Abstract
Polymorphonuclear leukocytes (PMNLs), nitric oxide (NO), calcium, and free radicals play an important role in hypoxia/ischemia and reoxygenation injury. In the present study, NO donors, sodium nitroprusside (SNP), and diethylamine-NO (DEA-NO) at low concentrations (10 and 100 nmol/L) potentiated, while higher (10 μmol/L to 10 mmol/L) concentrations inhibited free radical generation response in the rat PMNLs. Free radical generation response was found to be significantly augmented when hypoxic PMNLs were reoxygenated (hypoxia-reoxygenation [H-R]). This increase in free radical generation after reoxygenation or SNP (10 nmol/L) was blocked in the absence of extracellular calcium. SNP (10 nmol/L) or H-R–mediated increases in the free radical generation were prevented by the pretreatment of PMNLs with NO scavenger (hemoglobin), the polyadenine diphosphate (ADP)-ribosylation synthase inhibitor (benzamide) or the calcium channel antagonist (felodipine). A significant augmentation in the nitrite and intracellular calcium levels was observed during hypoxia. Hemoglobin pretreatment also blocked the increase in intracellular calcium levels due to SNP (10 nmol/L) or hypoxia. Thus, increased availability of NO during SNP treatment or H-R, may have led to an ADP-ribosylation–mediated increase in intracellular calcium, thereby increasing the free radical generation from the rat PMNLs.
THE ROLE OF polymorphonuclear leukocytes (PMNLs) in ischemia/hypoxia-reperfusion damage has been demonstrated in a variety of organs such as the heart, lungs, kidneys, brain, liver, and in striated muscles and the gastrointestinal tract.1-3Migration of PMNLs into the ischemic tissue as early as 10 minutes after ischemia and increases with time and reperfusion have been reported.4,5 Augmentation of reactive oxygen species (ROS) generation, chemotaxis, adherence, release of other cytotoxic substances, and upregulation of opsonic receptors in the activated PMNLs after reoxygenation has also been demonstrated.3 6
PMNLs, which play an important role in ischemic reoxygenation injury, synthesize nitric oxide (NO) in addition to ROS from L-arginine by the enzyme nitric oxide synthase (NOS).7 Peroxynitrite production in PMNLs has also been found to mediate cytotoxicity.7 By contrast, the involvement of NO in the inhibition and scavenging of free radicals has also been demonstrated.8 Recently, it has been shown that the low concentrations (nmol/L) of NO enhanced the free radical generation in PMNLs, while higher concentrations (μmol/L) inhibited it.9-11 NO in higher concentrations inhibits the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and scavenges free radicals.8-11 However, the mechanisms of the increase in ROS generation induced by NO are not clearly understood. Most of the biologic functions of NO are mediated by modification of the heme group, thiol groups, or by poly- adenine diphosphate (ADP)-ribosylation.12,13 NO in PMNLs has also been shown to cause poly ADP-ribosylation of various proteins and thus modulate enzyme activities.9
Protection against ischemia and reperfusion injury has been reported after pretreatment with NOS inhibitors, blockade of neutrophil adherence, or inhibition of free radicals.3,14,15 Recently Hewett et al16 have shown that the inducible NOS-mediated NO production in astrocytes potentiates N-methyl-D-aspartate (NMDA) receptor-dependent neuronal death after a hypoxic/ischemic insult. However, some reports also indicate a deleterious or negligible effect of NOS inhibitors.17 It is likely that the increased concentration of NO in ischemia/hypoxia due to decreased degradation18 or increased synthesis16 might modulate ROS generation from PMNLs during ischemia and reperfusion injury. Therefore, we investigated the involvement of NO in ROS generation from the normal and reoxygenated PMNLs after hypoxia.
MATERIALS AND METHODS
Materials.
Aminoguanidine, arachidonic acid (AA), catalase, dextran T-500, flavin adenine dinucleotide (FAD), hemoglobin (Hb), lactate dehydrogenase (LDH), luminol, lucigenin, Fura-2 AM, formyl-methionyl-leucyl-phenylalanine (FMLP), βNADPH-reduced form, N-(1 naphthyl) ethylene diamine, nitrate reductase, o-dianisidine, phorbol esters (phorbol myristate acetate [PMA]), phosphoric acid, sodium nitroprusside (SNP), sodium pyruvate, sulfanilamide, superoxide dismutase (SOD), zymosan particles, and Histopaque (1077 and 1119) were purchased from Sigma Chemical Co (St Louis, MO). Diethylamine nitric oxide complex sodium salt (DEA-NO), 7-nitroindazole, and benzamide were procured from Research Biochemicals International (Natick, MA). Felodipine was a gift from Astra IDL (Banglore, India). Sodium nitrite was obtained from Glaxo India (Bombay, India). All other chemicals used in the present study were of analytical grade (SRL, Bombay, India).
Preparation of the reagents.
Stock solutions of FMLP (100 μmol/L), PMA (1.625 mmol/L), felodipine (0.5 mmol/L), Fura-2 AM (5 mol/L) and luminol (100 mmol/L) were made in dimethyl sulfoxide (DMSO), while a stock solution of AA (5 × 10−2 mol/L) was prepared in alcohol and sodium carbonate; further dilutions were made in normal saline. Solutions of lucigenin (50 mmol/L) and aminoguanidine (0.5 mol/L) were prepared in water. Benzamide (0.2 mol/L) was dissolved in methanol. Opsonized zymosan (OZ) was made by treating washed zymosan particles with fresh autologous serum for 30 minutes at 37°C, then removing the serum by centrifugation. The particles were resuspended in Hanks’ Balanced Salt Solution (HBSS) and a stock solution of 12.5 mg/mL was stored in 100-μL aliquots at −70°C. Hemoglobin (0.5 mmol/L) solution was prepared in normal saline.
PMNLs isolation.
Rat blood PMNLs were obtained from male Sprague Dawley rats (130 to 150 g) by the Boyum method.19 Blood was collected by cardiac puncture in sodium citrate (0.129 mol/L, pH 6.5, 9:1, vol/vol) under ether anesthesia from normal animals. Platelet-rich plasma was removed by centrifugation at 250g for 20 minutes at 20°C (Sigma centrifuge, Osterode, Germany) and the buffy coat was subjected to dextran sedimentation as described previously.20 PMNLs were further purified on Histopaque-density gradient at 700g for 30 minutes at 25°C. The PMNL-rich layer was recovered at the interface of Histopaque 1119/1077 and was washed three times with HBSS (sodium chloride 138 mmol/L, potassium chloride 2.7 mmol/L, disodium hydrogen phosphate 8.1 mmol/L, potassium dihydrogen phosphate 1.5 mmol/L, magnesium chloride 0.6 mmol/L, calcium chloride 1.0 mmol/L, glucose 10 mmol/L, pH 7.4). The viability of the cells, tested by trypan blue exclusion, was never less than 95%. Before experiments, PMNLs were kept at 4°C for no more than 2 to 3 hours.
Estimation of LDH activity.
LDH activity was measured8 in the supernatant and in the PMNLs lysate after the disruption of PMNLs by sonication. The amount (%) of the enzyme released in each set was calculated relative to the total activity present in the supernatant and in the lysate.
Measurement of chemiluminescence response.
Free radical generation from PMNLs stimulated with various inducers such as AA (1 to 5 × 10−5 mol/L), FMLP (1 μmol/L), OZ (1.25 μg) or PMA (3 × 10−8mol/L) was measured at 37°C using a dual channel lumiaggregometer (Model 560; Chronolog Corp, Havertown, PA) with constant stirring at 900 rpm. Chemiluminescence is reported as units calculated from the maximum output (obtained from stimulated PMNLs) divided by the “gain setting” of the instrument.8 An assay mixture (1,000 μL) contained 1 to 5 × 106 PMNLs, 10 μmol/L luminol (LCL), or 50 μmol/L of lucigenin (LUCDCL), the test substance and FMLP (1 × 10−6 mol/L), AA (1 × 10−5 mol/L), OZ (1.25 μg) or PMA (3 × 10−8 mol/L).
The effect on the AA (1 × 10−5 mol/L)-induced chemiluminescence response of pretreatment with different concentrations of sodium nitroprusside (SNP, 10 nmol/L to 10 mmol/L), DEA-NO (10 nmol/L to 1 mmol/L), poly-ADP-ribosylation inhibitor (benzamide, 2 × 10−3 mol/L), hemoglobin (Hb, 5 or 10 μmol/L), calcium channel antagonist (felodipine, 5 × 10−6 mol/L), and NOS inhibitor (aminoguanidine, 5 × 10−3 mol/L) was studied. Chemiluminescence responses were also studied in the absence of extracellular calcium (a calcium-free medium).
Preparation of hypoxic and hypoxic-reoxygenated cells.
Rat PMNLs were subjected to hypoxic conditions by suspending the cells in HBSS, which was sponged with nitrogen gas for 30 minutes, and the partial pressure of oxygen was then measured to assess the oxygen depletion. Partial pressures of oxygen (pO2) and pH (7.3 ± 0.007) were measured in the cells suspended in HBSS before and after hypoxia (H) and reoxygenation (H-R) using a blood gas analyzer (Eschweiler System C 2000; Keil, Germany). PMNLs were incubated in the oxygen-depleted HBSS for 30 minutes at 37°C and pO2 was measured at the end of the incubation time (pO2 68.05 ± 3 mm Hg, n = 90). PMNLs were reoxygenated by suspending the cells in normoxic HBSS (pO2 120.9 ± 2.6 mm Hg, n = 90) after centrifugation, and pO2 was again measured (117.2 ± 4.2 mm Hg, n = 50). Viability of the cells was measured at the end of the experiment by a trypan blue exclusion test (96% ± 1.3%) and LDH release, which was 7.29% for normoxic cells, and was unchanged after hypoxia and hypoxia-reoxygenation.
Measurement of the myeloperoxidase (MPO) activity.
Experiments were performed to determine the AA (1 × 10−5 mol/L)-induced MPO release from PMNLs granules during H and H-R. Enzyme activity was estimated in the supernatant, and the remaining activity was estimated in the lysed PMNLs preparations by the methods described earlier.12 Briefly, 0.1 mL lysate or supernatant was incubated in a 2.5-mL phosphate buffer (pH 6.2) with 0.05% hydrogen peroxide and O-dianisidine (250 μg) at 37°C for 30 minutes. Reaction was stopped by adding sodium azide, and the absorbance of the solutions was read at 460 nm. The percentage of the enzyme released was calculated relative to the total activity (obtained in each set by adding the activities estimated in supernatant and in the lysate).
Nitrite measurement.
Nitrite contents in the normal control, H, and H-R PMNLs were measured by diazo formation.21 The cell suspensions (5 × 107 cells/mL) were incubated in normoxic, hypoxic, and reoxygenated conditions in the presence of superoxide dismutase and catalase (50 U/mL); the cells were then sonicated and supernatants were used for nitrite estimations. The supernatants were incubated with NADPH (40 μmol/L), FAD (4 μmol/L), and nitrate reductase (0.1 U) for 30 minutes at 37°C. The reaction mixture was then treated with lactate dehydrogenase (2 U/mL) and sodium pyruvate (5 μmol/L) and incubated for a further 20 minutes at 37°C. The color was developed by using Griess reagent, and absorbance was recorded at 548 nm after a 30-minute incubation at 37°C. The amount of nitrite was calculated from a standard curve of sodium nitrite.
Measurement of intracellular calcium levels.
The PMNLs suspended in calcium-free HBSS were loaded with Fura 2-AM (3 μmol/L) for 60 minutes at 4°C. After 60 minutes, the cells were diluted twofold with calcium-free HBSS and centrifuged, resuspended in normoxic or hypoxic HBSS, and incubated at 37°C for 30 minutes. [Ca+2]i was estimated by using the method of Grynkiewiez et al.22
Calculations and statistical analysis.
Each experimental group consisted of at least four or five sets of experiments. Results were expressed as the mean ± standard error (SE) for separate experiments, and comparisons were made by paired Student’s t-test or by one way analysis of variance for multiple comparisons of three or more groups. When F was significant, the differences between individual groups were compared witht-test results. The differences were considered to be statistically significant when P value was less than .05.
RESULTS
Effect of hypoxia-reoxygenation on the rat PMNLs chemiluminescence response induced by various agents.
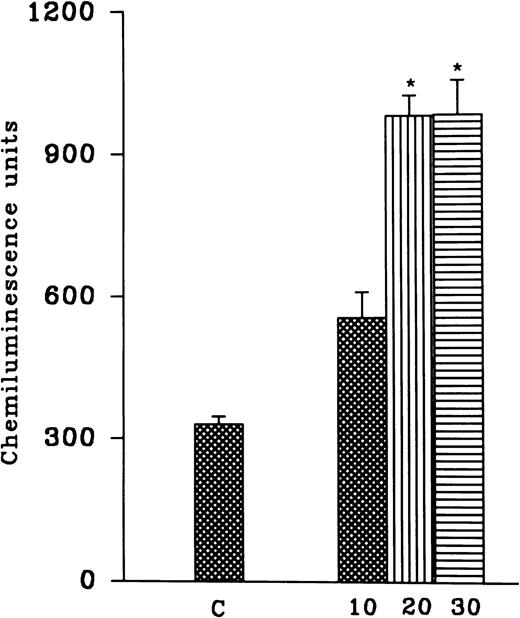
PMNLs were subjected to hypoxia for variable time intervals (10, 20, and 30 minutes) and then reoxygenated for 30 minutes. There was an increase in the AA-induced free radical generation after reoxygenation and no significant difference between hypoxia for 20 and 30 minutes was observed (Fig 1). In another set of experiments, cells were subjected to 10-, 20-, and 30-minute periods of reoxygenation after 15 and 30 minutes of hypoxia (Fig 1). Although 10 minutes of hypoxia followed by 30 minutes of reoxygenation was sufficient to induce an increase in free radical generation, we observed a significant and consistent increase at 30 minutes of hypoxia and 30 minutes of reoxygenation. Therefore, in all of the subsequent studies, 30 minutes of hypoxia followed by 30 minutes of reoxygenation was used (Fig 2A). A significant increase in the luminol, as well as the lucigenin-dependent chemiluminescence response induced by FMLP, OZ, or PMA, was also observed after reoxygenation (30 minutes) of the hypoxic (30 minutes) rat PMNLs (Table 1). AA-induced MPO release from the normoxic cells was 24% ± 6%, which was not altered after hypoxia (30% ± 5%) or H-R (31% ± 6%).
(Top) Effect of hypoxia for different time intervals (10, 20, and 30 minutes) and reoxygenation (30 minutes) on free radical generation. (Bottom) Effect of variable time of reoxygenation (10, 20, and 30 minutes) on hypoxic cells. Cells were subjected to hypoxia for 15 (solid bars) or 30 (empty bars) minutes. Free radical generation response was induced by AA (1 to 5 × 10−5 mol/L) after H-R. *P < .01 in comparison with the control.
(Top) Effect of hypoxia for different time intervals (10, 20, and 30 minutes) and reoxygenation (30 minutes) on free radical generation. (Bottom) Effect of variable time of reoxygenation (10, 20, and 30 minutes) on hypoxic cells. Cells were subjected to hypoxia for 15 (solid bars) or 30 (empty bars) minutes. Free radical generation response was induced by AA (1 to 5 × 10−5 mol/L) after H-R. *P < .01 in comparison with the control.
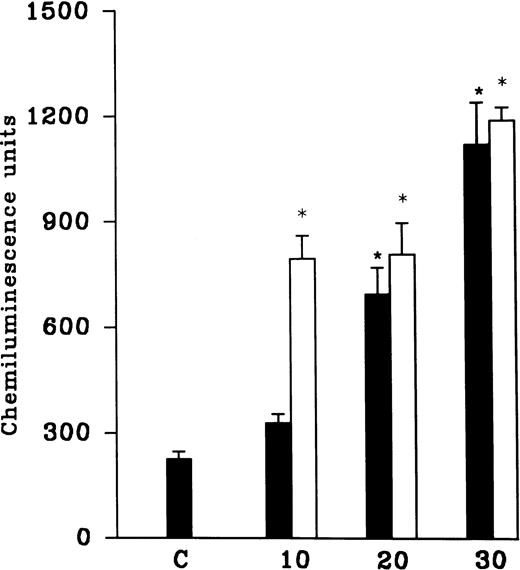
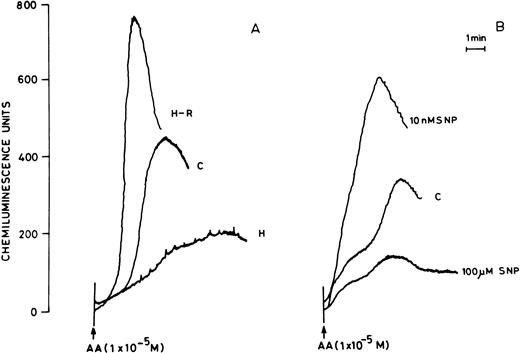
(A) AA-induced chemiluminescence response in normoxic (C), hypoxic (H), and reoxygenated (H-R) PMNLs (5 × 106cells). (B) AA-induced chemiluminescence response in SNP pretreated cells. SNP was used at 10 nmol/L and 100 μmol/L concentrations.
(A) AA-induced chemiluminescence response in normoxic (C), hypoxic (H), and reoxygenated (H-R) PMNLs (5 × 106cells). (B) AA-induced chemiluminescence response in SNP pretreated cells. SNP was used at 10 nmol/L and 100 μmol/L concentrations.
Effect of NO donors on AA induced chemiluminescence response of rat PMNLs.
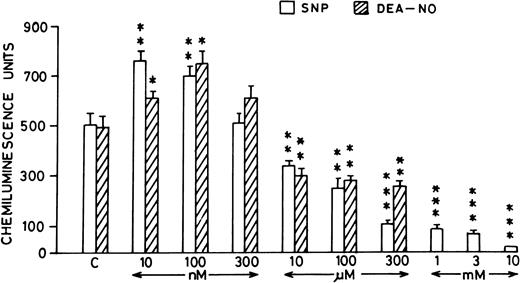
Pretreatment of rat peripheral PMNLs with SNP or DEA-NO for 10 to 20 minutes increased the AA-induced chemiluminescence response at 10 and 100 nmol/L concentrations (Figs 2B and 3). However, higher concentrations (10 μmol/L to 10 mmol/L) reduced the LCL response significantly (Figs 2B and 3).
Histogram showing effect of SNP (open bars) and DEA-NO (hatched bars) at different concentrations (10 nmol/L to 10 mmol/L) on the AA-induced PMNLs chemiluminescence response. *P < .05, **P < .01, and ***P < .001 in comparison with the control.
Histogram showing effect of SNP (open bars) and DEA-NO (hatched bars) at different concentrations (10 nmol/L to 10 mmol/L) on the AA-induced PMNLs chemiluminescence response. *P < .05, **P < .01, and ***P < .001 in comparison with the control.
Modulation of chemiluminescence response in the SNP pretreated cells by intracellular and extracellular calcium.
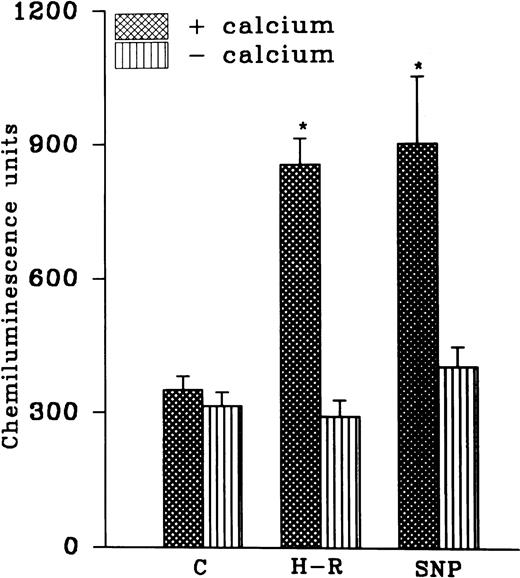
While AA-induced LCL response in the normoxic cells was not altered in the absence of extracellular calcium, potentiation in the AA-induced LCL response after H-R was not observed in the absence of extracellular calcium (Fig 4). Interestingly, augmentation in the AA-induced LCL response in the presence of the NO donor SNP (10 nmol/L) was also blocked when extracellular calcium was absent (Fig 4).
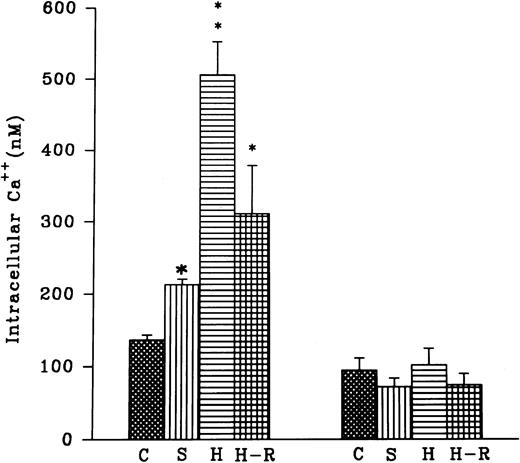
(Top) AA (1 × 10−5 mol/L) stimulated chemiluminescence response in normoxic, H-R, or SNP (10 nmol/L) pretreated cells in the presence and absence of calcium. (Bottom) Intracellular calcium concentration in the normoxic (C), SNP (S, 10 nmol/L) pretreated, hypoxic (H), and reoxygenated (H-R) PMNLs. Alteration in the cell calcium levels was estimated in absence of Hb (left set) and in the presence of Hb (10 μmol/L, right set). *P < .05 and **P < .01 in comparison with the control.
(Top) AA (1 × 10−5 mol/L) stimulated chemiluminescence response in normoxic, H-R, or SNP (10 nmol/L) pretreated cells in the presence and absence of calcium. (Bottom) Intracellular calcium concentration in the normoxic (C), SNP (S, 10 nmol/L) pretreated, hypoxic (H), and reoxygenated (H-R) PMNLs. Alteration in the cell calcium levels was estimated in absence of Hb (left set) and in the presence of Hb (10 μmol/L, right set). *P < .05 and **P < .01 in comparison with the control.
Effect of SNP pretreatment, hypoxia, or H-R on the intracellular calcium levels.
Intracellular calcium levels were found to be augmented after the addition of 10 nmol/L SNP to the rat PMNLs (Fig 4). A significant increase in intracellular calcium levels was also observed after hypoxia, and this increase did not revert back to the normal control level after reoxygenation (Fig 4). In the presence of hemoglobin (5 μmol/L) an increase in the intracellular calcium levels after SNP pretreatment or hypoxia was blocked (Fig 4). However, Hb had no significant effect on the basal calcium levels in the normoxic cells (Fig 4).
Effect of hypoxia and H-R on the PMNLs nitrite content.
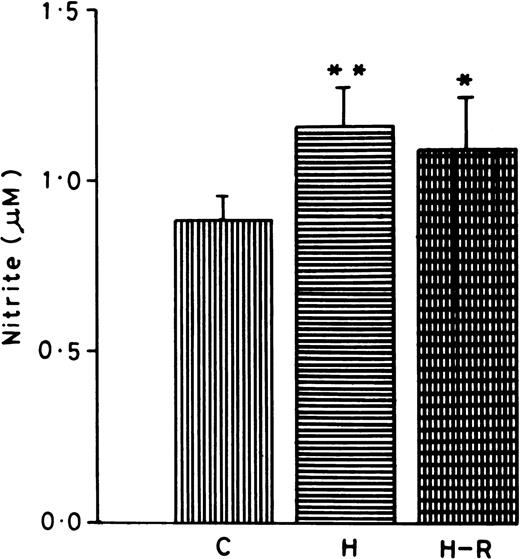
Nitrite content was measured in the normoxic, H, and H-R PMNLs cell suspensions. PMNLs nitrite concentrations (1.15 ± 0.07 μmol/L/5 × 107 cells) after hypoxia were found to be significantly augmented (42% ± 5%, P < .01) in comparison to the control (Fig 5). While AA stimulation also increased the nitrite content in the PMNLs, there was no significant difference in normoxic versus hypoxic or H-R cells (data not shown).
Nitrite content in normoxic (C), hypoxic (H), and reoxygenated (H-R) cell suspensions. Nitrite was estimated in 5 × 107 cells after a 30-minute incubation at 37°C. *P < .01 in comparison with the control.
Nitrite content in normoxic (C), hypoxic (H), and reoxygenated (H-R) cell suspensions. Nitrite was estimated in 5 × 107 cells after a 30-minute incubation at 37°C. *P < .01 in comparison with the control.
Effect of hemoglobin, benzamide, and felodipine pretreatment on SNP or H-R induced increases in the chemiluminescence response.
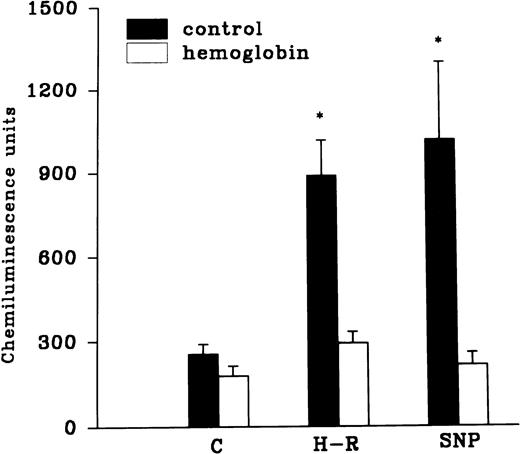
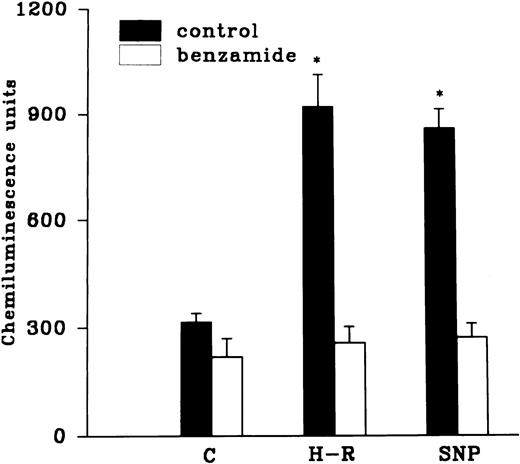
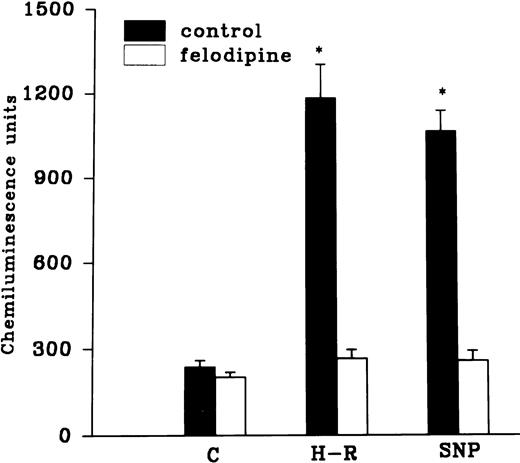
The H-R or SNP-induced potentiation of the AA-induced PMNLs free radical generation response was prevented by the addition of Hb to the hypoxic cells or by the pretreatment of cells with benzamide or felodipine (Figs 6 and7).
(Top) Effect of Hb (10 μmol/L) pretreatment on the chemiluminescence response after H-R and SNP (10 nmol/L) pretreatment. (Bottom) Histogram representing effect of benzamide (2 × 10−3 mol/L) on the AA-induced chemiluminescence response of H-R and SNP pretreated PMNLs. *P < .01 in comparison with the control.
(Top) Effect of Hb (10 μmol/L) pretreatment on the chemiluminescence response after H-R and SNP (10 nmol/L) pretreatment. (Bottom) Histogram representing effect of benzamide (2 × 10−3 mol/L) on the AA-induced chemiluminescence response of H-R and SNP pretreated PMNLs. *P < .01 in comparison with the control.
Effect of felodipine (5 × 10−6 mol/L; top) or aminoguanidine (5 × 10−3 mol/L; bottom) on the H-R– or SNP-mediated increases in the free radical generation. *P < .01 in comparison with the control.
Effect of felodipine (5 × 10−6 mol/L; top) or aminoguanidine (5 × 10−3 mol/L; bottom) on the H-R– or SNP-mediated increases in the free radical generation. *P < .01 in comparison with the control.
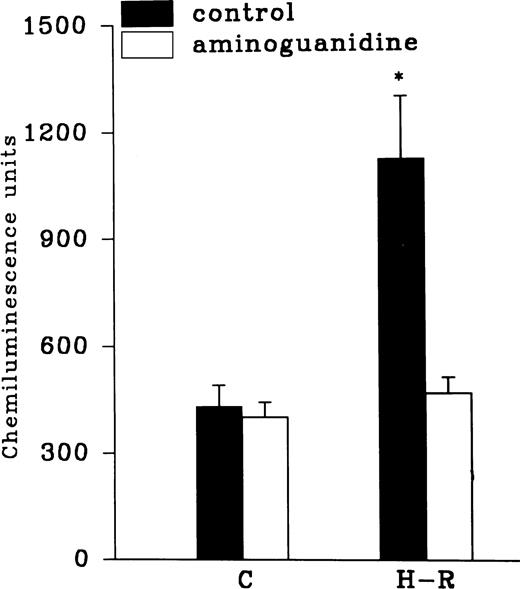
Effect of NOS inhibitors on the chemiluminescence response after H-R.
Aminoguanidine pretreatment prevented the enhancing effect of H-R on the AA-induced chemiluminescence response (Fig 7). Another NOS inhibitor, 7-nitroindazole (1 × 10−3 mol/L), was also used to assess the effect of NOS inhibition on H-R–mediated augmentation of OZ-induced chemiluminescence response (Table 1). OZ-induced LCL responses in normoxic and H-R PMNLs in the presence of 7-nitroindazole were 639.36 ± 93.47 and 671.56 ± 70.03 (n = 20) chemiluminescence units, respectively.
DISCUSSION
The results obtained in the present investigation suggest that NO donors had a biphasic effect on the rat PMNLs free radical generation response. Low concentrations (10 and 100 nmol/L) potentiated, but higher concentrations (10 μmol/L to 10 mmol/L) attenuated ROS generation from the rat PMNLs. We postulate that H-R increases the NO level causing ADP-ribosylation and increases the intracellular calcium level, which potentiates PMNLs free radical generation.
Rubanyi et al23 have demonstrated the scavenging role of NO on the PMNLs superoxide radicals. Other investigators have shown that augmentation in the exogenous or endogenous NO inhibits NADPH-oxidase activity and scavenges free radicals.8,24,25 An increase in the intracellular calcium levels in a guanosine 3′-5′ cyclic monophosphate (cGMP) independent manner by SNP in platelets, endothelial cells, and PMNLs has been observed.11,26,27 Consistent with these results, we also observed an increase in the intracellular calcium levels along with augmented chemiluminescence response in the presence of SNP at 10 nmol/L concentration. In contrast, higher concentrations (10 μmol/L to 10 mmol/L) inhibited free radical generation8 (Figs 2and 3). We also observed that benzamide, an ADP ribosylation inhibitor,12,28 and felodipine, an inhibitor of calcium channels on the PMNLs,29 inhibited SNP-mediated augmentation in the chemiluminescence response. SNP did not augment ROS generation in the calcium-deficient medium. Bromo cGMP (a cell permeable analogue of cGMP) did not modify the free radical generation response (data not shown). Thus, it appears that NO donors (SNP and DEA-NO), possibly by ADP-ribosylation–mediated mechanisms, raised the intracellular calcium, which augments ROS generation in the rat PMNLs.
Studies on inflammation, ischemia, and reperfusion have recognized PMNLs as the principal participant and effector cells in acute inflammatory conditions.1,2 The availability of NO during hypoxia/ischemia has been found to be augmented.16,18 To delineate the biological importance and relevance of NO-mediated increases in free radical generation, we investigated the role of NO in H-R–mediated increases in free radical generation response. Reoxygenation of PMNLs and endothelial cells in vitro after brief periods of hypoxia has been shown to augment free radical generation,6,8 a result replicated in the present study. We have found that nitrite contents in the hypoxic cell suspensions paralleled the concentrations of NO donors used, which also potentiated free radical generation response in the normal PMNLs. When the cells were incubated in the presence of Hb, a NO scavenger,30during hypoxia and reoxygenation, there was no increase in the free radical generation, suggesting involvement of NO. During hypoxia there was a significant increase in the intracellular calcium levels, which did not revert back to the control levels after reoxygenation. In the cerebellar granule cells, it was reported that hypoxia-induced elevation of the intracellular calcium was greatly attenuated by NOS inhibition.31 Interestingly, in the presence of the NO scavenger, Hb, we observed no increase in the intracellular calcium in the PMNLs subjected to H-R. Hb did not affect the basal calcium levels in the control cells (Fig 4). H-R–mediated augmentation in ROS generation was also totally prevented by pretreatment of the cells with a calcium antagonist, felodipine. Furthermore, the basal free radical generation response was found to be independent of the presence of extracellular calcium in the normal PMNLs. However, H-R–mediated potentiation in free radical generation response was totally dependent on the presence of extracellular calcium (Fig 4), thereby suggesting that NO mediates an increase in the intracellular calcium during H-R, which augments the ROS generation.
A significant reduction in the cerebral infarct size in nNOS (neuronal NOS) knock-out mice32 and augmentation in NO synthesis due to the induction of NOS after hypoxia/ischemia has been shown.18,33 It is interesting to observe that NOS inhibition in the nNOS containing cells protects against ischemia/hypoxia and reoxygenation injury.28,32 NOS in the PMNLs resembles nNOS, as shown by specific antibodies and DNA probes.34,35 However, it has not yet been demonstrated that there is any interrelationship between NO and calcium in the augmentation of free radical generation response after H-R in the PMNLs. Recently Volk et al36 have shown in human endothelial cells that the blockade of the receptor-mediated calcium entry or prevention of intracellular calcium release inhibits NO-induced interleukin-8 secretion, suggesting an interrelationship between NO and calcium. Our work shows for the first time that the NO-mediated increase in the intracellular calcium in the hypoxic cells is responsible for the increase in free radical generation after reoxygenation.
Previous research in our laboratory demonstrated the nonspecific interference of L-nitro arginine analogues with the chemiluminescence response.37 Chen and Mehta34 have also shown that L-arginine analogues do not inhibit PMNLs NOS activity. Therefore, we used 7-nitroindazole (an inhibitor of nNOS28) and aminoguanidine (a NOS inhibitor38), which inhibited PMNLs NOS (data not shown) and prevented H-R–mediated increases in the PMNLs free radical generation. Thus, inhibition of NO synthesis in PMNLs was protective against H-R–mediated increases in the free radical generation, indicating the involvement of NO in this response.
In addition, the role of granular MPO18 release in the augmentation of chemiluminescence response was also assessed. However, no significant change in MPO release after AA stimulation from normoxic versus H-R cells was observed. This indicates that the increase in ROS generation after H-R is not mediated by MPO. Furthermore, H-R–mediated augmentation in ROS generation was also prevented by the pretreatment of the cells with an ADP ribosylation inhibitor, benzamide.
Recently Thiemermann et al28 reported that ADP-ribosylation inhibitors reduced the infarct size caused by ischemia and reperfusion of rabbit myocardium and skeletal muscles. In the skeletal muscles, inhibition of nNOS by 7-nitroindazole also decreased the infarct size. However, they did not investigate the involvement of calcium in the ADP-ribosylation–mediated injury.28 NO-mediated ADP ribosylation of G protein has been demonstrated to regulate the intracellular second messenger level in the bovine ciliary body.39 Regulation of the mitochondrial calcium homeostasis in the isolated hepatocyte has also been shown to be controlled by the protein ADP-ribosylation.40 41 In the present study, we have achieved the blockade of H-R–mediated increase in the ROS generation by the calcium antagonist, NOS, and ADP-ribosylation inhibitors. It, therefore, appears that during H-R, NO induces ADP-ribosylation, which increased the intracellular calcium, which in turn, increases the ROS generation. The role of these mechanisms in hypoxia/ischemic injury to various organs needs further examination.
ACKNOWLEDGMENT
We are grateful to S.K. Mandal for statistical analysis of the data.
This is CDRI Communication No. 5742.
Supported by a financial grant to M.D. from the Department of Science and Technology, New Delhi, India, and an award of research fellowship to M.P.S. from the Council of Scientific and Industrial Research New Delhi, India.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Madhu Dikshit, PhD, Pharmacology Division, Central Drug Research Institute, Lucknow 226 001, India; e-mail: root@csdri.ren.nic.in.