Human platelets contained about 15 times lower amounts of Rho-kinase than Ca2+/calmodulin-dependent myosin light chain (MLC) kinase. Anti–myosin-binding subunit (MBS) antibody coimmunoprecipitated Rho-kinase of human platelets, and addition of GTPγS-RhoA stimulated phosphorylation of the 130-kD MBS of myosin phosphatase and consequently inactivated myosin phosphatase. Two kinds of selective Rho-kinase inhibitors, HA1077 and Y-27632, reduced both GTPγS-RhoA–dependent MBS phosphorylation and inactivation of the phosphatase activity. Activation of human platelets with thrombin, a stable thromboxane A2 analog STA2, epinephrine, and serotonin resulted in an increase in MBS phosphorylation, and the agonist-induced MBS phosphorylation was prevented by pretreatment with the respective receptor antagonist. HA1077 and Y-27632 inhibited MBS phosphorylation in platelets stimulated with these agonists. These compounds also blocked agonist-induced inactivation of myosin phosphatase in intact platelets. In addition, HA1077 and Y-27632 inhibited 20-kD MLC phosphorylation at Ser19 and ATP secretion of platelets stimulated with STA2, thrombin (0.05 U/mL), and simultaneous addition of serotonin and epinephrine, whereas these compounds did not affect MLC phosphorylation or ATP secretion when platelets were stimulated with more than 0.1 U/mL thrombin. Thus, activation of Rho-kinase and the resultant phosphorylation of MBS is likely to be the common pathway for platelet activation induced by various agonists. These results also suggest that Rho-kinase–mediated MLC phosphorylation contributes to a greater extent to the platelet secretion induced by relatively weak agonists.
PLATELETS CAN BE activated by a number of agonists, including thrombin, thromboxane A2, epinephrine, and serotonin.1,2 An agonist may be classified as either strong or weak, depending on whether it causes full activation, including the release reaction. Thrombin is the most potent agonist for human platelets, whereas serotonin and epinephrine are weak agonists. All these agonist receptors are linked to heterotrimeric G proteins, and the earliest event after receptor-ligand interaction is the activation of phospholipase C, which hydrolyzes phosphatidylinositol 4,5-bisphosphate to generate inositol 1,4,5-triphosphate (IP3) and 1,2-diacylglyceride.1,2IP3 is the primary intracellular stimulus for Ca2+ mobilization from intracellular storage sites. An increase in Ca2+ leads to phosphorylation of the 20-kD light chain of myosin (MLC) at Ser19 via the Ca2+/calmodulin-dependent MLC kinase: this phosphorylation increases an actomyosin contractile response that is involved in platelet shape change and secretion.3-5 The level of MLC phosphorylation is regulated not only positively by MLC kinase, but also negatively by a myosin phosphatase. We have shown that myosin phosphatase from human platelets is composed of a 38-kD protein phosphatase 1δ catalytic subunit, 130-kD myosin-binding subunit (MBS), and 20-kD regulatory subunit,6 as is the case for smooth muscle phosphatase.7-9
There is increasing evidence that a number of small G proteins are involved in signal transduction pathways at the plasma membrane.10 The small G protein Rho and its target Rho-kinase are implicated in physiological functions associated with actin-myosin filaments such as shape change, cell mortility, secretion, and smooth muscle contraction.11-14 A recent study15 with botulinum C3 exoenzyme has suggested that RhoA is involved in discrete outside-in signaling responses in fibrinogen-adherent platelets, most prominently the formation of focal adhesion, although RhoA does not appear to be involved in either agonist-induced affinity modulation of integrin αIIbβ3 or in primary aggregation. Trimeric G-protein–coupled receptors appear to be the major upstream pathway for Rho activation.11 Rho-kinase, when activated by GTP-RhoA, phosphorylates MBS and thereby inhibits the catalytic activity of smooth muscle myosin phosphatase,16 so that MLC phosphorylation is increased, an event that induces the consequent contraction of smooth muscle at a constant Ca2+concentration (referred to as Ca2+sensitization).11 In permeabilized platelets, the Ca2+ sensitivity of serotonin secretion can be enhanced by GTPγS, without activation of phospholipase C.17 We have recently shown that platelet MBS is an in vitro substrate for Rho-kinase and that phosphorylation of MBS decreases the activity of platelet myosin phosphatase.6 Moreover, treatment of intact platelets with a stable thromboxane A2 analog STA2 led to an increase in MBS phosphorylation and a decrease in the activity of myosin phosphatase.6 In addition, a recent report has shown that Rho-kinase directly phosphorylates the 20-kD MLC in vitro at the site that is phosphorylated by MLC kinase, which causes activation of myosin ATPase.18 Thus, MLC phosphorylation at Ser19induced by various stimuli in intact cells may be mediated by not only Ca2+/calmodulin-dependent MLC kinase, but also by Rho-kinase. A pyridine derivative Y-27632 and an isoquinolinesulfonamide derivative HA1077 have recently been shown to be relatively selective inhibitors of Rho-kinase.19 Y-27632 not only inhibited the Ca2+ sensitivity of vascular smooth muscle, but also reduced high blood pressure in laboratory animals.19 HA1077 inhibited both vascular contractions and MLC phosphorylation in response to a variety of agents20and has been clinically used in Japan in the treatment of the cerebral vasospasm after subarachnoid hemorrhage.21 Using these inhibitors, we asked if Rho-kinase–induced MBS phosphorylation occurs in intact platelets in response to other agonists such as thrombin, serotonin, and epinephrine, as is the case for STA2. In addition, we also investigated whether RhoA-mediated MLC phosphorylation contributes to platelet secretion induced by these agonists.
MATERIALS AND METHODS
Materials.
GST-RhoA was expressed and purified using a glutathione-Sepharose column.13 HA1077 [1-(5-isoquinolinesulphonyl) homopiperazine hydrochloride] was generously provided by Asahi Chemical Industry Co, Ltd (Tokyo, Japan). Y-27632 [(+)-(R)-trans-4(1-aminoethyl)-N-(4-pynidyl) cyclohexanecarboxamide dihydrochloride, monohydrate] was a kind gift from Yoshitomi Pharmaceutical Industries Ltd (Osaka, Japan). Other drugs and suppliers were as follows: M-1 [(±)-3-dimethylamino 1-(o-(m-methoxypheneytyl) phenoxyl)-2-propanol] from Mitsubishi Kasei Corp, (Yokahama, Japan) STA2 [9, 11-epithio-11, 12-methano-thromboxane A2] and ONO-3708 [(9, 11), (11, 12)-dideoxa-9α, 11α-dimetylmethano-11, 12-methano-13, 14-dihydro-13-aza-14-oxo-15-cyclopentyl-16, 17, 18, 19, 20-pentanor-15-epi-thromboxane A2] from Ono Pharmaceutical Co, Ltd (Osaka, Japan); KT5926 [(8R*, 9S*, 11S*)-(−)-9-hydroxy-9-methoxycarbonyl-8-methyl-14-n-propoxy-2, 3, 9, 10-tetrahydro-8, 11-epoxy, 1H, 8H, 11H-2, 7b, 11a-triazadibenzo[a,g]cycloocta[cde]trinden-1-one] from Kyowa Medex Co, Ltd (Tokyo, Japan); acetoxymethyl ester of 5,5′-dimethyl-bis-(-o-aminophenoxy)-ethane-N,N,N,N,-tetraacetic acid (BAPTA-AM) from Dojindo Laboratories (Kumamoto, Japan); W-7 [N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride] from Seikagaku Corp (Tokyo, Japan); yohimbine from Wako Pure Chemical Industries (Osaka, Japan); and GTPγS from Boehringer Mannheim GmbH (Mannheim, Germany). [γ-32P]ATP (111 τBq/mmol) was from NEN Life Science Products, Inc (Boston, MA). [32P]orthophosphate was from ICN Pharmaceuticals, Inc (Costa Mesa, CA).
Measurement of Rho-kinase and MLC kinase in human platelets.
Amounts of Rho-kinase and MLC kinase in human platelets were estimated by immunoblot analysis using polyclonal antibodies specific against human platelet MLC kinase and Rho-kinase. Antisera against MLC kinase and Rho-kinase were obtained by immunizing rabbits with the purified MLC kinase from human platelets22 and with the synthesized fragment of Rho-kinase,13 respectively, and used after purification. Quantitative estimation of the levels of MLC kinase and Rho-kinase in human platelets was performed densitometrically by scanning the immunoreactive band after immediately photographing the visualized band.23 The signal was then compared with that of known amounts of the purified platelet MLC kinase or purified recombinant Rho-kinase.
Immunoprecipitation of platelet myosin phosphatase.
Immunoprecipitation of platelet myosin phosphatase was performed as described,6 with a few modifications. Platelets (800 μL) were dissolved in the lysis buffer (1% Nonidet P-40, 20 mmol/L Tris-HCl, pH 7.5, 0.15 mol/L NaCl, 4 mmol/L EDTA, 4 mmol/L phenylmethylsulfonyl fluoride [PMSF], 200 μg/mL leupeptin, 2 mmol/L sodium orthovanadate). The lysate was centrifuged at 15,000 ×g for 15 minutes. The soluble fraction precleared with Protein A Sepharose CL-4B (Pharmacia Biotech AB, Uppsala, Sweden) was incubated with 10 μL of anti-MBS antibody at 4°C for 1 hour. The immune complex was precipitated by adding protein A-Sepharose CL-4B, incubating for an additional 1 hour at 4°C, and then washing the beads three times with the lysis buffer. The immune complex contains three components of myosin phosphatase, namely MBS, PP1δ catalytic subunit, and the 20-kD subunit.6 We used these preparations to detect the phosphorylation and for assay of phosphatase activity.
In vitro MBS phosphorylation by Rho-kinase.
Immunoprecipitate with anti-MBS antibody from human platelets was used for in vitro MBS phosphorylation by Rho-kinase. The kinase reaction for Rho-kinase was performed in 50 μL of the reaction mixture (20 mmol/L Tris-HCl, pH 7.5, 2 mmol/L EDTA, 6 mmol/L MgCl2, 1 mmol/L dithiothreitol, 0.5 μmol/L okadaic acid, 25 μL of the immunoprecipitate, 5 μL of a dilution of drug, 10 μmol/L [γ-P32] ATP) and 1 μmol/L GTPγS·GST-RhoA or 1 μmol/L GDP·GST-RhoA.16 18 The kinase reaction was initiated by adding the ATP solution. Reactions were performed at 30°C for 30 seconds and terminated by the addition of sodium dodecyl sulfate (SDS) sample buffer. The samples were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and MBS phosphorylation was analyzed by autoradiography. To analyze the phosphatase activities, the kinase reaction was performed in 50 μL of the reaction mixture (20 mmol/L Tris-HCl, pH 7.5, 2 mmol/L EDTA, 6 mmol/L MgCl2, 1 mmol/L dithiothreitol, 25 μL of the immunoprecipitate, 5 μL of a dilution of drug, and 10 μmol/L ATP) at 30°C for 30 seconds. The reaction was terminated by adding 10 mmol/L EDTA, and phosphatase activity was measured immediately.
Preparation of human platelet suspension and measurements of ATP secretion.
Venous blood was freshly drawn from a healthy donor who had not taken any drugs for at least 2 weeks previously. Platelets (109/mL) were finally resuspended in a modified Tyrode-HEPES buffer that contained a final concentration of 0.14 mol/L NaCl, 2.7 mmol/L KCl, 1 mmol/L MgCl2, 0.1%D-glucose, 3.75 mmol/L NaH2PO4, and 15 mmol/L HEPES, pH 7.5. ATP secretion was measured using a Lumi-aggregometer (Chrono-Log Corp, Havertown, PA), as described.3 24 The standard platelet reaction mixtures consisted of 400 μL platelet suspension, 50 μL of Chrono-Lumi luciferase luciferin reagent, 50 μL of a dilution of drug (or saline), and an aggregating agent. Washed platelet suspension preincubated with the drug at 37°C for 3 minutes was activated by various agonists, under conditions of nonstirring. Epinephrine and/or serotonin was reacted in the Tyrode-HEPES buffer containing 1 mmol/L CaCl2.
Measurement of the phosphorylation of myosin phosphatase in intact platelets.
Washed platelets (800 μL) prelabeled with 18.5 MBq/mL [32P]orthophosphate (at 30°C for 60 minutes) were stimulated with the agonists, including thrombin, STA2, and epinephrine/serotonin. The reaction was terminated by adding one third volume of ×4 lysis buffer with a final concentration of 1 μmol/L calyculin A (Wako Pure Chemicals). Myosin phosphatase was precipitated by immunoprecipitation using an anti-MBS antibody, as mentioned above. Phosphoproteins were separated by SDS-PAGE. The gel was stained with Coomassie Blue, dried, and subjected to autoradiography. The radiolabeled bands were visualized using a Bio Imaging Analyzer BAS2000 (Fuji Photo Film Co, Ltd, Tokyo, Japan) and then exposed to Kodak X-Omat AR film (Eastman Kodak, Co, Rochester, NY) with an intensifying screen at −80°C. Quantitative estimation of the level of phosphorylation was performed densitometrically, using a Molecular Dynamics Scanning Densitometer (Sunnyvale, CA) in conjugation with the ImageQuant program run on a Dell Personal Computer (Austin, TX) by scanning the radioactive bands.23 The area of an individual peak was measured above background in densitometric tracing and was estimated as an arbitrary unit.
Measurement of myosin phosphatase activity in intact platelets.
Activities of myosin phosphatase were determined using [32P]-phosphorylated myosin light chain from chicken gizzards as a substrate.6 Myosin phosphatase was precipitated by immunoprecipitation using anti-MBS antibody from platelets activated by 1 μmol/L STA2. PP1 activity of the sample was measured in a reaction mixture (50 μL) containing 5 μL of immunoprecipitate, 20 mmol/L Tris-HCl, pH 7.4, 50 mmol/L NaCl, 0.1 mmol/L EGTA, 2 mmol/L sodium orthovanadate, 10 nmol/L okadaic acid, a heat stable phosphatase inhibitor-2, 4 mmol/L PMSF, and 200 μg/mL leupeptin. PP1 activity can be taken as the activity that is sensitive to the inhibitor-223 and was regarded as myosin phosphatase activity.6
Detection of 20-kD MLC monophosphorylated at Ser19 in intact platelets.
The monoclonal antibody specific against monophosphorylated 20-kD MLC at Ser19 was prepared as described elsewhere.25Washed platelets preincubated with various concentrations of HA1077 or Y-27632 in the presence or absence of 7.5 μmol/L BAPTA-AM for 3 minutes at 37°C were stimulated with thrombin, STA2, or serotonin plus epinephrine for 30 seconds, without stirring. Precipitates treated with 10% trichloroacetic acid from platelets were subjected to SDS-PAGE to assay the 20-kD MLC phosphorylation at Ser19 and to glycerol-PAGE to determine the extent of 20-kD MLC phosphorylation. Immunoblot analysis with the antimonophosphorylated MLC antibody was performed to detect the phosphorylation of 20-kD MLC. The extent of 20-kD MLC phosphorylation was expressed as percentages of 20-kD MLC in the monophosphorylated form.20,25 26
RESULTS
Levels of Rho-kinase and MLC kinase in human platelets.
We determined the amount of Rho-kinase and MLC kinase in whole platelets by immunoblot analysis, using purified recombinant Rho-kinase or the MLC kinase purified from human platelets,22respectively, as standards. The amount of Rho-kinase in whole platelets was 1.11 ± 0.325 ng/107 platelets (0.047 ± 0.014 μmol/L; mean ± SD; n = 4). The amount of platelet MLC kinase was 10.4 ± 2.05 ng/107 platelets (0.695 ± 0.137 μmol/L; n = 3). The amount of platelet Rho-kinase was about 15 times lower than that of MLC kinase.
Effects of Rho-kinase inhibitors on in vitro phosphorylation of platelet MBS.
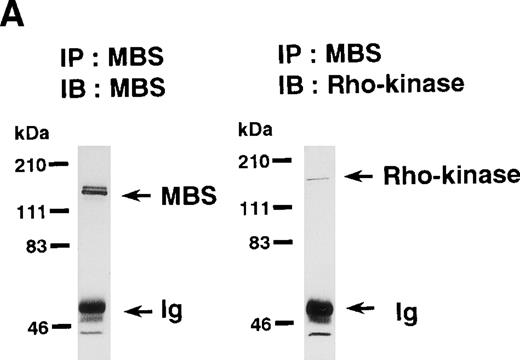
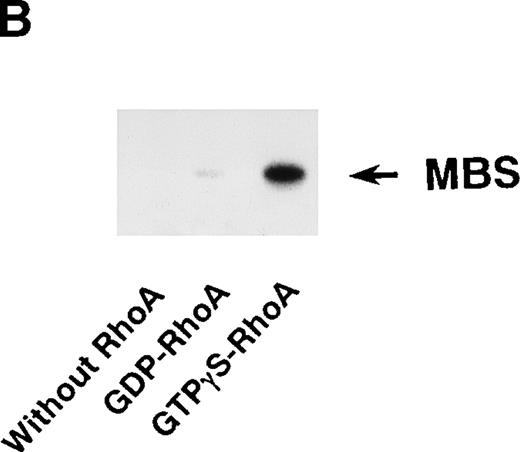
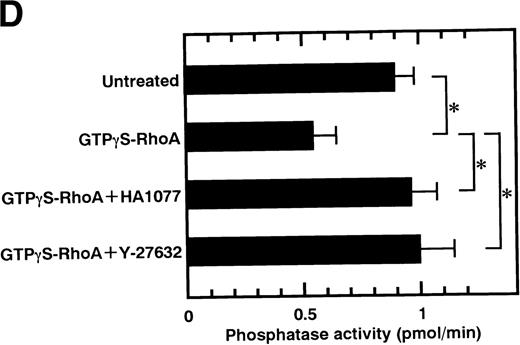
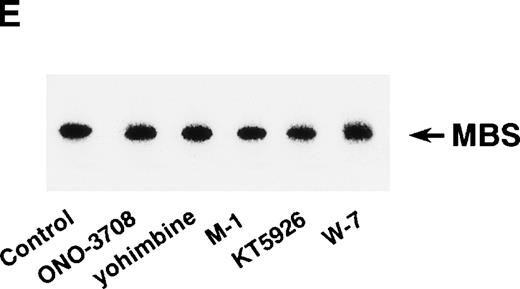
Rho-kinase was detected by immunoblot analysis in the anti-MBS immunoprecipitate (Fig 1A), as we reported.6 We did not detect protein kinase N in the anti-MBS immunoprecipitates, although activated Rho does interact with protein kinase N and Rho-kinase and stimulates their kinase activities.12-14,27,28 We then asked if phosphorylation of MBS by Rho-kinase was GTP-RhoA dependent, determined using the anti-MBS immunoprecipitates. RhoA has GDP-bound inactive and GTP-bound active forms that are interconvertible by GDP-GTP exchange and GTPase reactions.29,30 We made use of GTPγS (a nonhydrolyzable GTP analog) for activation of RhoA and thereby stimulation of Rho-kinase activity. As shown in Fig 1B, phosphorylation of MBS was prominent in the presence of GTPγS-RhoA, whereas GDP-RhoA had a much weaker effect. Phosphorylation was nil in the absence of Rho. These findings indicate that the phosphorylation of MBS in the immunoprecipitate is completely dependent on GTPγS-RhoA. To confirm whether the MBS phosphorylation induced by GTPγS-RhoA is catalyzed by Rho-kinase, we examined effects of Rho-kinase inhibitors. MBS phosphorylation was inhibited by HA1077 or Y-27632 in a dose-dependent manner (Fig 1C). IC50 values of HA1077 and Y-27632 for the inhibition of MBS phosphorylation were 10 and 0.3 μmol/L, respectively. We then investigated whether the inhibition of MBS phosphorylation by HA1077 and Y-27632 was associated with the phosphatase activity, tested using anti-MBS immunoprecipitates (Fig1D). Incubation with GTPγS-RhoA produced a significant decrease in the phosphatase activity of anti-MBS immunoprecipitates and the addition of HA1077 or Y-27632 restored the phosphatase activity. HA1077 and Y-27632 did not affect the phosphatase activity of protein phosphatase 1, 2A, 2B, or 2C (data not shown). GTPγS-RhoA–dependent phosphorylation of MBS was not inhibited by receptor antagonists such as ONO-3708 (thromboxane A2 receptor antagonist31), yohimbine (α2 receptor antagonist32), and M-1 (S2-serotonergic receptor antagonist33), the MLC kinase inhibitor KT5926,34 or the calmodulin antagonist W-73(Fig 1E).
In vitro phosphorylation of platelet MBS and inactivation of myosin phosphatase by Rho-kinase. (A) Coimmunoprecipitation of Rho-kinase with platelet MBS. Immunoprecipitates with anti-MBS antibodies were immunoblotted with antibodies against MBS (left) and Rho-kinase (right). IP, immunoprecipitation antibodies used; IB, immunoblotting antibodies used; Ig, cross-reacted Ig. (B) In vitro phosphorylation of platelet MBS. MBS immunoprecipitates from platelet lysates were incubated without RhoA, with GDP-RhoA, and with GTPγS-RhoA for 30 seconds, as described in Materials and Methods. Protein phosphorylation was analyzed by SDS-PAGE, followed by autoradiography. (C) Inhibitory effect of HA1077 and Y-27632 on GTPγS-RhoA–dependent phosphorylation of platelet MBS. Different concentrations of HA1077 (left panel) or Y-27632 (right panel) were included in the reaction mixture, and MBS phosphorylation was analyzed, as described in Materials and Methods. Results were expressed as the percentage of the value, without the addition of compounds. The results are representative of three independent experiments. (D) Effects of HA1077 and Y-27632 on the activity of myosin phosphatase derived from MBS immunoprecipitates in the presence of GTPγS-RhoA. MBS immunoprecipitates were incubated with 20 μmol/L HA1077 or 10 μmol/L Y-27632 in the presence of GTPγS-RhoA for 30 seconds, and the activity of myosin phosphatase was determined immediately. The value shows the mean ± SE from three experiments. *P < .05 (E) Effects of various compounds on GTPγS-RhoA–dependent phosphorylation of platelet MBS. Immunoprecipitates with anti-MBS antibody from platelet lysates were incubated for 30 seconds with 10 nmol/L M-1, 10 nmol/L yohimbine, 100 nmol/L ONO-3708, 100 nmol/L KT5926, or 50 μmol/L W-7 in the presence of GTPγS-RhoA, as described in Materials and Methods. Protein phosphorylation was analyzed by SDS-PAGE, followed by autoradiography. Similar results were obtained in three other experiments.
In vitro phosphorylation of platelet MBS and inactivation of myosin phosphatase by Rho-kinase. (A) Coimmunoprecipitation of Rho-kinase with platelet MBS. Immunoprecipitates with anti-MBS antibodies were immunoblotted with antibodies against MBS (left) and Rho-kinase (right). IP, immunoprecipitation antibodies used; IB, immunoblotting antibodies used; Ig, cross-reacted Ig. (B) In vitro phosphorylation of platelet MBS. MBS immunoprecipitates from platelet lysates were incubated without RhoA, with GDP-RhoA, and with GTPγS-RhoA for 30 seconds, as described in Materials and Methods. Protein phosphorylation was analyzed by SDS-PAGE, followed by autoradiography. (C) Inhibitory effect of HA1077 and Y-27632 on GTPγS-RhoA–dependent phosphorylation of platelet MBS. Different concentrations of HA1077 (left panel) or Y-27632 (right panel) were included in the reaction mixture, and MBS phosphorylation was analyzed, as described in Materials and Methods. Results were expressed as the percentage of the value, without the addition of compounds. The results are representative of three independent experiments. (D) Effects of HA1077 and Y-27632 on the activity of myosin phosphatase derived from MBS immunoprecipitates in the presence of GTPγS-RhoA. MBS immunoprecipitates were incubated with 20 μmol/L HA1077 or 10 μmol/L Y-27632 in the presence of GTPγS-RhoA for 30 seconds, and the activity of myosin phosphatase was determined immediately. The value shows the mean ± SE from three experiments. *P < .05 (E) Effects of various compounds on GTPγS-RhoA–dependent phosphorylation of platelet MBS. Immunoprecipitates with anti-MBS antibody from platelet lysates were incubated for 30 seconds with 10 nmol/L M-1, 10 nmol/L yohimbine, 100 nmol/L ONO-3708, 100 nmol/L KT5926, or 50 μmol/L W-7 in the presence of GTPγS-RhoA, as described in Materials and Methods. Protein phosphorylation was analyzed by SDS-PAGE, followed by autoradiography. Similar results were obtained in three other experiments.
Effects of Rho-kinase inhibitors on agonist-induced phosphorylation of MBS in intact platelets.
We reported that treatment of intact platelets with STA2led to an increase in phosphorylation of MBS and to a decrease in the activity of myosin phosphatase.6 To determine if phosphorylation of MBS occurs in platelets stimulated by agonists other than STA2, we examined immunoprecipitates using anti-MBS antibody of [32P]Pi-labeled platelets, before and after stimulation with STA2, thrombin, serotonin, and epinephrine. As shown in Fig 2A, a phosphorylated band at 130-kD was detected in the immunoprecipitates, and the level of MBS phosphorylation increased rapidly for up to 1 minute after exposure to each agonist, in nonstirred platelets. Amounts of precipitated MBS remained unchanged after stimulation (data not shown). Another phosphorylated protein with a molecular weight of 120-kD was detected in the case of either thrombin or STA2; the function was not investigated. To determine whether agonist-induced phosphorylation of MBS is catalyzed by Rho-kinase, we studied the effects of the Rho-kinase inhibitors, HA1077 and Y-27632, on STA2-induced MBS phosphorylation in intact platelets. Both HA1077 and Y-27632 produced a dose-dependent inhibition of STA2-induced phosphorylation of MBS (Fig 2B). We then examined effects of these Rho-kinase inhibitors on STA2-induced inactivation of myosin phosphatase in intact platelets (Fig 2C). Pretreatment of intact platelets with HA1077 or Y-27632 blocked the inactivation of myosin phosphatase induced by STA2. These data support the idea that Rho-kinase activation is involved in agonist-induced MBS phosphorylation with the resultant inactivation of myosin phosphatase in human platelets. These soluble agonists have been shown to activate respective G-protein–coupled receptors in platelets,1,2 which leads to cytoskeletal rearrangements, granule secretion, fibrinogen receptor activation, and aggregation. The signaling events that evoke these receptors are unclear, but there is general agreement that low molecular weight GTP-binding proteins play a central role. We therefore determined whether Rho-kinase activation is linked to G-protein–coupled receptors, and for this we used respective receptor antagonists, including ONO-3708, M-1, and yohimbine. Pretreatment of intact platelets with these antagonists inhibited the MBS phosphorylation induced by respective agonists, as shown in Fig 2D. The S2-serotonergic receptor antagonist M-1, at concentrations (10 nmol/L) blocking the aggregatory effect of simultaneous addition of serotonin and collagen,33 inhibited the secretory response of platelets evoked by both serotonin and epinephrine. The finding of complete inhibition by M-1 of synergic responses of serotonin and epinephrine is in accordance with previous observations by other investigators who used the S2-serotonergic receptor antagonist ketanserin.35 36 M-1 also inhibited the MBS phosphorylation induced by the simultaneous addition of serotonin and epinephrine (Fig 2D). Both HA1077 and Y-27632 abolished MBS phosphorylation induced by simultaneous addition of serotonin and epinephrine (Fig 2D) and by 0.1 U/mL thrombin (Fig 2E). These results suggest that activation of Rho-kinase and the resultant phosphorylation of MBS is likely to be the common pathway for the platelet activation induced by various agonists.
Agonist-induced phosphorylation of MBS and the inactivation of myosin phosphatase in intact platelets. (A) Agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were stimulated for the indicated time with either 1 μmol/L STA2, 0.1 U/mL thrombin, 1 μmol/L serotonin, or 1 μmol/L epinephrine. *An unknown protein of 120 kD. (B) Inhibition by HA1077 and Y-27632 of STA2-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with various concentrations of HA1077 (left panel) or Y-27632 (right panel) were stimulated for 30 seconds with 1 μmol/L STA2, without stirring. Results were expressed as the percentage of the value without the addition of compounds. Similar results were obtained in three other experiments, using different donor platelets. (C) Effects of HA1077 and Y-27632 on STA2-induced inactivation of myosin phosphatase in intact platelets. Human platelets incubated with saline (control; □), 20 μmol/L HA1077 (▪), or 10 μmol/L Y-27632 (▦) for 5 minutes were activated with 1 μmol/L STA2without stirring. MBS was immunoprecipitated with anti-MBS antibody and the activity of myosin phosphatase was determined immediately, as described in Materials and Methods. Results are expressed as the percentage of the value, without the addition of compounds. Data represent the mean ± SE of four experiments. *P < .05, **P < .01 (D) The inhibitory effect of receptor antagonists on agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were pretreated with 0.1 μmol/L ONO-3708, 10 nmol/L M-1, 10 nmol/L yohimbine, 10 μmol/L Y-27632, or 20 μmol/L HA1077 for 5 minutes at 37°C and were activated with 1 μmol/L STA2, 1 μmol/L serotonin, or 1 μmol/L epinephrine for 30 seconds, without stirring. Similar results were obtained in three other experiments, using different donor platelets. (E) Inhibition by HA1077 and Y-27632 of thrombin-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with 20 μmol/L HA1077 or 10 μmol/L Y-27632 were stimulated for 30 seconds with 0.1 U/mL thrombin without stirring. Similar results were obtained in three other experiments, using different donor platelets.
Agonist-induced phosphorylation of MBS and the inactivation of myosin phosphatase in intact platelets. (A) Agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were stimulated for the indicated time with either 1 μmol/L STA2, 0.1 U/mL thrombin, 1 μmol/L serotonin, or 1 μmol/L epinephrine. *An unknown protein of 120 kD. (B) Inhibition by HA1077 and Y-27632 of STA2-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with various concentrations of HA1077 (left panel) or Y-27632 (right panel) were stimulated for 30 seconds with 1 μmol/L STA2, without stirring. Results were expressed as the percentage of the value without the addition of compounds. Similar results were obtained in three other experiments, using different donor platelets. (C) Effects of HA1077 and Y-27632 on STA2-induced inactivation of myosin phosphatase in intact platelets. Human platelets incubated with saline (control; □), 20 μmol/L HA1077 (▪), or 10 μmol/L Y-27632 (▦) for 5 minutes were activated with 1 μmol/L STA2without stirring. MBS was immunoprecipitated with anti-MBS antibody and the activity of myosin phosphatase was determined immediately, as described in Materials and Methods. Results are expressed as the percentage of the value, without the addition of compounds. Data represent the mean ± SE of four experiments. *P < .05, **P < .01 (D) The inhibitory effect of receptor antagonists on agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were pretreated with 0.1 μmol/L ONO-3708, 10 nmol/L M-1, 10 nmol/L yohimbine, 10 μmol/L Y-27632, or 20 μmol/L HA1077 for 5 minutes at 37°C and were activated with 1 μmol/L STA2, 1 μmol/L serotonin, or 1 μmol/L epinephrine for 30 seconds, without stirring. Similar results were obtained in three other experiments, using different donor platelets. (E) Inhibition by HA1077 and Y-27632 of thrombin-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with 20 μmol/L HA1077 or 10 μmol/L Y-27632 were stimulated for 30 seconds with 0.1 U/mL thrombin without stirring. Similar results were obtained in three other experiments, using different donor platelets.
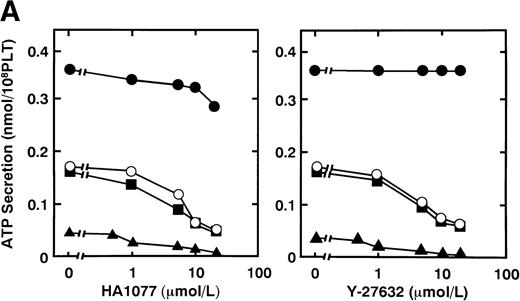
Effects on agonist-induced platelet secretion and phosphorylation of 20-kD MLC by two kinds of Rho-kinase inhibitors, HA1077 and Y-27632.
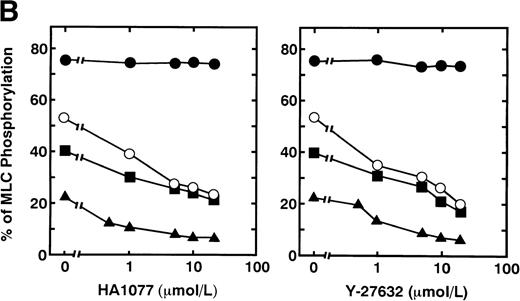
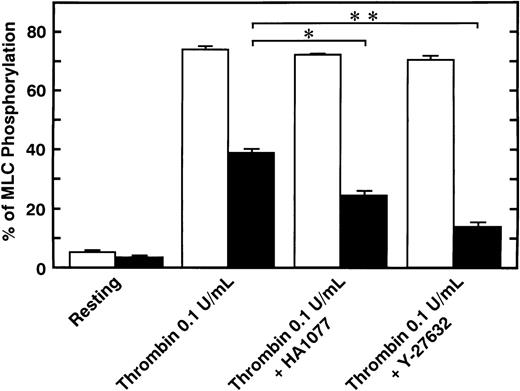
Serotonin and epinephrine are weak agonists that by themselves activate platelets with only weak potency and to a limited degree. These agonists by themselves did not induce secretion and aggregation of washed human platelets, unlike thrombin and STA2, but, when combined, ATP secretion occurs to some extent, under conditions of no stirring. As shown in Fig 3A, ATP secretion was more extensive with 0.1 U/mL thrombin, was to a lesser extent with 0.05 U/mL thrombin or 1 μmol/L STA2, and was least with the simultaneous addition of 1 μmol/L epinephrine and 1 μmol/L serotonin. We then examined the effects of two kinds of Rho-kinase inhibitors, HA1077 and Y-27632, on platelet secretion stimulated with various agonists such as thrombin, STA2 and a combination of serotonin with epinephrine. HA1077 and Y-27632 inhibited dose-dependently the ATP secretion induced by STA2, a low level (0.05 U/mL) of thrombin, and a simultaneous addition of serotonin and epinephrine (Fig 3A). IC50 values producing a 50% inhibition of ATP secretion of HA1077 and Y-27632 for STA2-induced secretion were 5.48 ± 1.80 and 11.2 ± 1.92 μmol/L, respectively. IC50values of HA1077 and Y-27632 for secretion induced by a low level (0.05 U/mL) of thrombin were 8.75 ± 3.25 and 7.55 ± 1.45, respectively. IC50 values of HA1077 and Y-27632 for secretion induced by a combination of serotonin plus epinephrine were 1.67 ± 0.440 and 4.83 ± 1.09 μmol/L, respectively. On the other hand, these compounds did not inhibit a high level (0.1 U/mL) of thrombin-induced platelet secretion. We then examined effects of HA1077 and Y-27632 on agonist-induced phosphorylation of 20-kD MLC in intact platelets (Fig 3B). MLC phosphorylation was analyzed by immunoblot analysis using an antibody specific for the 20-kD MLC phosphorylated at Ser19, the objective being to exclude MLC phosphorylation at other sites (eg, by protein kinase C37). The extent of MLC phosphorylation at Ser19 depended on strength of the agonist: most prominent phosphorylation (75%) with 0.1 U/mL thrombin, a lesser extent (53%) with 0.05 U/mL thrombin, a following extent (40%) with 1 μmol/L STA2, and to the least extent (22%) with the simultaneous addition of 1 μmol/L epinephrine and 1 μmol/L serotonin. The extent of ATP secretion by these agonists appeared to parallel the extent of MLC phosphorylation at Ser19. HA1077 and Y-27632 inhibited dose-dependently the phosphorylation of 20-kD MLC stimulated with a low level (0.05 U/mL) of thrombin, STA2 and the simultaneous addition of serotonin and epinephrine. These compounds did not inhibit a high level (0.1 U/mL) of thrombin-induced phosphorylation of 20-kD MLC. In the presence of a higher concentration of thrombin (>0.1 U/mL), neither platelet secretion nor 20-kD MLC phosphorylation was affected by these inhibitors (data not shown). Thrombin (0.1 U/mL) stimulation of platelets is associated with a dramatic increase in intracellular Ca2+ concentration.38 39 To further clarify the role of Rho-kinase in mediating MLC phosphorylation in the case of 0.1 U/mL thrombin stimulation, we examined the effect of intracellular Ca2+ chelator BAPTA-AM on inhibition by Rho-kinase inhibitors of thrombin-induced MLC phosphorylation. As shown in Fig 4, thrombin (0.1 U/mL)-induced increase in MLC phosphorylation was inhibited by preincubation with BAPTA-AM, and subsequent treatment with HA1077 or Y-27632 resulted in further inhibition of the thrombin-induced MLC phosphorylation.
Effects of HA1077 and Y-27632 on agonist-induced ATP secretion of human platelets and MLC phosphorylation at Ser19 in intact platelets. (A) Effects of HA1077 and Y-27632 on agonist-induced ATP secretion. Human platelets incubated for 3 minutes in the aggregometer with various concentrations of HA1077 (left panel) or Y-27632 (right panel) were stimulated with 0.1U/mL thrombin (•), 0.05 U/mL thrombin (○), 1 μmol/L STA2(▪), or a mixture of 1 μmol/L serotonin and 1 μmol/L epinephrine (▴) for 30 seconds, without stirring. Control levels of ATP secretion induced by 0.1 U/mL thrombin, 0.05 U/mL thrombin, STA2, and a mixture of serotonin and epinephrine were 354 ± 23.1, 184 ± 15.5, 173 ± 13.1, and 39.6 ± 2.58 pmol/108 platelets (n = 3), respectively. The values represent the average of three experiments (SD <10%). (B) Effects of HA1077 and Y-27632 on agonist-induced MLC phosphorylation at Ser19 in intact platelets. Washed platelets preincubated with various concentrations of HA1077 (left panel) or Y-27632 (right panel) were stimulated with 0.1 U/mL thrombin (•), 0.05 U/mL thrombin (○), 1 μmol/L STA2 (▪), or a mixture of 1 μmol/L serotonin and 1 μmol/L epinephrine (▴), without stirring. The extent of 20-kD MLC phosphorylation was expressed as the percentage of 20-kD MLC in the monophosphorylated form. The values represent the average of three experiments (SD <10%)
Effects of HA1077 and Y-27632 on agonist-induced ATP secretion of human platelets and MLC phosphorylation at Ser19 in intact platelets. (A) Effects of HA1077 and Y-27632 on agonist-induced ATP secretion. Human platelets incubated for 3 minutes in the aggregometer with various concentrations of HA1077 (left panel) or Y-27632 (right panel) were stimulated with 0.1U/mL thrombin (•), 0.05 U/mL thrombin (○), 1 μmol/L STA2(▪), or a mixture of 1 μmol/L serotonin and 1 μmol/L epinephrine (▴) for 30 seconds, without stirring. Control levels of ATP secretion induced by 0.1 U/mL thrombin, 0.05 U/mL thrombin, STA2, and a mixture of serotonin and epinephrine were 354 ± 23.1, 184 ± 15.5, 173 ± 13.1, and 39.6 ± 2.58 pmol/108 platelets (n = 3), respectively. The values represent the average of three experiments (SD <10%). (B) Effects of HA1077 and Y-27632 on agonist-induced MLC phosphorylation at Ser19 in intact platelets. Washed platelets preincubated with various concentrations of HA1077 (left panel) or Y-27632 (right panel) were stimulated with 0.1 U/mL thrombin (•), 0.05 U/mL thrombin (○), 1 μmol/L STA2 (▪), or a mixture of 1 μmol/L serotonin and 1 μmol/L epinephrine (▴), without stirring. The extent of 20-kD MLC phosphorylation was expressed as the percentage of 20-kD MLC in the monophosphorylated form. The values represent the average of three experiments (SD <10%)
Effect of BAPTA-AM on the inhibition by HA1077 and Y-27632 on thrombin (0.1 U/mL)-induced MLC phosphorylation in intact platelets. Platelets pretreated with vehicle (□) or 7.5 μmol/L BAPTA-AM (▪) for 3 minutes were treated with 20 μmol/L HA1077 or 10 μmol/L Y-27632 for 5 minutes. Platelets were stimulated with 0.1 U/mL thrombin for 20 seconds without stirring, and MLC phosphorylation was analyzed, as described for Fig 3. Data represent the mean ± SE of three experiments. *P < .05, **P < .01.
Effect of BAPTA-AM on the inhibition by HA1077 and Y-27632 on thrombin (0.1 U/mL)-induced MLC phosphorylation in intact platelets. Platelets pretreated with vehicle (□) or 7.5 μmol/L BAPTA-AM (▪) for 3 minutes were treated with 20 μmol/L HA1077 or 10 μmol/L Y-27632 for 5 minutes. Platelets were stimulated with 0.1 U/mL thrombin for 20 seconds without stirring, and MLC phosphorylation was analyzed, as described for Fig 3. Data represent the mean ± SE of three experiments. *P < .05, **P < .01.
DISCUSSION
HA1077 and Y-27632 were reported to be relatively selective inhibitors of Rho-kinase, compared with their inhibition of MLC kinase and protein kinase C.19 Y-27632 inhibits Rho-kinase in vitro with a Ki value of 0.14 μmol/L, a value about 185 times lower than that for protein kinase C (Ki = 26 μmol/L), and it does not significantly inhibit MLC kinase (Ki > 250 μmol/L). HA1077 is also a more potent inhibitor of Rho-kinase (Ki = 0.33 μmol/L) than against protein kinase C (Ki = 7.7 μmol/L) and MLC kinase (Ki = 170 μmol/L). We show here that MBS was phosphorylated in a GTPγS-RhoA–dependent manner and that HA1077 and Y-27632 inhibited, dose-dependently, GTPγS-RhoA–induced MBS phosphorylation, determined using anti-MBS immunoprecipitates as sources of platelet MBS and Rho-kinase. However, GTPγS-RhoA–dependent MBS phosphorylation was not inhibited by the agonist receptor antagonist, the calmodulin antagonist W-7, or the MLC kinase inhibitor KT5926. Moreover, HA1077 and Y-27632 also inhibited GTPγS-RhoA–induced inactivation of myosin phosphatase activity in anti-MBS immunoprecipitates. These results suggest that HA1077 and Y-27632 inhibited in platelets both Rho-kinase–induced MBS phosphorylation and the resultant attenuation of myosin phosphatase activity.
Phosphorylation of MBS occurred in intact platelets in response to agonists such as thrombin, STA2, epinephrine, and serotonin. Two kinds of Rho-kinase inhibitors produced a significant inhibition of MBS phosphorylation induced by not only a combination of the weak agonists serotonin and epinephrine, but also by 0.1 U/mL of, a strong agonist, thrombin in intact platelets. HA1077 and Y-27632 inhibited STA2-induced MBS phosphorylation with similar potency (IC50 values: 2 μmol/L with HA1077 and 8 μmol/L with Y-27632), whereas the IC50 value for HA1077 (10 μmol/L) in inhibiting GTPγS-RhoA–dependent MBS phosphorylation was higher than that for Y-27632 (0.3 μmol/L). The concentrations of both compounds required to affect MBS phosphorylation and ATP secretion in intact platelets were similar to those in other reports.19,20 One possible explanation of discrepancy found in a cell-free system vis-à-vis intact platelets would be the difference in penetration of the compound through the platelet membrane, and this aspect is now being investigated. The agonist-induced MBS phosphorylation was also inhibited by receptor antagonists such as an S2-serotonergic receptor antagonist M-1, a thromboxane A2 receptor antagonist ONO-3708, and a α2-adrenergic receptor antagonist yohimbine. These data indicate that MBS phosphorylation appears to be downstream of the agonist-receptor interaction, although the molecular mechanisms of signal transduction between the trimeric G-protein–coupled receptor and RhoA remain to be identified. In addition, HA1077 and Y-27632 significantly prevented the STA2-induced decrease in the myosin phosphatase activity in intact platelets. These results suggest that the Rho-kinase–induced MBS phosphorylation pathway and the resultant inactivation of myosin phosphatase are the common pathway in platelet activation stimulated by a variety of agonists. Rho-kinase and myosin phosphatase have been shown to regulate phosphorylation of α-adducin, a membrane cytoskeletal protein that participates in assembly of the spectrin-actin network.40 Therefore, Rho-kinase and myosin phosphatase may be involved in cell functions in addition to the regulation of the contractile cytoskeleton.
The extent of platelet ATP secretion depended on strength of the agonist; a high level (0.1U/mL) of thrombin > a low level (0.05 U/mL) of thrombin ≈ STA2 > simultaneous stimulation of epinephrine and serotonin. Moreover, the extent of secretion also appeared to parallel the extent of MLC phosphorylation at Ser19. RhoA appears to regulate MLC phosphorylation via two pathways: one is the direct phosphorylation of myosin at Ser19 by Rho-kinase,18 and the second is inactivation of myosin phosphatase through MBS phosphorylation by Rho-kinase.16 Both Rho-kinase–mediated pathways might be important for an increase in MLC phosphorylation, because both pathways (as well as the MLC kinase pathway) result in increased phosphorylation of Ser19 in the MLC and therefore activate myosin ATPase activity. Another target for Rho, protein kinase N did not phosphorylate MBS nor the 20-kD MLC at Ser19 (data not shown). Rho-kinase inhibitors should have an inhibitory effect on both of these Rho-kinase–mediated pathways in intact platelets but not MLC kinase phosphorylation. Uehata et al19 reported that Y-27632 inhibits the contraction of vascular and tracheal smooth muscles induced by various agonists such as phenylephrine, histamine, serotonin, endothelin, and a thromboxane agonist, U-46619. Interestingly, HA1077 and Y-27632 inhibited MLC phosphorylation at Ser19 and ATP secretion of human platelets induced by a low level (0.05 U/mL) of thrombin, STA2, and the simultaneous stimulation with epinephrine and serotonin, whereas these compounds affected neither MLC phosphorylation at Ser19 nor ATP secretion stimulated by a high level (0.1 U/mL) of thrombin. Increases in intracellular Ca2+concentration in platelets have been observed in response to agonists such as thrombin, STA2, and a simultaneous addition of serotonin and epinephrine, determined using the fluorescent Ca2+ probe quin-2 and fura-2.38,39 However, the magnitude of the peak and the duration of the Ca2+ levels depend on the strength of the agonist. After thrombin stimulation (0.1 U/mL), the intracellular Ca2+ level increased from about 100 nmol/L to greater than 1,000 nmol/L in human platelets.38,39 STA2 treatment led to an increase in the intracellular Ca2+ level, but the peak level (∼200 to 300 nmol/L) was lower than that seen with thrombin stimulation.41 Although our data suggest that either epinephrine or serotonin by itself can activate Rho-kinase and phosphorylate MBS in intact platelets, these weak agonists do not induce aggregation or ATP secretion in washed platelets suspensions.35 A simultaneous stimulation with serotonin and epinephrine induced to a certain extent intracellular Ca2+ mobilization and ATP secretion. The peak level of intracellular Ca2+ by the simultaneous stimulation was about 150 to 200 nmol/L,35 which is is substantially lower than that induced with thrombin (0.1 U/mL) stimulation. We have shown that human platelets contain approximately 15 times lower amounts of Rho-kinase than MLC kinase, on a molar basis. In addition, a previous report has shown that the molecular activity of Rho-kinase for MLC is about three times lower than that of MLC kinase,18 although the apparent Km value (0.91 μmol/L) of Rho-kinase for the MLC is lower than that (52 μmol/L) of MLC kinase. Therefore, the Ca2+/calmodulin-dependent MLC kinase is thought to be the primary regulator for MLC phosphorylation at Ser19 in human platelets. Collectively, our data suggest the possibility that relatively high levels (>0.1 U/mL) of thrombin that lead to rapid mobilization of intracellular Ca2+induce phosphorylation of the 20-kD MLC by MLC kinase and thereby trigger ATP secretion. Under these circumstances, the contribution of Rho-kinase–mediated MLC phosphorylation appears to be minimal, even though Rho-kinase–induced MBS phosphorylation is evident. This hypothesis is supported by the experiments with BAPTA-AM–preincubated platelets showing that chelation of cytoplasmic Ca2+ made apparent the inhibition by HA1077 and Y-27632 of 0.1 U/mL thrombin-induced MLC phosphorylation, presumably due to less activation of Ca2+-dependent MLC kinase. On the other hand, our pharmacological studies suggested that Rho-kinase mediated MLC phosphorylation (both directly and indirectly through MBS phosphorylation), in addition to MLC kinase-dependent phosphorylation, may be necessary for platelet secretion induced by relatively weak agonist, STA2, and a lower level (<0.05 U/mL) of thrombin, all of which presumably cause a submaximal elevation of intracellular Ca2+ concentration.42 43
Platelet-activating factor and thrombin have been shown to induce MLC phosphorylation in intact platelets, even when cytoplasmic Ca2+ concentration remains at, or close to, resting levels44; the observation suggests the existence of Ca2+-independent pathway that might synergize with Ca2+ to produce MLC phosphorylation. In permeabilized platelets, GTPγS and phorbol ester significantly enhance the Ca2+ sensitivity of serotonin secretion, without detectable phosphatidylinositol hydrolysis.17,45 However, the augmentation of MLC phosphorylation by GTPγS at submaximal Ca2+ concentration was slight, therefore the dominant factor regulating MLC phosphorylation appears to be the Ca2+ concentration in permeabilized platelets.17 These findings are consistent with the hypothesis that Rho-kinase activated MLC phosphorylation is involved in mediating platelet secretion induced by relatively weak stimuli, although Ca2+/calmodulin-dependent MLC kinase is thought to be the essential regulator for MLC phosphorylation at Ser19. Additional elements, such as protein kinase C,17 also appear to be involved in the GTPγS-induced increase in Ca2+ sensitivity that accompanies secretion. In conclusion, Rho-kinase–mediated MLC phosphorylation is involved in agonist-induced secretion in human platelets at submaximal Ca2+ concentrations, in synergy with the Ca2+-dependent MLC phosphorylation, possibly with the cooperation of other signaling systems such as protein kinase C.
ACKNOWLEDGMENT
The authors are grateful to Dr R.S. Adelstein (NIH) for helpful discussions, to E. Imai for technical assistance, and to M. Ohara for helpful comments.
Supported in part by grants for research from the Ministry of Education, Science, Sports and Culture of Japan and by grants from the Mie Medical Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Masakatsu Nishikawa, MD, PhD, The 2nd Department of Internal Medicine, Mie University School of Medicine, 2-174 Edobashi, Tsu, Mie 514-8507, Japan; e-mail:nisikawa@clin.medic.mie-u.ac.jp.


![Fig. 2. Agonist-induced phosphorylation of MBS and the inactivation of myosin phosphatase in intact platelets. (A) Agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were stimulated for the indicated time with either 1 μmol/L STA2, 0.1 U/mL thrombin, 1 μmol/L serotonin, or 1 μmol/L epinephrine. *An unknown protein of 120 kD. (B) Inhibition by HA1077 and Y-27632 of STA2-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with various concentrations of HA1077 (left panel) or Y-27632 (right panel) were stimulated for 30 seconds with 1 μmol/L STA2, without stirring. Results were expressed as the percentage of the value without the addition of compounds. Similar results were obtained in three other experiments, using different donor platelets. (C) Effects of HA1077 and Y-27632 on STA2-induced inactivation of myosin phosphatase in intact platelets. Human platelets incubated with saline (control; □), 20 μmol/L HA1077 (▪), or 10 μmol/L Y-27632 (▦) for 5 minutes were activated with 1 μmol/L STA2without stirring. MBS was immunoprecipitated with anti-MBS antibody and the activity of myosin phosphatase was determined immediately, as described in Materials and Methods. Results are expressed as the percentage of the value, without the addition of compounds. Data represent the mean ± SE of four experiments. *P < .05, **P < .01 (D) The inhibitory effect of receptor antagonists on agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were pretreated with 0.1 μmol/L ONO-3708, 10 nmol/L M-1, 10 nmol/L yohimbine, 10 μmol/L Y-27632, or 20 μmol/L HA1077 for 5 minutes at 37°C and were activated with 1 μmol/L STA2, 1 μmol/L serotonin, or 1 μmol/L epinephrine for 30 seconds, without stirring. Similar results were obtained in three other experiments, using different donor platelets. (E) Inhibition by HA1077 and Y-27632 of thrombin-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with 20 μmol/L HA1077 or 10 μmol/L Y-27632 were stimulated for 30 seconds with 0.1 U/mL thrombin without stirring. Similar results were obtained in three other experiments, using different donor platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/10/10.1182_blood.v93.10.3408.410k37_3408_3417/5/m_blod41037002aw.jpeg?Expires=1769087625&Signature=FvOMb0TgdqGjZJX~6c671NXDi6N2PWbsY6ldroP5yRWiLDO4QOP3E0pA9eu1iVY~PX5hLMxEEXK0FQpOE7KtNzDMczCBEf1CP6Hv13IeZSS2jz6UEd2Qi83IwWFm2JahKLRSQspMIqlpXvHdvypOMRyLX6ZdV6-M5xT72ifXvaLKLqhx5iDEzT6x0sFMyw~DSibpeZl~wfh7QkUK~EjZ8bOz-ctG8JqhURpltzSqJIwriRAaD8LgvQTmajUBl2TaWaRuokQnMTf1qU1TGFKHrlJAvbEbikfsJLg0N0AOUH3LYc2HhSxPRcnZA5XGwXLpEV1hy2qFInikaRuNZa5B0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Agonist-induced phosphorylation of MBS and the inactivation of myosin phosphatase in intact platelets. (A) Agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were stimulated for the indicated time with either 1 μmol/L STA2, 0.1 U/mL thrombin, 1 μmol/L serotonin, or 1 μmol/L epinephrine. *An unknown protein of 120 kD. (B) Inhibition by HA1077 and Y-27632 of STA2-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with various concentrations of HA1077 (left panel) or Y-27632 (right panel) were stimulated for 30 seconds with 1 μmol/L STA2, without stirring. Results were expressed as the percentage of the value without the addition of compounds. Similar results were obtained in three other experiments, using different donor platelets. (C) Effects of HA1077 and Y-27632 on STA2-induced inactivation of myosin phosphatase in intact platelets. Human platelets incubated with saline (control; □), 20 μmol/L HA1077 (▪), or 10 μmol/L Y-27632 (▦) for 5 minutes were activated with 1 μmol/L STA2without stirring. MBS was immunoprecipitated with anti-MBS antibody and the activity of myosin phosphatase was determined immediately, as described in Materials and Methods. Results are expressed as the percentage of the value, without the addition of compounds. Data represent the mean ± SE of four experiments. *P < .05, **P < .01 (D) The inhibitory effect of receptor antagonists on agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were pretreated with 0.1 μmol/L ONO-3708, 10 nmol/L M-1, 10 nmol/L yohimbine, 10 μmol/L Y-27632, or 20 μmol/L HA1077 for 5 minutes at 37°C and were activated with 1 μmol/L STA2, 1 μmol/L serotonin, or 1 μmol/L epinephrine for 30 seconds, without stirring. Similar results were obtained in three other experiments, using different donor platelets. (E) Inhibition by HA1077 and Y-27632 of thrombin-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with 20 μmol/L HA1077 or 10 μmol/L Y-27632 were stimulated for 30 seconds with 0.1 U/mL thrombin without stirring. Similar results were obtained in three other experiments, using different donor platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/10/10.1182_blood.v93.10.3408.410k37_3408_3417/5/m_blod41037002bw.jpeg?Expires=1769087625&Signature=zZTJ501eS3uoKoKoIy-U14G5q-~eut5iMCnoQ8ia1tiRisTXtcoxRcgUNk9JNp86CD04jVxta1Madm8RZZWWTxFrUhmQTFKoC8Z~t4h3jqiDNyqvGpfvXWrUYxmVXZeMTzMmEZklmUJSxIqXuYu0lU5kYu7j2M-4l9FjCoQNk2aaDz02ytJAcoWexf2wP1rD8uOBJuT88ZkSVEllxTt9gYyKXI3ceXvBDA0QO0B~jrj9~qWjNwDwPHM257Mh9OWmS56jz44BXmA6WHx2o17WcHCoks25bK2orRRFk-TronG9ov5fla4Rri7dtByG0IIkrzy87u6wKlPTtTM6XOocEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Agonist-induced phosphorylation of MBS and the inactivation of myosin phosphatase in intact platelets. (A) Agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were stimulated for the indicated time with either 1 μmol/L STA2, 0.1 U/mL thrombin, 1 μmol/L serotonin, or 1 μmol/L epinephrine. *An unknown protein of 120 kD. (B) Inhibition by HA1077 and Y-27632 of STA2-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with various concentrations of HA1077 (left panel) or Y-27632 (right panel) were stimulated for 30 seconds with 1 μmol/L STA2, without stirring. Results were expressed as the percentage of the value without the addition of compounds. Similar results were obtained in three other experiments, using different donor platelets. (C) Effects of HA1077 and Y-27632 on STA2-induced inactivation of myosin phosphatase in intact platelets. Human platelets incubated with saline (control; □), 20 μmol/L HA1077 (▪), or 10 μmol/L Y-27632 (▦) for 5 minutes were activated with 1 μmol/L STA2without stirring. MBS was immunoprecipitated with anti-MBS antibody and the activity of myosin phosphatase was determined immediately, as described in Materials and Methods. Results are expressed as the percentage of the value, without the addition of compounds. Data represent the mean ± SE of four experiments. *P < .05, **P < .01 (D) The inhibitory effect of receptor antagonists on agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were pretreated with 0.1 μmol/L ONO-3708, 10 nmol/L M-1, 10 nmol/L yohimbine, 10 μmol/L Y-27632, or 20 μmol/L HA1077 for 5 minutes at 37°C and were activated with 1 μmol/L STA2, 1 μmol/L serotonin, or 1 μmol/L epinephrine for 30 seconds, without stirring. Similar results were obtained in three other experiments, using different donor platelets. (E) Inhibition by HA1077 and Y-27632 of thrombin-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with 20 μmol/L HA1077 or 10 μmol/L Y-27632 were stimulated for 30 seconds with 0.1 U/mL thrombin without stirring. Similar results were obtained in three other experiments, using different donor platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/10/10.1182_blood.v93.10.3408.410k37_3408_3417/5/m_blod41037002cx.jpeg?Expires=1769087625&Signature=1U5zuULby5qWPmX5e3IIyWX~BrGixbwUfOSA5ousAvM14UbuwSzb3RjAQvNqY46q-ja3rmx24OMB-6n2tgMfVFyCEFHLL11drFW4TZUsiY8A6yO6FBIq-dafXQ7CW9KM33l-q4CN5yPtaQ1-Xq9uDrSuTpg1d7LyQLP3XiL1kS8pZ5-eiiijwU-~GF~MySSkZctQRMmFSmwNnVHVSmvfdFpqUpwvIi~It7F4aAJhIeqQwAf2dR-0g3XSQvFVrrYr7l-gqBbvVG-AbtH0HChyrGPIEGfKsVgD-KIre6fvVQJlKB2DR7zmTtiAC4018tUppDBmC0fgtYyzQPtcWDt~3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Agonist-induced phosphorylation of MBS and the inactivation of myosin phosphatase in intact platelets. (A) Agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were stimulated for the indicated time with either 1 μmol/L STA2, 0.1 U/mL thrombin, 1 μmol/L serotonin, or 1 μmol/L epinephrine. *An unknown protein of 120 kD. (B) Inhibition by HA1077 and Y-27632 of STA2-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with various concentrations of HA1077 (left panel) or Y-27632 (right panel) were stimulated for 30 seconds with 1 μmol/L STA2, without stirring. Results were expressed as the percentage of the value without the addition of compounds. Similar results were obtained in three other experiments, using different donor platelets. (C) Effects of HA1077 and Y-27632 on STA2-induced inactivation of myosin phosphatase in intact platelets. Human platelets incubated with saline (control; □), 20 μmol/L HA1077 (▪), or 10 μmol/L Y-27632 (▦) for 5 minutes were activated with 1 μmol/L STA2without stirring. MBS was immunoprecipitated with anti-MBS antibody and the activity of myosin phosphatase was determined immediately, as described in Materials and Methods. Results are expressed as the percentage of the value, without the addition of compounds. Data represent the mean ± SE of four experiments. *P < .05, **P < .01 (D) The inhibitory effect of receptor antagonists on agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were pretreated with 0.1 μmol/L ONO-3708, 10 nmol/L M-1, 10 nmol/L yohimbine, 10 μmol/L Y-27632, or 20 μmol/L HA1077 for 5 minutes at 37°C and were activated with 1 μmol/L STA2, 1 μmol/L serotonin, or 1 μmol/L epinephrine for 30 seconds, without stirring. Similar results were obtained in three other experiments, using different donor platelets. (E) Inhibition by HA1077 and Y-27632 of thrombin-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with 20 μmol/L HA1077 or 10 μmol/L Y-27632 were stimulated for 30 seconds with 0.1 U/mL thrombin without stirring. Similar results were obtained in three other experiments, using different donor platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/10/10.1182_blood.v93.10.3408.410k37_3408_3417/5/m_blod41037002dw.jpeg?Expires=1769087625&Signature=k-7onHa4ZO7pAMm3sFPCce55lpo6lkRTkOwhduOkcWermjdLRNG49601pTyFmxQ0H47phmSTShxD6xYAdfWkY4hShzilF7SXDzi8DQnwitkXp4BNMc42yOsfQH3WBvcQcdcOEDQE4So3ZlkcKPRMWmqrUPC2kwgzeGqpZJNiW7e4IFLeTgm2RUoLlWzLBsI-OltnxHeuAEbGokd5~kzkxFxKCmSxSBmzUzzKJ0qOqM9tCYYCuQZasdwF3Rd~10TLwzh8ovyDv0WOeLOQA2bZdq5WFAmzR0xSXL66k6cY8~PZN2k8k6WR7EjCEhcEh3ooSDqa7BdrCcFOnb3fA17-zw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Agonist-induced phosphorylation of MBS and the inactivation of myosin phosphatase in intact platelets. (A) Agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were stimulated for the indicated time with either 1 μmol/L STA2, 0.1 U/mL thrombin, 1 μmol/L serotonin, or 1 μmol/L epinephrine. *An unknown protein of 120 kD. (B) Inhibition by HA1077 and Y-27632 of STA2-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with various concentrations of HA1077 (left panel) or Y-27632 (right panel) were stimulated for 30 seconds with 1 μmol/L STA2, without stirring. Results were expressed as the percentage of the value without the addition of compounds. Similar results were obtained in three other experiments, using different donor platelets. (C) Effects of HA1077 and Y-27632 on STA2-induced inactivation of myosin phosphatase in intact platelets. Human platelets incubated with saline (control; □), 20 μmol/L HA1077 (▪), or 10 μmol/L Y-27632 (▦) for 5 minutes were activated with 1 μmol/L STA2without stirring. MBS was immunoprecipitated with anti-MBS antibody and the activity of myosin phosphatase was determined immediately, as described in Materials and Methods. Results are expressed as the percentage of the value, without the addition of compounds. Data represent the mean ± SE of four experiments. *P < .05, **P < .01 (D) The inhibitory effect of receptor antagonists on agonist-induced phosphorylation of MBS in intact platelets. [32P]Pi-labeled platelets were pretreated with 0.1 μmol/L ONO-3708, 10 nmol/L M-1, 10 nmol/L yohimbine, 10 μmol/L Y-27632, or 20 μmol/L HA1077 for 5 minutes at 37°C and were activated with 1 μmol/L STA2, 1 μmol/L serotonin, or 1 μmol/L epinephrine for 30 seconds, without stirring. Similar results were obtained in three other experiments, using different donor platelets. (E) Inhibition by HA1077 and Y-27632 of thrombin-induced phosphorylation of MBS. [32P]Pi-labeled platelets incubated for 5 minutes with 20 μmol/L HA1077 or 10 μmol/L Y-27632 were stimulated for 30 seconds with 0.1 U/mL thrombin without stirring. Similar results were obtained in three other experiments, using different donor platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/10/10.1182_blood.v93.10.3408.410k37_3408_3417/5/m_blod41037002ew.jpeg?Expires=1769087625&Signature=SVnSS5kwfR60lRSQL59qHNKitbp9X731kFv3KILpQHp~9sNMImO-L9zzUVgo~I~V4D2n-hJtULAtDsr8oJsE3mgU6wIT4T81bd9VF9nENOxGRx6L0S5YCNqBsDFdoZNppYWVvDjJKQamScjbgcF2HyONYNkPftDS0VNAXr3jNy61inEJO6ONcb0FX97diqxSxg~AvyEhklHMx8BoAXHRMpKWtF9nSMJsDAzBRWH1P07gQvP5NbircLmSXkUW6iNeaMoTaS6Ilxb9DHRN1EM254Rch9UerHyhHOZKytj~GdfGIlsJhJAmBByZio8gI-FgxtGA9z5yW16WUqyOASZRTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)