Abstract
The granulocyte colony-stimulating factor receptor (G-CSF-R) activates multiple STAT proteins. Although the membrane-proximal cytoplasmic region of the G-CSF-R is necessary and sufficient for activation of STAT1 and STAT5, activation of STAT3 requires the membrane distal region that contains four tyrosines. Although one of these (Y704) has previously been shown to be involved in STAT3 activation from a truncated G-CSF-R derived from a patient with severe chronic neutropenia (SCN), this tyrosine is not required for STAT3 activation by the full-length G-CSF-R. To investigate possible alternative mechanisms of STAT3 activation, we generated a series of Ba/F3 cell transfectants expressing the wild-type G-CSF-R or mutant receptors that either completely lack tyrosines or retain just one of the four cytoplasmic tyrosines of the G-CSF-R. We show that, at saturating G-CSF concentrations, STAT3 activation from the full-length G-CSF-R is efficiently mediated by the C-terminal domain in a manner independent of receptor tyrosines. In contrast, at low G-CSF concentrations, Y704 and Y744 of the G-CSF-R play a major role in STAT3 activation. Both tyrosine-dependent and -independent mechanisms of STAT3 activation are sensitive to the Jak2 inhibitor AG-490, follow similar kinetics, and lead to transactivation of a STAT3 reporter construct, indicating functional equivalence. STAT3 activation is also impaired, particularly at nonsaturating G-CSF concentrations, in bone marrow cells from mice expressing a truncated G-CSF-R (gcsfr-▵715). These findings suggest that G-CSF–induced STAT3 activation during basal granulopoiesis (low G-CSF) and “emergency” granulopoiesis (high G-CSF) are differentially controlled. In addition, the data establish the importance of the G-CSF-R C-terminus in STAT3 activation in primary cells, which has implications for understanding why truncated G-CSF-R derived from SCN patients are defective in maturation signaling.
GRANULOCYTE COLONY-STIMULATING factor (G-CSF) plays a crucial role in the regulation of granulopoiesis by stimulating the proliferation, survival, and maturation of myeloid progenitor cells.1-4 The various biological effects of G-CSF are mediated through a receptor (G-CSF-R) of the hematopoietin receptor superfamily, which forms homo-oligomeric complexes upon ligand binding.5,6 The cytoplasmic domain of the human G-CSF-R contains four conserved tyrosine residues (Y704, Y729, Y744, and Y764) that serve as potential docking sites for Src homology 2 (SH2) domains of signaling proteins.7 Deletion studies have shown that the membrane-proximal cytoplasmic region of the G-CSF-R, lacking all four tyrosines, is indispensable for transduction of growth signals, whereas the carboxy-terminal region, including three tyrosines, is involved in the induction of neutrophilic maturation.8-10
We have previously identified mutations in the G-CSF-R that delete the carboxy-terminal domain in approximately 20% to 25% of cases of severe congenital neutropenia (SCN) and an even higher proportion of cases of acute myeloid leukemia (AML) preceded by SCN, indicating a positive role for this region in modulating G-CSF signaling in primary cells.11-13 When expressed in myeloid cells these truncated receptors transduce a strong growth signal but are defective in maturation signaling.12 We recently generated mice with a similar mutation in the gcsfr gene (gcsfr-Δ715 mice) that showed a basal neutropenia.14 However, the molecular basis for the defective maturation signaling from these truncated receptors has remained unclear.
Like other hematopoietin receptors, the G-CSF-R lacks intrinsic tyrosine kinase activity but activates cytoplasmic tyrosine kinases.2,5 Signal transduction pathways that involve activation of Janus tyrosine kinases (Jaks), Jak1, Jak2, and Tyk2, and signal transducer and activator of transcription (STAT) proteins, STAT1, STAT3, and STAT5, have been linked to the G-CSF-R.15-22 Jaks associate with the membrane-proximal cytoplasmic region of the G-CSF-R and become activated upon ligand-induced receptor homo-oligomerization.18,19,22,23Jak activation leads to tyrosine phosphorylation of a conserved tyrosine residue in the C-terminus of the STAT proteins. Subsequently, STAT proteins form stable homodimers and heterodimers by interactions between the SH2 domain of one STAT protein and the phosphotyrosine of another STAT protein before translocation to the nucleus, where they influence transcription of target genes by binding to specific regulatory sequences.24 A crucial question regards how the STATs are recruited to the receptor/Jak complexes to mediate their activation. It has been proposed that receptor-associated Jaks phosphorylate specific STATs via their recruitment to particular cytoplasmic domains of each receptor. For example, interleukin-4 (IL-4)–induced activation of STAT6 is mediated by tyrosines 578 and 606 of IL-4-R.25 Similarly, multiple tyrosine residues in the cytoplasmic domain of gp130 and LIF receptor mediate STAT3 activation,26 whereas tyrosine 440 in the cytoplasmic domain of the interferon-γ (IFN-γ) receptor α chain is required for STAT1 phosphorylation and activation, presumably through specific interaction with the SH2 domain of STAT1.27 28
The membrane-proximal region of the G-CSF-R, containing the conserved box 1 and box 2 subdomains, is sufficient for G-CSF–induced activation of STAT1 and STAT5, but not for STAT3.18,20 Importantly, activation of STAT3 has recently been linked to G-CSF–mediated differentiation.29 Determining the mechanisms of STAT3 activation is vital, therefore, for understanding how the G-CSF-R controls the processes of proliferation and differentiation. In this study, we show that different mechanisms of STAT3 activation operate depending on ligand concentration. At saturating levels of G-CSF, STAT3 activation is effectively mediated by a mechanism involving the C-terminal region of the G-CSF-R, which does not require the presence of phosphorylated receptor tyrosine residues to provide docking sites. In contrast, at low G-CSF concentrations, tyrosine-dependent docking via Y704 and Y744 plays a major role in the activation of STAT3. Both tyrosine-dependent and -independent mechanisms of STAT3 activation require Jak2 activity, follow similar kinetics, and result in transcriptionally active STAT3 complexes. In addition, we show reduced STAT3 activation from truncated G-CSF-R derived from SCN patients, even at saturating G-CSF concentrations. Moreover, there is an altered dose response in STAT3 compared with STAT5, which exacerbates this reduced activation, such that at lower G-CSF concentrations the STAT3 deficiency is amplified compared with other G-CSF signals. These findings reveal an unexpected heterogeneity in the signaling function of the G-CSF-R under different receptor saturation conditions that typically occur in vivo during basal granulopoiesis (low G-CSF) or during “emergency” conditions, such as bacterial infections (high G-CSF), and suggest that decreased STAT3 activation may contribute to the defective maturation signaling from truncated G-CSF receptors derived from patients with SCN.
MATERIALS AND METHODS
Cells and cell culture.
The IL-3–dependent murine pro–B-cell line Ba/F330 and a subline of the IL-3–dependent murine myeloid cell line 32Dcl3,31 called 32D.cl8.6, were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 10 ng of murine IL-3 per mL at 37°C and 5% CO2. Mice containing the gcsfr-Δ715 “knock-in” mutation, leading to a truncation of the G-CSF-R C-terminus, have been described.14 To obtain bone marrow cell suspensions, femurs and tibias were crushed in a mortar in HBSS/10% FCS. Cells were passed through a 100-μm sieve, spun down, and resuspended, resulting in monocellular suspensions containing 98% to 99% viable cells, as determined by Trypan blue exclusion.
Site-directed mutagenesis.
The pLNCX expression clones of human G-CSF-R wild-type (WT), the deletion mutant mDA (d715), the single tyrosine-to-phenylalanine (Y → F) substitution mutants Y704F, Y729F, Y744F, and Y764F, and the combined mutant mDAF (d715-Y704F) have been described previously.8,32 Double Y → F mutants were created from the single mutants using site-directed mutagenesis, as described.32 A series of triple Y → F mutants (mA, mB, mC, and mD) and a quadruple Y → F “null” mutant (mO) were constructed from the various double and single mutants by recombinant polymerase chain reaction (PCR), using the following oligonucleotide primers: GRRV14 (5′-CCTGGGCTTGTGGGGCTGC), GRFR11 (5′-TGCTGGGCAGCCCCACAAG), LNCXRV (5′-CCCTTACTTTCTGGGGTGGACATC), and GRFR7 (5′-GTCCTCACCCTGATGACC). The 5′ segment of each mutant was amplified using GRFR7 and GRRV14, and the 3′ segment using GRFR11 and LNCXRV. Products of the primary PCR were isolated, mixed 1:1, and used as a template for a secondary PCR with GRFR7 and LNCXRV. The product was digested with HpaI and BglII and cloned into pLNCX containing G-CSF-R WT, which had also been digested with these enzymes. The authenticity of all mutants was verified by restriction enzyme analysis and DNA sequencing.
Transfections and analysis.
For stable transfections, Ba/F3 and 32D cells were electroporated with 10 μg PvuI–digested pLCNX clones, using a Progenetor (Hoefer Scientific Instruments, San Francisco, CA) apparatus set at 230 V, 100 μF, and 1 second. After 24 hours of incubation, cells were selected with G418 (GIBCO-BRL, Breda, the Netherlands) at a concentration of 1.2 and 0.8 mg/mL, respectively. Multiple clones were expanded for further analysis. To determine G-CSF-R expression levels, cells were incubated at 4°C for 60 minutes sequentially with 10 μg/mL of biotinylated mouse anti-human G-CSF-R monoclonal antibody LMM741 (PharMingen, San Diego, CA), 5 μg/mL of phycoerythrin (PE)-conjugated streptavidin, 5 μg/mL of biotinylated anti-streptavidin antibody, and finally 2 μg/mL of PE-conjugated streptavidin, with washing between each antibody step. Samples were analyzed by flow cytometry using a FACScan (Becton Dickinson, San Jose, CA). At least three independently derived cell lines of each construct with homogeneous receptor expression, as determined by equivalent mean fluorescence following fluorescence-activated cell sorter (FACS) analysis, were selected for further study. For transient transfections, Ba/F3 cells were electroporated with 50 μg of a STAT3(m67)-luciferase construct described previously33 at 260 V, 490 μF, and 1 second. After 18 hours of recovery on IL-3, cells were washed and divided into two portions, which were incubated separately for a further 8 hours with either IL-3 or G-CSF, before harvesting for luciferase assays.
Preparation of cell lysates, immunoprecipitation, and Western blotting.
Cells were deprived of serum and factors for 4 hours at 37°C in RPMI 1640 medium at a density of 4 × 106 per mL and then stimulated with either RPMI 1640 medium alone or in the presence of 100 ng/mL human G-CSF. At different time points, 10 volumes of ice-cold phosphate-buffered saline (PBS) supplemented with 10 μmol/L Na3VO4 were added. Subsequently, cells were pelleted and lysed by incubation for 30 minutes at 4°C in lysis buffer (20 mmol/L Tris-HCl pH 8.0, 137 mmol/L NaCl, 10 mmol/L EDTA, 100 mmol/L NaF, 1% Nonidet P-40, 10% glycerol, 2 mmol/L Na3VO4, 1 mmol/L Pefabloc SC, 50 μg/mL aprotinin, 50 μg/mL leupeptin, 50 μg/mL bacitracin, and 50 μg/mL iodoacetamide). Insoluble materials were removed by centrifugation at 4°C for 15 minutes at 15,000g. Immunoprecipitations were performed on the clarified cell lysates by incubation overnight at 4°C with anti-STAT3 antibodies (sc-482; Santa Cruz Biotechnology Inc, Santa Cruz, CA). Protein A-Sepharose beads (Pharmacia, Uppsala, Sweden) were then added for 1 hour at 4°C. After washing the beads with ice-cold lysis buffer 4 times and once with PBS, bound proteins were eluted by boiling for 5 minutes in sodium dodecyl sulphate (SDS) sample buffer. Following SDS-polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred onto nitrocellulose (0.2 μm; Schleicher & Schuell, Dassel, Germany). Filters were blocked by incubation in TBST (10 mmol/L Tris-HCl pH 7.4, 150 mmol/L NaCl, 0.05% [vol/vol] Tween-20) containing 0.6% (wt/vol) bovine serum albumin (BSA). Antibodies used for Western blotting were anti-phosphotyrosine antibody 4G10 (Upstate Biotechnology Inc, Lake Placid, NY), anti–G-CSF-R (sc-693; Santa Cruz), and anti-STAT3 described above, and were diluted in TBST containing 0.6% (wt/vol) BSA. After washing with TBST, immune complexes were detected with horseradish peroxidase–conjugated species-specific antiserum (DAKO, Glostrup, Denmark), followed by enhanced chemiluminescence reaction (DuPont, Boston, MA). In some instances, membranes were stripped in 62.5 mmol/L Tris-HCl pH 6.7, 2% SDS, and 100 mmol/L β-mercaptoethanol at 50°C for 30 minutes, reblocked, washed, and reprobed. For signal quantification, Western blots were scanned with an ArcusII Color Scanner (Agfa, The Hague, the Netherlands) and the data processed using ImageQuant Software (Molecular Dynamics, Sunnyvale, CA).
Preparation of nuclear extracts.
Cells were stimulated as described above then pelleted and resuspended in ice-cold hypotonic buffer (20 mmol/L HEPES pH 7.8, 20 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L Na4P2O7, 1 mmol/L DTT, 1 mmol/L EDTA, 1 mmol/L EGTA, 0.2% Tween-20, 0.125 μmol/L okadaic acid, 1 mmol/L Pefabloc SC, 50 μg/mL aprotinin, 50 μg/mL leupeptin, 50 μg/mL bacitracin, and 50 μg/mL iodoacetamide).34 Cells were vortexed for 10 seconds and the nuclei pelleted by centrifugation at 15,000g for 30 seconds. Nuclear extracts were prepared by resuspension of the nuclei in high-salt buffer (hypotonic buffer with 420 mmol/L NaCl and 20% glycerol) and extraction of proteins by rocking for 30 minutes at 4°C. Insoluble materials were removed by centrifugation at 4°C for 15 minutes at 15,000g. For the Jak2 inhibition studies, cells were preincubated with the specific inhibitor AG-49035 at 50 μmol/L for 1 hour before stimulation.
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts of approximately 0.4 to 0.5 × 106cells were incubated for 20 minutes at room temperature with 0.2 ng of32P-labeled double-stranded oligonucleotide (5 to 10 × 103 cpm) and 2 μg of poly(dI-dC) in 20 μL of binding buffer (13 mmol/L HEPES, pH 7.8, 80 mmol/L NaCl, 3 mmol/L NaF, 3 mmol/L NaMoO4, 1 mmol/L DTT, 0.15 mmol/L EDTA, 0.15 mmol/L EGTA, and 8% glycerol).36 The oligonucleotide probes used in this study were m67 (5′-CATTTCCCGTAAATC), a high-affinity mutant of the sis-inducible element (SIE) of the human c-fos gene,37 which binds STAT1 and STAT3, and β-cas (5′-AGATTTCTAGGAATTCAATCC), derived from the 5′ region of the β-casein gene,38 which binds STAT5 and STAT1. In some experiments, phosphotyrosine peptides specific for the murine G-CSF-R were added to the binding reaction 1 hour before the addition of the radiolabeled probe. The peptides had the following sequences: LVQApY703VLQG, DQVLpY729GQVL, GVMQpY743IRSD, and SPKSpY763ENIW, and they were purchased from Chiron Mimotopes (Clayton, Victoria, Australia). The DNA-protein complexes were separated by electrophoresis on 5% polyacrylamide gels containing 5% glycerol in 0.25 × Tris buffered EDTA (TBE). The gels were dried and subsequently analyzed by autoradiography. For quantification, gels were exposed to phosphoimager screens and analyzed with ImageQuant software (Molecular Dynamics).
Luciferase assays.
IL-3 and G-CS–treated cells were lysed in a minimal volume of luciferase lysis buffer (25 mmol/L Tris phosphate pH 7.8, 15% glycerol, 1% Triton X-100, 1 mmol/L DTT, 8 mmol/L MgCl2) and, after removal of cell debris by centrifugation, the supernatant was assayed on a Biocounter M2500 luminometer (Lumac, Landgraaf, The Netherlands) using an equal volume of luciferin solution (1 mmol/L luciferin, 1 mmol/L adenosine triphosphate [ATP], 8 mmol/L MgCl2) as a substrate. Because IL-3 activates equivalent levels of STAT3 in each of the clones analyzed (data not shown), luciferase activity in IL-3–treated cells was taken as an internal control. To match for variations in the transfection efficiency of the reporter construct, the G-CSF–mediated effects on the reporter constructs are expressed as the ratio of G-CSF–mediated transactivation to IL-3–mediated transactivation.
In vitro binding analysis.
The cytoplasmic domain of the human G-CSF-R and the SH2 domain of STAT3 were cloned into pET-15b (Novagen, Madison, WI) and pGEX-2TK (Pharmacia), respectively, using standard PCR protocols to amplify the appropriate coding regions. For the production of tyrosine-phosphorylated G-CSF-R cytoplasmic domain, the pET clone was introduced into the Escherichia coli strain TKB1 (Stratagene, La Jolla, CA), which contains an inducible tyrosine kinase, and fusion protein produced according to the manufacturer’s instructions. For production of GST and GST-STAT3(SH2), plasmids were transformed into XL-1 Blue (Stratagene), with proteins expressed and purified on glutathione-Sepharose 4B beads as described.39 Equivalent amounts of bead-bound GST or GST-STAT3(SH2) were incubated with tyrosine-phosphorylated G-CSF-R cytoplasmic domain in TBST containing 10% glycerol and 1 mmol/L DTT at 4°C for 1 hour, before extensive washing with the same buffer. To detect bound G-CSF-R, beads were boiled in SDS-sample buffer and subjected to Western blot analysis with anti–G-CSF-R antibody, as described earlier.
RESULTS
Construction and expression of different forms of the human G-CSF-R.
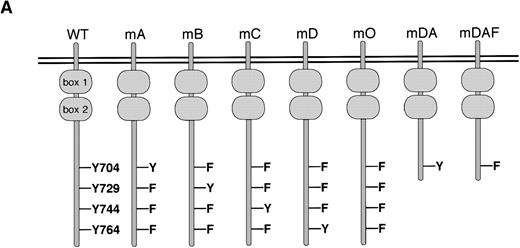
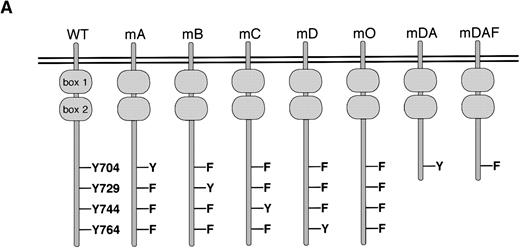
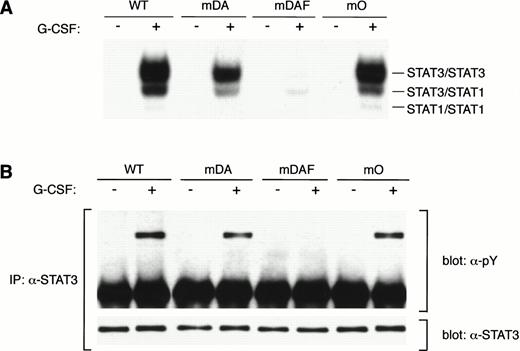
Ba/F3 cells have provided a convenient model for the study of specific signaling pathways from the G-CSF-R.15,19,20,23,32 To investigate the mechanisms of STAT3 activation via the G-CSF-R, a series of triple Y → F mutants, which each retain a single cytoplasmic tyrosine (mA, mB, mC and mD), and a quadruple “null” mutant with no cytoplasmic tyrosines (mO) were constructed. Expression vectors encoding the WT G-CSF-R, the various tyrosine mutants, and the previously described mutants mDA and mDAF8 32(Fig 1A) were then introduced into Ba/F3 cells. Surface expression of the G-CSF-R was determined using FACS analysis, and cell lines expressing equivalent levels of receptor were selected for further analysis, with at least three independent clones studied for each mutation. Examples of clones expressing WT or mutant G-CSF-R proteins are shown in Fig 1B.
Expression of mutant G-CSF-Rs in Ba/F3 cells. (A) Schematic representation of G-CSF-R proteins. Boxes 1 and 2 denote subdomains conserved in members of the hematopoietin receptor superfamily. Y, tyrosine; F, phenylalanine. (B) Flow cytometric analysis of G-CSF-R expression on parental Ba/F3 cells and Ba/F3 transfectants. Cells were either stained with biotinylated mouse anti-human G-CSF-R antibodies, followed by PE-conjugated streptavidin, biotinylated anti-streptavidin, and finally PE-conjugated streptavidin (unfilled), or without the anti–G-CSF-R step (filled).
Expression of mutant G-CSF-Rs in Ba/F3 cells. (A) Schematic representation of G-CSF-R proteins. Boxes 1 and 2 denote subdomains conserved in members of the hematopoietin receptor superfamily. Y, tyrosine; F, phenylalanine. (B) Flow cytometric analysis of G-CSF-R expression on parental Ba/F3 cells and Ba/F3 transfectants. Cells were either stained with biotinylated mouse anti-human G-CSF-R antibodies, followed by PE-conjugated streptavidin, biotinylated anti-streptavidin, and finally PE-conjugated streptavidin (unfilled), or without the anti–G-CSF-R step (filled).
G-CSF–induced activation of STAT3 can occur in the complete absence of tyrosines in the cytoplasmic domain of the G-CSF-R.
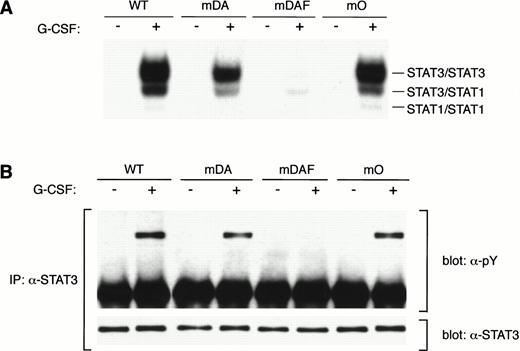
Tyrosine 704 of the G-CSF-R was assumed to be the major docking site for STAT3 because it fits the consensus STAT3 activation sequence, YxxQ.26 However, while Y704 is essential for STAT3 activation by the truncated form of the receptor,20substitution of this or other tyrosines in the full-length G-CSF-R had little effect on STAT3 activation, suggesting the involvement of other mechanisms.20 To investigate this further, we compared STAT3 activation from the WT G-CSF-R with the mutants mDA, truncated at position 715; mDAF, truncated at position 715 with a Y704F mutation; and mO, which represents the full-length receptor “null mutant” with no tyrosines (Fig 1A). As described previously,20 the WT G-CSF-R activates a large amount of STAT3 homodimer (upper band) as well as STAT3:STAT1 heterodimer and some STAT1 homodimer (Fig 2A). Interestingly, in this direct comparison, the truncation mutant mDA also activates the same complexes, although clearly at a reduced level, further suggesting STAT3 activation pathway(s) from the receptor C-terminus. Mutant mDAF, on the other hand, only induces very minor activation of STAT3, probably via indirect mechanisms involving STAT1.20Strikingly, mutant mO activated STAT3 almost to the same level as the WT receptor. This indicates that although Y704 is essential for STAT3 activation from truncated receptors, receptor tyrosines are not required for robust STAT3 activation from the full-length receptor. Analysis of STAT3 tyrosine phosphorylation in response to G-CSF showed a similar reduction from truncated receptors (Fig 2B). This confirms that the WT receptor activates more STAT3 than truncated forms, and rules out differences in nuclear transport or binding affinity as a cause of the decreased STAT3 binding observed by EMSA.
STAT3 activation in the absence of receptor tyrosines. (A) EMSA of nuclear extracts from Ba/F3 cells expressing WT G-CSF-R or mutants. Growth factor–deprived cells were incubated for 10 minutes at 37°C without factor (−) or with 100 ng/mL G-CSF (+). Nuclear extracts were prepared and incubated with32P-labeled double-stranded m67 oligonucleotide. (B) STAT3 immunoprecipitation from lysates from Ba/F3 cells expressing WT or mutant G-CSF-R proteins. Serum- and growth factor–starved cells were incubated for 10 minutes at 37°C without factor (−) or with 100 ng/mL G-CSF (+). The Western blot was hybridized with anti-phosphotyrosine antibodies 4G10, before stripping and reprobing with anti-STAT3 antibodies to confirm equal loading of STAT3. Multiple analyses of at least three independent clones of each mutant gave equivalent results.
STAT3 activation in the absence of receptor tyrosines. (A) EMSA of nuclear extracts from Ba/F3 cells expressing WT G-CSF-R or mutants. Growth factor–deprived cells were incubated for 10 minutes at 37°C without factor (−) or with 100 ng/mL G-CSF (+). Nuclear extracts were prepared and incubated with32P-labeled double-stranded m67 oligonucleotide. (B) STAT3 immunoprecipitation from lysates from Ba/F3 cells expressing WT or mutant G-CSF-R proteins. Serum- and growth factor–starved cells were incubated for 10 minutes at 37°C without factor (−) or with 100 ng/mL G-CSF (+). The Western blot was hybridized with anti-phosphotyrosine antibodies 4G10, before stripping and reprobing with anti-STAT3 antibodies to confirm equal loading of STAT3. Multiple analyses of at least three independent clones of each mutant gave equivalent results.
Tyrosine-dependent STAT3 activation from the G-CSF-R predominates at low G-CSF concentrations.
Given the already strong activation of STAT3 from mO, we then investigated what additional, and perhaps more subtle, role the G-CSF-R tyrosine residues may play in STAT3 activation from the full-length G-CSF-R. Therefore, we compared STAT3 activation by the WT G-CSF-R, the triple mutants, and the null mutant (Fig3A). At 100 ng/mL G-CSF (∼4 to 5 nmol/L), a concentration approximately 10-fold above the receptor Kd,5WT G-CSF-R induced the highest level of STAT3 tyrosine phosphorylation, followed by mA and mC, which activated approximately 80% of this level. Compared with WT G-CSF-R, activation of STAT3 by mB, mD, and mO was reduced to approximately 60%. Analysis of STAT3 activation at this G-CSF concentration by EMSA produced similar results (Fig 3B). Strikingly, however, at a concentration of 1 ng/mL G-CSF, which is approximately 10-fold below the Kd of the G-CSF-R, these differences become much more pronounced. At this level of receptor saturation, STAT3 activation by mutants mB, mD, and mO was several fold below that of the WT G-CSF-R, whereas mA and mC showed an intermediate level. Serial dilution of the nuclear extracts from cells stimulated at 100 ng/mL G-CSF confirmed that the EMSA was in the linear range using extracts stimulated with this ligand concentration (Fig 3C and D), ruling out probe depletion in the EMSA as the cause of the difference between stimulation with high and low G-CSF concentrations.
STAT3 activation by G-CSF-R tyrosine mutants. (A) STAT3 immunoprecipitation from lysates of Ba/F3 cells expressing WT or mutant G-CSF-R proteins, as described in Fig 2B. Multiple analyses of at least three independent clones of each mutant gave equivalent results. (B) EMSA of nuclear extracts from Ba/F3 cells expressing WT G-CSF-R (WT) or mutants, as described in Fig 2A, at the concentrations of G-CSF shown. (C) EMSA of serially diluted nuclear extracts from Ba/F3 cells expressing WT or mA receptors stimulated with 100 ng/mL G-CSF. Extracts from the equivalent of 4, 2, 1, and 0.5 × 105 cells were used. (D) Quantitative analysis of EMSA shown in (C), setting STAT3 binding from 4 × 105 Ba/F3[WT] cells at 100%. Results from dilution of Ba/F3[WT] extracts are shown with filled squares, and from Ba/F3[mA] extracts with open diamonds.
STAT3 activation by G-CSF-R tyrosine mutants. (A) STAT3 immunoprecipitation from lysates of Ba/F3 cells expressing WT or mutant G-CSF-R proteins, as described in Fig 2B. Multiple analyses of at least three independent clones of each mutant gave equivalent results. (B) EMSA of nuclear extracts from Ba/F3 cells expressing WT G-CSF-R (WT) or mutants, as described in Fig 2A, at the concentrations of G-CSF shown. (C) EMSA of serially diluted nuclear extracts from Ba/F3 cells expressing WT or mA receptors stimulated with 100 ng/mL G-CSF. Extracts from the equivalent of 4, 2, 1, and 0.5 × 105 cells were used. (D) Quantitative analysis of EMSA shown in (C), setting STAT3 binding from 4 × 105 Ba/F3[WT] cells at 100%. Results from dilution of Ba/F3[WT] extracts are shown with filled squares, and from Ba/F3[mA] extracts with open diamonds.
STAT3 docks directly to Y704 and Y744 of the G-CSF-R.
To determine if the activation mediated by Y704 and Y744 is due to direct binding of STAT3, as reported for other cytokine receptors,26,27,40 41 we added phosphopeptides spanning each of the four cytoplasmic tyrosines of the murine G-CSF-R to STAT binding reactions. If the SH2 domain of STAT3 has specificity for the phosphotyrosine-containing sequence, then the equivalent peptide should be able to disrupt preformed STAT3 dimers, leading to a reduction in binding by EMSA. The addition of phosphopeptides covering either Y703 or Y743 of the murine G-CSF-R, equivalent to Y704 or Y744 of the human receptor, respectively, successfully competed for STAT3 dimer formation, as determined by DNA binding (Fig 4A). This suggests direct docking of STAT3 to these phosphorylated tyrosines on the activated receptor. The Y743 peptide competed less efficiently for STAT3 binding than Y703, indicating a possible difference in relative affinity for STAT3 (Fig4B). However, unlike the Y703 peptide, which contains the same YxxQ motif found in the human G-CSF-R, the Y743 peptide contains a YxxS motif rather than the YxxC sequence found in the equivalent position of the human receptor, so whether the same difference in affinity occurs with the human receptor is as yet unclear. To confirm that direct binding occurs, we examined interactions between the isolated STAT3 SH2 domain and tyrosine-phosphorylated G-CSF-R cytoplasmic domain in vitro (Fig 4C). Binding of the G-CSF-R was observed with the GST-STAT3(SH2), but not to GST alone. Interestingly, only specific forms of the tyrosine-phosphorylated G-CSF-R cytoplasmic domain bound, presumably because only these forms contain Y704 and/or Y744 in a tyrosine-phosphorylated form. No specific binding was observed if a non–tyrosine-phosphorylated G-CSF-R was used as a probe (data not shown).
Characterization of STAT3/G-CSF-R interactions. (A) Competition of STAT3 containing EMSA complexes with phosphopeptides specific for each of the cytoplasmic tyrosines of the murine G-CSF-R receptor. Nuclear extracts from Ba/F3[WT] cells stimulated with G-CSF for 10 minutes were incubated either without peptide (−) or with the indicated phosphopeptides at a concentration of 500 μmol/L for 60 minutes, followed by EMSA. (B) Peptide competition as described in (A), with no peptide (−) or with Y703 and Y743 peptides at concentrations of 500, 200, and 50 μmol/L. (C) Direct binding of the STAT3 SH2 domain to tyrosine-phosphorylated G-CSF-R in vitro. Purified GST proteins immobilized on glutathione-Sepharose beads were incubated with purified, tyrosine-phosphorylated G-CSF-R cytoplasmic domain and washed extensively. Bound G-CSF-R was determined by boiling the beads in SDS sample buffer and subjecting the supernatant, along with the input G-CSF-R, to Western blot analysis with anti–G-CSF-R (–G-CSF-R) or anti-phosphotyrosine (-pY) antibodies, as indicated.
Characterization of STAT3/G-CSF-R interactions. (A) Competition of STAT3 containing EMSA complexes with phosphopeptides specific for each of the cytoplasmic tyrosines of the murine G-CSF-R receptor. Nuclear extracts from Ba/F3[WT] cells stimulated with G-CSF for 10 minutes were incubated either without peptide (−) or with the indicated phosphopeptides at a concentration of 500 μmol/L for 60 minutes, followed by EMSA. (B) Peptide competition as described in (A), with no peptide (−) or with Y703 and Y743 peptides at concentrations of 500, 200, and 50 μmol/L. (C) Direct binding of the STAT3 SH2 domain to tyrosine-phosphorylated G-CSF-R in vitro. Purified GST proteins immobilized on glutathione-Sepharose beads were incubated with purified, tyrosine-phosphorylated G-CSF-R cytoplasmic domain and washed extensively. Bound G-CSF-R was determined by boiling the beads in SDS sample buffer and subjecting the supernatant, along with the input G-CSF-R, to Western blot analysis with anti–G-CSF-R (–G-CSF-R) or anti-phosphotyrosine (-pY) antibodies, as indicated.
Tyrosine-dependent and -independent routes of STAT3 activation are functionally equivalent.
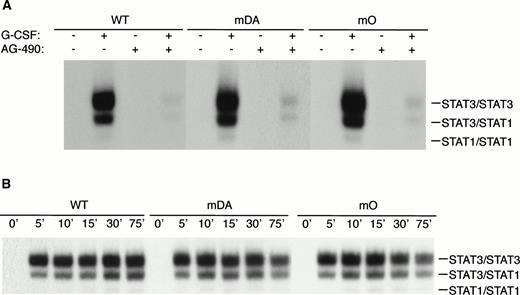
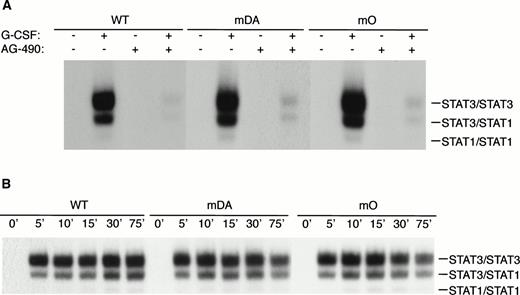
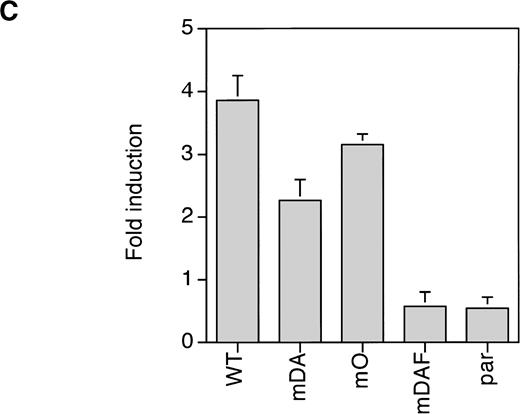
Because mDA almost exclusively uses a receptor tyrosine-dependent route of STAT3 activation (via Y704), whereas mO relies solely on a receptor tyrosine-independent route, comparison of mDA and mO provides an opportunity to separately investigate these different routes of STAT3 activation from the G-CSF-R. To analyze whether STAT3 activation in both cases depends on Jak activity, we repeated the EMSA with cells pretreated with the specific Jak2 inhibitor AG-490.35Activation of STAT3 by both mDA and mO was sensitive to the Jak2 inhibitor (Fig 5A), indicating that Jak2 is required for tyrosine-dependent and -independent activation routes. Furthermore, time-course studies showed that, although mDA and mO activated STAT3 at slightly different levels, time kinetics of activation were similar (Fig 5B). Finally, to determine if the STAT3 complexes activated by the alternate routes had comparable transactivating properties, we investigated the ability of the different mutants to activate transcription of a STAT3-luciferase reporter construct.33 Eighteen hours after transfection, cells were incubated with G-CSF or IL-3 for 8 hours and lysates assayed for luciferase activity (Fig 5C). Clones expressing the WT G-CSF-R, or the mDA and mO mutants, all activated the reporter to an extent comparable to their STAT3 DNA binding as analyzed by EMSA.
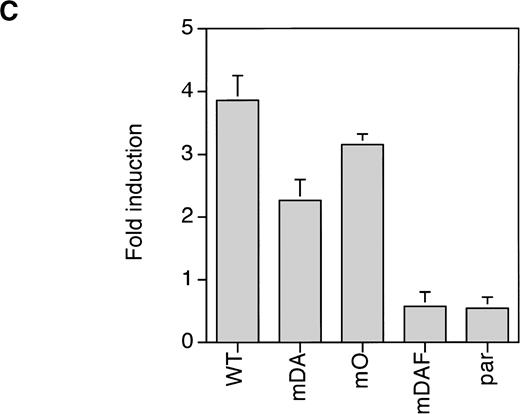
Properties of tyrosine-dependent and -independent pathways of STAT3 activation. (A) Sensitivity of STAT3 activation to the Jak2 inhibitor AG-490 from WT, mDA, and mO G-CSF-Rs. Cells were starved as described in the presence (+) or absence (−) of AG-490 before stimulation for 10 minutes with (+) or without (−) G-CSF. To aid the interpretation of the result, exposures were adjusted so that G-CSF–treated samples were approximately equal. (B) Kinetics of STAT3 activation in Ba/F3 cells expressing WT, mDA, and mO G-CSF-Rs. Serum- and growth factor–deprived cells were incubated with 100 ng/mL G-CSF for the times indicated. Nuclear extracts were prepared and incubated with 32P-labeled double-stranded m67 oligonucleotide. (C) Transactivation of STAT3(m67)-luciferase reporter by parental Ba/F3 cells (par), or Ba/F3 cells expressing WT or mutant G-CSF-Rs. Luciferase activity was assayed after incubation of cells from the same transfection with either G-CSF or IL-3. Activity is expressed as fold induction by G-CSF compared with by IL-3 to standardize for transfection efficiency of the reporter construct. Data represent the mean of at least three independent analyses, with the standard error indicated.
Properties of tyrosine-dependent and -independent pathways of STAT3 activation. (A) Sensitivity of STAT3 activation to the Jak2 inhibitor AG-490 from WT, mDA, and mO G-CSF-Rs. Cells were starved as described in the presence (+) or absence (−) of AG-490 before stimulation for 10 minutes with (+) or without (−) G-CSF. To aid the interpretation of the result, exposures were adjusted so that G-CSF–treated samples were approximately equal. (B) Kinetics of STAT3 activation in Ba/F3 cells expressing WT, mDA, and mO G-CSF-Rs. Serum- and growth factor–deprived cells were incubated with 100 ng/mL G-CSF for the times indicated. Nuclear extracts were prepared and incubated with 32P-labeled double-stranded m67 oligonucleotide. (C) Transactivation of STAT3(m67)-luciferase reporter by parental Ba/F3 cells (par), or Ba/F3 cells expressing WT or mutant G-CSF-Rs. Luciferase activity was assayed after incubation of cells from the same transfection with either G-CSF or IL-3. Activity is expressed as fold induction by G-CSF compared with by IL-3 to standardize for transfection efficiency of the reporter construct. Data represent the mean of at least three independent analyses, with the standard error indicated.
Reduced STAT3 activation from truncated G-CSF receptors found in severe chronic neutropenia.
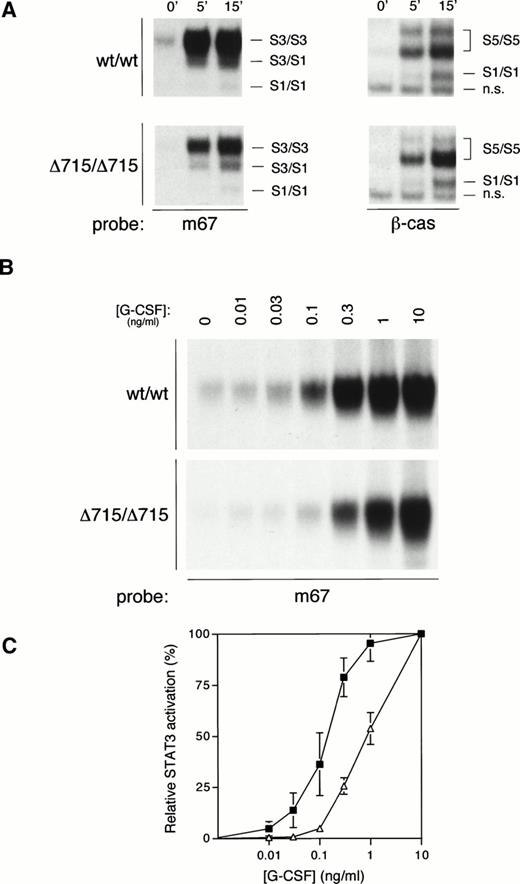
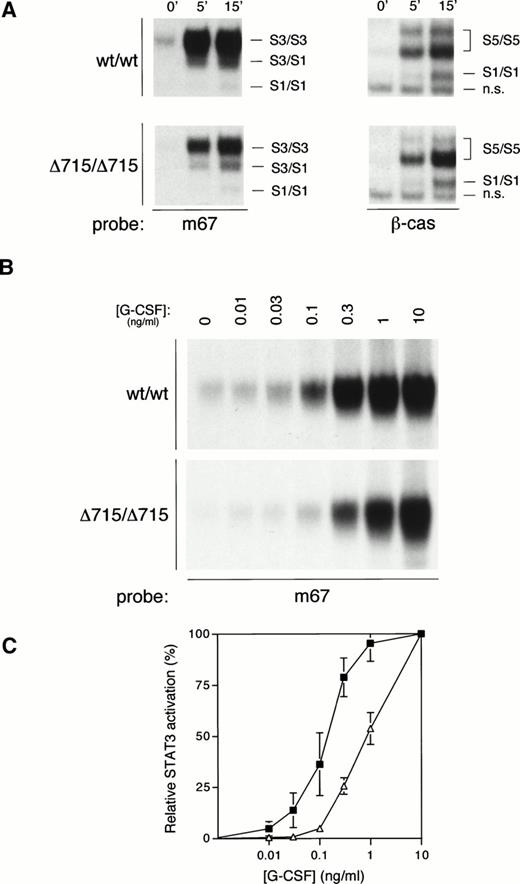
We have recently generated mice with a mutation in the gcsfrgene (gcsfr-Δ715 mice) that express a truncated form of the G-CSF-R found in SCN patients, and we have shown that both homozygous and heterozygous mutant mice have significantly reduced numbers of circulating neutrophils as compared with their WT littermates, suggesting defective G-CSF–mediated maturation.14 Given both the data presented above and the important role for STAT3 in differentiation signaling from the G-CSF-R,29 we sought to investigate whether compromised G-CSF–induced maturation induction from such truncated receptors might correlate with defective STAT3 activation. We therefore examined G-CSF–mediated STAT activation in bone marrow cells from WT mice (wt/wt) or mice harboring a truncated G-CSF-R (Δ715/Δ715) (Fig 6A). Activation of STAT5 was equivalent between WT and mutant mice. However, STAT3 activation in bone marrow cells from Δ715/Δ715 mice was significantly reduced, even at the highest G-CSF concentration used (100 ng/mL). Because the in vivo levels of G-CSF that lead to the observed basal neutropenia in these mice are considerably lower, we also studied STAT3 activation at lower concentrations of G-CSF. At reduced G-CSF concentrations, this deficiency in STAT3 activation is exacerbated (Fig 6B). Even if the maximal STAT3 activation in both genotypes is set at 100%, there is a clear right-shift in the dose-response in mutant animals (Fig 6C). Bone marrow cells from heterozygous mice gave an intermediate result (data not shown). Although these data already strongly suggest that the reduced STAT3 activation might be a direct result of receptor truncation, it cannot be excluded that the differences observed might be due to a disparity in bone marrow composition between genotypes. Therefore, we also examined this phenomenon in 32D cells expressing WT or truncated (mDA) receptors.12 Similar to the primary bone marrow samples, 32D[mDA] cells also showed normal activation of STAT5, but reduced activation of STAT3 compared with 32D[WT] cells (Fig 7A), which was again more pronounced at low concentrations of G-CSF (Fig 7B and C). Ba/F3 cells expressing WT or mDA receptors showed a similar picture of activation (data not shown). These data clearly show that C-terminal truncation of the G-CSF-R alters the dose-response of STAT3 activation.
STAT activation from mice with a targeted deletion in the G-CSF-R derived from SCN. (A) EMSA of nuclear extracts from bone marrow cells of homozygous WT (wt/wt) and mutant “knock-in” (▵715/▵715) mice, as described in Fig 2A, except that both m67 and β-cas oligonucleotides were used, as indicated. The position of STAT1 (S1), STAT3 (S3), STAT5 (S5), and nonspecific (n.s.) complexes are indicated. (B) EMSA of nuclear extracts from bone marrow cells from homozygous WT (wt/wt) and mutant “knock-in” (▵715/▵715) mice with m67 oligonucleotide, as described in Fig 2A, except cells were incubated for 15 minutes with the indicated concentrations of G-CSF. (C) Dose response of STAT3 activation from WT (filled squares) and “knock-in” (open triangles) mice. Quantitative analysis of EMSAs performed as described in (B), setting maximal STAT3 activation at 100%, and basal activation at 0%. The graph shows the mean and range of two independent experiments.
STAT activation from mice with a targeted deletion in the G-CSF-R derived from SCN. (A) EMSA of nuclear extracts from bone marrow cells of homozygous WT (wt/wt) and mutant “knock-in” (▵715/▵715) mice, as described in Fig 2A, except that both m67 and β-cas oligonucleotides were used, as indicated. The position of STAT1 (S1), STAT3 (S3), STAT5 (S5), and nonspecific (n.s.) complexes are indicated. (B) EMSA of nuclear extracts from bone marrow cells from homozygous WT (wt/wt) and mutant “knock-in” (▵715/▵715) mice with m67 oligonucleotide, as described in Fig 2A, except cells were incubated for 15 minutes with the indicated concentrations of G-CSF. (C) Dose response of STAT3 activation from WT (filled squares) and “knock-in” (open triangles) mice. Quantitative analysis of EMSAs performed as described in (B), setting maximal STAT3 activation at 100%, and basal activation at 0%. The graph shows the mean and range of two independent experiments.
Dose response of STAT activation from truncated G-CSF-Rs. (A) EMSA of nuclear extracts from 32D cells expressing WT and truncated (mDA) receptors, as described in Fig 6A. (B) EMSA of nuclear extracts from 32D cells expressing WT and truncated (mDA) receptors, as described in Fig 6B, except that both m67 and β-cas oligonucleotides were used, as indicated. (C) Quantitative analysis of EMSA results shown in (B), as described in Fig 6C. 32D[WT] responses are shown with filled squares and 32D[mDA] responses with open triangles.
Dose response of STAT activation from truncated G-CSF-Rs. (A) EMSA of nuclear extracts from 32D cells expressing WT and truncated (mDA) receptors, as described in Fig 6A. (B) EMSA of nuclear extracts from 32D cells expressing WT and truncated (mDA) receptors, as described in Fig 6B, except that both m67 and β-cas oligonucleotides were used, as indicated. (C) Quantitative analysis of EMSA results shown in (B), as described in Fig 6C. 32D[WT] responses are shown with filled squares and 32D[mDA] responses with open triangles.
DISCUSSION
The data presented here reveal multiple mechanisms of STAT3 activation by the G-CSF-R. Firstly, we have shown that activation from the full-length receptor at saturating ligand concentrations is effectively mediated by the C-terminus in a non–tyrosine-dependent manner. Presumably this is achieved by docking through an intermediate molecule associated with the C-terminal domain of the G-CSF-R. Secondly, activation of STAT3 can also occur via Y704 or Y744, which appears to be the major mechanism of activation at low G-CSF concentrations. In contrast, Y704 alone is the major mechanism for a C-terminal truncated mutant of the G-CSF-R found in patients with severe congenital neutropenia.11 Finally, previous studies have shown that STAT3 can also be activated to a small extent indirectly, via heterodimer formation with STAT1 or STAT5.20 21
Several examples of STAT activation not requiring docking to receptor tyrosines have been reported. For instance, activation of STAT1 by G-CSF or growth hormone and of STAT5 by G-CSF or granulocyte-macrophage colony-stimulating factor (GM-CSF) occurs in the complete absence of receptor tyrosines.20,42,43 It has been proposed that Jak1 and Jak2 specifically recruit and phosphorylate STAT1 and STAT5, respectively.40,44 In these cases, it is possible that STATs associate with tyrosine-phosphorylated Jaks through their SH2 domains. However, while we show that Jak activity is necessary for STAT3 activation, the Jaks bind to the membrane-proximal region of the G-CSF-R,19,23 which is sufficient for activation of STATs 1 and 5, but not STAT3.18 Although we cannot exclude direct, non–SH2-mediated STAT3 binding to the G-CSF-R C-terminus, we have been unable to detect any such interaction by either in vitro binding studies or coimmunoprecipitation (data not shown). We consider it more likely that an intermediate molecule, docking to the C-terminal region of the receptor in a non–tyrosine-dependent manner, provides a phosphotyrosine binding site for the STAT3 SH2 domain. One obvious candidate would be Lyn, which is constitutively associated with the G-CSF-R complex and activated by G-CSF.45 Lyn also contains a consensus STAT3 YxxQ docking motif at its C-terminus in a context known to be phosphorylated.46 This explanation is plausible given that STAT3 can become activated through its association with the Lyn-related kinase, Src.47,48 However, we have been unable to show such an interaction by coimmunoprecipitation (data not shown). Importantly, the receptor tyrosine-independent route of activation is not restricted to Ba/F3 cells, because it was recently reported that in a Jak-1–deficient cell line, in which G-CSF-R phosphorylation is absent, G-CSF–mediated STAT3 activation is only marginally reduced.22
In addition to this novel receptor tyrosine-independent mechanism of STAT3 activation, we obtained evidence for activation routes involving Y704 and Y744 of the G-CSF-R. Competition studies with phosphotyrosine peptides corresponding to the murine G-CSF-R confirmed that the YxxQ motif at Y704 (Y703 of the murine receptor) is indeed a direct docking site for STAT3. Competition was also seen with the murine Y743 peptide, equivalent to Y744 of the human receptor, although to a slightly lesser extent, suggesting direct docking also occurs at this site. Indeed the sequence at this position, YIRS in the mouse and YLRC in the human, bears some homology to two other known interaction sites for the STAT3 SH2 domain, its homodimerization motif, YLKT, and its heterodimerization motif with STAT1, YIKT,49 50 which also supports this conclusion. Furthermore, we present in vitro binding studies that show that the SH2 domain of STAT3 is sufficient for direct interaction with the tyrosine-phosphorylated G-CSF-R cytoplasmic domain.
An important question to address is what specific role each route of STAT3 activation might play in normal physiological responses to G-CSF. At first glance, the mechanisms seem rather similar in the sense that their activation relies on Jak2 and results in functionally active STAT3 complexes, as assayed with a STAT3(m67)-luciferase reporter construct. This is consistent with recent data from mutant cell lines, showing that Jak1 is required for recruitment of STAT3 to the G-CSF-R, and Jak2 for tyrosine-phosphorylation of STAT3.22 However, the fact that the distinct mechanisms of STAT3 activation are differentially utilized depending on ligand concentration may have significant implications for the regulation of basal versus “emergency” granulopoiesis. Typically, the effects mediated by G-CSF on basal granulopoiesis3,4 occur at low ligand concentration,51 at which STAT3 activation will depend largely on the Y704/Y744-dependent mechanism. However, during “emergency” granulopoiesis resulting from bacterial infections, G-CSF treatment, or hematopoietic recovery after myelosuppressive treatment, serum G-CSF levels rise drastically,52 and STAT3 activation is likely to proceed efficiently independent of access to specific receptor phosphotyrosines. There are at least two possible biochemical explanations for the preference for different mechanisms at high and low G-CSF concentrations. Firstly, it may be that at low ligand concentrations, Y704 and Y744 act as the major docking sites due to more efficient phosphorylation or higher affinity than the putative intermediate docking protein, whereas at high G-CSF concentration these differences are less important. Secondly, there may be alternate receptor complexes formed at different ligand concentrations, with those formed at low G-CSF concentrations favoring tyrosine-dependent docking, while those at high G-CSF hindering direct access to the Y-residues, thereby favoring the tyrosine-independent mechanism of STAT3 activation. These two possibilities are not mutually exclusive. Given the importance of STAT3 in the control of G-CSF–mediated cell cycle progression and differentiation,29 these different mechanisms potentially provide the opportunity for fine tuning of the proliferation/differentiation balance during basal versus “emergency” granulopoiesis, for instance, through differential phosphatase sensitivity, or control of the expression of the putative adapter protein.
Using bone marrow cells from mice harboring a targeted G-CSF-R truncation (gcsfr-Δ715),14 we showed that STAT3 activation from the truncated G-CSF-R is reduced, even at saturating G-CSF concentrations. In addition, there is an altered dose-response of STAT3 activation such that at lower G-CSF concentrations the STAT3 deficiency is even more pronounced, a result confirmed in myeloid 32D cells. Given the relative dose-response properties, this would seem to be primarily due to loss of the Y744-dependent route of STAT3 activation. Because STAT3 has been shown to be a vital factor in G-CSF–dependent differentiation,29 this defective STAT3 activation might be an important factor in the basal neutropenia seen in gcsfr-Δ715 mice, as well as in SCN patients harboring similar truncated forms of G-CSF-Rs. This is partially supported by the observation that heterozygous gcsfr-Δ715 mice, which mimic SCN patients, showed both a deficiency in STAT3 activation and a reduction in basal neutrophil numbers that was intermediate to that of homozygous animals.14 In addition, an altered dose response of STAT3 activation compared with STAT5 activation was observed. It has recently been established that STAT5-deficient bone marrow cells showed reduced proliferative responses to G-CSF.53 In contrast, expression studies with a dominant-negative STAT3 molecule suggest that STAT3 activation is inhibitory to G-CSF proliferation.29 We would, therefore, propose that a reduced STAT3:STAT5 ratio in cells with truncated receptors at low G-CSF concentrations may contribute to shifting the balance of proliferation-maturation toward proliferation, which might also provide an explanation for the hypersensitivity to G-CSF of these cells.8 12
ACKNOWLEDGMENT
We thank M. Parren-van Amelsvoort and S. Oomen for excellent technical advice and assistance, M. von Lindern and T. de Koning-Ward for critical reading of the manuscript, A. Yoshimura and T. Hirano for plasmids, A. Levitzki for the Jak2 inhibitor, and K. van Rooyen for exquisite graphical work.
Supported by an EMBO Long Term Fellowship (A.C.W.) and grants from the N.W.O. and the Dutch Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Alister C. Ward, PhD, Institute of Hematology, Erasmus University (Room H Ee 1314), P.O. Box 1738, 3000 DR Rotterdam, The Netherlands; e-mail: ward@hema.fgg.eur.nl.

![Fig. 3. STAT3 activation by G-CSF-R tyrosine mutants. (A) STAT3 immunoprecipitation from lysates of Ba/F3 cells expressing WT or mutant G-CSF-R proteins, as described in Fig 2B. Multiple analyses of at least three independent clones of each mutant gave equivalent results. (B) EMSA of nuclear extracts from Ba/F3 cells expressing WT G-CSF-R (WT) or mutants, as described in Fig 2A, at the concentrations of G-CSF shown. (C) EMSA of serially diluted nuclear extracts from Ba/F3 cells expressing WT or mA receptors stimulated with 100 ng/mL G-CSF. Extracts from the equivalent of 4, 2, 1, and 0.5 × 105 cells were used. (D) Quantitative analysis of EMSA shown in (C), setting STAT3 binding from 4 × 105 Ba/F3[WT] cells at 100%. Results from dilution of Ba/F3[WT] extracts are shown with filled squares, and from Ba/F3[mA] extracts with open diamonds.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.113/5/m_blod40133003w.jpeg?Expires=1766089311&Signature=kbedd1HmRedXMLAJsx8xcGnfl6hS0VhyP3o18YQcgyBLhOHFpaE9t6rIdw4QNj9CRGy4WllBX~5YddoWTV6CnZpt81wHzJEQro0t9UzpHJFcj4-BbYKPCS9naa9RCe9M-l-N5uzcW3qS6KzPYBDfjJs03bQ0UoxDwQYEAMk59rxSYxAFYVLXZSbSAfxiJ7bHhgnHfQudtJNNp8~BlHbVR6QGKpfP8sr9-oBK1xn9g2LNAsFLYywzQO984slAqBv3wlteaisF5XUV2H4arMAd18CFdBfO~gTIqhf5yIE2AF8WMOE5SbwqaTkZ1LlIx2KpUWF-8ybRpjm2El5jrZNtkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Characterization of STAT3/G-CSF-R interactions. (A) Competition of STAT3 containing EMSA complexes with phosphopeptides specific for each of the cytoplasmic tyrosines of the murine G-CSF-R receptor. Nuclear extracts from Ba/F3[WT] cells stimulated with G-CSF for 10 minutes were incubated either without peptide (−) or with the indicated phosphopeptides at a concentration of 500 μmol/L for 60 minutes, followed by EMSA. (B) Peptide competition as described in (A), with no peptide (−) or with Y703 and Y743 peptides at concentrations of 500, 200, and 50 μmol/L. (C) Direct binding of the STAT3 SH2 domain to tyrosine-phosphorylated G-CSF-R in vitro. Purified GST proteins immobilized on glutathione-Sepharose beads were incubated with purified, tyrosine-phosphorylated G-CSF-R cytoplasmic domain and washed extensively. Bound G-CSF-R was determined by boiling the beads in SDS sample buffer and subjecting the supernatant, along with the input G-CSF-R, to Western blot analysis with anti–G-CSF-R (–G-CSF-R) or anti-phosphotyrosine (-pY) antibodies, as indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.113/5/m_blod40133004w.jpeg?Expires=1766089311&Signature=VEQZWo2q2n7L1pk-fCHkscL6gxdTiLgBVLw2kG6fb8qcdu9Sj82pfIlV5o0laAjgscgmEFR4TTA~3WkicRV9-vaBVXUCiCrXlE8ZP0kfwYCzqu-J5b9HYeN9dR2K~4yYYkSQpmhh9OcyWxoucwWLOJl4MQIWJtxH4PNSj4IgOxAzpd46annkEhC2RK-AhQM9UKs5SR10xwPLqWG~Ed7nnWLnYu4HXGCPh46iiP4E8JUzePFUankdvVr~oVFgEhf9bG8FnQ1HgWyCOvXWLuTwD3CFfW3Ag0loseFGl7qufrMxkSliW8CujpNFuba~banOGZ0Mzp6rXPMGnXlszPhHDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Dose response of STAT activation from truncated G-CSF-Rs. (A) EMSA of nuclear extracts from 32D cells expressing WT and truncated (mDA) receptors, as described in Fig 6A. (B) EMSA of nuclear extracts from 32D cells expressing WT and truncated (mDA) receptors, as described in Fig 6B, except that both m67 and β-cas oligonucleotides were used, as indicated. (C) Quantitative analysis of EMSA results shown in (B), as described in Fig 6C. 32D[WT] responses are shown with filled squares and 32D[mDA] responses with open triangles.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.113/5/m_blod40133007w.jpeg?Expires=1766089311&Signature=lsOKogDPcxMJ~JwurFwIHsEU-X3FBjWDRjNlSbAVA5T5V3bnnnUt9BeXcflCX6xnukrG9I5yOMyxPsI0Za8XcYq3Oa2KuC46Hmu-MtE7LxSHQFg3tFL6AF2l8q6SteSDePb3X7g44DG4Tb6YgSCem6DwF5O1oFwLBKxkfvFgQs1Fku7cIGTIVVsIRHGBaWErgkzbY3isIjMIkjHmpZ-qjZwNla-qGTCIKTGdr5JdULkoXRP39uEdeIz3v7X6VTsUDXyY93oSfGhVB9APkqxNa2menOuJyUaJ6oA6LzWkEO5YgpDLvTt6fxG10RR62qVbfuerWrZputVhPjRIFmu0IQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 3. STAT3 activation by G-CSF-R tyrosine mutants. (A) STAT3 immunoprecipitation from lysates of Ba/F3 cells expressing WT or mutant G-CSF-R proteins, as described in Fig 2B. Multiple analyses of at least three independent clones of each mutant gave equivalent results. (B) EMSA of nuclear extracts from Ba/F3 cells expressing WT G-CSF-R (WT) or mutants, as described in Fig 2A, at the concentrations of G-CSF shown. (C) EMSA of serially diluted nuclear extracts from Ba/F3 cells expressing WT or mA receptors stimulated with 100 ng/mL G-CSF. Extracts from the equivalent of 4, 2, 1, and 0.5 × 105 cells were used. (D) Quantitative analysis of EMSA shown in (C), setting STAT3 binding from 4 × 105 Ba/F3[WT] cells at 100%. Results from dilution of Ba/F3[WT] extracts are shown with filled squares, and from Ba/F3[mA] extracts with open diamonds.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.113/5/m_blod40133003w.jpeg?Expires=1766287831&Signature=iSogGEQ3iZzQJBvR7-XLAqfbIgcjNdpjmksiFNKnmYNfJ-tkCKON1K55yTwuRMdKKvU6bUKXZ3Uye239pTFvOnnCnhAhMekFeZcNgM1OeJ9mkwM4yjdzVJoPUIkUiyaBJHi4WdqOxHDmSmWHH69~391p8F--VGhmP-q3R203Kq3NhLte4N0jZ4WCw5zmpcM-JDfw8f6KHLOTtnmMfLnKvDEkbZpgQAOKlbtTEuf8jRXLi71R4Yd4m0q65q0QBJbFn3ga1Qr-04W3nC-RBg2F0k9OWW106vBTSUdEkh2axIlmJLN082b1IVD7XjWD5Z8MYAR3I0J6e3T2nK~chx7IhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Characterization of STAT3/G-CSF-R interactions. (A) Competition of STAT3 containing EMSA complexes with phosphopeptides specific for each of the cytoplasmic tyrosines of the murine G-CSF-R receptor. Nuclear extracts from Ba/F3[WT] cells stimulated with G-CSF for 10 minutes were incubated either without peptide (−) or with the indicated phosphopeptides at a concentration of 500 μmol/L for 60 minutes, followed by EMSA. (B) Peptide competition as described in (A), with no peptide (−) or with Y703 and Y743 peptides at concentrations of 500, 200, and 50 μmol/L. (C) Direct binding of the STAT3 SH2 domain to tyrosine-phosphorylated G-CSF-R in vitro. Purified GST proteins immobilized on glutathione-Sepharose beads were incubated with purified, tyrosine-phosphorylated G-CSF-R cytoplasmic domain and washed extensively. Bound G-CSF-R was determined by boiling the beads in SDS sample buffer and subjecting the supernatant, along with the input G-CSF-R, to Western blot analysis with anti–G-CSF-R (–G-CSF-R) or anti-phosphotyrosine (-pY) antibodies, as indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.113/5/m_blod40133004w.jpeg?Expires=1766287831&Signature=gx4Cp82ghIh0p0b9kDDYtMveAOaGn5JRubURz2szHigqUF2y-Ylg5dL~bLjR2V4eehu0F~ESrX~6tr9NPWO1HZ6cdaoXO~Z3tCEX9-dDuGUdTRODXgwxGr1dLhNXpEUKWmCU-DgD51yShauehJH9dlY~sSRyc48H25zpmp~GDWUMx8224sfzFycCMocYn~xnR9gvtxMgp~ptX33OhTK2r7mupnQYFIUUMqNMwmpSM6HiTjqmw6dHMwrxr73aZz1NaqNBNtbW2r-hRKr-1jiD2ysPks55IwyW64Gb~PFj9mokEvXGL1NDuudp-ZVkrwNzpFMdAch4fNoPSnbhe4TdhQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Dose response of STAT activation from truncated G-CSF-Rs. (A) EMSA of nuclear extracts from 32D cells expressing WT and truncated (mDA) receptors, as described in Fig 6A. (B) EMSA of nuclear extracts from 32D cells expressing WT and truncated (mDA) receptors, as described in Fig 6B, except that both m67 and β-cas oligonucleotides were used, as indicated. (C) Quantitative analysis of EMSA results shown in (B), as described in Fig 6C. 32D[WT] responses are shown with filled squares and 32D[mDA] responses with open triangles.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.113/5/m_blod40133007w.jpeg?Expires=1766287831&Signature=o8anxJFAAPi5oqoo23rH6AcYerDYUBk2iAQ6zlJobWZf5kXkqLALBVD0c5Z7rvRyYqC2X33aLqKy9IH1Vai5mOXfCweDlyVJXvJwcFVLkSPojOOW0LjBunLIbmohZ4TnIrEwBmO6cUKLIvJoW6ff~j-O4HQ0RCNDoEPuug56q6uZeki9jxRkC3kdbTx9DLWqTfa51blA6sz8S0-DMG4AHMS48CIcxHObH5DF20tR-Gs3g40oXA1r-DwZtWSp~byvWT~AJ-ylGq8xb1MEFeH1JSzL~upiYshI93sHtVtB7tJMF-8vuWYqW2p0jqWR2ccQY6hqtC2e1yaMojp1QMSsoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)