Abstract
Marrow stromal cultures support adult CD34+/Lin−/HLA-DR− or CD34+/Lin−/CD38− cell differentiation into natural killer (NK) or myeloid cells, but unlike committed lymphoid progenitors (CD34+/Lin−/CD45RA+/CD10+), no B cells are generated. We tested whether different microenvironments could establish a developmental link between the NK and B-cell lineages. Progenitors were cultured in limiting dilutions with interleukin-7 (IL-7), flt3 ligand (FL), c-kit ligand (KL), IL-3, IL-2, and AFT024, a murine fetal liver line, which supports culture of transplantable murine stem cells. NK cells, CD10+/CD19+ B-lineage cells and dendritic cells (DC) developed from the same starting population and IL-7, FL, and KL were required in this process. Single cell deposition of 3,872 CD34+/Lin−/CD38− cells onto AFT024 with IL-7, FL, KL, IL-2, and IL-3 showed that a one time addition of IL-3 at culture initiation was essential for multilineage differentiation from single cells. Single and double lineage progeny were frequently detected, but more importantly, 2% of single cells could give rise to at least three lineages (NK cells, B-lineage cells, and DC or myeloid cells) providing direct evidence that NK and B-lineage differentiation derive from a common lymphomyeloid hematopoietic progenitor under the same conditions. This study provides new insights into the role of the microenvironment niche, which governs the earliest events in lymphoid development.
THERE HAS BEEN MUCH interest in mechanisms by which the marrow microenvironment governs lymphoid differentiation through its supportive extracellular matrix, cell surface ligands, and production of soluble cytokines and proteoglycans. We have shown that culture of CD34+/Lin−/DR− cells from adult marrow will induce differentiation into phenotypic and functional natural killer (NK) cells if progenitors are grown in direct contact with normal allogeneic stroma and interleukin-2 (IL-2).1 In mice, the ability of stroma to induce differentiation is, at least in part, regulated by the transcription factor interferon-regulatory factor-1 (IRF-1).2 IRF-1 knockout mice exhibit a severe NK deficiency, which is mediated by failure of transcriptional regulation of IL-15. In human studies, IL-15 made by stroma and monocytes plays a role in NK development and survival by interaction with components of the IL-2 receptor.3,4 The production of IL-15 by monocytes may explain why NK differentiation can occur in the absence of exogenous IL-15 or stroma.5 However, stroma still provides other factors to induce the most primitive adult marrow progenitors to develop along the NK lineage. The requirement for direct contact with intact stroma distinguishes this population from more committed progenitors (CD34+/CD7+), which do not require direct contact with stroma for differentiation.6
Culture of human primitive progenitors with IL-2 and stroma results in terminal differentiation of NK cells. Progeny of long-term NK cultures cannot initiate secondary long-term NK cultures or support other lymphoid or myeloid lineages. The role of other defined cytokines in NK cell commitment and differentiation was assessed by studies using CD34+/CD33− progenitors after 14-day culture in IL-3 and macrophage inflammatory protein (MIP)-1α, which are highly clonogenic for myeloid long-term culture-initiating cells (LTC-IC).7,8CD34+/CD33− cells from these cultures do not differentiate into NK cells when cultured with IL-2 and stroma until IL-7 and c-kit ligand (KL) were added.9 We have also been exploring the molecular events, which may occur using in vitro culture. Fresh double sorted CD34+/Lin−/DR− cells do not express transcripts for RAG-2, CD3γ, CD3δ, or CD3ζ. However, culture with stromal-conditioned media, IL-7, FL, KL, IL-2, and IL-3 not only induced CD3γ, CD3δ, or CD3ζ, but also RAG-2 suggesting possible early T-or B-cell development.10 The role of FL in lymphopoiesis is further highlighted by defects identified in flt3-deficient mice,11 and recently, the overlapping and distinct roles of FL and KL in hematopoiesis have been reviewed.12
There are many similarities between NK cell and B-cell development. The importance of stroma for B-lymphoid progenitor differentiation has been described for several lymphoid culture systems and is the basis for the Whitlock-Witte culture.13 The control of B lymphopoiesis requires survival, proliferation, and differentiation signals from the bone marrow microenvironment.13-15 In the absence of stroma, immature B-cell progenitors rapidly die by apoptosis.16 Normal B-cell progenitors adhere to stroma through α4β1 integrin.17 Disruption of this adhesive interaction results in decreased B-cell differentiation. Normal human B-cell progenitors cultured while physically separated from stroma by a microporous membrane lead to decreased proliferation of CD34+/CD10+ pro-B cells suggesting the importance of direct stromal contact.18 Similar experiments with B-cell acute lymphocytic leukemia demonstrated decreased cell survival by an apoptotic mechanism when stroma contact was prohibited.19 Moreover, maximal proliferation of murine pre-B cells in a M2-10B4–dependent culture occurs through RGD-dependent binding of B-cell precursors to fibronectin, which is lost when differentiation occurs.20
In this report, we questioned whether an alternate microenvironment could induce human adult marrow progenitors to differentiate along the B-cell lineage, which is not found when culturing CD34+/Lin−/DR−progenitors on primary adult stromal layers using current conditions and multiple cytokines. Given the heterogeneity of human stroma containing fibroblasts, adipocytes, macrophages, and endothelial cells, we focused on murine stromal cell lines known to support murine primitive cells. M2-10B4 has been characterized for the ability to support human myeloid LTC-IC.21 More recently, Moore et al22 have developed a cell line, called AFT024, which is derived from day 14 gestational fetal liver adherent cells immortalized by introduction of a retrovirus containing a temperature-sensitive SV40 T antigen. Culture of as few as 100 highly purified murine marrow or fetal liver stem cells on AFT024 for 4 to 7 weeks resulted in multilineage reconstitution after transplantation into irradiated mice. In vitro, AFT024 induced proliferation of primitive progenitors and expansion of pro-B–cell progenitors suggesting a novel role for AFT024 in expansion and differentiation of murine hematopoietic progenitors. Therefore, we use the murine AFT024 cell line and defined cytokines to study human hematopoiesis in vitro.
MATERIALS AND METHODS
Normal bone marrow.
Bone marrow was obtained from normal donors after informed consent using guidelines approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota. Bone marrow mononuclear cells were obtained by Ficoll-Hypaque (specific gravity, 1.077) (Sigma Diagnostics, St Louis, MO) density gradient centrifugation.
Purification of primitive progenitors.
Bone marrow mononuclear cells were enriched for CD34+ cells using an avidin-biotin column as recommended by the manufacturer (Cellpro, Bothel, WA). Resultant cells were stained with CD34-biotin (Cellpro) for multicolor sorting, as previously described.10 23 Fluorescein isothiocyanate (FITC)-conjugated antibodies against CD2, CD3, CD4, CD5, CD7, CD8, CD10, and CD19 were used for the lineage (Lin) cocktail (Becton Dickinson [BD], San Jose, CA). Phycoerythrin (PE)-conjugated anti–HLA-DR (BD) or anti-CD38 (BD) was used and streptavidin SA670 (GIBCO-BRL, Grand Island, NY) as the third fluorescent color. Single CD34+/Lin−/DR− or CD34+/Lin−/CD38− were sorted directly into 96-well plates using the Automatic Cell Deposition Unit of the FACS Star Plus (BD). For single cell deposition experiments, the Automatic Cell Deposition Unit was set up in a low event “through-put” (200 events/second) and single droplet sorting was used instead of packet sorting to insure deposition of single cells.
Stromal cell lines.
Murine stromal cell lines were grown to confluency in 96-well plates and then irradiated (2,000 rad) before use. The M2-10B4 cell line was cultured as described.21,24 The AFT024 was cloned from murine fetal liver using described techniques25 and supports the ex vivo culture of murine transplantable stem cells.22 AFT024 was maintained at 33°C in Dulbecco’s Modified Eagle’s Medium (DMEM) (GIBCO Laboratories, Grand Island, NY) supplemented with 20% fetal calf serum (HyClone Laboratories, Logan, UT) and 50 μmol/L 2-mercaptoethanol (Bio-Rad, Hercules, CA) and subcultured in 96-well plates precoated with 0.1% gelatin (Specialty Media, Lavalette, NJ).
Culture of hematopoietic progenitors.
CD34+/Lin−/DR− or CD34+/Lin−/CD38− cells were plated in a 2:1 (vol/vol) mix of DMEM/Ham’s F12-based medium without stroma or in direct contact with stromal cell lines as indicated. The DMEM/F12-based medium (DMEM and Ham’s F12 were obtained from GIBCO Laboratories), developed to maximize NK cell growth,26 was supplemented with 24 μmol/L 2-mercaptoethanol, 50 μmol/L ethanolamine, 20 mg/L L-ascorbic acid, 5 μg/L sodium selenite (Na2SeO3), 100 U/mL penicillin, 100 U/mL streptomycin (GIBCO), 20% heat inactivated human AB serum (North American Biologicals, Miami, FL) at culture initiation reduced to 10% for subsequent media changes. Progenitors were plated in limiting dilution assays (22 replicates at four dilutions: 1,000 to 1,200, 300 to 400, 100 to 130, and 33 to 45 cells/well) or by single cell deposition in 96-well plates. The cloning frequency of NK, B, and dendritic cell progenitors was determined by immunophenotyping and was calculated as the reciprocal of the concentration of cells that resulted in 37% negative wells using Poisson statistics and the weighted mean method.27 28 In limiting dilution experiments, multiple wells were analyzed and reported irrespective of cell growth. In single cell deposition experiments, only those wells which exhibited visual cell growth (>100 cells/well) were analyzed further with three-color immunophenotyping, the remaining wells were considered as having no growth. Cultures were maintained in a humidified atmosphere at 37°C and 5% CO2 and medium was half changed weekly with the indicated cytokines. Cytokines were supplemented as indicated with 1,000 U/mL IL-2 (a gift from Amgen, Thousand Oaks, CA), 10 ng/mL flt3 ligand (FL, a gift from Immunex, Seattle, WA), 20 ng/mL c-kit ligand (KL or stem cell factor, a gift from Amgen), 20 ng/mL IL-7 (R&D Systems, Minneapolis, MN), and 5 ng/mL IL-3 (R&D Systems). Secondary dendritic cell conditions contained 10 ng/mL tumor necrosis factor (TNF) (R&D Systems), 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Immunex, Seattle, WA), and 5 ng/mL IL-4 (R&D Systems). Cytokines were added weekly or only once at the time of culture initiation as indicated.
Phenotype, cell quantitation, cytotoxicity, and allogeneic mixed lymphocyte reaction.
FITC- and PE-coupled control immunoglobulins or specific antibodies directed at CD2, CD3, CD4, CD7, CD8, CD10, CD11c, CD34, CD19, HLA-DR, kappa light chain, lambda light chain, IgM (all from BD), and mu heavy chain (clone ATTC HB57 from T. LeBien, University of Minnesota Cancer Center, Minneapolis, MN) were used to evaluate progeny of long-term cultures. Three-color phenotype analysis was used to determine multilineage progeny of starting progenitors using CD56-PE, CD19-PerCP, and either CD1a-FITC (PharMingen, San Diego, CA), CD14-FITC (BD), or CD15-FITC (BD). Absolute cell numbers were determined by addition of 3 × 105 polystyrene microspheres (Polysciences, Warrington, PA) to the total progeny of a culture well and after gating out debris, absolute cell numbers were calculated using the method described by Pribyl et al.29,30 The absolute number of cells/well was calculated as: [(total number of beads added/well)/(number of beads collected) × (number of cells in the phenotype gate of interest)]. The relationship between polystyrene microspheres and absolute cell numbers using this technique was linear between 2.1 × 102 cells/well and 3.3 × 105 cells/well. All analyses were performed with a FACSCalibur (BD) and CELLQuest software (BD). Cytotoxicity assays were performed from progeny of single cells in triplicate using the K562 (American Type Culture Collection, Rockville, MD) cell lines in a 4-hour Cr51 release assay.31Allogeneic mixed lymphocyte reactions were performed as described with modifications.32 Briefly, 105 allogeneic monocyte-depleted mononuclear cells were incubated in round bottom 96-well tissue culture plates with graded doses of stimulators (30,000 to 3,300) irradiated at 3,000 rads. After 5 days, cultures were pulsed with 1 μCi/well of 3H-thymidine (New England Nuclear, Boston, MA) for 18 hours before harvesting and counting.
Terminal deoxynucleotidyl transferase (TdT) determination by in situ staining.
Cytospin preparations of sorted CD19+ or CD56+cells were fixed for 30 minutes in absolute methanol at 4°C immediately before staining. Rabbit anti-TdT (Supertechs, Bethesda, MD) is diluted 1:10 in phosphate-buffered saline (PBS) buffer and 20 μL is applied to each slide for 30 minutes. After washing, an equal amount of a 1:10 dilution of secondary FITC-goat antirabbit (Supertechs) is applied. TdT positivity is determined within 24 hours of staining using a fluorescent microscope.
TdT determination by PCR.
After determining the phenotypic presence of CD19 positive cells by flow cytometry, the remaining progeny of single cell cultures were used for determination of TdT by reverse transcriptase-polymerase chain reaction (RT-PCR). Total mRNA was extracted using RNeasy spin columns according to the manufacturer’s recommendations (Qiagen, Santa Clarita, CA). Reverse transcription was performed as previously described.10 Briefly, samples were subjected to 40 cycles of denaturation at 95°C for 20 seconds, annealing at 55°C for 15 seconds, and extension at 72°C for 1 minute in a Perkin Elmer 480 thermal cycler (Applied Biosystems, Foster City, CA). Oligonucleotide primer sequences were: TdT 5′ primer: 5′-ACACGAATGCAGAAAGCAGGA-3′; TdT 3′ primer: 5′-AGGCAACCTGAGCTTTTCAAA-3′ (provided by Dr T. LeBien); β-actin 5′ primer: 5′-TACCTCATGAAGATCCTCA-3′; β-actin 3′ primer: 5′-TTCGTGGATGCCACAGGAC-3′. Amplified products were size separated on 1.5% agarose gels and transferred to Hybond N+ nucleic acid transfer membranes (Amersham, Arlington Heights, IL). Probes were labeled with32P-deoxyadenosine triphosphate (dATP) using a TdT 3′-end labeling kit (Boehringer Mannheim, Indianapolis, IN) using probe sequences: TdT 5′-ACACGAATGCAGAAAGCAGGA-3′ (provided by Dr T. LeBien); β-actin 5′-CCATCTCTT-GCTCGAAGTC-3′.
Statistics.
Results of experimental points obtained from multiple experiments were reported as mean ± 1 standard error of the mean (SEM). Significance levels were determined by two sided Student’s t-test analysis.
RESULTS
Although primitive CD34+/Lin−/DR−progenitors can be induced to differentiate along the NK cell lineage when cultured with IL-2 in contact with primary adult marrow allogeneic stroma, these cultures result in terminal differentiation and do not support B-lineage cells or other lineages. Therefore, we designed experiments to assess stromal feeder cell lines, which may support differentiation along the NK and B-cell lineage to establish a link between these two lymphoid lineages where differentiation is known to occur in the marrow microenvironment. CD34+/Lin−/DR− cells were isolated from adult marrow and plated with multiple cytokines at culture initiation (IL-7, FL, KL, and IL-2) in the absence of stroma or with two murine feeder cell lines, M2-10B4 and AFT024 (n = 3). Analysis of progenitors cultured without stroma showed little growth and complete absence of NK cell or B-cell progeny. M2-10B4, which supports proliferation of mature NK cells and primitive myeloid cells,21 33 inefficiently gave rise to NK cell progeny (36% of wells positive at 1,200 cells/well) and no B-lineage cells could be identified. In contrast, coculture of CD34+/Lin−/DR− cells on AFT024 in the presence of IL-7, FL, KL only at culture initiation and IL-2 throughout culture resulted in both NK and B-cell progeny from the same wells (Table 1). Culture of a population of lymphoid-committed cells already expressing one of the lymphoid antigens (CD34+/Lin+) also gave rise to both NK and B-lineage cell progeny (data not shown). In contrast to the committed CD34+/Lin+ population where lymphoid progeny were identified by 14 days, differentiation of the more primitive CD34+/Lin−/DR−population required longer (>28 days) culture intervals.
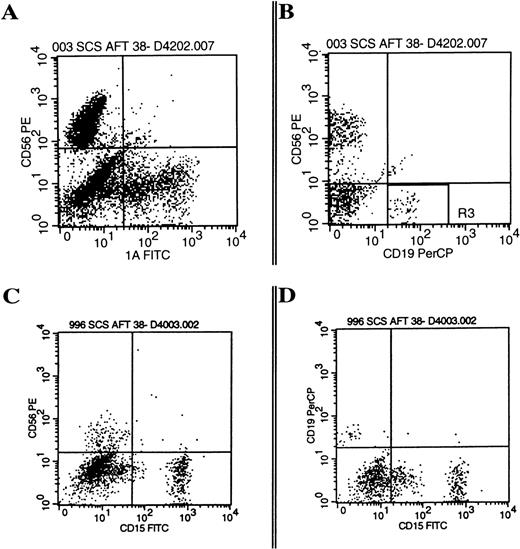
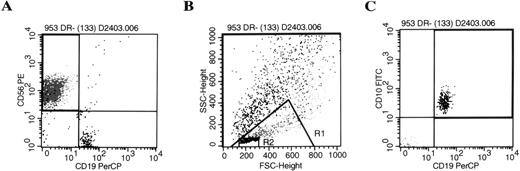
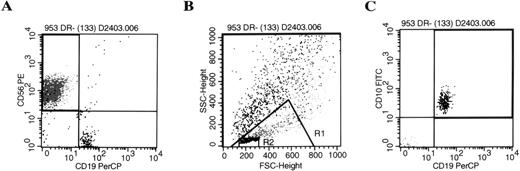
B-lineage cells from the AFT024 cultures were CD19 and CD10 positive and were found to have very low forward and side scatter, significantly smaller than NK cells within the same cultured population (Fig 1). Phenotype analysis showed that the B-lineage cells were negative for CD34 (n = 20), IgM (n = 8), mu (n = 30), kappa light chains (n = 20), and lambda light chains (n = 20), irrespective of the cytokine combination used. Small numbers of CD19+ B-lineage cells were positive for CD20 (18% ± 5.5%, n = 6; P =.005) and CD21 (14% ± 2.9%, n = 13;P = .009) compared with isotype controls (4.3% ± 1.5%). Given this immature phenotype, cells were analyzed further for TdT in situ fluorescent staining. CD56+ NK cells were essentially negative (<2%) for characteristic nuclear TdT staining by fluorescence microscopy (n = 4). In contrast, CD19+B-lineage cells from the same cultured population were strongly positive in 23% to 55% of cells (n = 4).
Progeny of AFT024 cultures give rise to NK cells and CD10+/CD19+ B-lineage cells. CD34+/Lin−/DR− cells were cultured in limiting dilutions on AFT024 with IL-7, FL, KL, and IL-2 added at culture initiation. Media was half changed weekly with fresh media supplemented with IL-2 alone. Individual wells were harvested and analyzed by flow cytometry after three-color staining (CD56-PE, CD10-FITC, CD19-PerCP) using appropriate isotype controls. The representative example shown is from a culture well initiated with 130 cells. (A) Shows the two-color analysis of CD56 and CD19. CD19 positive cells were then backgated onto the forward and side scatter plot (B). The CD19+ B-lineage cells (black dots) were significantly smaller than NK cells (medium gray) found within the lymphoid window of the same culture. Backgating to find B-lineage cells in this very small lymphocyte gate (R2) was used as an absolute criteria for all cultures determined positive for B-lineage cells. (C) Shows a representative example of the CD10+/CD19+ B-lineage cells based on the R2 gate.
Progeny of AFT024 cultures give rise to NK cells and CD10+/CD19+ B-lineage cells. CD34+/Lin−/DR− cells were cultured in limiting dilutions on AFT024 with IL-7, FL, KL, and IL-2 added at culture initiation. Media was half changed weekly with fresh media supplemented with IL-2 alone. Individual wells were harvested and analyzed by flow cytometry after three-color staining (CD56-PE, CD10-FITC, CD19-PerCP) using appropriate isotype controls. The representative example shown is from a culture well initiated with 130 cells. (A) Shows the two-color analysis of CD56 and CD19. CD19 positive cells were then backgated onto the forward and side scatter plot (B). The CD19+ B-lineage cells (black dots) were significantly smaller than NK cells (medium gray) found within the lymphoid window of the same culture. Backgating to find B-lineage cells in this very small lymphocyte gate (R2) was used as an absolute criteria for all cultures determined positive for B-lineage cells. (C) Shows a representative example of the CD10+/CD19+ B-lineage cells based on the R2 gate.
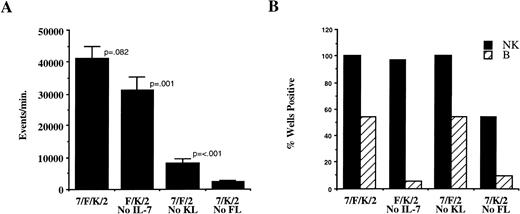
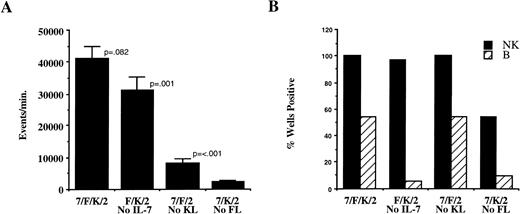
The precise role of the individual cytokines was assessed by sequential deletion of single cytokines from the four-cytokine cocktail (IL-7, FL, KL, IL-2) using sorted CD34+/Lin−/DR− cells. Comparative cell growth was estimated by flow cytometry as the number of events (per minute) from progeny of each well. Omitting IL-2 from the four-cytokine combination significantly decreased total cell proliferation from 14,660 ± 3,197 to 3,561 ± 312 events per minute (n = 22 wells initiated with 330 cells/well from two donors; P = < .001). However, the number of wells positive for NK cells and B-lineage cells was not significantly different, suggesting that IL-2 is not critical for either NK or B-lineage cell differentiation in the presence of IL-7, FL, and KL. IL-7, FL, and KL were then each omitted from the four-cytokine combination. Deletion of IL-7 slightly decreased relative proliferation (P = .082), did not effect the number of wells with NK cell growth, but significantly decreased the number of wells with B-lineage cell progeny (Fig 2). Although elimination of KL significantly affected relative proliferation, there was no difference in the percent of wells positive for either NK or B-lineage cells. In contrast, omitting FL significantly decreased relative proliferation and the frequency of wells with NK and B-lineage cells (Fig 2), suggesting critical roles for FL and IL-7 in lymphoid differentiation on AFT024. Weekly addition of cytokines lead to increased NK and B-lineage cell proliferation (data not shown), therefore weekly cytokines were used in all subsequent experiments.
Proliferation and differentiation in AFT024 cultures depends on addition of defined cytokines. CD34+/Lin−/DR− cells (1,000 cells/well) were cultured on AFT024 in 96-well plates with the cytokine combinations indicated. Cytokines were added only once at culture initiation and weekly half media changes contained fresh IL-2 alone. Abbreviations for cytokines in all figures are as follows: 7, IL-7; F, FL (Flt3 ligand); K, KL (c-kit ligand); 2, IL-2; 3, IL-3. After 28 to 35 days in culture, 42 wells per condition from cells derived from three donors were analyzed for proliferation and presence or absence of NK and B-lineage cells. (A) Relative proliferation was determined by flow cytometry as the number of events analyzed per minute. Data are the mean ± SEM for replicate wells analyzed in parallel with the total contents of a harvested well analyzed in a constant volume of approximately 180 μL. The P values shown are comparisons between adjacent cytokine combinations. (B) The percentage of positive wells (of 42 wells analyzed per condition) is shown for NK cells (black bars) and B-lineage cells (hatched bars) for each cytokine combination.
Proliferation and differentiation in AFT024 cultures depends on addition of defined cytokines. CD34+/Lin−/DR− cells (1,000 cells/well) were cultured on AFT024 in 96-well plates with the cytokine combinations indicated. Cytokines were added only once at culture initiation and weekly half media changes contained fresh IL-2 alone. Abbreviations for cytokines in all figures are as follows: 7, IL-7; F, FL (Flt3 ligand); K, KL (c-kit ligand); 2, IL-2; 3, IL-3. After 28 to 35 days in culture, 42 wells per condition from cells derived from three donors were analyzed for proliferation and presence or absence of NK and B-lineage cells. (A) Relative proliferation was determined by flow cytometry as the number of events analyzed per minute. Data are the mean ± SEM for replicate wells analyzed in parallel with the total contents of a harvested well analyzed in a constant volume of approximately 180 μL. The P values shown are comparisons between adjacent cytokine combinations. (B) The percentage of positive wells (of 42 wells analyzed per condition) is shown for NK cells (black bars) and B-lineage cells (hatched bars) for each cytokine combination.
Having identified a role for IL-7 and FL, experiments were then performed to determine cloning frequency and lineage-specific cell proliferation by sequential addition of IL-7, FL, KL, and IL-2. IL-3 was also added to this analysis to determine if this primitive acting cytokine would increase cloning frequency and proliferation or decrease lymphoid capacity, as has been suggested by others.34 A known number of polystyrene microspheres was added to progeny of each well,30 allowing precise quantitation of the absolute number of cells by flow cytometry. For these experiments, cytokines were added weekly except for IL-3, which was only added once at culture initiation, because weekly addition of IL-3 lead to cell death from myeloid overgrowth (data not shown). Total cell number (all lineages) was determined for cultures inoculated with 130 CD34+/Lin−/DR− cells on AFT024 with or without sequential addition of cytokines (n = 20 to 38 wells per condition from cells derived from four normal donors). After 35 to 42 days, proliferation was poor with either no cytokines (653 ± 29 cells/well) or IL-7 alone (512 ± 21 cells/well). However, addition of IL-7 + FL (2,717 ± 407 cells/well), IL-7 + FL + KL (21,454 ± 2,390 cells/well), IL-7 + FL + KL + IL-2 (59,595 ± 10,612 cells/well), and IL-7 + FL + KL + IL-2 + IL-3 (108,380 ± 11,712 cells/well) lead to a significant increase in total cell proliferation with each additional cytokine (P = < .03 for each added cytokine).
Based on phenotype studies, a proportion of cells derived from AFT024 cultures were neither NK or B-lineage cells, suggesting the presence of other lineages. Wright-Giemsa staining showed the presence of myeloid cells (from promyelocyte to neutrophils), monocyte/macrophage cells, and cells with abundant cytoplasmic projections distinct from other cells. Sorting of CD15+ cells from cultures and subsequent cytospin staining showed that all cells were myeloperoxidase positive, further verifying their myeloid origin. Sorting of CD14+cells from a 4-week culture showed mixed morphologic monocytes, macrophages, and some myeloid cells. CD14+ cells from cultures greater than 8 weeks old showed only macrophages that were myeloperoxidase negative. Sorting CD1a+ cells identified cells with cytoplasmic projections morphologically consistent with dendritic cells (DC). These cells were CD15− and CD14−/dim+. Transfer of progeny from 5 week AFT024 culture into media containing GM-CSF, TNF, and IL-4 further enriched for a population of cells, which were CD1a+, CD11c+, HLA-DR+, CD4+, CD15−, and CD14− consistent with the phenotype of cultured DC.32 35 Cultured cells exhibited characteristic DC function, as primary progeny of AFT024 cultures or progeny of secondary cultures supplemented with GM-CSF, TNF, and IL-4 were capable of stimulating allogeneic T cells in mixed lymphocyte reaction (Fig 3). We could not detect any CD3+ T cells or CD4+/CD8+ T cells in cultured progeny of AFT024 cultures with any of the cytokine combinations tested. Despite the identification of NK, B-lineage, myeloid, and DC, there was always a population of AFT024 cultured progeny, which was negative for any of the antigens tested, raising the possibility that other lineages may be present as well.
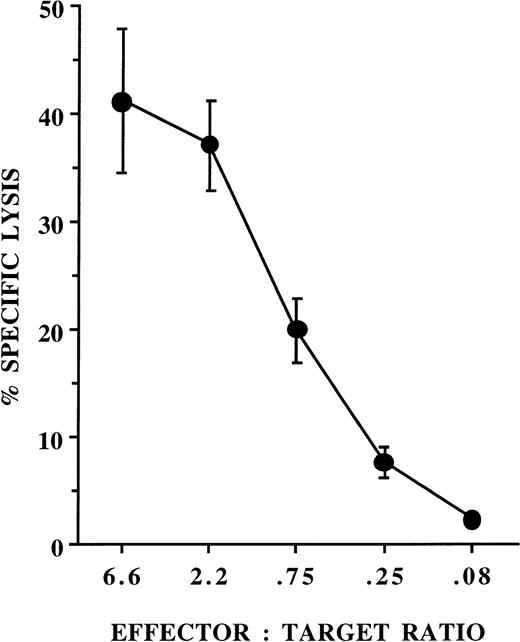
Progeny of AFT024 cultures function to stimulate allogeneic mixed lymphocyte reactions. Monocyte-depleted peripheral blood mononuclear cells (105) from the same donor were mixed with irradiated (3,000 rads) autologous CD14+ fresh monocytes (▪), 6-week progeny of AFT024 cultures (•), and 5-week progeny of AFT024 cultures transferred for an additional week to media containing TNF, GM-CSF, and IL-4 (○). Proliferation (counts per minute [cpm]) was assessed after 5 days by pulsing for 18 hours with3H-thymidine and data are presented as the mean ± SEM cpm of triplicate wells from a representative experiment.
Progeny of AFT024 cultures function to stimulate allogeneic mixed lymphocyte reactions. Monocyte-depleted peripheral blood mononuclear cells (105) from the same donor were mixed with irradiated (3,000 rads) autologous CD14+ fresh monocytes (▪), 6-week progeny of AFT024 cultures (•), and 5-week progeny of AFT024 cultures transferred for an additional week to media containing TNF, GM-CSF, and IL-4 (○). Proliferation (counts per minute [cpm]) was assessed after 5 days by pulsing for 18 hours with3H-thymidine and data are presented as the mean ± SEM cpm of triplicate wells from a representative experiment.
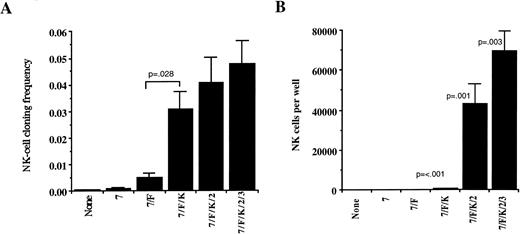
The role of cytokines (added weekly except for IL-3) was then evaluated in AFT024 cultures by plating CD34+/Lin−/DR− in limiting dilutions to determine the cloning frequency for NK cells, B-lineage cells, and DC. From these same cultures, the absolute number of cells derived from each positive well could also be calculated. The NK cell cloning frequency of CD34+/Lin−/DR− cells grown without cytokines or with IL-7 alone was less than 0.2% and only slightly increased to 0.5% when IL-7 and FL were combined. However, addition of KL to IL-7 and FL significantly increased (P = .028) the NK cloning frequency to over 3% (Fig 4A). Further addition of IL-2 and IL-3 to IL-7, FL, and KL did not change cloning frequency. The role of cytokines on proliferation differed from the role of cytokines on cloning frequency. Proliferation was determined from wells started with 130 CD34+/Lin−/DR−cells cultured for 35 to 42 days (Fig 4B). Even though IL-7, FL, and KL had the greatest impact on cloning frequency, the number of absolute NK cells per well was low (507 ± 100, n = 36). In contrast, addition of IL-2 and further addition of IL-3 at culture initiation significantly increased NK proliferation. The absolute number of NK cells derived from 130 cells when all cytokines were used was 69,354 ± 10,108 NK cells/well (n = 38).
NK cell cloning frequency and absolute NK cell proliferation in AFT024 cultures is dependent on the addition of exogenous cytokines. CD34+/Lin−/DR− cells were plated in limiting dilutions (replicates of 1,200 cells/well, 400 cells/well, 130 cells/well, and 45 cells/well) on AFT024 in 96-well plates with the cytokine combinations indicated. Cultures were maintained with weekly half media changes and fresh cytokines were added weekly except for IL-3, which was only added once at culture initiation. (A) After 35 to 42 days of culture, wells were analyzed using three-color flow cytometry for the presence of CD56+ NK cells to calculate the cloning frequency of initially plated CD34+/Lin−/DR− cells. Cells were gated on viable cells and any well containing greater than 20 absolute CD56+ cells was counted as positive. Each bar represents the mean ± SEM cloning frequency from four donors. (B) The absolute number of NK cells per positive well initiated with 130 CD34+/Lin−/DR− cells is shown for each cytokine combination. Absolute cell counts per harvested well was determined by addition of a known number of polystyrene microspheres to each sample before analysis by flow cytometry as described in Materials and Methods. Each condition represents the mean ± SEM of 20 to 38 individual wells initiated with CD34+/Lin−/DR− cells derived from four donors. P values listed are for comparisons between adjacent conditions.
NK cell cloning frequency and absolute NK cell proliferation in AFT024 cultures is dependent on the addition of exogenous cytokines. CD34+/Lin−/DR− cells were plated in limiting dilutions (replicates of 1,200 cells/well, 400 cells/well, 130 cells/well, and 45 cells/well) on AFT024 in 96-well plates with the cytokine combinations indicated. Cultures were maintained with weekly half media changes and fresh cytokines were added weekly except for IL-3, which was only added once at culture initiation. (A) After 35 to 42 days of culture, wells were analyzed using three-color flow cytometry for the presence of CD56+ NK cells to calculate the cloning frequency of initially plated CD34+/Lin−/DR− cells. Cells were gated on viable cells and any well containing greater than 20 absolute CD56+ cells was counted as positive. Each bar represents the mean ± SEM cloning frequency from four donors. (B) The absolute number of NK cells per positive well initiated with 130 CD34+/Lin−/DR− cells is shown for each cytokine combination. Absolute cell counts per harvested well was determined by addition of a known number of polystyrene microspheres to each sample before analysis by flow cytometry as described in Materials and Methods. Each condition represents the mean ± SEM of 20 to 38 individual wells initiated with CD34+/Lin−/DR− cells derived from four donors. P values listed are for comparisons between adjacent conditions.
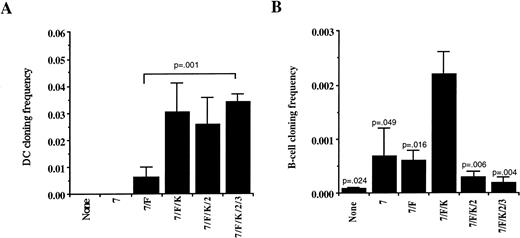
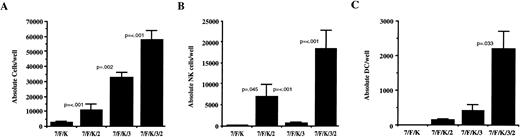
The cloning frequency of CD1a positive DC was similar to that of NK cells when a minimum of IL-7, FL, and KL were added to cultures (Fig 5A). This did not change when IL-2 or IL-3 were added. Although the addition of all cytokines consistently resulted in the highest number of absolute DC (2,546 ± 492, n = 37) derived from 130 CD34+/Lin−/DR− cells, individual cytokine combinations had less influence on proliferation until only IL-7 and FL were added, which resulted in 278 ± 122 DC per positive well (n = 20). B-lineage cell cloning frequency and proliferation was significantly less than either NK cells or DC. Culture of CD34+/Lin−/DR− cells in limiting dilutions on AFT024 with IL-7, FL, and KL (n = 8) resulted in a significantly higher B-lineage cell cloning frequency than any other cytokine combination tested (Fig 5B). Although this same combination induced the highest number of B-lineage cells per well (89 ± 19), given the low overall cloning frequency, the B-lineage cell proliferation did not change significantly with the different cytokine combinations.
DC and B-lineage cell cloning frequency in AFT024 cultures is dependent on the addition of exogenous cytokines. CD34+/Lin−/DR− cells were plated in limiting dilutions on AFT024 in 96-well plates with the cytokine combinations indicated for 35 to 42 days. (A) Multiple replicates were analyzed using three-color flow cytometry for the presence of CD1a+ cells to calculate the cloning frequency of initially plated CD34+/Lin−/DR− cells. Cells were gated on viable cells and any well containing greater than 20 absolute CD1a+ cells was counted as positive. Each bar represents the mean ± SEM cloning frequency from four donors. (B) Replicates were analyzed for the presence of CD19+ cells to calculate the B-lineage cell cloning frequency. Cells were gated on a very low forward and side scatter (Fig 1B designated R2) and any well containing greater than 10 absolute CD19+ cells was counted as positive. Each bar represents the mean and SEM cloning frequency from four donors except for the condition using IL-7, FL, and KL (7/F/K) where eight donors were used. All P values shown are compared with the frequency using IL-7, FL, and KL.
DC and B-lineage cell cloning frequency in AFT024 cultures is dependent on the addition of exogenous cytokines. CD34+/Lin−/DR− cells were plated in limiting dilutions on AFT024 in 96-well plates with the cytokine combinations indicated for 35 to 42 days. (A) Multiple replicates were analyzed using three-color flow cytometry for the presence of CD1a+ cells to calculate the cloning frequency of initially plated CD34+/Lin−/DR− cells. Cells were gated on viable cells and any well containing greater than 20 absolute CD1a+ cells was counted as positive. Each bar represents the mean ± SEM cloning frequency from four donors. (B) Replicates were analyzed for the presence of CD19+ cells to calculate the B-lineage cell cloning frequency. Cells were gated on a very low forward and side scatter (Fig 1B designated R2) and any well containing greater than 10 absolute CD19+ cells was counted as positive. Each bar represents the mean and SEM cloning frequency from four donors except for the condition using IL-7, FL, and KL (7/F/K) where eight donors were used. All P values shown are compared with the frequency using IL-7, FL, and KL.
Bulk cultures identified conditions optimal for inducing NK cell, B-lineage cell, and DC differentiation and proliferation from adult marrow primitive progenitors. However, we still could not conclude that all of these cell lineages were derived from a single cell. We also questioned whether the role of certain cytokines may be more important at the single cell level rather than in bulk cultures. To answer these questions, experiments were performed by single cell deposition of primitive progenitors from four normal donors onto AFT024 stromal layers using flow cytometry. To maximize outgrowth of primitive progenitors, single cells were sorted CD34+/Lin−/CD38− based on findings that this population may contain a higher frequency of the primitive human progenitors than the CD34+/Lin−/DR−population. Sorting windows were chosen so that the CD34+/Lin−/CD38−population accounted for 2.4% ± 1.2% of total CD34+cells.
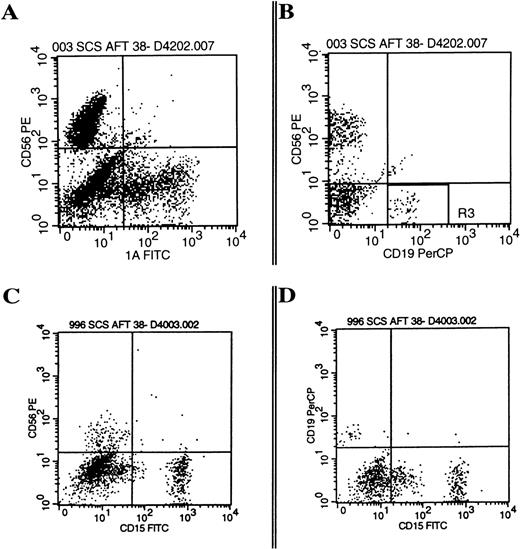
A total of 3,872 single CD34+/Lin−/CD38− cells were sorted onto AFT024 with IL-7, FL, and KL with or without the addition of IL-2, IL-3, or the combination (Table 2). CD34+/Lin−/CD38− cells plated without IL-3 exhibited significantly less single cell growth with 6.3% positive wells versus 27% when IL-3 was included. There was only one positive well of 1,584 plated without IL-3 that gave rise to B-lineage cells compared with 46 of 2,288 wells positive for B-lineage cells when IL-3 was included only at culture initiation. More than 10% of wells with IL-3 at culture initiation gave rise to NK cells and either DC or myeloid cells. Most importantly, 1.5% to 2.0% of single CD34+/Lin−/CD38− cells plated under optimal conditions gave rise to three lineages (NK cells, B-lineage cells, and either CD1a+ DC, CD15+myeloid cells, or CD14+ monocytes) demonstrating origin from the same cell (Fig 6).
Multiple lineages are present from the cultured progeny of single cells. Single CD34+/Lin−/CD38− cells were cultured on AFT024 for 42 days with IL-7, FL, KL, IL-2, and IL-3 (A and B are from progeny of the same single cell) or with IL-7, FL, KL, and IL-3 (C and D are from progeny of the same single cell). Data are examples of the three-color phenotypes summarized in Table 2 showing the multilineage differentiation, which resulted from single cell cultures gated on all viable cells (panel A and C) or a smaller lymphocyte gate (panel B and D).
Multiple lineages are present from the cultured progeny of single cells. Single CD34+/Lin−/CD38− cells were cultured on AFT024 for 42 days with IL-7, FL, KL, IL-2, and IL-3 (A and B are from progeny of the same single cell) or with IL-7, FL, KL, and IL-3 (C and D are from progeny of the same single cell). Data are examples of the three-color phenotypes summarized in Table 2 showing the multilineage differentiation, which resulted from single cell cultures gated on all viable cells (panel A and C) or a smaller lymphocyte gate (panel B and D).
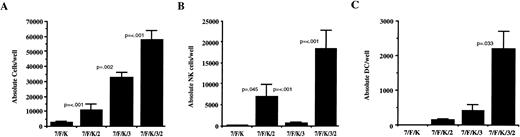
Single-cell proliferation was analyzed using data from Table 2. Exogenous cytokines played a significant role in cell proliferation from single cells (Fig 7A). Although the frequency of single cells giving rise to any progeny was most influenced by initial addition of IL-3, analysis for the absolute number of cells derived from single cells showed contributions to NK cell and DC proliferation from both IL-2 and IL-3 (Fig 7B and C). The absolute number of B-lineage cells in positive wells cultured with IL-7, FL, KL, IL-3, and IL-2 was 190 ± 83.
Proliferation of single sorted CD34+/Lin−/CD38− cells is optimal with IL-7, FL, KL, IL-2, and IL-3. The absolute number of total cells (A), NK cells (B), and DC (C) were analyzed from progeny of 2,640 single sorted CD34+/Lin−/CD38− cells summarized in Table 2. Results in (A) are from all wells evaluated per cytokine condition. Results in (B) and (C) are from wells determined positive for NK cell or DC progeny, respectively. The P values listed are comparisons between adjacent bars where significant.
Proliferation of single sorted CD34+/Lin−/CD38− cells is optimal with IL-7, FL, KL, IL-2, and IL-3. The absolute number of total cells (A), NK cells (B), and DC (C) were analyzed from progeny of 2,640 single sorted CD34+/Lin−/CD38− cells summarized in Table 2. Results in (A) are from all wells evaluated per cytokine condition. Results in (B) and (C) are from wells determined positive for NK cell or DC progeny, respectively. The P values listed are comparisons between adjacent bars where significant.
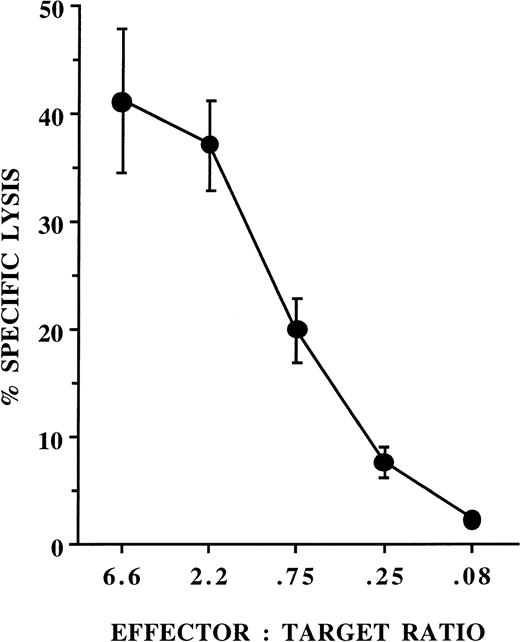
To further evaluate the B-lineage cells from AFT024 cultures, progeny of single cells were evaluated for TdT mRNA by RT-PCR. Progeny of single CD34+/Lin−/CD38−cells were analyzed by three-color flow cytometry for the presence of NK cells, B-lineage cells, and other lineages. The remaining cells were used for mRNA extraction after identifying the phenotypic presence or absence of CD19+ cells. All of the single cell progeny (n = 9), which were phenotypically positive for B-lineage cells were positive for TdT transcripts. In contrast, in wells where B-lineage cells could not be phenotypically identified, seven of 17 were still positive for TdT. The finding of TdT in 40% of phenotypic B-lineage cell negative populations suggests that the number of wells giving rise to B-lineage cells may be underscored by phenotypic identification alone. Finally, after 42 days of culture with IL-7, FL, KL, IL-3, and IL-2, progeny of single CD34+/Lin−/CD38− cells cocultured with AFT024 were selected based on growth for expansion in 24-well plates with IL-2 alone to obtain enough NK cells for functional and NK cell subset analysis. The 16 populations derived from single CD34+/Lin−/CD38− cells gave rise to an average of 8.3 × 105 cells (range, 3 to 22 × 105) of which 94% ± 1.0% were CD56+/CD3−, 11% ± 2.0% were CD56+/CD2+, and 21% ± 5% were CD56+/CD7+. These NK cells exhibited characteristic function against K562 targets in cytotoxicity assays (Fig 8).
NK cells derived from single cells exhibit cytotoxic activity against K562 targets. Single CD34+/Lin−/CD38− progenitors were cultured on AFT024 with IL-7, FL, KL, IL-2, and IL-3 (added only at culture initiation) for 42 days and wells with the highest proliferation were transferred to 24-well plates in media containing IL-2 alone for an additional 14 days. Cells were then counted and tested for cytotoxicity against chromium-labeled K562 targets. Results are from 13 NK cell populations derived from progeny of 13 single cells from two normal donors (data represent the mean ± SEM of the average of triplicate wells from each population).
NK cells derived from single cells exhibit cytotoxic activity against K562 targets. Single CD34+/Lin−/CD38− progenitors were cultured on AFT024 with IL-7, FL, KL, IL-2, and IL-3 (added only at culture initiation) for 42 days and wells with the highest proliferation were transferred to 24-well plates in media containing IL-2 alone for an additional 14 days. Cells were then counted and tested for cytotoxicity against chromium-labeled K562 targets. Results are from 13 NK cell populations derived from progeny of 13 single cells from two normal donors (data represent the mean ± SEM of the average of triplicate wells from each population).
DISCUSSION
We have developed a novel long-term culture assay using the murine fetal stromal cell line, AFT024, and human cytokines (IL-7, FL, KL, IL-2, and IL-3) to induce multilineage lymphoid and myeloid differentiation from adult human marrow progenitors. The multilineage potential of this murine stromal-based system using one culture condition was striking. In addition to inducing NK differentiation more efficiently than found previously with adult human allogeneic stroma,6 CD10+/CD19+ B-lineage cells were also generated from the same starting cells. Limiting dilution assays using three-color immunophenotype analysis and absolute quantitation of various cell types, determined the role of cytokines on NK cell, B-lineage cell, and DC cloning frequency and proliferation. Single cell sorting of individual CD34+/Lin−/CD38− cells verified that not only NK cells and B-lineage cells derive from the same cell, but DC and mixed myeloid cells (granulocytic/monocytic) as well. Finding that most single cells that resulted in B-lineage cell differentiation also resulted in NK cell and DC or myeloid differentiation (at least three lineages from one cell) suggests origin from a very primitive progenitor.
In experiments eliminating single cytokines, the B-lineage cell differentiating capacity of this culture was dependent on FL, IL-7, and stromal ligands in agreement with previous reports in human and murine lymphopoiesis showing a role for one or more of these factors.36-41 Although the B-lineage cell development from CD34+/Lin−/DR− cells occurred without KL, its addition optimizes growth for readout detection. IL-7, FL, and KL were required to induce development along the NK cell lineage. This is in agreement with Silva et al,5 who demonstrate that NK differentiation is independent of IL-2 as long as IL-7 is present. The finding that KL potentiates outgrowth fits with the finding of c-kit receptor on lymphoid progenitors and more primitive CD56+bright blood NK cells.42,43 The ability of AFT024 to induce NK cell, B-lineage cell, and other myeloid lineages from the same cell depends on properties of the murine AFT024 fetal cell line. M2-10B4 produces soluble factors and provides contact-mediated growth promoting ligands to mature NK cells.24,33 Although M2-10B4 can support primitive myelopoiesis,21 it poorly supports NK cell differentiation and does not support B-cell development from primitive human progenitors. The capacity of the AFT024 cell line to support lymphoid differentiation may be, in part, related to its developmental fetal origin. Pribyl and LeBien44 have shown that human fetal stroma can differentiate fetal CD34++/Lin− cells into mu/kappa or mu/lambda expressing B cells and differentiation was IL-7–independent. Alternatively, there may be unique properties of murine stromal cell lines to support B-cell differentiation such as the S17 cell line reported by Rawlings et al45,46 or by the MS-5 cell line described by Berardi et al.47 However, the cytokine-independent differentiation observed with S17 and MS-5 is in contrast to results shown here with the AFT024 cell line. In addition to different murine stroma cell lines, results may be explained in part by our use of adult marrow progenitors contrasted to progenitors from cord blood, which may contain more immature stem cells with different cytokine requirements than adult bone marrow.48
The B-lineage cells, which developed from adult marrow progenitors, were developmentally blocked at the pro-B cell stage by the absence of surface heavy and light chains and the presence of TdT. The detection of CD20 and CD21 on a small proportion of CD19+ cells may suggest early progression to B-cell maturation with D-J gene rearrangement but the absence of surface IgM shows they are not able to develop into mature B cells.49,50 The phenotype of B-lineage cells resulting from AFT024 cultures is similar to that shown by Rawlings et al45 with the exception that less than 3% of their S17 cultured B-lineage cells were TdT positive, while more AFT024 B-lineage cell progeny were positive for TdT by both in situ staining and RT-PCR.
Single-cell experiments differed from bulk cultures in the absolute requirement of a one time addition of 5 ng/mL of IL-3 at culture initiation. Whether this is required for mere survival or proliferation induction is uncertain. The role of early acting cytokines can alter the self-renewal, viability, or proliferation of single CD34+CD38− cells.51 For example, in studies of single CD34+/CD38−progenitors, 60 ng/mL IL-3 in addition to 300 ng/mL of FL and KL were necessary to obtain optimal amplification of myeloid LTC-IC and colony-forming cells (CFC).52 However, when 10 ng/mL of FL and KL were used, the same concentration of IL-3 decreased LTC-IC expansion and increased CFC expansion, suggesting that the net effect of multiple cytokines is determined not only by the cytokines themselves, but also their relative concentrations. The requirement for IL-3 early in the culture period differs from several murine studies by Ogawa’s group demonstrating that IL-3 is inhibitory to B cell, T-cell, and NK cell development.34,42,53,54However, the concentration of IL-3 in our cultures was low and distant (4 to 6 weeks) from the readout of lymphoid progeny. In addition, IL-3–induced lymphoid suppression may not be absolute, as the addition of IL-4 in combination with IL-11 or IL-6 reversed the IL-3–induced inhibition on early B-cell development.55 The sequential, low concentration, one time addition of IL-3 clearly increased NK proliferation, possibly by upregulation of IL-2 receptors by simultaneous exposure to KL, as proposed by Shibuya et al.56
The cloning frequency from the single cell experiments was consistently higher than from the limiting dilution assays. This may be explained by competitive interactions within the AFT024 cultures. In addition to the interaction with AFT024, the contribution of human progeny cell interactions induced by AFT024 may be critical to the resultant multilineage lymphoid and myeloid differentiation. Differentiation of DC progeny expressing costimulatory molecules or monocyte progeny secreting IL-15 may interact with B-lineage cells and NK cells, respectively. Similarly, transforming growth factor-β (TGF-β), a potent costimulator of FL-induced DC growth,57 may be present in culture by activation of latent TGF-β in serum by the extracellular matrix component thrombospondin or by production of TGF-β from developing NK cells.33 The complexities of these interactions will need further study.
In summary, using the murine fetal liver cell line, AFT024, and defined cytokines, long-term in vitro culture can differentiate single adult human CD34+/Lin−/CD38−cells into NK cells, B-lineage cells, DC, and myeloid lineages using a single culture condition. Our results are consistent with the NK cell, B-cell and DC differentiation pattern derived from the more committed CD34+/Lin−/CD45RA+/CD10+adult marrow cell reported by Galy et al.58 However, the additional myeloid lineage differentiation in AFT024 cultures, not found from CD34+/Lin−/CD45RA+/CD10+cells, distinguishes our system and suggests multilineage differentiation from a more primitive cell. The AFT024 cell line and IL-7, FL, KL, IL-2, and IL-3 are critical to this process. This in vitro hematopoietic culture gives quantitative information, which will be useful in comparing hematopoietic stem cell sources, and lends itself to easy manipulation of in vitro culture. The ability to efficiently induce differentiation from single primitive cells identifiable from adult marrow will provide new insights into mechanisms governing the earliest steps in lymphoid differentiation.
ACKNOWLEDGMENT
The authors thank Brad Anderson for his help with flow cytometry, Jeanne Lund for her excellent technical help in PCR assays, and Dr Tucker Lebien for his review of this manuscript. We also thank the special hematology staff at the University of Minnesota for performing the in situ TdT stains and Dr Naheed Mitha for the analysis.
Supported in part by National Institutes of Health Grants No. R29-HL-55417, R01-HL-54039, and PO1-CA-65493. M.P. was supported by a grant from the Deutscher Akademischer Austauschdienst. We also acknowledge the support of the University of Minnesota Bone Marrow Transplant Research Fund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jeffrey S. Miller, MD, University of Minnesota Cancer Center, Box 806, Division of Hematology, Oncology and Transplantation, Harvard St at East River Rd, Minneapolis, MN 55455; e-mail: mille011@tc.umn.edu.

![Fig. 3. Progeny of AFT024 cultures function to stimulate allogeneic mixed lymphocyte reactions. Monocyte-depleted peripheral blood mononuclear cells (105) from the same donor were mixed with irradiated (3,000 rads) autologous CD14+ fresh monocytes (▪), 6-week progeny of AFT024 cultures (•), and 5-week progeny of AFT024 cultures transferred for an additional week to media containing TNF, GM-CSF, and IL-4 (○). Proliferation (counts per minute [cpm]) was assessed after 5 days by pulsing for 18 hours with3H-thymidine and data are presented as the mean ± SEM cpm of triplicate wells from a representative experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.96/5/m_blod40113003x.jpeg?Expires=1769554035&Signature=cwog7ljy175rGPMUjHrmCUV4jCn72GJyqCZT640nI0t2o6GDrJ4ZoqBC6KhJflDcSTr6iuJ-phHZ29lzEj6BCraJjp4Zx2FG8zRyQwiU4PvYP-sAmLjydi21ggWY8KBgm38CHSHzSosgAj-VFWKSLOEAM39ad3JK-sVzffbWYStIgU41LPnrb9G0-f2bVt16tOPGfo39v4YeHJOdP2OhT-dIeef-PANEmKAS5eipomJUww4-zmrf1gRVbvql0isRLbW7GcEQ7yOq-2-xOZgmUNodrHm8eADJ7qwtDPio7TgYX3fNKgytqNh70kWKys5AbVyF38NVt-dnBGinMgkhhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 3. Progeny of AFT024 cultures function to stimulate allogeneic mixed lymphocyte reactions. Monocyte-depleted peripheral blood mononuclear cells (105) from the same donor were mixed with irradiated (3,000 rads) autologous CD14+ fresh monocytes (▪), 6-week progeny of AFT024 cultures (•), and 5-week progeny of AFT024 cultures transferred for an additional week to media containing TNF, GM-CSF, and IL-4 (○). Proliferation (counts per minute [cpm]) was assessed after 5 days by pulsing for 18 hours with3H-thymidine and data are presented as the mean ± SEM cpm of triplicate wells from a representative experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.96/5/m_blod40113003x.jpeg?Expires=1769554036&Signature=TWftZegzxKNvm-gHj18i4JEuG~TWLV6VVZFE9zgeIgk4iY4oOcwIec8TVVZ8S0RhohhJy0yJQ4xl33cL~MVcX3c8s1-MMcXIA4aPI8LpvGvTlhtBazQ6MDkVUf3U-boAZfSYZs3t6D72mAwCw7S4ONLaFFkP~Bzv5EVejaEAUbRVjXbhOjseFT4unrZ9d1DwQ~lvYXn0g7pXLKHAOUk-e1VACCsWijboBRNDgYEqapeQ8pqnX0ouEsxpadD5BZjU-3P~7idiD2smUxCKHSc6DxFbKtTWV00jCSVx4hHyQdydW-ShoPoSvV7wQ4NG7WMR1ivWKHQw26G~2~IHHH3AWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)