using γ-32P-dATP and T4 polynucleotide kinase. ddF reactions were performed in a total volume of 8.0 μL containing 20 mmol/L Tris-HCl, pH 8.4; 50 mmol/L KCl; 2 mmol/L MgCl2; 20 μmol/L of each dNTP; 100 μmol/L of dideoxy-GTP; 1 pmol of32P-labeled primer; 0.25 U of Taq DNA Polymerase; and 5 to 15 ng of purified PCR fragment. The reactions were performed on the Perkin Elmer model 460 DNA Thermal Cycler using the following cycling parameters: denaturation at 94°C for 45 seconds, annealing at 60°C for 30 seconds, and elongation at 72°C for 1 minute for 30 cycles. Subsequently, each reaction was mixed with 16 μL of gel-loading buffer (98% formamide, 10 mmol/L EDTA with bromphenol blue and xylene cyanol) and subjected to electrophoresis at 30 W for 3 hours at room temperature using a Sequi-Gen GT Sequencing Cell system (Bio-Rad, Hercules, CA) containing a 0.5× MDE gel (prepared according to the manufacturer’s specifications) in 0.5× conventional Tris-boric acid-EDTA buffer. Gels were dried and exposed to X-ray film overnight. PCR fragments that differed from their normal counterparts on the ddF analysis were subjected to dideoxy-chain termination sequencing using a Thermo Sequenase radiolabeled terminator cycle sequencing kit according to the manufacturer’s instructions. Each PCR fragment was sequenced in both directions using the oligonucleotide primers originally used to produce the PCR fragment (Table 2).
Allele-Specific Analysis for Factor XI Mutations
To determine whether the Q226R and S248Q mutations in patient one are on the same or opposite alleles the following analysis was performed. A 4-kb fragment of the factor XI gene encompassing exons 7 through 9 was amplified by PCR from genomic DNA isolated from patient one. The 5′-primer from exon 7 and the 3′-primer from exon 9 were used for the amplification (Table 2). The PCR product was cloned into plasmid pBluescript and subsequently used to transform XL-1 blue E coli. Plasmid DNA was isolated from individual clones of transformed bacteria and subjected to restriction endonuclease digestion and DNA sequencing analysis. The Q226R mutation in exon 7 was detected by SmaI restriction enzyme analysis. The S248Q mutation was detected by sequencing exon 8 as described above.
Frequencies of Neutral Polymorphisms in a Group of Normal Volunteer Donors
Genomic DNA isolated from the blood of normal volunteers was subjected to ddF analysis for PCR fragments representing exons 5, 8-9, and 11. In addition, the exon 5 PCR fragments underwent restriction endonuclease digestion analysis with the enzyme AatII using the conditions recommended by the manufacturer. Fragments were run on a 2% conventional agarose gel, stained with ethidium bromide, and photographed.
In Vitro Expression and Analysis of Factor XI
Complementary DNAs for human factor XI containing the Q226R and S248Q mutations were prepared using the Chameleon double-stranded site-directed mutagenesis kit. The oligonucleotides used to introduce the mutations were 5′-GGGCCATTCCCGGGAAAAGAAGG-3′ for Q226R and 5′-TTAATGCGTGTATTGGGCAATCCAC-3′ for S248Q, where the underlined nucleotide indicates the location of the introduced mutation. The wild-type and mutant cDNAs were introduced into the mammalian expression vector pJVCMV,26 and the constructs were used in transient transfection assays with 293 human fetal kidney fibroblasts, using a conventional calcium phosphate precipitation technique.27 Twenty-four hours after transfection the medium was replaced by serum-free medium (Cell-gro Complete). Conditioned medium was collected on day 4 and the concentration of recombinant factor XI was determined by ELISA (see above) using known amounts of purified recombinant wild-type factor XI26 to construct a control curve. Fifty-microliter samples of conditioned media were size fractionated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis on 10% gels, followed by Western immunoblot analysis using a 1:1,000 dilution of the same goat anti-human factor XI polyclonal antibody used for the ELISA. Detection was with a rabbit anti-goat IgG polyclonal antibody conjugated to alkaline phosphatase as previously described.23
Stable transfectants for wild-type factor XI and the two mutants were established in 293 cells by a previously published method.26 Serum-free conditioned medium was collected and the recombinant factor XI was purified by monoclonal antibody affinity chromatography.26 Specific activities for the recombinant proteins were determined as follows: factor XI protein was diluted to 5 μg/mL in 50 mmol/L Tris-HCl, pH 7.5; 150 mmol/L NaCl; 0.1% bovine serum albumin (TBSA); and serial 1:2 dilutions of these preparations were made in TBSA. Sixty micoliters of each recombinant protein dilution was mixed with equal volumes of factor XI–deficient plasma and aPTT reagent.23 After 5 minutes of incubation at 37°C, 60 μL of 25 mmol/L CaCl2 was added, and the time to clot formation was determined on the Dataclot 2 fibrometer. Results were compared with a standard curve constructed with plasma-derived factor XI that has a specific activity of 200 U/mg.
RESULTS
ddF
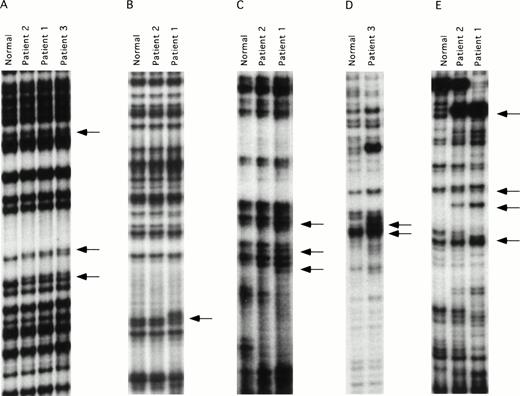
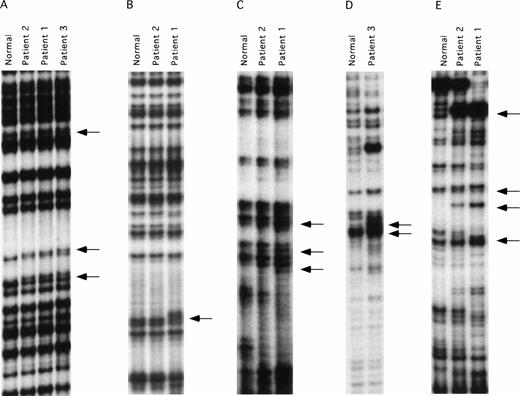
ddF is a hybrid technique using features of SSCP analysis and dideoxy-chain termination sequencing.21,22 The technique has been shown to have improved sensitivity compared with conventional SSCP, and is easier to perform because it is not necessary to establish critical temperature ranges for each fragment tested. DNA mutations may be identified by ddF either as a shift in the position of bands (the SSCP component) or as the loss or gain of a band (dideoxy-sequencing component) on the autoradiograph. ddF was performed on exons 3 through 15 of the factor XI gene on the three patients and a pair of randomly selected normal controls. Exon 1 of the factor XI gene is not translated, whereas exon 2 codes for a signal peptide that is not present on the mature circulating protein.25 28 The ddF patterns of the two controls were identical for all exons tested, and these patterns were assumed to represent wild-type sequence. The validity of this assumption was confirmed by subsequent analysis of a larger sample of control individuals (data not shown). Results for exons in which abnormal ddF patterns were detected are shown in Fig 1. Samples from patient one showed abnormal ddF patterns for PCR fragments representing exons 5, 7, 8-9, and 11, and patient two had a similar abnormality in exon 5. Whereas patient two also showed abnormalities in fragments 8-9 and 11, the patterns differed from those of her son, and her exon 7 pattern did not differ from the normal controls. Patient three showed abnormal ddF patterns for exons 5, 8-9, and 9-10 that did not resemble the patterns of either patient one or two.
ddF analysis of the factor XI gene. Autoradiographs of ddF analysis are shown for exons that gave discordant results between the patients and the control individuals designated as normal. (A) exon 5, (B) exon 7, (C) exon 8-9, (D) exon 9-10, and (E) exon 11. The arrows identify the positions of new or shifted bands in the patient samples that indicate the presence of nucleotide changes.
ddF analysis of the factor XI gene. Autoradiographs of ddF analysis are shown for exons that gave discordant results between the patients and the control individuals designated as normal. (A) exon 5, (B) exon 7, (C) exon 8-9, (D) exon 9-10, and (E) exon 11. The arrows identify the positions of new or shifted bands in the patient samples that indicate the presence of nucleotide changes.
DNA Sequencing
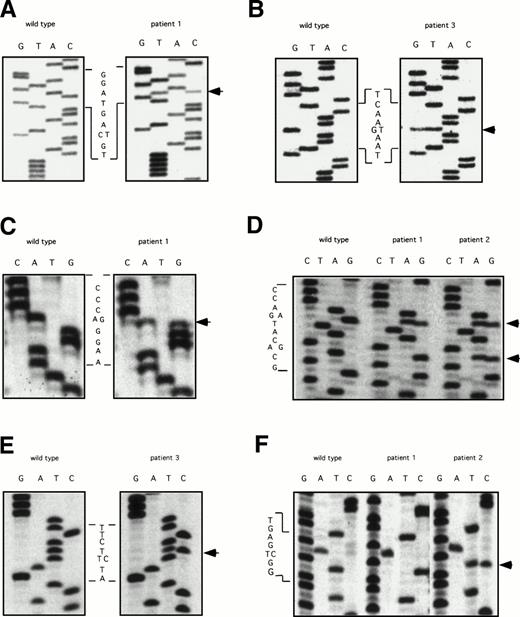
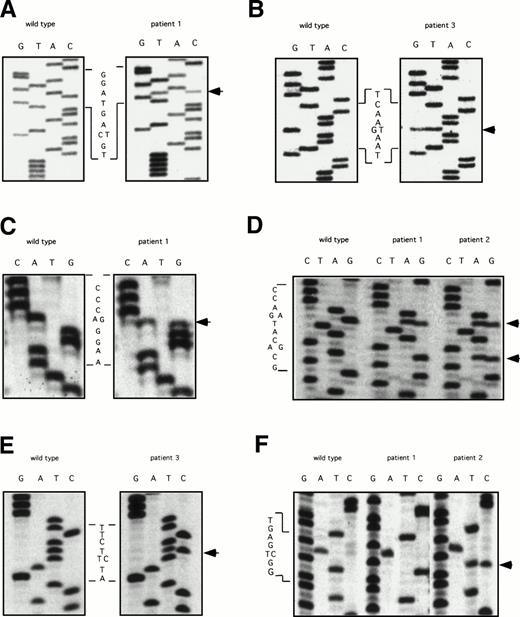
The ddF results were followed by conventional dideoxy-chain termination based sequencing of PCR fragments for exons 5, 7, 8-9, 9-10, and 11. Results are shown in Fig 2. The factor XI cDNA nucleotide numbering system is based on the published sequence reported by Fujikawa et al.28 All three patients were found to be heterozygous for a C to T change in exon 5 at nucleotide 472 that does not result in a change in amino acid sequence (Fig 2A). In addition, patient three has a G to T mutation at bp 446, changing glutamic acid 117 to a stop codon (Fig 2B). This is the factor XI type II mutation that is commonly found in patients of Jewish ancestry.2 18
DNA sequencing of PCR products with abnormal ddF patterns. The arrows indicate the locations of nucleotides that differ from the published sequence for wild-type human factor XI.28 (A) exon 5: C to T at nucleotide 472, no amino acid change; (B) exon 5: G to T at nucleotide 446, changing glutamic acid 117 to a stop codon; (C) exon 7: A to G at nucleotide 774, changing glutamine 226 to arginine; (D) exon 8: G to A (top arrow) at nucleotide 840, changing serine 248 to glutamine and A to G (bottom arrow) at nucleotide 844, no amino acid change; (E) exon 9: T to C at nucelotide 944, changing phenylalanine 283 to leucine; and (F) exon 11: T to C at nucleotide 1234, no amino acid change.
DNA sequencing of PCR products with abnormal ddF patterns. The arrows indicate the locations of nucleotides that differ from the published sequence for wild-type human factor XI.28 (A) exon 5: C to T at nucleotide 472, no amino acid change; (B) exon 5: G to T at nucleotide 446, changing glutamic acid 117 to a stop codon; (C) exon 7: A to G at nucleotide 774, changing glutamine 226 to arginine; (D) exon 8: G to A (top arrow) at nucleotide 840, changing serine 248 to glutamine and A to G (bottom arrow) at nucleotide 844, no amino acid change; (E) exon 9: T to C at nucelotide 944, changing phenylalanine 283 to leucine; and (F) exon 11: T to C at nucleotide 1234, no amino acid change.
Analysis of exon 7 showed that patient one is heterozygous for an A to G change at nucleotide 774, resulting in arginine substituting for the normal glutamine residue at amino acid position 226 (Q226R, Fig 2C). This mutation was not identified in his mother, patient two. Analysis of the fragment representing exons 8 and 9 showed that patients one and two are heterozygous for a G to A change at nucleotide 840 that changes serine 248 to glutamine (S248Q, Fig 2D). In addition, patient one is homozygous and patient two is heterozygous for an A to G change at nucleotide 844 that does not affect amino acid sequence (Fig 2D). This latter finding explains the difference in ddF patterns between patient one and patient two for the exon 8-9 fragment. The abnormal patterns for fragments 8-9 and 9-10 for patient three were due to a T to C change at bp 944 in exon 9, changing phenylalanine 283 to leucine (Fig2E). This is the factor XI type III mutation that is also common in the Ashkenazi Jewish population.2 18 Finally, analysis of exon 11 (Fig 2F) determined that patient one is homozygous and patient two is heterozygous for a T to C change at nucleotide 1234 that does not affect the amino acid sequence.
In summary, the DNA sequencing analysis identified an amino acid substitution in exon 8, S248Q, in one copy of the factor XI gene in patient one that seems to have been inherited from his mother, patient two. Patient one has an additional mutation in exon 7, Q226R, not found in his mother. Three neutral polymorphisms were also identified in exons 5, 8, and 11. The analysis shows that the factor XI genes of patient one differ at 7 bp locations from the published sequence, whereas those of patient two differ at four locations. Finally, ddF identified patient three as a compound heterozygote for the factor XI type II and type III mutations. This is a frequent finding in severe factor XI deficiency in individuals of Jewish ancestry, and the mutations correlate well with his factor XI level of 2% to 3% of normal plasma levels.2 Patient three is also heterozygous for the exon 5 neutral polymorphism identified in patients one and two.
Allele Analysis for Factor XI Mutations in Patient One
Although the Q226R mutation was not identified in the genomic DNA of patient two, a mutation event during oogenesis could have caused the mutation on the same allele that contained the S248Q mutation, which was subsequently passed on to patient one. To determine if the Q226R and S248Q mutations in patient one reside on the same or different alleles, a 4-kb fragment of the factor XI gene including exons 7 through 9 was amplified by PCR, cloned into plasmid pBluescript, and used to transform E coli. Plasmid DNA from individual clones was subjected to restriction enzyme analysis and DNA sequencing. The Q226R mutation in exon 7 creates a new SmaI restriction site, allowing the identification of clones containing this mutation by simple restriction enzyme analysis. Results of the restriction digest analysis were subsequently confirmed by sequencing of exon 7. DNA sequence analysis of exon 8 for clones containing the new SmaI site (Q226R) did not identify the S248Q mutation, whereas sequencing of clones lacking the SmaI site showed the S248Q mutation. Therefore, the two mutations reside on different alleles and patient one is a compound heterozygote for mutations in both copies of the factor XI gene.
Frequency of Factor XI Polymorphisms in Healthy Volunteers
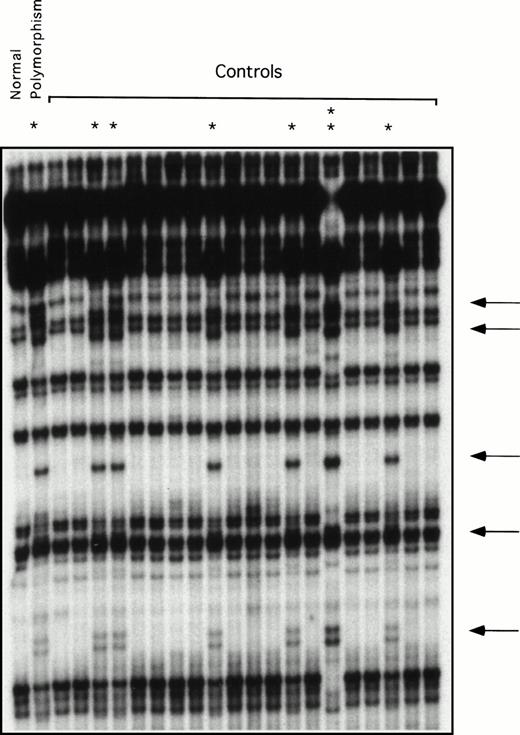
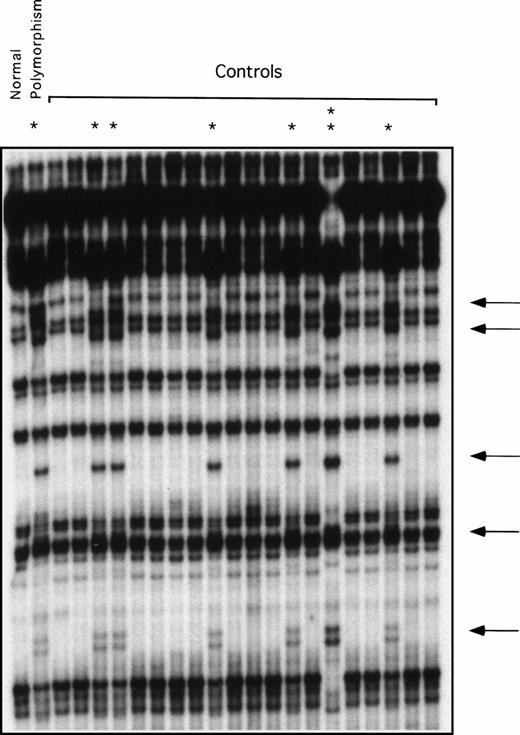
Restriction enzyme analysis and ddF were used to determine the frequency of the neutral polymorphisms identified in exons 5, 8, and 11 in a random sample of normal individuals unrelated to the patients. The change at bp 472 in exon 5 destroys an AatII restriction endonuclease site. Using restriction enzyme analysis, this change was identified in 4 of 54 alleles tested (frequency, 7.4%). Using ddF, the polymorphism at bp 844 in exon 8 was found in 8 of 42 alleles (data not shown) and at bp 1234 of exon 11 in 8 of 44 alleles tested (Fig 3), yielding frequencies of 19% and 18%, respectively. The racial backgrounds of individuals in the normal volunteer pool are not known, because samples were collected anonymously.
ddF analysis of exon 11 in a group of normal volunteers. ddF was performed as described in Materials and Methods. The asterisk indicates samples in which the presence of the neutral T to C change at nucleotide 1234 was identified by the appearance of new or shifted bands on the autoradiograph (indicated by the arrows). The sample indicated by the double asterisk comes from a person who is homozygous for the polymorphism, whereas the samples indicated by the single asterisk are from heterozygotes.
ddF analysis of exon 11 in a group of normal volunteers. ddF was performed as described in Materials and Methods. The asterisk indicates samples in which the presence of the neutral T to C change at nucleotide 1234 was identified by the appearance of new or shifted bands on the autoradiograph (indicated by the arrows). The sample indicated by the double asterisk comes from a person who is homozygous for the polymorphism, whereas the samples indicated by the single asterisk are from heterozygotes.
Expression of Recombinant Wild-Type and Mutant Factor XI
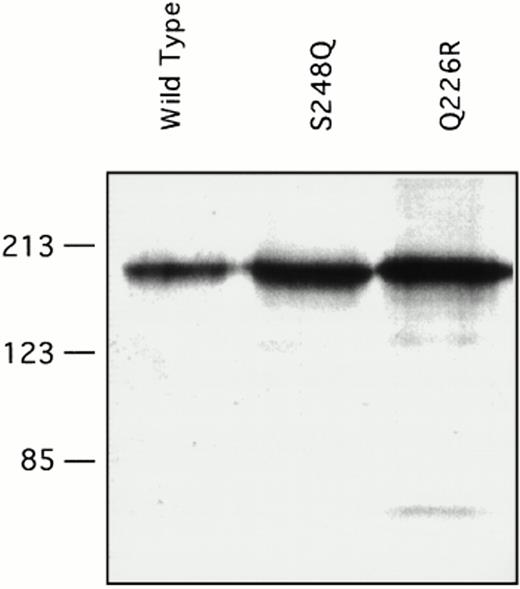
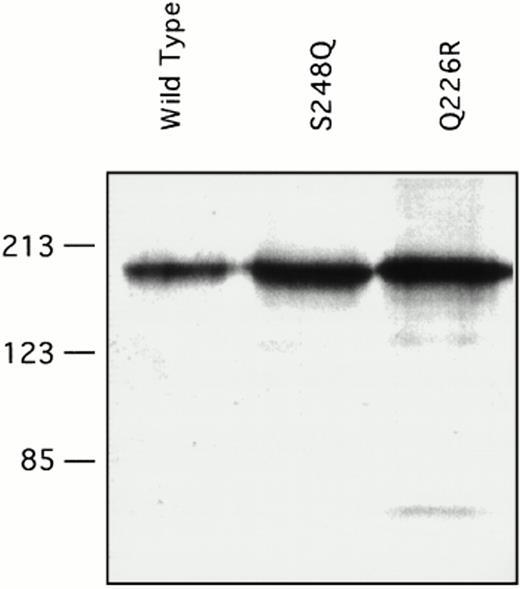
Recombinant factor XI was expressed in mammalian tissue culture using a vector containing the cytomegalovirus promoter.26 Our experience with other factor XI mutations using this system is that virtually all amino acid changes associated with decreased factor XI antigen in vivo cause decreased expression of the protein in the in vitro system. Transient transfection with a wild-type factor XI construct and constructs containing either the Q226R or S248Q mutation showed that all three proteins are expressed at similar levels in the 293-cell system. Protein concentrations as measured by ELISA ranged from 1 to 3 μg/mL in multiple transfections for wild-type factor XI and both mutants. This is consistent with the observation that patients one and two have substantial factor XI antigen present in their plasma. Samples of conditioned media were size fractionated by SDS-polyacrylamide gel electrophoresis and analyzed by Western immunoblotting (Fig 4). The two mutant proteins ran similarly to the wild-type protein on SDS-polyacrylamide gels, indicating that the mutations did not cause gross abnormalities in protein structure or interfere with dimer formation. Specific activities of purified recombinant proteins were determined in an aPTT-based assay, and showed that wild-type recombinant protein had a specific activity of approximately 200 U/mg, which is the same as the plasma-derived factor XI control. The specific activity of the Q226R mutation was slightly less (180 U/mg), whereas that of the S248Q mutation was modestly reduced (140 U/mg) compared with the wild-type protein.
Western immunoblots of conditioned media for recombinant factor XI protein. Fifty microliters of conditioned media from transient transfections in 293 fibroblasts for the indicated factor XI constructs were evaluated by Western immunoblotting as described in Materials and Methods. The position of molecular weight standards in kilodaltons is shown at the left of the figure.
Western immunoblots of conditioned media for recombinant factor XI protein. Fifty microliters of conditioned media from transient transfections in 293 fibroblasts for the indicated factor XI constructs were evaluated by Western immunoblotting as described in Materials and Methods. The position of molecular weight standards in kilodaltons is shown at the left of the figure.
DISCUSSION
The highly variable nature of the bleeding diathesis in factor XI deficiency has been puzzling since the first description of the disorder more than forty years ago.29 Hemorrhage is usually mild to moderate and typically follows trauma or surgery, particularly when involving tissues with high fibrinolytic activities such as the oral cavity and the urinary tract.2,4-6 There has been long-standing disagreement concerning bleeding tendencies in patients with severe versus mild factor XI deficiency. Many investigators maintain that the risk of hemorrhage is greatest for those with factor XI activities of <15% of normal and that bleeding is usually not significant with levels >25% of normal.2,5,10,11However, there are numerous instances of patients with severe deficiency not experiencing abnormal hemostasis, and symptoms correlate poorly with results of conventional coagulation assays.2,8,9 Moreover, some groups have reported difficulty in distinguishing patients with severe deficiency from those with mild deficiency based on propensity to bleed,8,9 and there are reports of families in which mild factor XI deficiency and bleeding symptoms are apparently inherited in an autosomal-dominant manner.12-14 These disparate results are likely to be explained, at least in part, by differing criteria for excessive bleeding as well as by the variable nature of the hemostatic challenges responsible for bleeding. However, in some cases the discrepancy is probably due to the nature of the genetic abnormality underlying the deficiency.
Hereditary deficiency of factor XI is a rare condition in the general population; however, it is a common disorder in persons of Ashkenazi Jewish descent, in whom abnormal allele frequencies as high as 13.4% have been reported.2,3 Two point mutations have been identified with equal frequency in this population. The type II mutation introduces a termination codon in exon 5, whereas the type III mutation causes a phenylalanine to leucine substitution at amino acid 283 resulting in severely reduced protein secretion.18,19Patient three in our study was identified by ddF as a compound heterozygote for these mutations, a common cause of severe factor XI deficiency in the Jewish population. Factor XI deficiency has been reported sporadically in other ethnic groups8,9,17; however, there are few reports of black patients with the disorder.17 No single mutation seems to predominate in factor XI–deficient patients who are not of Jewish ancestry,20,30-34 and the type II and III mutations are infrequently found in this group. Virtually all identified mutations in the factor XI gene associated with deficiency seem to either prevent or greatly reduce protein expression.18,20 30-33 Therefore, most cases of severe deficiency are the result of homozygosity or compound heterozygosity for mutations in the factor XI gene. Individuals with mild deficiency presumably have one normal copy of the gene that is responsible for protein production and one dysfunctional allele that does not contribute significantly to plasma protein.
The propositus in our study, an individual with excessive bleeding and mild factor XI deficiency by conventional assays, is a compound heterozygote for two mutations, both of which map to the third apple domain of the factor XI heavy (noncatalytic) chain.28 A series of recent reports have implicated this area of the molecule in a variety of important functions including binding to platelets,35 glycosaminoglycans,36 and factor IX.26 In fact, the S248Q mutation is within a recently mapped platelet-binding sequence.35 The mother of the propositus, a heterozygote for the S248Q mutation, has normal factor XI levels but also has a history of abnormal hemostasis consistent with this mutation contributing to bleeding. The standard activity and antigen assays for factor XI indicate, at most, moderate defects in these two individuals and the specific activities of the mutant recombinant proteins (determined by an aPTT based assay) indicate a relatively modest decrease in clotting activity. However, it is possible that results of conventional aPTT-based measurements of factor XI activity could be misleading in some cases of factor XI deficiency. Factor XI is a critical component for contact activation–initiated fibrin clot formation through the intrinsic pathway,1 and its deficiency results in a marked prolongation of in vitro clotting assays, such as the aPTT, which depend on this mechanism.37Unlike the situation for classic hemophilia (deficiency of factors VIII or IX), bleeding in patients with factor XI deficiency correlates poorly with factor XI activity levels determined by aPTT-based assays.2,4,8,9 It is now thought that the primary function of factor XI is to sustain thrombin production after clot formation has been achieved.6,38,39 Clinical observations agree with this hypothesis and indicate that this activity is most important after major hemostatic challenges, particularly when involving tissues where clot integrity is threatened by high levels of fibrinolytic activity.2,4-6 This role differs substantially from that of factor XI in the aPTT assay, where the protein must be activated by factor XIIa and in turn activate factor IX at a sufficient rate to drive initial formation of the clot.1 Indeed, the activation of factor XI in some models of hemostasis occurs by factor XII–independent mechanisms.38 39 Therefore, poor correlation between factor levels determined in vitro and clinical bleeding symptoms in vivo may in part be due to the very different roles the protein serves in the two systems. The defects identified in our patients may affect critical functions of the factor XI molecule that are not assessed well by the aPTT assay. Consistent with this premise are preliminary results indicating that the S248Q mutant has a defect in platelet binding, a property not required for normal activity in the aPTT assay (Ho D, Walsh PN, Gailani D, unpublished observation, March 1998). Standard aPTT-based assays may, in fact, miss abnormalities in factor XI function that are not directly related to the activity of the protein in the classic intrinsic pathway. It is important to note that although the S248Q mutation, common to patients one and two, is associated with modest defects in in vitro aPTT and platelet-binding assays, the significance of these abnormalities are as yet unclear. Other unidentified abnormalities of factor XI function, or factors unrelated to factor XI, could be contributing to the bleeding in these patients.
The zymogen of human factor XI, unlike those of other coagulation proteases, is a disulfide bond–linked dimer of two identical polypeptide chains, capable of expressing two catalytic sites on activation.15 The dimeric nature of the molecule indicates that the genetics of factor XI deficiency may be more complex than for the other monomeric components of the hemostatic mechanism. Assuming that the two factor XI alleles are equally expressed in a liver cell, an individual with one normal and one abnormal factor XI allele might produce homodimers of normal polypeptides, heterodimers of normal and abnormal polypeptides, and homodimers of abnormal polypeptides in a 1:2:1 ratio, respectively. Patient two in our study may be an example of this situation, with three-quarters of her circulating factor XI possessing at least one abnormal chain. In contrast, in situations where the abnormal allele contains a mutation that prevents protein expression (such as the type II mutation found in Jewish patients18) only normal homodimers may be produced. These two situations may result in different propensities to bleed, with our patient two having a significant amount of factor XI protein, only a small amount of which is normal, whereas the heterozygote for the type II mutation would have moderately decreased total protein, but it would all be normal. The first case could present as a bleeding disorder with an autosomal-dominant mode of transmission, whereas the second may seem to be an asymptomatic carrier. This situation is similar to that of a number of reported variants of von Willebrand factor, another multimeric protein.16 In the case of patient one, due to compound heterozygosity for two mutations, all of the factor XI in his plasma is composed of molecules with two abnormal chains. It is likely that the two abnormal molecules are forming dimers in different combinations, and the effects of this phenomenon on in vivo hemostasis are difficult to predict.
Three neutral polymorphisms were identified in the three patients in this study, suggesting that there may be considerable heterogeneity in the factor XI gene. The polymorphisms in exons 5 and 8 were previously reported in abstract form by Ventura et al32 studying the population in Portugal. Our study, although not directly addressing the issue of prevalence by racial or ethnic group, determined that these polymorphisms are common in normal volunteers at our hospital, with allele frequencies of 7.4% and 19%, respectively. More work will be required to determine frequencies of these alleles in various ethnic groups; however, the identification of the same DNA base pair changes in African Americans and Europeans suggests that these polymorphisms may be very old and widely spread. In addition, a previously unreported polymorphism in exon 11 was noted in 18% of alleles from our normal volunteers. These polymorphisms are common enough that they should prove useful in genetic studies of factor XI deficiency.
In summary, ddF, a powerful screening technique for DNA mutations, was used to identify nucleotide base pair changes associated with amino acid substitutions and neutral polymorphisms in the factor XI genes of three patients with abnormal hemostasis. The amino acid substitutions in patient one and two are the first examples, to our knowledge, of changes in this protein that do not result in severely decreased expression and therefore may be consistent with an autosomal-dominant form of bleeding abnormality. Most mutations in the factor XI gene cause decreased protein expression and would probably result in a bleeding disorder with an autosomal recessive mode of transmission. However, the fact that factor XI is dimeric suggests that bleeding abnormalities with a more complex genetic mechanism could arise when mutations do not significantly reduce protein expression.
Supported by National Institutes of Health Grants HL02917 and HL58837.
Address reprint requests to David Gailani, MD, Division of Hematology, Vanderbilt University, 538 Medical Research Bldg II, 2220 Pierce Ave, Nashville, TN 37232-6305; e-mail:dave.gailani@mcmail.vanderbilt.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.