Abstract
To further examine the potential clinical usefulness of the hexadentate phenolic aminocarboxylate iron chelatorN,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid (HBED) for the chronic treatment of transfusional iron overload, we performed a subchronic toxicity study of the HBED monosodium salt in rodents and have evaluated the iron excretion in primates induced by HBED. The HBED-induced iron excretion was determined for the monohydrochloride dihydrate that was first dissolved in a 0.1-mmol/L sodium phosphate buffer at pH 7.6 and administered to the primates either orally (PO) at a dose of 324 μmol/kg (149.3 mg/kg, n = 5), subcutaneously (sc) at a dose of 81 μmol/kg (37.3 mg/kg, n = 5), sc at 324 μmol/kg (n = 5), and sc at 162 μmol/kg (74.7 mg/kg) for 2 consecutive days for a total dose of 324 μmol/kg (n = 3). In addition, the monosodium salt of HBED in saline was administered to the monkeys sc at a single dose of 150 μmol/kg (64.9 mg/kg, n = 5) or at a dose of 75 μmol/kg every other day for three doses, for a total dose of 225 μmol/kg (n = 4). For comparative purposes, we have also administered deferoxamine (DFO) PO and sc in aqueous solution at a dose of 300 μmol/kg (200 mg/kg). In the iron-loadedCebus apella monkey, whereas the PO administration of DFO or HBED even at a dose of 300 to 324 μmol/kg was ineffective, the sc injection of HBED in buffer or its monosodium salt, 75 to 324 μmol/kg, produced a net iron excretion that was nearly three times that observed after similar doses of sc DFO. In patients with transfusional iron overload, sc injections of HBED may provide a much needed alternative to the use of prolonged parenteral infusions of DFO. Note: After the publication of our previous paper (Blood, 91:1446, 1998) and the completion of the studies described here, it was discovered that the HBED obtained from Strem Chemical Co (Newburyport, MA) that was labeled and sold as a dihydrochloride dihydrate was in fact the monohydrochloride dihydrate. Therefore, the actual administered doses were 81, 162, or 324 μmol/kg; not 75, 150, or 300 μmol/kg as was previously reported. The new data have been recalculated accordingly, and the data from our earlier study, corrected where applicable, are shown in parentheses.
BECAUSE THERE IS NO mechanism for the excretion of iron, patients with transfusion-dependent chronic hemolytic anemias will ultimately develop toxic iron overload, and without adequate chelation therapy, will frequently die in their second decade.1-3 For the past 30 years, deferoxamine B (DFO), produced in large-scale fermentation by a strain of Streptomyces pilosus,4 has been regarded as the drug of choice for the treatment of transfusional iron overload. DFO mesylate (Fig 1) has been shown to be a generally safe and efficacious means of controlling body iron that can prolong survival and prevent or ameliorate organ dysfunction.3,5-9Although the drug’s efficacy and long-term tolerability have been well documented, there are still problems associated with treatment with DFO. Because DFO is poorly absorbed from the gastrointestinal tract and rapidly eliminated from the circulation, prolonged parenteral infusion is needed; effective therapy usually requires subcutaneous (sc) or intravenous administration by a portable infusion pump for 9 to 12 hours daily.10-12
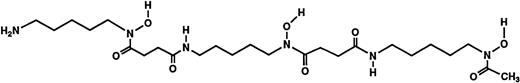
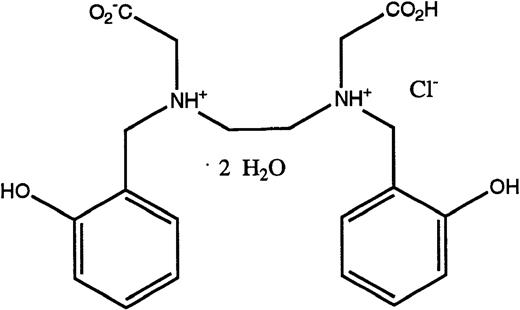
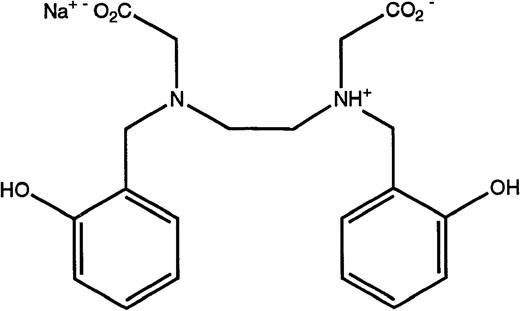
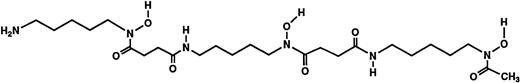
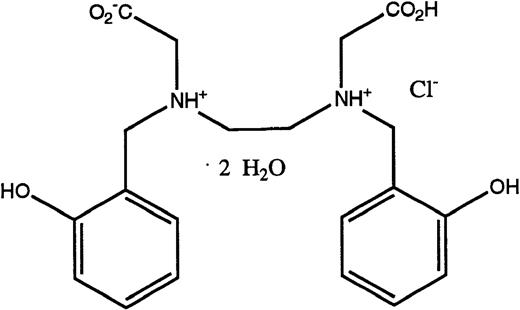
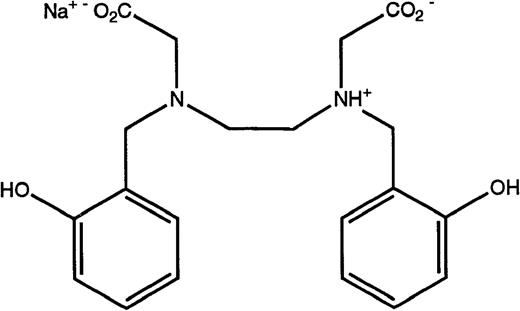
Structures of the iron chelators chosen for evaluation: DFO (top), HBED monohydrochloride dihydrate (middle), and HBED monosodium salt (bottom).
Structures of the iron chelators chosen for evaluation: DFO (top), HBED monohydrochloride dihydrate (middle), and HBED monosodium salt (bottom).
Although the experience with DFO has shown that adequate control of body iron can avert complications of transfusional iron overload, drug-related toxicities13-19 and patient compliance have prompted an ongoing search for safe, inexpensive alternatives to DFO. To date, most efforts to develop substitutes for DFO for iron-chelating therapy have concentrated on bi- or tridentate iron-chelating agents that remain active after oral administration.20,21 However, because of concerns that these partial ligands might exacerbate iron toxicity and the realization that oral formulations are likely to require taking a large number of tablets on multiple occasions each day, we have examined the possibility of the administration of a hexadentate iron chelator,N,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid22 (HBED) by sc injection. HBED is a synthetic hexadentate ligand that, like DFO, forms a 1:1 complex with iron with high affinity and selectivity. Because HBED is a synthetic product, problems of local reactions to potential fermentation by-products not removed during purification would be avoided. For patients allergic to DFO,23-25 HBED, a member of a different family of chelators, would be unlikely to provoke a similar response.
In a recently published study,26 we showed that HBED administered sc to iron-loaded monkeys consistently induced iron clearance that was two to three times greater than that induced by a similar dose of DFO. No adverse effects of chelator administration were noted in the monkeys; all hematologic and biochemical tests remained within normal ranges. On the basis of those findings, we have now expanded the preclinical investigation of the compound in both rodent and primate models. In the studies described here, we have performed a 2-week toxicity study in rodents and have assessed the iron excretion induced by additional dosages and formulations of both orally (PO) and sc administered HBED to monkeys. Based on the data available thus far, sc HBED has emerged as a strong candidate for a much needed alternative to DFO and could potentially provide patients with a clinically effective form of iron-chelating therapy.
MATERIALS AND METHODS
Materials.
DFO in the form of the methanesulfonate salt, Desferal, was obtained from Ciba-Geigy Ltd (Basel, Switzerland). HBED monohydrochloride dihydrate (Fig 1) was obtained from Strem Chemical Co (Newburyport, MA). Conversion to its monosodium salt (Fig 1) was performed by SRI International (Menlo Park, CA). Sprague-Dawley rats (Crl:CD[SD]BR-CD) were purchased from Charles River (Wilmington, MA). Cebus apellamonkeys were obtained from World Wide Primates (Miami, FL). All reagents and standard iron solutions were obtained from Aldrich Chemical Co (Milwaukee, WI). Atomic absorption (AA) measurements were made on a Perkin-Elmer model 5100 PC (Norwalk, CT). Ultrapure salts were obtained from Johnson Matthey Electronics (Royston, UK). All hematologic and biochemical studies27 were performed by Antech Diagnostics (Tampa, FL). Histological evaluation of necropsy tissues was performed by Florida Animal Resources (Ocala, FL).
Rodent toxicity studies.
Male Sprague-Dawley rats averaging 450 g were housed in polycarbonate cages with Beta-chips (Northeastern Products Corp, Warrensburg, NY) provided as bedding. Before the first drug administration, the rats were weighed and evaluated for their general condition. To allow for better visualization of the injection sites, the dorsal region of each rat was shaved free of hair. The injection sites were rotated and were closely monitored for any signs of inflammation or irritation. The HBED monosodium salt was put into solution with sterile normal saline and filtered via a 0.2-μ syringe filter. The drug was administered at a concentration of 60 mg/mL, and the solution had an unadjusted pH of 7.3. The rats (n = 5/group) were administered the HBED at a dose of 75, 150, or 300 μmol/kg (32.4, 64.9, or 129.7 mg/kg, respectively) as a single sc injection every other day for 14 days (7 doses). Control rats were administered saline by sc injection. Before each drug administration, the rats were weighed and evaluated for their general condition. Food and water intake were determined, and a necropsy was performed 1 to 2 days after the last dose of the drug had been administered. Extensive tissues, obtained from all animals, included the following: adrenal gland, aorta, bone marrow, brain, eye, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, testicle, heart, kidney, liver, lung, mesenteric lymph node, pancreas, prostate, mandibular salivary gland, skeletal muscle, spleen, thymus, thyroid, trachea, bladder, and skin from injection and noninjection sites. The tissues from the control animals and the high-dose (300 μmol/kg) group were examined by an outside pathologist.
Iron loading of C apella monkeys.
The monkeys were iron overloaded as previously described to provide about 500 mg iron/kg body weight.28 After administration of iron dextran, the serum transferrin iron saturation rose to between 70% and 80%. The serum half-life of iron dextran in humans is 2.5 to 3.0 days.29 We waited at least 20 half-lives, 60 days, before using any of the animals in experiments evaluating iron-chelating agents.
Iron-balance studies in C apella monkeys.
Seven days before the administration of the drug, the animals were placed in metabolic cages30 and started on a low-iron liquid diet.27 The monkeys were maintained on the low-iron liquid diet for the duration of the experiment. They were administered food according to their body weight, and intake was very carefully monitored.
The total amount of iron intake was compared with the total amount of iron excreted. Net iron balance = dietary iron intake − (urinary + fecal iron excretion); animals in a negative iron balance are excreting more iron than they are absorbing.
Primate fecal and urine samples.
Fecal and urine samples were collected at 24-hour intervals. The collections began 4 days before the administration of the test drug and continued for an additional 5 to 7 days after the first dose of the drug was administered. Fecal samples were assayed for the presence of occult blood, weighed, and mixed with distilled deionized water and autoclaved for 30 minutes. The mixture was then freeze-dried, and a known portion of the powder was mixed with low-iron nitric acid and refluxed for 48 hours. Once any particulate matter in the digested samples was removed by centrifugation, iron concentrations were determined by flame AA. Monkey urine samples were acidified and reconstituted to initial volume after sterilization, if necessary.
Drug preparation and administration.
Rodents were administered the HBED monosodium salt in saline at a concentration of 60 mg/mL. The rats (n = 5/group) were administered the HBED at a dose of 75, 150, or 300 μmol/kg (32.4, 64.9, or 129.7 mg/kg, respectively) as a single sc injection every other day for 14 days (seven doses). In the primates, DFO was administered PO or sc in sterile water for injection at a dose of 300 μmol/kg (129.7 mg/kg). The HBED monohydrochloride dihydrate was first dissolved in a phosphate buffer and administered (1) PO at a dose of 324 μmol/kg (149.3 mg/kg, n = 5), (2) sc at a dose of 81 μmol/kg (37.3 mg/kg, n = 5), (3) sc at 162 μmol/kg (74.7 mg/kg) for 2 consecutive days for a total dose of 324 μmol/kg (n = 3), and (4) 324 μmol/kg as a single sc injection (149.3 mg/kg, n = 5). In addition, the monosodium salt of HBED in saline was administered to the monkeys sc at a single dose of 150 μmol/kg (64.9 mg/kg, n = 5) or at a dose of 75 μmol/kg (32.4 mg/kg) every other day for three doses, for a total dose of 225 μmol/kg (n = 4). Before each drug administration the monkeys were anesthetized with Telazol (Elkins-Sinn, Inc, Cherry Hill, NJ), 0.03 mg/kg intramuscularly (IM), and given a single injection of atropine, 0.1 mg/kg IM.
Calculation of iron chelator efficiency.
The efficiency of each chelator was calculated on the basis of a 1:1 ligand-iron complex. For animals administered a single dose, the numbers were generated by averaging the iron output for 4 days before the administration of the drug, subtracting these numbers from the 2-day iron clearance after the administration of the drug, and then dividing by the theoretical output; the result is expressed as a percentage. If two or more doses were administered, the efficiency was calculated by averaging the iron output for 4 days before the administration of the drug, subtracting these numbers from the daily iron clearance after the administration of the drug, and then dividing by the theoretical output; the result is expressed as a percentage.
Statistical analysis.
Data are presented as the mean ± standard error of the mean. For comparisons of the means of two groups, the two-sample t-test (without the assumption of equality of variances) was used for analyzing the primate data. All tests were two-tailed, and a significance level of P < .05 was used.
RESULTS
Rodent drug toxicity.
A subchronic toxicity study of the monosodium salt of HBED was performed in normal rodents. The animals were administered the drug as a single sc injection at a dose of 75, 150, or 300 μmol/kg every other day for 14 days (seven doses). Control animals were administered sc injections of saline. At the doses investigated, no toxicity was noted in any of the animals. All the animals ate and drank well and gained weight at a rate that was indistinguishable from the control animals. In addition, no erythema was noted at any of the injection sites, either grossly or histologically. At necropsy, all tissues (see above list) appeared grossly normal; histological evaluation of tissues from HBED 300 μmol/kg-treated versus control animals showed no drug-related abnormalities.
Chelator-induced iron excretion in C apella monkeys.
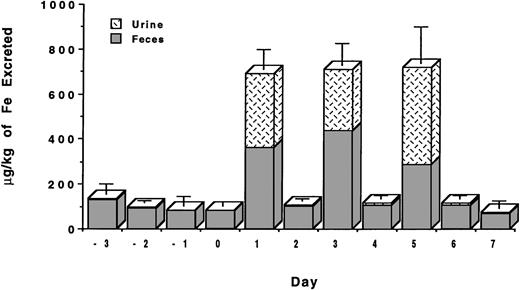
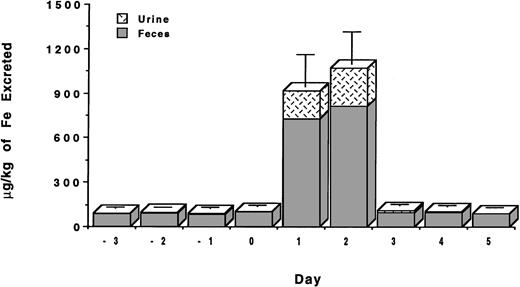
The studies were conducted with C apella monkeys who had been administered intravenous iron dextran to provide about 500 mg iron/kg body weight.28 We recently reported that DFO administered sc in aqueous solution at a dose of 150 μmol/kg was found to have an efficiency of 5.1% ± 1.3% and induced the excretion of 435 ± 115 μg iron/kg body weight.26 About 65% of the chelator-induced iron excretion was in the stool and about 35% in the urine. For comparative purposes, we have now also administered DFO in aqueous solution both PO and sc at a dose of 300 μmol/kg (Table 1). When DFO is administered PO at 300 μmol/kg, very little iron is excreted in either the urine or the feces (Fig 2). The efficiency of the drug by this route is only 0.1% ± 0.4%. However, when the drug is administered sc at 300 μmol/kg, it induced the excretion of 716 ± 244 μg/kg of iron (Fig 2) and had an efficiency of 4.2% ± 1.4%. The majority of iron, 60%, was excreted in the feces, and about 40% was excreted in the urine.
Mean net iron excretion in C apella monkeys with iron overload (see text) after administration of DFO or HBED. The primates were administered (A) DFO 300 μmol/kg PO, (B) HBED 324 μmol/kg PO, (C) DFO 150 μmol/kg sc, (D) HBED 150 μmol/kg sc, (E) DFO 300 μmol/kg sc, (F) HBED 324 μmol/kg sc, (G) HBED 81 μmol/kg sc, (H) HBED 75 μmol/kg sc for three doses (225 μmol/kg total), and (I) HBED 162 μmol/kg sc for two doses (324 μmol/kg total). Animals in groups B, F, G, and I received the HBED monohydrochloride dihydrate in 0.1 mmol/L sodium phosphate buffer at pH 7.6, whereas those in groups D and H were administered the HBED monosodium salt in saline at pH 7.3. Excretion is shown as μg iron/kg body weight on the scale of the left vertical axis. For comparative purposes, the result of our previously published study26 of the sc administration of DFO 150 μmol/kg to C apella monkeys with a similar magnitude of iron overload is shown and is indicated by an asterisk.
Mean net iron excretion in C apella monkeys with iron overload (see text) after administration of DFO or HBED. The primates were administered (A) DFO 300 μmol/kg PO, (B) HBED 324 μmol/kg PO, (C) DFO 150 μmol/kg sc, (D) HBED 150 μmol/kg sc, (E) DFO 300 μmol/kg sc, (F) HBED 324 μmol/kg sc, (G) HBED 81 μmol/kg sc, (H) HBED 75 μmol/kg sc for three doses (225 μmol/kg total), and (I) HBED 162 μmol/kg sc for two doses (324 μmol/kg total). Animals in groups B, F, G, and I received the HBED monohydrochloride dihydrate in 0.1 mmol/L sodium phosphate buffer at pH 7.6, whereas those in groups D and H were administered the HBED monosodium salt in saline at pH 7.3. Excretion is shown as μg iron/kg body weight on the scale of the left vertical axis. For comparative purposes, the result of our previously published study26 of the sc administration of DFO 150 μmol/kg to C apella monkeys with a similar magnitude of iron overload is shown and is indicated by an asterisk.
Because of its poor solubility, the HBED monohydrochloride dihydrate was first dissolved in a 0.1-mmol/L sodium phosphate buffer adjusted to pH 7.6 (no Cremophor vehicle was used) and was administered to the primates as described in Materials and Methods. Although HBED administered PO at a dose of 150 μmol/kg was ineffective, only 0.5% ± 0.5% efficient,31 we had hoped that increasing the dose to 324 μmol/kg would result in the excretion of a clinically beneficial amount of iron. Unfortunately, very little iron was excreted (Fig 2); the efficiency of the drug at this dose was only 0.3% ± 0.3%. However, the sc injection of HBED in a buffer at a dose of 81 μmol/kg did result in the excretion of a significant amount of iron. In this case, the drug induced the excretion of 608 ± 175 μg/kg of iron (Fig 2) and had an efficiency of 13.0% ± 4.6% (Table 1). This is very similar to what was observed when HBED-Cremophor was administered sc to the primates at this same dose26; HBED-Cremophor induced the clearance of 793 ± 410 μg/kg of iron and had an efficiency of 18.4% ± 9.1% (17.5% ± 9.1%) (P > .3). In both experiments, the majority of the iron was excreted in the feces—78% when the drug was administered in buffer and 92% when it was administered in Cremophor.
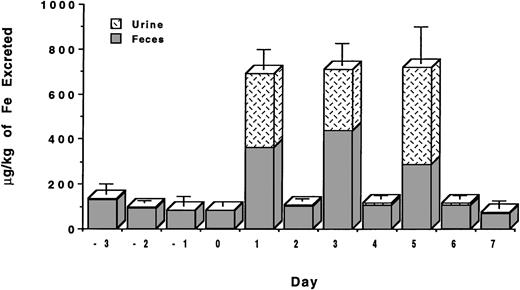
To more closely mimic potential clinical applications, we have also performed a study in which the HBED monohydrochloride dihydrate in buffer was administered sc at a dose of 162 μmol/kg on 2 consecutive days for a total dose of 324 μmol/kg (Fig3). In this study, the first dose of the drug induced the excretion of 820 ± 215 μg/kg of iron with a 24-hour efficiency of 9.1% ± 2.4%. The injection of the same dose of the drug on the following day induced the excretion of an additional 978 ± 213 μg/kg of iron with a 24-hour efficiency of 10.8% ± 2.4%. The total iron excreted over the 2-day period was 1,798 ± 210 μg/kg of iron (Fig2) with an overall efficiency of 9.9% ± 2.1% (Table 1). These values are very similar to what is observed when the drug is administered as a single dose of 324 μmol/kg; in this case, the iron excretion is 2,400 ± 808 μg/kg of iron (Fig 2) with an efficiency of 13.3% ± 4.5% (P > .2). In each experiment, the majority of the iron was excreted in the feces, 65% to 75%, with the remainder being excreted in the urine.
Urinary and fecal iron excretion (μg/kg) induced by the sc administration of HBED monohydrochloride dihydrate in 0.1 mmol/L sodium phosphate buffer at pH 7.6 at a dose of 162 μmol/kg on 2 consecutive days for a total dose of 324 μmol/kg. The baseline iron levels in the urine and stool have not been subtracted.
Urinary and fecal iron excretion (μg/kg) induced by the sc administration of HBED monohydrochloride dihydrate in 0.1 mmol/L sodium phosphate buffer at pH 7.6 at a dose of 162 μmol/kg on 2 consecutive days for a total dose of 324 μmol/kg. The baseline iron levels in the urine and stool have not been subtracted.
Because the poor solubility of the HBED monohydrochloride dihydrate and the necessary manipulations to prepare it for injection could be problematic in a clinical setting, we have also examined the iron clearing efficiency of the HBED monosodium salt. The salt is much more soluble than the monohydrochloride dihydrate, with a solubility in water that is in excess of 30% (wt/vol). In addition, when the drug is dissolved in saline the unadjusted pH of the resulting solution is 7.3. When the drug was administered to the primates at a single dose of 150 μmol/kg, it induced the excretion of 1,139 ± 383 μg/kg of iron (Fig 2) and had an efficiency of 13.6% ± 4.5% (Table 1). This is well within error of the efficiency observed after the sc injection of 162 μmol/kg of the HBED monohydrochloride dihydrate in buffer or in Cremophor, 10.7% ± 2.3% (9.9% ± 2.1%)(P > .2) and 16.1% ± 5.6% (14.9% ± 5.2%) (P > .7), respectively.26
Finally, we have also administered the HBED monosodium salt at a dose of 75 μmol/kg every other day for three doses (Fig 4), for a total dose of 225 μmol/kg (n = 4). In this study, the first dose of the drug induced the excretion of 597 ± 91 μg/kg of iron with a 24-hour efficiency of 14.2% ± 2.2%. The second injection of the drug induced the excretion of 616 ± 90 μg/kg of iron with a 24-hour efficiency of 14.7% ± 2.2%. The third injection of the same dose of the drug induced the excretion of 625 ± 156 μg/kg of iron with a 24-hour efficiency of 14.9% ± 3.7%. The total iron excreted as a result of the three injections was 1,837 ± 301 μg/kg of iron (Fig 2) with an overall efficiency of 14.6% ± 2.4% (Table 1). These data are virtually identical to the iron excretion induced after a single sc injection of HBED in a buffer administered at a dose of 81 μmol/kg, 608 ± 175 μg/kg, and an efficiency of 13.0% ± 4.6% (P > .6, P > .5, and P > .5 for doses 1 through 3, respectively).
Urinary and fecal iron excretion (μg/kg) induced by the sc administration of HBED monosodium salt, 75 μmol/kg for three doses (225 μmol/kg total). Drug was administered on days 0, 2, and 4. Note the prompt return to baseline levels within 24 hours of each dose; baseline iron levels in the urine and stool have not been subtracted.
Urinary and fecal iron excretion (μg/kg) induced by the sc administration of HBED monosodium salt, 75 μmol/kg for three doses (225 μmol/kg total). Drug was administered on days 0, 2, and 4. Note the prompt return to baseline levels within 24 hours of each dose; baseline iron levels in the urine and stool have not been subtracted.
Interestingly, with the monosodium salt, a greater proportion of the iron was excreted in the urine than was observed with the monohydrochloride dihydrate administered in a buffer. At a single sc dose of 150 μmol/kg HBED monosodium salt, 32% of the iron was excreted in the urine with the remaining 68% being excreted in the feces. When the drug was administered sc at a dose of 75 μmol/kg every other day for three doses, the amount of iron excreted via the urine was even higher, 54%, 43%, and 68% for doses 1 through 3, respectively. The apparent difference in urinary versus biliary excretion may be caused by individual animal variability, or it may be that the more water-soluble monosodium salt is more readily cleared by the kidneys than the much less soluble monohydrochloride dihydrate.
Primate iron balance studies.
The total amount of iron intake was compared with the total amount of iron excreted. Net iron balance = dietary iron intake − (urinary + fecal iron excretion); animals in a negative iron balance are excreting more iron than they are absorbing. We have previously shown that monkeys treated with sc DFO 150 μmol/kg, or HBED 75 (81) or 150 (162) μmol/kg sc with or without the Cremophor vehicle were able to hold the monkeys in negative iron balance.26
In the current studies, whereas the doubling of the sc dose of DFO to 300 μmol/kg resulted in the excretion of 711 ± 230 μg/kg more iron than the primates absorbed (Table 1), the PO administration of 300 μmol/kg of DFO or 324 μmol/kg HBED to the monkeys did not result in a negative iron balance (data not shown). However, animals treated with a single sc dose of 81 μmol/kg of HBED in buffer excreted 259 ± 209 μg/kg more iron than they absorbed. This is in good agreement with what we previously observed with the administration of the same sc dose of HBED-Cremophor, 606 ± 406 μg/kg more iron than they absorbed (P > .1).26 The sc administration of HBED, 162 μmol/kg/day for 2 consecutive days, also resulted in a negative iron balance. The animals excreted 1,607 ± 372 μg/kg of iron more than they absorbed, which is within error of what is observed after a single sc dose of HBED of 324 μmol/kg, 2,346 ± 830 μg/kg more iron than they absorbed (P > .2).
The sc administration of the monosodium salt of HBED was also able to hold the monkeys in a negative iron balance (Table 1). Monkeys treated with the drug at a single dose of 150 μmol/kg excreted 899 ± 365 μg/kg of iron more than they absorbed. This is in keeping with what we have noted previously when the HBED was administered sc to the primates in either a buffer or in Cremophor at a similar dose26; 1,141 ± 456 μg/kg more than they absorbed when the compound was dosed with the Cremophor vehicle (P > .4), and 689 ± 158 μg/kg when the drug was administered without the Cremophor vehicle (P > .3). Finally, the HBED monosodium salt administered sc every other day for three doses (75 μmol/kg/dose) also resulted in a negative iron balance (Table 1). The iron excreted during a 7-day period after the administration of the first dose amounted to 1,578 ± 345 μg/kg of iron more than they absorbed.
As was observed with the urinary and fecal iron clearance data, animals treated with sc HBED consistently have a negative iron balance that is two to three times greater than that observed with DFO. The results of these studies clearly indicate that although PO administered DFO and HBED are unable to hold the monkeys in a negative iron balance (data not shown), both sc DFO and HBED administered sc as its monohydrochloride dihydrate in a buffer or as its monosodium salt can hold the monkeys in a negative iron balance (Table 1).
DISCUSSION
HBED (Mr 388) is a phenolic aminocarboxylate chelator that, like DFO, forms a 1:1 hexadentate complex with ferric iron. It was first synthesized by L’Eplattenier et al22 some 3 decades ago, and, in rats, has an LD50 (PO or intraperitoneally [IP]) that is in excess of 800 mg/kg.32 The ligand was originally chosen for further development as an iron-chelating agent after studies in rodents suggested that (1) it cleared radiolabeled iron from overloaded animals when administered parenterally,33,34 and (2) it was well absorbed from the gastrointestinal tract and remained active as an iron chelator after PO administration.32 Unfortunately, subsequent evaluations in both iron-loaded primates31 and human volunteers35 36 showed that although the PO administration of the drug did result in the excretion of some iron, the amount was insufficient for clinical use in transfusional iron overload.
The potential therapeutic usefulness of HBED administered parenterally was evaluated in the monkeys26 after recalling that IP injection of the drug in the hypertranfused rat produced an iron excretion that was significantly greater than that after injection of DFO.33 Recently, we showed that the iron excretion induced by sc injection of HBED in monkeys was at least twice that induced by sc injection of DFO.26 We reported the iron clearance values for the drug administered sc in 40% Cremophor at doses of 75 (81) and 150 (162) μmol/kg, as well as the efficiency of the drug administered PO or sc in a phosphate buffer dosed at 150 or 162 μmol/kg, respectively.26,31 At a dose of 75 (81) μmol/kg, HBED-Cremophor sc induced the clearance of 793 ± 410 μg/kg of iron and had an efficiency of 18.4% ± 9.1% (17.5% ± 9.1%).26 Increasing the sc dose to 150 (162) μmol/kg resulted in the clearance of 1,349 ± 475 μg/kg of iron and an efficiency of 16.1% ± 5.6% (14.9% ± 5.2%).26 However, HBED administered PO in a phosphate buffer (no Cremophor) at a dose of 150 μmol/kg had an efficiency of only 0.5% ± 0.5%,31 whereas a similar dose of the drug administered sc in the same vehicle induced the excretion of 899 ± 193 μg iron/kg body weight and was found to have an efficiency of 10.7% ± 2.3% (9.9% ± 2.1%).26 In all three sc experiments, approximately 90% of the iron was excreted in the feces with only 10% being excreted in the urine. At the dose of 150 (162) μmol/kg, no significant difference (corrected, P > .1) was found between the mean net iron excretion induced by HBED prepared in phosphate buffer or in Cremophor.
Given these encouraging results, additional experiments were clearly in order. Accordingly, in the current studies, we have greatly expanded the preclinical testing of HBED to include subchronic rodent toxicity studies, as well as more clinically applicable dosing regimens in the primates. HBED monosodium salt administered sc to rodents at doses up to 300 μmol/kg every other day for 2 weeks was found to be virtually nontoxic, ie, no drug-related toxicities were identifiable either grossly or histologically. In addition, the net iron excretion after sc HBED (monohydrochloride dihydrate in phosphate buffer or monosodium salt) in the primate model with iron overload produced iron excretion that was at least twice that observed after sc DFO and the compound retained its effectiveness when administered under a multiple-dosing protocol.
In patients with a high transfusion regimen, erythropoiesis is suppressed and iron absorption may be near normal, but each unit of transfused red blood cells contains 200 to 250 mg of iron. Most patients with thalassemia major require 200 to 300 mL/kg/year of blood, an amount equivalent to 250 to 400 μg iron/kg/day.37Thus, to maintain iron balance, a chelator must be able to remove a minimum of between 250 and 400 μg of iron/kg/day. The iron excretion observed after a single sc 150 μmol/kg injection of DFO in the C apella monkey with iron overload, 435 ± 115 μg iron/kg body weight, is consistent with the established ability of the daily use of DFO to control body iron. Under the same experimental conditions, sc HBED induces the excretion of more than twice as much iron as sc DFO, ie, 1,139 ± 383 μg/kg versus 435 ± 115 μg/kg of iron when both drugs are administered at 150 μmol/kg, and 2,400 ± 808 μg/kg versus 716 ± 244 μg/kg of iron when they are administered at a dose of 324 μmol/kg and 300 μmol/kg, respectively. Although the iron-loaded primate model26-28,30,31,38 has proven itself to be an excellent predictor of a potential chelator’s effectiveness in a clinical setting, caution is clearly needed in extrapolating from the primate model to patients with iron overload. For example, the C apella monkey with iron overload has normal erythropoiesis, whereas in patients with thalassemia major, erythropoietic activity is increased, even with regular transfusion. This difference could influence the relative proportions of iron excretion via the urinary and biliary routes, because at least with DFO,11 erythroid hyperplasia is associated with enhanced urinary iron excretion. Nevertheless, the overall pattern of iron excretion (urinary v fecal) in the primate model seems to be a reliable indicator of iron excretion in patients. Accordingly, the increased iron excretion observed after single or multiple sc injections of HBED in a phosphate buffer or as its monosodium salt suggests that a regimen in which sc HBED was used every other day (75 to 150 μmol/kg) might be as effective in maintaining iron balance as daily sc infusions of DFO. The prospect of using higher doses of HBED administered less frequently to maintain iron balance, eg, 300 to 324 μmol/kg only once or twice weekly, is also a possibility. Overall, these findings suggest that prompt completion of preclinical evaluation of parenteral HBED is in order; iron balance studies in human volunteers would be a subsequent step. The sc injection of HBED may provide patients with transfusional iron overload with a more effective, less demanding alternative iron chelation therapy to the use of prolonged parenteral infusions of DFO.
Supported in part by research grants from the National Institutes of Health (DK49108, AI35827, and HL61219) and by SunPharm Corporation, Jacksonville, FL.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Raymond J. Bergeron, PhD, Box 100485 JHMHC, Department of Medicinal Chemistry, University of Florida, Gainesville, FL 32610.