Abstract
Chronic myelogenous leukemia (CML) is characterized by the Philadelphia (Ph) translocation and BCR/ABL gene rearrangement which occur in a pluripotent hematopoietic progenitor cell. Ph-negative (Ph−) hematopoiesis can be restored in vivo after treatment with -interferon or intensive chemotherapy, suggesting that normal stem and progenitor cells coexist with the Ph+ clone. We have previously shown that Ph− progenitors are highly enriched in the CD34+HLA-DR− fraction from early chronic phase (ECP) CML patients. Previous studies have suggested that the Ph-translocation represents a secondary clonal hit occurring in an already clonally mutated Ph− progenitor or stem cells, leaving the unanswered question whether Ph−CD34+HLA-DR- progenitors are normal. To show the clonal nature of Ph−CD34+HLA-DR− CML progenitors, we have compared the expression of BCR/ABL mRNA with X-chromosome inactivation patterns (HUMARA) in mononuclear cells and in CD34+HLA-DR+ and CD34+HLA-DR− progenitors in marrow and blood obtained from 11 female CML patients (8 in chronic phase and 3 in accelerated phase [AP] disease). Steady-state marrow-derived BCR/ABL mRNA−, CD34+HLA-DR−progenitors had polyclonal X-chromosome inactivation patterns in 2 of 2 patients. The same polyclonal pattern was found in the progeny of CD34+HLA-DR− derived long-term culture-initiating cells. Mobilization with intensive chemotherapy induced a Ph−, BCR/ABL mRNA−and polyclonal state in the CD34+HLA-DR−and CD34+HLA-DR+ progenitors from 2 ECP patients. In a third ECP patient, polyclonal CD34+ cells could only be found in the first peripheral blood collection. In contrast to ECP CML, steady-state marrow progenitors in late chronic phase and AP disease were mostly Ph+, BCR/ABL mRNA+, and clonal. Further, in the majority of these patients, a Ph−, polyclonal state could not be restored despite mobilization with intensive chemotherapy. We conclude from these studies that CD34+HLA-DR− cells that are Ph− and BCR/ABL mRNA− are polyclonal and therefore benign. This population is suitable for autografting in CML.
CHRONIC MYELOGENOUS leukemia (CML) is a clonal myeloproliferative disease characterized in more than 90% of patients by the Philadelphia (Ph) chromosome which involves a reciprocal translocation between the c-abl gene on chromosome 9 and the breakpoint cluster region (bcr) locus on chromosome 22 [t(9;22) (q34.11;q11.1)].1 The BCR/ABL mRNA gives rise to a 210-kD (p210BCR/ABL) tyrosine kinase. Compared with the endogenous c-abl, p210BCR/ABL has increased kinase activity and binds significantly more to the actin cytoskeleton. This affects the regulation of cell adhesion, cell survival, and proliferation,2-4 all of which are thought to play a crucial role in the pathogenesis of CML.
The clonal Ph-positive (Ph+) pattern can be found in cells of the myelomonocytic, erythroid, and megakaryocytic lineages,5,6 indicating that the BCR/ABL mutation occurs in an early hematopoietic progenitor. In the chronic phase of the disease, most T lymphocytes7-9 and some of the B lymphocytes10 are polyclonal and Ph-negative (Ph−). At diagnosis, Ph−progenitors can be detected in steady-state marrow from some CML patients.11 Further, a Ph− state can be restored, at least temporarily, in marrow or blood cells after in vivo treatment with either α-interferon (IFN-α)12 or intensive chemotherapy,13,14 or after ex vivo “purging” by growth in long-term cultures,15 exposure to IFN,16 cytotoxic drugs,17 or antisense oligonucleotides.18 Finally, we and others have shown that BCR/ABL− progenitors are enriched in an immature precursor fraction expressing the CD34 antigen, but lacking HLA-DR antigens (CD34+HLA-DR−), but not in CD34+ cells coexpressing the HLA-DR antigen (CD34+HLA-DR+),19,20 or other immature phenotypes.21 All of these studies thus indicate that Ph− progenitors coexist with the Ph+, malignant progenitor pool.
Previous studies using glucose-6-phosphate dehydrogenase (G6PD) polymorphisms as a marker for clonality have shown that clonal Ph− B cells could be detected in some Ph+patients,22-24 suggesting that a clonal Ph− stage may precede the Ph+ state in some CML patients. Because autografts using Ph−progenitors, obtained either by in vivo administration of chemotherapy13,14,25,26 or by ex vivo selection27,28 are currently being done or are contemplated, it is crucial to determine the clonal nature of Ph− CML progenitors. We compared the presence of BCR/ABL mRNA in mononuclear cells, CD34+HLA-DR−, and CD34+HLA-DR+ cells from female CML patients in chronic and AP with their respective X-chromosome inactivation (XCI) patterns. XCI analysis is based on the Lyon hypothesis that inactivation of one of both X-chromosomes occurs in each female somatic cell early in embryogenesis.29 This inactivation pattern is stably inherited by all daughter cells.30 Therefore, all cells in a clonal cell population have the same X-chromosome inactivated, whereas polyclonal tissues are mosaics for X-linked loci. We have used the trimeric CAG polymorphism in the 5′-region of the human androgen-receptor (HUMARA) gene31 to determine the clonal nature of BCR/ABL− and BCR/ABL+ cell fractions from CML patients with chronic phase and accelerated phase (AP) disease. Because the HUMARA gene has a high heterozygosity frequency, stable methylation patterns, and polymerase chain reaction (PCR) accessibility, this assay is applicable to low cell numbers such as sorted progenitor fractions and individual cell colonies.32
We show that BCR/ABL− subpopulations in steady-state marrow or in mobilized peripheral blood (PB) collections are polyclonal. In contrast, marrow or blood populations that express the BCR/ABL mRNA are almost always clonal. We conclude from these studies that Ph−, BCR/ABL− progenitors present in CML blood or marrow are polyclonal and, therefore, more than likely benign.
MATERIALS AND METHODS
Sample Characteristics
Bone marrow (BM), steady-state PB, and mobilized PB (PBPC) specimens were sampled from female CML patients. Diagnosis of CML and determination of disease stages was performed according to standard criteria.12 Patients with early chronic phase (ECP) disease had CML diagnosed less than 1 year before analysis and no signs of AP disease. AP was diagnosed when 2 or more of the following characteristics were present: systemic symptoms previously well controlled with single-agent chemotherapy, splenomegaly, leukocytosis, thrombocytopenia and/or anemia, greater than 10% basophils in the PB, cytogenetic evolution, and/or severe reticulin fibrosis of the marrow. Patients’ characteristics are listed in Table 1. Therapy before enrollment in this study was IFN-α alone (n = 4), hydroxyurea alone (n = 4), or a combination of both (n = 3). Unique patient numbers (UPNs) 6, 7, 8, and 11 had received IFN-α for at least 12 months before enrollment was done, whereas UPNs 3 and 9 had been treated with IFN-α for 3 months and UPN 2 for 6 months. Mobilization was done using the MAC-G protocol: mitoxantrone (8 mg/m2 on days 1 and 2), cytosine-arabinoside (1,000 mg/m2 twice daily on days 1 and 2), and cyclophosphamide (4 g/m2 on day 1). Granulocyte colony-stimulating factor (G-CSF) was started on day +5 at 5 μg/kg/d until the last day of PBPC collection. Alternatively, PBPCs were mobilized by administration of 1 dose of cyclophosphamide (4 g/m2) followed by G-CSF (5 μg/kg/d) on day +5 after chemotherapy. PBPC harvesting was started when the absolute neutrophil counts reached 0.8 to 1.0 × 109/L.
In addition, between 50 and 100 mL of BM was collected from 11 healthy female volunteer donors (mean age, 23 years; range, 22 to 25) heterozygous for the HUMARA polymorphism. All samples were obtained with informed consent according to guidelines of the Human Subjects Committee at the University of Minnesota and Leuven, Belgium. Samples were collected in preservative-free heparin for further processing.
Cell Purification
BM and PB mononuclear cells (MNC) were recovered after centrifugation for 30 minutes at 1,650 rpm over a Ficoll gradient (1.077 g/mL; Nycomed Pharma AS, Oslo, Norway). Cells were washed twice in phosphate-buffered saline (PBS) supplemented with 0.3% bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO) and kept on ice for subsequent CD34 selection. Enrichment of CD34+ progenitors from MNC was performed using either CeprateLC columns (CellPro Inc, Bothell, WA) or the MiniMACS CD34 isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. CD34+ fractions were then labeled with fluorescein isothiocyanate (FITC)-conjugated anti-CD34 antibody (HPCA-2) and phycoerythrin (PE)-conjugated anti–HLA-DR (Becton Dickinson Immunocytometry Systems, San Jose, CA). After labeling, cells were washed, resuspended in Iscove’s medium supplemented with 20% fetal bovine serum (Hyclone, Logan, UT), and sorted on a FACStar-Plus flow cytometer supplemented with Lysis II software (Becton Dickinson). Sort gates were drawn around cell populations with intermediate forward scatter (FSC), low to intermediate side scatter (SSC), CD34-positivity and high (CD34+HLA-DR+) or absent (CD34+HLA-DR−) levels of HLA-DR coexpression. Isotype-matched conjugated mouse IgG for both CD34 and HLA-DR (Becton Dickinson) were used as controls. Sorted populations were at least 97% pure after reanalysis. A median number of 106 MNC, 104CD34+HLA-DR+, and 4 × 103CD34+HLA-DR− cells were removed for clonality analysis from each patient sample and kept frozen in liquid nitrogen in the presence of 20% fetal calf serum (FCS) and 10% dimethylsulfoxide (DMSO; Merck, Darmstadt, Germany). At time of analysis, samples were rapidly thawed in a 37°C waterbath, washed once with ice-cold PBS, and equally split for DNA and RNA analysis. For controls, 2 × 104 MNC, CD34+HLA-DR+, and CD34+HLA-DR− cells were isolated and freshly processed.
Reverse Transcriptase (RT)-PCR for BCR/ABL and β-Actin
RNA was extracted using RNeasy spin columns (Qiagen, Chatsworth, CA) and diluted in 30 μL of RNAse-free H2O. cDNA was subsequently synthesized from 12 μL of this RNA in a 30-μL reaction mixture containing 400 U of Moloney murine leukemia virus (M-MLV) reverse transcriptase, 0.1 mmol/L dithiothreitol (DTT), 6 μL of 5× First Strand Buffer (all from GIBCO BRL-Life Technologies, Gaithersburg, MD), 40 U of RNAsin (Promega, Madison, WI), 2 mmol/L of dNTPs (Pharmacia Biotech, Uppsala, Sweden), and 20 pmol/L of primer A (5′-GGAGCTGCAGATGCTGACCAAC-3′) for BCR-ABL or primer BA (5′-TACCTCATGAAGATCCTCA-3′) for β-actin. After incubation for 60 minutes at 42°C, the RT was inactivated by heating for 10 minutes at 80°C. Nested PCR for BCR/ABL was performed as previously described.11 For β-actin, a single-step PCR was performed: 7.5 μL of cDNA was added to 42.5 μL of PCR mix containing 5 μL of 10× PCR buffer, 1.5 mmol/L MgCl2, 2.5 U Taq Polymerase (all from Promega), 200 μmol/L dNTPs, and 20 pmol/L of primer BA and BB (5′-TTCGTGGATGCCACAGGAC-3′). Denaturing was performed for 45 minutes at 95°C, annealing for 30 minutes at 55°C, and extension for 45 minutes at 72°C for 44 cycles in a Perkin Elmer Thermal Cycler (Perkin Elmer Cetus Corp, Emeryville, CA). Several precautions were taken to avoid false-positive results. Each RT-PCR reaction included a positive (K562 cell line) and a negative (Raji cell line) control for BCR/ABL expression as well as another negative control containing all components of the reaction, except for the target nucleic acids; finally, all PCR reactions were performed in duplicate. BCR/ABL and β-actin PCR products were loaded on a 3% Nusieve agarose gel (Life Sciences International Ltd, Cheshire, UK) and electrophoresis was performed for 2 hours at 150 V. After ethidium-bromide staining, gels were photographed and subsequently immersed at room temperature in denaturing buffer (3 mol/L NaCl, 0.4 mol/L NaOH) on an orbital shaker for 60 minutes. DNA was blotted onto nylon membranes by downward alkaline transfer for 2 hours using a Turboblotter (Schleicher & Schuell, Keene, NH). After transfer, membranes were washed, UV-crosslinked, and prehybridized for 30 minutes in prewarmed Rapid-hyb buffer (Amersham Life Science, Arlington Heights, IL). Before hybridization, 10 pmol/L probe B3A2 (5′-GCTGAAGGGCTTTTGAACTCTGCTTA-3′) , B2A2 (5′-GCTGAAGGGCTTCTTCCTTATTGATG-3′) for detection of BCR/ABL, and BAP (5′-CCATCTCTTGCTCGAAGTC-3′) for detection of β-actin was 5′-labeled with 50 μCi of 32P γATP (4,500 Ci/mmol) (ICN Pharmaceuticals Inc, Irvine, CA) for 40 minutes at 37°C using 10 U of Polynucleotide kinase (Boehringer Mannheim, Indianapolis, IN). Hybridization was performed for 60 minutes at 42°C. After hybridization, blots were washed for 20 minutes in 5× SSC, 0.1% (wt/vol) sodium dodecyl sulfate (SDS) at room temperature and 2 times 15 minutes in 1.0 to 0.1× SSC, 0.1% (wt/vol) SDS at 42°C. Blots were dried, stored overnight on phosphorscreens, and analyzed on a Phosporimager (Molecular Dynamics, Sunnyvale, CA).
Clonality Analysis With the HUMARA Assay
Restriction enzyme digest and PCR were performed as previously described.32 Briefly, DNA was extracted from individual cell fractions and resuspended in 40 μL of H2O. One half of this sample was incubated overnight at 37°C with 40 U ofHpaII and 10 U of Cfo I (Hha I) (Boehringer Mannheim) for the digestion of unmethylated (or active) alleles. The other half of the DNA was treated similarly but without restriction enzymes. After restriction enzyme digest, residual DNA was amplified in a nested PCR procedure with one of the inner primers 5′ labeled with 32P and separated on a 4% denaturing 19:1 acrylamide/bisacrylamide gel for 4 hours at 60 W. Gels were dried and scored on a phosphorimager (Molecular Dynamics) after overnight exposition to phosphorscreens. For each sample, a corrected ratio (CrR) was calculated by dividing the ratio of the predigested sample (upper/lower allele) by the ratio of the non-predigested sample using ImageQuant software (Molecular Dynamics). This CrR compensates for preferential amplification of the shorter allele when the number of PCR cycles increases.32 A clonal population is defined as a cell population with greater than 75% expression of one of both X-linked alleles. This corresponds to CrR values of <0.33 or >3.33 34
RESULTS
Steady-State BM
CML patients.
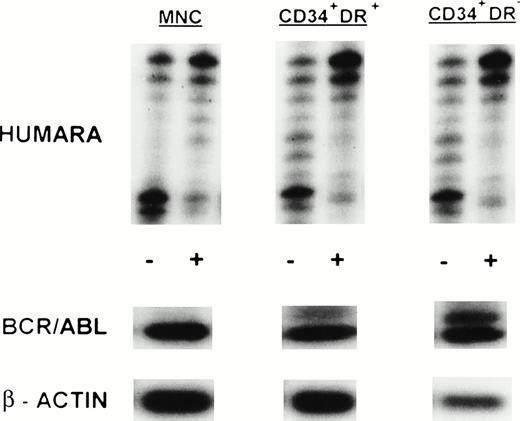
We studied steady-state marrow samples obtained from 10 female patients with CML in whom a polymorphism for the HUMARA locus could be detected (Table 2). Three patients had ECP CML, 4 patients late CP CML, and 3 patients AP disease. Mononuclear cells and fluorescence-activated cell sorting (FACS)-selected CD34+HLA-DR+ and CD34+HLA-DR− cells were examined by RT-PCR for BCR/ABL mRNA expression and by the HUMARA assay for clonality analysis. BCR/ABL RT-PCR of the MNC fraction was positive in all 10 patients in two independent determinations. In 7 of 10 patients, BCR/ABL expression was associated with skewing of the HUMARA assay toward the upper (UPNs 5, 7, 8, and 9) or lower (UPNs 1, 2, and 10) allele, showing the predominance of one single clone in these cell populations. In two patients, UPN 6 and UPN 11, the HUMARA assay on MNC showed a more polyclonal pattern. CD34+HLA-DR+cells from 9 of 10 patients showed presence of the BCR/ABL mRNA, whereas CD34+HLA-DR+ cells from UPN 3 were BCR/ABL mRNA negative in two independent determinations despite the presence of β-actin in the RT-PCR reactions. Seven of these 9 BCR/ABL mRNA+ fractions were examined with the HUMARA assay to determine their clonal origin. In 4 patients, 2 in late chronic phase (LCP) and 2 in AP disease, CD34+HLA-DR+ cells were clonal (CrR of 6.28 [UPN 7], 24.20 [UPN 8], 29.74 [UPN 9], and 0.01 [UPN 10]). In the other three patients (1 ECP CML [UPN 1], 1 LCP CML [UPN 6], and 1 AP CML patient treated to a partial Ph− state with IFN-α but with cytogenetic evolution [UPN 11]), CD34+HLA-DR+ cells were nonclonal (CrR of 0.41, 0.43, and 1.71, respectively) even though RT-PCR analysis showed the presence of the BCR/ABL mRNA. In the only patient in whom CD34+HLA-DR+ cells did not contain BCR/ABL mRNA, the HUMARA assay confirmed polyclonality (CrR = 1.47). We also studied FACS-selected CD34+HLA-DR− cells. Consistent with previous studies from our group, CD34+HLA-DR− cells from 2 of 3 ECP CML patients were BCR/ABL mRNA− (UPN 1 and UPN 3). These cells were polyclonal by HUMARA (CrR = 0.63 and 1.34, respectively) (Fig 1A). In contrast, CD34+HLA-DR− cells from the other ECP CML patient, 2 LCP CML, and 1 AP CML patient were BCR/ABL mRNA+. In these patients, CD34+HLA-DR− cells were clonal (CrR of 0.13 [UPN 2], 3.35 [UPN 5], 16.92 [UPN 8], 34.24 [UPN 9]) (Fig 2). CD34+HLA-DR− cells from the AP patient treated to partial cytogenetic remission with IFN-α but with cytogenetic evolution (UPN 11) were BCR/ABL mRNA− and nonclonal (CrR = 1.41).
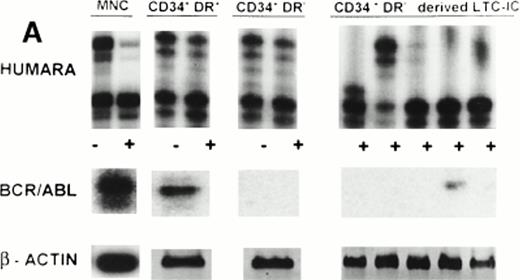
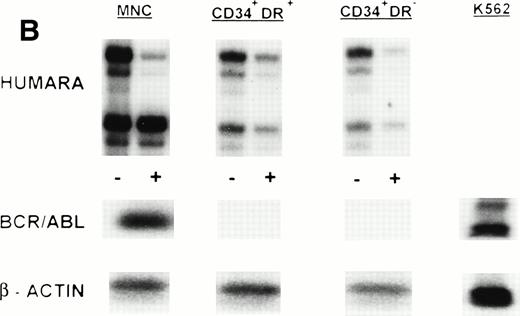
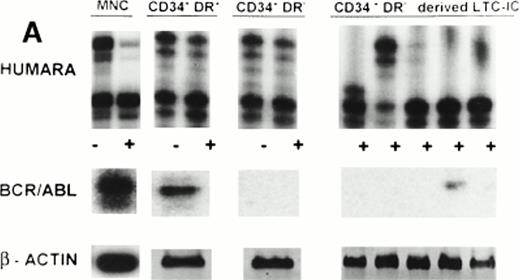
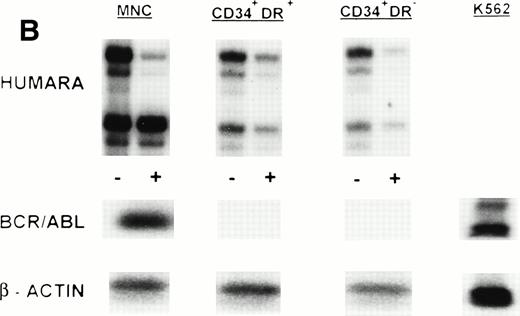
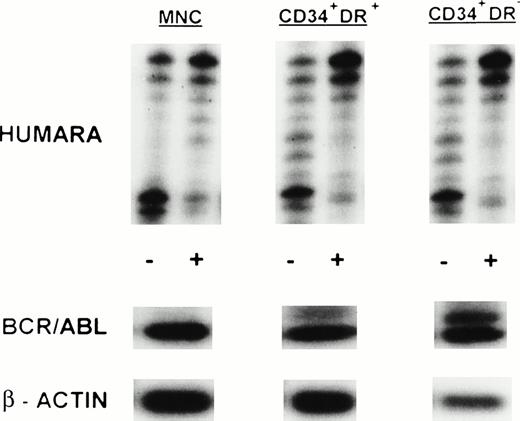
(A) X-inactivation patterns with the HUMARA assay and BCR/ABL mRNA expression in MNC, CD34+HLA-DR+, CD34+HLA-DR−, and DR− derived LTC-IC in steady-state marrow in ECP CML. HUMARA PCR and BCR/ABL and β-actin RT-PCR reactions were performed as described in Materials and Methods. Results are shown for one experiment in marrow from UPN 1. HUMARA PCRs are shown without (−) and with (+) prior exposure toHpaII and Cfo I restriction endonucleases. Shadow bands are due to slippage of Taq polymerase. BCR/ABL yielded positive signals in the MNC and DR+ fraction and in one LTC-IC–derived colony, after hybridization with a B3A2 probe. No positive signal was detected in the other samples. The housekeeping gene β-actin was used as an internal control for the presence of mRNA. (B) HUMARA analysis and BCR/ABL mRNA expression in mobilized PB in ECP CML. Results are shown for day 3 harvest from UPN 1. HUMARA PCR and BCR/ABL and β-actin RT-PCR reactions were performed as described in Materials and Methods. HUMARA was performed without (−) and with (+) predigestion with the methylation-sensitive endonucleases HpaII andCfo I. MNC displayed monoclonal patterns and BCR/ABL mRNA positivity, whereas CD34+HLA-DR+ and CD34+HLA-DR− progenitors were polyclonal and BCR/ABL mRNA−. The erythroleukemic cell line K562 served as a positive control for BCR/ABL (B3A2) expression. The housekeeping gene β-actin was used as a control for the presence of mRNA.
(A) X-inactivation patterns with the HUMARA assay and BCR/ABL mRNA expression in MNC, CD34+HLA-DR+, CD34+HLA-DR−, and DR− derived LTC-IC in steady-state marrow in ECP CML. HUMARA PCR and BCR/ABL and β-actin RT-PCR reactions were performed as described in Materials and Methods. Results are shown for one experiment in marrow from UPN 1. HUMARA PCRs are shown without (−) and with (+) prior exposure toHpaII and Cfo I restriction endonucleases. Shadow bands are due to slippage of Taq polymerase. BCR/ABL yielded positive signals in the MNC and DR+ fraction and in one LTC-IC–derived colony, after hybridization with a B3A2 probe. No positive signal was detected in the other samples. The housekeeping gene β-actin was used as an internal control for the presence of mRNA. (B) HUMARA analysis and BCR/ABL mRNA expression in mobilized PB in ECP CML. Results are shown for day 3 harvest from UPN 1. HUMARA PCR and BCR/ABL and β-actin RT-PCR reactions were performed as described in Materials and Methods. HUMARA was performed without (−) and with (+) predigestion with the methylation-sensitive endonucleases HpaII andCfo I. MNC displayed monoclonal patterns and BCR/ABL mRNA positivity, whereas CD34+HLA-DR+ and CD34+HLA-DR− progenitors were polyclonal and BCR/ABL mRNA−. The erythroleukemic cell line K562 served as a positive control for BCR/ABL (B3A2) expression. The housekeeping gene β-actin was used as a control for the presence of mRNA.
X-inactivation patterns with the HUMARA assay and BCR/ABL mRNA expression in MNC, CD34+HLA-DR+, and CD34+HLA-DR− progenitors in steady-state marrow in AP CML. Results are shown for marrow from UPN 9. HUMARA PCR and BCR/ABL and β-actin RT-PCR reactions were performed as described in Materials and Methods. HUMARA was performed without (−) and with (+) predigestion with the methylation-sensitive endonucleasesHpaII and Cfo I. All samples show monoclonality. BCR/ABL results are shown after hybridization with the B3A2 probe. The housekeeping gene β-actin was used as a control for the presence of mRNA.
X-inactivation patterns with the HUMARA assay and BCR/ABL mRNA expression in MNC, CD34+HLA-DR+, and CD34+HLA-DR− progenitors in steady-state marrow in AP CML. Results are shown for marrow from UPN 9. HUMARA PCR and BCR/ABL and β-actin RT-PCR reactions were performed as described in Materials and Methods. HUMARA was performed without (−) and with (+) predigestion with the methylation-sensitive endonucleasesHpaII and Cfo I. All samples show monoclonality. BCR/ABL results are shown after hybridization with the B3A2 probe. The housekeeping gene β-actin was used as a control for the presence of mRNA.
Control population.
CrR values of MNC, CD34+HLA-DR−, and CD34+HLA-DR+ fractions were found to be polyclonal in 9 of the 11 female marrow donors (Table 3). CrR values ranged from 0.43 to 2.28. Only 2 of 11 controls (8 and 10) had monoclonal allelic ratios and were therefore considered as constitutionally skewed. XCI patterns, expressed as allelic ratios, were highly correlated between MNC and CD34+HLA-DR+(r = .96) and CD34+HLA-DR−(r = .97) fractions.
Mobilized PB Progenitors
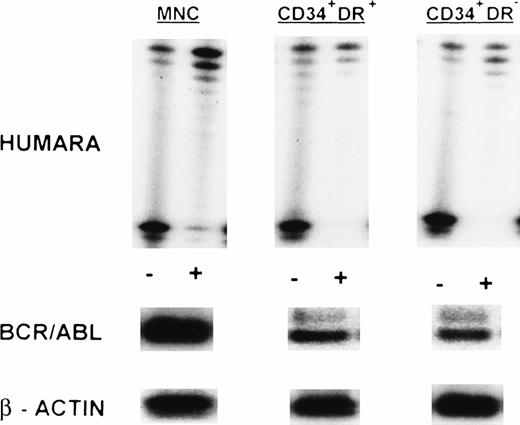
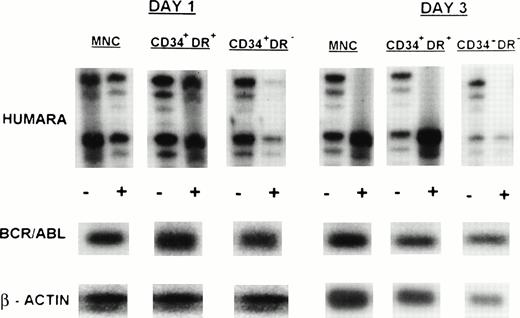
MNC, CD34+HLA-DR+, and CD34+HLA-DR− cells selected from one or more of the PB progenitor collections obtained in 7 patients (3 ECP CML, 3 LCP CML, 1 AP CML) after in vivo mobilization with intensive chemotherapy and growth factor support, were analyzed by RT-PCR for BCR/ABL expression and by HUMARA for clonality (Table 4). Cytogenetics on PBPC collections showed that a major cytogenetic response was obtained with the in vivo mobilization regimen in 2 ECP and 1 LCP CML patients whereas between 80% and 100% of metaphases in 1 ECP, 1 LCP, and the AP CML patient were still Ph+. RT-PCR analysis of the MNC fraction of all patients showed presence of the BCR/ABL mRNA. MNC were clonal in UPNs 1, 5, 6, 7, and 9, but polyclonal in UPN 2 and UPN 4 on day 1 of PBPC collections. Except for patient 2, showing a shift from monoclonality to polyclonality in the MNC fraction, these findings were similar to the observations in steady-state BM. CD34+HLA-DR+ and CD34+HLA-DR− cells selected from 2 ECP CML patients (UPNs 1 and 2) did not express detectable amounts of BCR/ABL mRNA in two independent PCR reactions. This was consistent with the nonclonal character of these cells as determined by HUMARA (Fig1B). Patient 4, with a complex Ph translocation involving chromosomes 9, 17, and 22 still had, despite BCR/ABL positivity, residual nonclonal CD34+HLA-DR+ and CD34+HLA-DR− progenitors at day 1 of the PBPC harvest. However, by day 3 both the CD34+HLA-DR− and the CD34+HLA-DR+ compartments had shifted toward full monoclonality (Fig 3). CD34+HLA-DR+ and CD34+HLA-DR− cells from mobilized PBPC of all LCP and the AP CML patient were BCR/ABL mRNA+. CD34+HLA-DR+ cells were clonal in all 4 patients whereas CD34+HLA-DR− cells from UPNs 5 and 7 (LCP CML) and UPN 9 (AP CML), but not UPN 6 (LCP CML), were clonal in two independent HUMARA assays (Fig 4). For all patients except UPN 4, we did not find significant differences in BCR/ABL mRNA expression and HUMARA ratios between PBPC collections from different days.
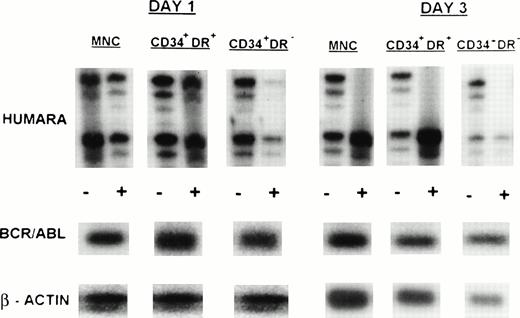
Clonal evolution in PBPC collections from a patient with early chronic phase CML (UPN 4). Results are shown for day 1 and day 3 PBPC harvests. HUMARA, BCR/ABL, and β-actin PCRs were performed as described in Materials and Methods. All cell fractions switch from polyclonality on day 1 to monoclonality on day 3. (−), No prior digest with HpaII and Cfo I; (+), prior digest withHpaII and Cfo I.
Clonal evolution in PBPC collections from a patient with early chronic phase CML (UPN 4). Results are shown for day 1 and day 3 PBPC harvests. HUMARA, BCR/ABL, and β-actin PCRs were performed as described in Materials and Methods. All cell fractions switch from polyclonality on day 1 to monoclonality on day 3. (−), No prior digest with HpaII and Cfo I; (+), prior digest withHpaII and Cfo I.
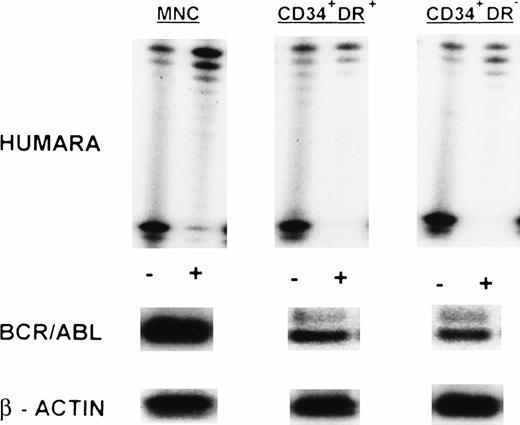
HUMARA analysis and BCR/ABL mRNA expression in mobilized PB in LCP CML. Results are shown for day 1 harvest from UPN 5. HUMARA PCR and BCR/ABL and β-actin RT-PCR reactions were performed as described in Materials and Methods. MNC, CD34+HLA-DR+, and CD34+HLA-DR− progenitors were monoclonal and BCR/ABL mRNA+. Shadow bands in the HUMARA PCR are caused by slippage of Taq polymerase. (−), No prior digest withHpaII and Cfo I; (+), prior digest with HpaII and Cfo I.
HUMARA analysis and BCR/ABL mRNA expression in mobilized PB in LCP CML. Results are shown for day 1 harvest from UPN 5. HUMARA PCR and BCR/ABL and β-actin RT-PCR reactions were performed as described in Materials and Methods. MNC, CD34+HLA-DR+, and CD34+HLA-DR− progenitors were monoclonal and BCR/ABL mRNA+. Shadow bands in the HUMARA PCR are caused by slippage of Taq polymerase. (−), No prior digest withHpaII and Cfo I; (+), prior digest with HpaII and Cfo I.
Clonal or Nonclonal Nature of DR+ and DR− Long-Term Culture-Initiating Cells (LTC-IC)
To further ascertain that CD34+HLA-DR−cells are polyclonal in patients in whom RT-PCR analysis does not reveal BCR/ABL mRNA, we examined single LTC-IC–derived colony-forming cells (CFC) from UPN 1 by RT-PCR and HUMARA. CD34+HLA-DR− cells selected from steady-state marrow were cultured in limiting dilutions onto M2-10B4 feeders (kindly provided by Dr C. Eaves, Vancouver, Canada) for 5 weeks. Stromal feeders and hematopoietic progeny were then overlaid with cytokine-containing methylcellulose medium and CFC from positive wells at the lower cell doses collected 14 days later.35Four of five DR− LTC-IC–derived CFC were BCR/ABL mRNA− and the different colonies had either the upper or lower allele active, further confirming the polyclonal origin of these BCR/ABL mRNA−, CD34+HLA-DR− cells and LTC-IC (Fig1A).
DISCUSSION
In this article we report that CD34+ cells in marrow and blood from patients with CML, which are BCR/ABL mRNA−and Ph−, are polyclonal and therefore presumably benign. X-chromosome inactivation analysis using the HUMARA assay shows that highly purified, FACS-selected CD34+HLA-DR+ or CD34+HLA-DR− cells and LTC-IC within these populations that do not express BCR/ABL mRNA are nonclonal. In contrast, BCR/ABL mRNA containing CD34+ subpopulations are clonal in the majority of samples analyzed.
Previous studies using G6PD polymorphisms22-24 have suggested that immature progenitors that are clonal but do not have the Ph chromosome may be found in marrow or blood of some patients with CML. This would suggest that acquisition of the BCR/ABL gene rearrangement is a secondary genetic abnormality and results from subclonal expansion in an already clonal, but Ph−, hematopoietic stem or progenitor cell. However, clinical studies using DNA-based X-chromosome inactivation assays have indicated that CML patients treated to cytogenetic remission with IFN-α usually have restoration of polyclonal hematopoiesis.36,37 Likewise, patients that have regenerated Ph− hematopoiesis after transplantation with peripheral blood progenitors obtained after in vivo therapy with polychemotherapy and G-CSF have polyclonal mature cells and progenitors in blood and marrow.38 These and other studies39 indicate that polyclonal Ph− progenitors coexist with the BCR/ABL+, Ph+ clone. Moreover, a number of animal studies suggest that the BCR/ABL gene rearrangement is not only necessary but also sufficient for the development of the clinical CML syndrome.2 Although this does not preclude the possibility that acquisition of the BCR/ABL gene rearrangement is a secondary genetic event leading to CML, the animal models as well as the clinical experience in humans make the hypothesis that BCR/ABL is a secondary hit leading to CML less likely.
Because the only curative therapy for CML, allogeneic transplantation, is available for less than 50% of patients afflicted with this disease,12 increasing numbers of patients failing IFN-α treatment are being considered for autologous transplantation with blood- or marrow-derived progenitors. We have identified the CD34+HLA-DR− cell population, selected from steady-state marrow or mobilized PB of a fraction of patients with early chronic phase CML, to be BCR/ABL mRNA−.19 Determination of the monoclonal or polyclonal nature of CD34+ progenitors which are BCR/ABL− and Ph− is important. Monoclonal patterns would confirm that CD34+ progenitors in CML originate from a clonally mutated preleukemic cell which does not yet express BCR/ABL. In contrast, polyclonal patterns, as can be found in normal hematopoiesis, would suggest that the BCR/ABL− compartment consists of normal progenitors.
We used RT-PCR followed by Southern blot analysis to detect the BCR/ABL gene rearrangement and HUMARA to evaluate X-chromosome inactivation patterns. Products of the BCR/ABL RT-PCR reactions were considered positive if at least one of two independent RT-PCR reactions yielded a BCR/ABL cDNA and the β-actin cDNA was visualized. Only those RT-PCR reactions in which two independent experiments did not yield BCR/ABL cDNA, despite visualization of β-actin cDNA, were considered negative. The sensitivity of this RT-PCR reaction is 1/105cells. Duplicate RT-PCR reactions were performed to decrease the likelihood of obtaining false-negative results when low copy numbers of BCR/ABL mRNA are present in immature CD34+ cell populations.40 The sensitivity of the HUMARA assay, assessed by mixing MNC from two healthy male volunteers with different numbers of CAG repeats, has been previously reported by our group.32 Use of phosphorimaging can increase the sensitivity of detection to values between 90% and 95%. This enables detection of a cell clone contributing only 5% to 10% to the cell mixture. CrR values were calculated to semiquantify the X-inactivation patterns. To ascertain accuracy, all HUMARA assays were performed in duplicate. This yielded a high reproducibility (r2> .90) between two PCR reactions independently performed on one sample.
Using both BCR/ABL RT-PCR and the HUMARA assay on the same cell fraction, we found that CML progenitors derived from steady-state marrow or mobilized PB with no detectable BCR/ABL mRNA transcripts were polyclonal, because their CrR values were within the range found in normal control subjects. These polyclonal BCR/ABL mRNA− progenitors are particularly enriched in the CD34+HLA-DR− fractions. Further, LTC-IC present in this CD34+HLA-DR− population of one ECP CML patient were also polyclonal. Because “steady-state” patients with polyclonal progenitors were already being treated with hydroxyurea and/or IFN-α, the precise role of these treatment regimens on clonality of CML progenitors could not be evaluated. However, previous reports have shown restoration of polyclonality in CML by IFN-α.36 Nevertheless, our results confirm previous studies from our group and other investigators19 20 that CD34+HLA-DR− cells are enriched for BCR/ABL− primitive progenitors in some patients with ECP CML. In addition, our HUMARA studies support the concept that BCR/ABL− progenitors in CML are “benign” and that the Ph translocation is likely the initial genetic abnormality occuring in a very primitive progenitor. If a clonal event would precede the acquisition of the Ph translocation, then BCR/ABL mRNA− CD34+HLA-DR−cells and their LTC-IC would be derived from a single clone, a hypothesis that is not supported by our studies. Although our current methodology of X-inactivation analysis does not permit detection of a minor monoclonal Ph− population that coexists with polyclonal Ph− progenitors, its contribution to the total progenitor compartment is low and it does not seem to have any proliferation advantage.
Despite the presence of clonal Ph+ progenitors in steady-state marrow, treatment with intensive chemotherapy can restore a Ph−, BCR/ABL mRNA− and polyclonal progenitor compartment as has been shown in UPNs 1 and 2. However, this residual pool of Ph−, polyclonal progenitors decreases over time and with acceleration of the disease, as a polyclonal population could no longer be mobilized in patients with LCP and AP disease. Moreover, as can be learned from the clonality studies on the PBPC collections from UPN 4, these polyclonal progenitors are preferentially mobilized during early recovery after chemotherapy. This observation clearly shows the coexistence of a polyclonal progenitor compartment together with clonal BCR/ABL mRNA+ precursors, where the clonal Ph+ population rapidly suppresses the residual polyclonal hematopoiesis. Although transplantation with nonclonal progenitor cells may be superior to transplantation with clonally mutated preleukemic progenitors, future studies will be needed to demonstrate a survival benefit.
The observation that some of the cell fractions have a polyclonal nature despite the presence of detectable amounts of BCR/ABL mRNA transcripts is not a contradiction, but can be explained by taking the sensitivity of both the BCR/ABL RT-PCR and HUMARA PCR into account. Because BCR/ABL RT-PCR reactions are up to 5,000-fold more sensitive as the HUMARA assay, the apparent discrepancy between the HUMARA and RT-PCR assays indicates that the degree of contamination of these CD34+HLA-DR+ cell populations with BCR/ABL+ cells is relatively low. This implies that polyclonal BCR/ABL mRNA+ progenitor cell fractions are composed of a mixture of polyclonal BCR/ABL mRNA−precursors and a smaller fraction of clonal BCR/ABL mRNA+leukemic precursors. Additionally, nonclonal allelic ratios of the MNC might be attributable to the presence of a sizable population of nonclonal, nonmyeloid cells, such as B cells and T cells, which are usually not part of the Ph+ clone in chronic phase CML.7-10
Are our results in contradiction with previously reported studies in which the clonal origin of Ph− cells in CML was examined with G6PD-based clonality assays? As for the DNA-based HUMARA assay, the isoenzyme G6PD-based clonality studies have reported a sensitivity of 95%. This makes the nondetection of a residual nonclonal population in the G6PD-based clonality studies unlikely. The major difference between the studies reported by Fialkow et al22,24 and our studies are the cell populations analyzed. Fialkow et al have examined the clonal origin of Ph− Epstein-Barr virus (EBV)-derived lymphoblastoid cell lines obtained from blood of patients with Ph+ chronic phase CML. In chronic phase CML, both T and B lymphocytes, which are long-lived, are usually Ph−.7-10Therefore, it seems reasonable to examine the clonal origin of these lymphoid cells to determine if a clonal, but still Ph−, population exists in CML. Although EBV is a polyclonal stimulator of B lymphocytes, there are no data available on the comparison of clonal patterns of freshly sorted B lymphocytes and their progeny after EBV-transformation. Additionally, T lymphocytes are also reported to be Ph−. But in contrast to B lymphocytes, they seem to display more often polyclonal X-inactivation patterns.9 Finally, Fialkow et al have tried to correct for excessive constitutional skewing or “extreme Lyonization” in the clonal Ph− fractions by comparing the clonal patterns of B lymphocytes with these of fibroblasts and skin. However, it has been recently shown that hematopoietic tissue is more prone to acquired skewing compared with other somatic tissues.33 34 We have studied BCR/ABL expression and X-inactivation patterns on highly purified immature CD34+HLA-DR−progenitors and their more committed CD34+HLA-DR+ counterparts. We did not have access to nonhematopoietic tissues to exclude excessive skewing. Therefore, we cannot discriminate between monoclonality and constitutional or acquired skewing in patients with more extreme allelic ratios (CrR <0.3 or >3). However, because Ph− BCR/ABL− progenitors were polyclonal, skewing does not influence the interpretation of these data nor does it bias our conclusions. We show here that BCR/ABL−CD34+HLA-DR−cells are polyclonal even when recovered from steady-state marrow of patients whose MNC are 100% Ph+, BCR/ABL mRNA+, and monoclonal. In addition, in those patients in whom CD34+HLA-DR− or CD34+HLA-DR+ cells were monoclonal in steady-state marrow, polyclonal cell populations can be recovered at the time of cytogenetic remissions after in vivo chemotherapy mobilization. Finally, the rapid suppression of polyclonal progenitors by clonal precursor cells during PBPC harvesting in one patient confirms the coexistence of clonal and polyclonal progenitors. These data allow the conclusion that a polyclonal Ph−, BCR/ABL mRNA− population exists in the marrow and blood of some patients with CML. Therefore, such CD34+HLA-DR− cells should be suitable for autografting in CML.
ACKNOWLEDGMENT
The authors thank Scott Wissink, Brad Anderson, and Victor Van Duppen for excellent technical help.
M.D. is a Research Fellow of the Flander’s Fund for Scientific Research (FWO). C.M.V. is a Scholar of the Leukemia Society of America.
Supported in part by National Institutes of Health Grants No. PO1-CA-65493 and PO1-CA-21737, Leukemia Society of America Translational Research Grant No. 6377-96, and the University of Minnesota Hospitals and Clinics.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Michel Delforge, MD, Department of Hematology, University Hospital Gasthuisberg, Herestraat 49, B-3000 Leuven, Belgium; e-mail: Michel.Delforge@uz.kuleuven.ac.be.