Abstract
Leukemic blast cells express the CD33 antigen in most patients with acute myeloid leukemia (AML), but this antigen is not expressed by hematopoietic stem cells. We conducted a study to determine whether normal hematopoiesis could be restored in patients with AML by selective ablation of cells expressing the CD33 antigen. In a dose escalation study, 40 patients with relapsed or refractory CD33+ AML were treated with an immunoconjugate (CMA-676) consisting of humanized anti-CD33 antibody linked to the potent antitumor antibiotic calicheamicin. The capacity of leukemic cells to efflux 3,3’-diethyloxacarbocyanine iodide (DiOC2) was used to estimate pretreatment functional drug resistance. Leukemia was eliminated from the blood and marrow of 8 (20%) of the 40 patients; blood counts returned to normal in three (8%) patients. A high rate of clinical response was observed in leukemias characterized by low dye efflux in vitro. Infusions of CMA-676 were generally well tolerated, and a postinfusion syndrome of fever and chills was the most common toxic effect. Two patients who were treated at the highest dose level (9 mg/m2) were neutropenic >5 weeks after the last dose of CMA-676. These results show that an immunoconjugate targeted to CD33 can selectively ablate malignant hematopoiesis in some patients with AML.
PATIENTS WITH refractory or recurrent acute myeloid leukemia (AML) have a dismal prognosis. Toxic effects associated with additional conventional chemotherapy are often life-threatening, and few patients achieve a complete remission (CR). A therapy that more specifically targets leukemia is likely to be safer, and possibly more effective than current nonspecific chemotherapeutic agents.
During hematopoietic development, stem cells capable of establishing long-term multilineage hematopoiesis give rise to progenitors with diminished self-renewal capacity and a greater degree of differentiation. During this process, hematopoietic cells express distinct cell surface antigens that are also expressed by their malignant counterparts. Some of these antigens (eg, CD33) are present on maturing normal hematopoietic cells and on AML cells, but not on normal hematopoietic stem cells.1-4 These findings raise the possibility that an antibody can be used to deliver cytotoxic agents to selectively ablate malignant myeloid and immature normal cells while sparing normal stem cells.
The myeloid cell surface antigen CD33 is an attractive target for this approach, as it is expressed on AML blast cells from about 90% of patients.2,3 CD33 also may be expressed by most, if not all, of the malignant precursors in at least some cases of AML, as precursor cells that lack CD33 from some patients give rise to normal granulocyte/monocyte precursors in a marrow long-term culture system.5,6 The possibility that anti-CD33 antibody could be used to deliver a cytotoxic agent to malignant cells was suggested by studies showing rapid saturation of leukemic blast cells in peripheral blood and marrow after intravenous administration of approximately 5 mg/m2 radioiodinated anti-CD33 antibodies.7,8Further, in vitro studies showed rapid internalization of the antibody by the target cell.9 To test the concept of selective ablation of malignant hematopoiesis and to evaluate the safety of this approach, we treated patients with relapsed or refractory AML with escalating doses of CMA-676, antibody-targeted chemotherapy consisting of an engineered human anti-CD33 antibody linked with the potent antitumor antibiotic calicheamicin.10
MATERIALS AND METHODS
Patients.
Patients were required to have AML that was either refractory to standard therapy or that had recurred after remission. Patients whose leukemia relapsed after a marrow or peripheral blood stem cell transplant were required to have had successful engraftment (platelets ≥20 × 103/μL without transfusion and an absolute neutrophil count [ANC] ≥500/μL) before relapse. Each prospective patient underwent a bone marrow aspirate to confirm that morphologic evidence of leukemia was present and to document leukemic blast cell surface expression of the CD33 antigen. Patients were required to have a Karnofsky performance status ≥60%, a white blood cell count ≤30 × 103/μL, serum creatinine ≤2.0 mg/dL, and serum bilirubin ≤1.5 mg/dL, to have recovered from toxic effects of previous antineoplastic therapy, and to be able to give informed consent. Patients were ineligible if they had previously received anti-CD33 antibody treatment, were pregnant or nursing, had a prior malignancy, or had active symptomatic central nervous system leukemia or an uncontrolled life-threatening infection.
Recombinant humanized anti-CD33-calicheamicin drug conjugate.
CMA-676 was produced and provided for clinical study by Wyeth-Ayerst Research (Radnor, PA) under an Investigational New Drug Application. Celltech Therapeutics, Ltd (Slough, UK) transformed the murine-derived IgG1 monoclonal antibody (p67.6)8 into an engineered human IgG4 antibody (hP67.6), which was then conjugated to the enediyne calicheamicin10 using a Nac-gamma linker molecule (Hamann et al, manuscript in preparation). Calicheamicin binds in a sequence-specific manner to the minor groove of DNA and induces double-strand DNA breaks that ultimately induce cell death.10 Specific cytotoxicity of CMA-676 against CD33+ leukemia cells was documented in colony-forming assays in vitro, against leukemic cell lines in vitro, and in vivo in nude mice (data not shown).
Protocol design.
In this open, single-arm, phase I dose escalation study, CMA-676 was administered to patients with relapsed or refractory CD33+AML at two study sites. Informed consent was obtained from all patients in accordance with the institutional review boards of the participating institutions. In a single-dose tolerance study with CMA-676 in chimpanzees, an intravenous dose of 0.5 mg/m2 (twice the initial clinical dose of 0.25 mg/m2) was well tolerated, with no compound-related clinical signs of clinical pathology changes evident throughout a 15-day postinfusion period (data not shown). In previous clinical studies in patients using radiolabeled mP67.6 antibody,8 dose-limiting toxicity was not observed at doses up to and including 17 mg/m2 antibody trace-labeled with131I. However, saturation of CD33 binding sites was achieved at a dose level of approximately 5 mg/m2. Hence, at least three patients were treated at each dose level of CMA-676 with up to three infusions of 0.25, 0.5, 1, 2, 4, 5, 6, and 9 mg protein per square meter of body surface area. If one or two of the first three of these patients experienced a grade III toxic effect (see below), three additional patients were to be treated at the same dose. The maximum tolerated dose was defined as one dose level below the dose that was associated with unacceptable CMA-676–related toxicity. Dose-limiting toxicity was defined as severe marrow hypoplasia of more than 6 weeks duration after a single dose of CMA-676, one grade IV study drug-related toxic effect or two grade IV toxic conditions of ambiguous relationship to CMA-676.
CMA-676 was administered as a single 2-hour intravenous infusion. All cytokines or chemotherapeutic agents were withdrawn before treatment. Patients without leukemic progression and drug-related, nonhematologic toxic effects judged to be grade III or less could receive one or two subsequent cycles of CMA-676 with at least 14 days between cycles. Patients were treated with acetaminophen 650 mg orally and diphenhydramine 25 to 50 mg intravenously 15 to 30 minutes before infusion of CMA-676. Patients who achieved a CR and subsequently had a relapse of their leukemia could receive retreatment at the dosage level being evaluated at the time of retreatment.
Patient evaluation.
Patients were examined for acute toxic effects in accordance with the World Health Organization toxic effect grades. Peripheral blood specimens were obtained to study the pharmacokinetics of CMA-676, to evaluate for evidence of CMA-676 binding to CD33-positive cells, and to measure the effects of CMA-676 on hematologic variables and serum chemistry parameters. A complete blood count was obtained before the initial dose and each day for 2 weeks thereafter, or until recovery of granulocyte and platelet levels to the prestudy levels. Hepatic and renal function were measured before initial dose administration, three times per week thereafter, and on day 28 after the last dose of CMA-676.
Bone marrow aspirates were obtained before initial dose administration with CMA-676 on days 1, 7, and 14 of each treatment cycle and on days 1, 7, 14, and 28 of the last treatment cycle. All marrow specimens were examined by light microscopy to estimate cellularity and to detect any residual leukemic blast cells. Patients with progressive disease were removed from the study. Disappearance of leukemia was defined as the absence of peripheral blasts and the presence of ≤5% blast cells in the bone marrow by light microscopic evaluation. CR was defined as disappearance of leukemia in addition to an ANC >1,500/μL and a platelet count >100 × 103/μL, without transfusions.
Laboratory investigations.
Total hP67.6 antibody concentrations in plasma samples were determined using an enzyme-linked immunosorbent assay (ELISA). Formation of antigen-antibody CMA-676 bound to peripheral blood mononuclear cells was detected by flow microfluorimetry. Cells were incubated with biotinylated goat monoclonal anti-human IgG4, followed by avidin-fluorescein isothiocyanate. Cells incubated with avidin-fluorescein isothiocyanate alone comprised the negative control. The saturation percentage was defined as 100 times the ratio of the fluorescence intensity of patient mononuclear cells (minus the negative control) over the maximum fluorescence intensity (minus the negative control). Maximum achievable saturation was determined by incubating patient mononuclear cells from the same time point with saturating amounts of CMA-676 in vitro before the addition of the anti-human IgG4 antibody. The efflux of 3,3’-diethyloxacarbocyanine iodide (DiOC2) from CD33-positive blast cells was measured as an indication of functional drug efflux.11 Serum samples obtained from each patient before CMA-676 administration, on day 7 after initial doses, and on days 7, 14, 21, and 28 after administration of the final dose of CMA-676 were analyzed for anti-hP67.6 (humanized mouse antibody) or anti-calicheamicin/linker immune response by ELISA.
RESULTS
Patient characteristics.
A total of 40 patients with relapsed or refractory AML were enrolled. Patient characteristics are shown in Table1. Three to eight patients were treated at each of eight dose levels of CMA-676: 0.25, 0.5, 1, 2, 4, 5, 6, and 9 mg protein per square meter of body surface area.
Nonhematologic toxicity.
The most frequently reported drug-related adverse events are summarized in Table 2. The most commonly observed nonhematologic side effect, a syndrome of fever and chills, occurred in 32 of 40 (80%) patients beginning 2 to 4 hours after the start of the 2-hour intravenous infusion. The syndrome was limited to grade I to II except in three instances. Grade III fever and chills occurred in two patients after receiving CMA-676 at 6 and 9 mg/m2, respectively. The third patient (COH-008) who had asymptomatic hypotension and was receiving low-dose dopamine before CMA-676 administration, had a temperature >40°C and reversible symptomatic hypotension 5 hours after the initiation of the CMA-676 infusion at 6 mg/m2. Another patient (FH-007) developed transient shortness of breath in association with retreatment with CMA-676 after his leukemia relapsed, presumably caused by an immune reaction to CMA-676 (see below). Nausea and fatigue were among other less frequent toxic effects thought possibly related to CMA-676.
Reversible, possibly drug-related hepatic transaminase elevations from 5 to 10 times the normal range were observed in eight patients (Table2). One additional patient with documented concurrent cholelithiasis had a pretreatment alanine serum transaminase (AST) of 13 U/L that increased to 304 U/L 4 days after receiving the first dose of CMA-676, and rapidly declined to the normal range within a week. Immediately before receipt of the second dose of CMA-676, her AST increased to 1,804 U/L and again rapidly returned to the normal range. In this patient, elevated enzymes were thought to be primarily due to the patient’s cholelithiasis. No patient had a drug-related bilirubin elevation of greater than five times the normal range. No significant study drug-related central nervous system, cardiac, or renal toxic effects were observed. With regard to nonhematologic toxic effects, a maximum tolerated dose was not reached, and dose-limiting toxicity was not observed.
Hematologic toxicity.
Because most patients had concurrent neutropenia and thrombocytopenia caused by active AML at the time of enrollment, whether hematologic toxic effects were directly attributable to CMA-676 was often impossible to ascertain. However, among nine patients with neutrophil counts >1,500 cells/μL before treatment, eight had severe neutropenia (<200 cells/μL) within 14 days of CMA-676 infusion regardless of dose level. Figure 1 shows the decline and recovery of the neutrophil count from a representative patient during the 2 weeks after each infusion. Although dose-limiting toxicity was not formally observed, two of seven evaluable patients had prolonged drug-related neutropenia after treatment at a dose level of 9 mg/m2. The first patient (FH-023) required 38 days to recover to an ANC >500 cells/μL after the third dose of CMA-676, and the second patient (COH-015) did not achieve neutrophil recovery and died of sepsis 50 days after receiving the second dose (Table 3).
Relationship between hematologic parameters and time for a representative patient (FH-012) who received CMA-676 at 4 mg/m2 per dose. Arrows denote infusions of CMA-676. All counts refer to peripheral blood counts.
Relationship between hematologic parameters and time for a representative patient (FH-012) who received CMA-676 at 4 mg/m2 per dose. Arrows denote infusions of CMA-676. All counts refer to peripheral blood counts.
Elimination of blast cells from the peripheral blood and bone marrow.
Blast cells were identified on a peripheral blood smear in 31 (78%) patients before infusion of CMA-676. One week after treatment, 22 (71%) of these patients had fewer blast cells and in six (19%) of these patients, blast cells had completely disappeared from the peripheral blood smears. The proportion of patients who experienced reductions in peripheral blast cell counts was highest among those who received higher doses of CMA-676. Reduced numbers of peripheral blast cells were observed in 10 of 11 patients treated with either 6 or 9 mg/m2 of CMA-676 compared with 12 of 20 patients treated at dose levels from 0.25 to 5 mg/m2.
Morphologic evidence of leukemia within the bone marrow (>5% blast cells) was seen in all patients before treatment. After treatment with one to three doses of the drug conjugate, 8 (20%) of 40 patients had <5% leukemic blast cells on morphologic examination of bone marrow aspirate and biopsy specimens (Table 3). In one of these patients (FH-007), blast cells were also eliminated from the bone marrow on a second occasion after retreatment with CMA-676 (see below).
Recovery of normal blood counts.
Recovery of blood neutrophil counts to greater than 1,500 cells/μL was observed in five of eight patients who had <5% leukemic blasts by morphologic examination of bone marrow aspirate and biopsy specimens (Table 3). In addition, three of these five patients achieved normal platelet counts. The first patient, FH-007, achieved a complete hematologic and cytogenetic remission 7 days after receiving the third dose of CMA-676 at 1 mg/m2. After 140 days, local leukemia recurrences in his femurs and iliac wing were identified by magnetic resonance imaging and confirmed by surgical biopsy. He was treated with conventional chemotherapy and local radiation and achieved another CR. He experienced relapse again 293 days after completing the first course of CMA-676, was treated with two additional doses of CMA-676 at 6 mg/m2, and his leukemia disappeared again for 56 days. His leukemic cells expressed CD33 at the time of each of his relapses.
The second patient, FH-012, achieved a complete hematologic and cytogenetic remission 35 days after the third dose of 4 mg/m2. In an attempt to consolidate his remission, he was given an infusion of lymphocytes from his bone marrow donor 40 days after infusion of the third dose of CMA-676. He remained in CR for 214 days after treatment until testicular and central nervous system relapses occurred.
The third patient, FH-023, received three doses of CMA-676 at 9 mg/m2, and his ANC recovered to >1,500/μL 42 days after he received the third dose of CMA-676. His platelet count reached a peak of 36 × 103/μL 23 days after he received the third dose of CMA-676, but the patient subsequently became platelet transfusion-dependent for approximately 3 weeks. Evidence of antibody on the patient’s platelet surfaces was documented. Megakaryocytes were present and platelet maturation appeared normal on examination of the marrow aspirate. In an attempt to consolidate his remission, he was given an infusion of lymphocytes from his bone marrow donor 54 days after receiving the third dose, and his platelet count eventually rose to >100 × 103/μL 106 days later. At the time of this report, he remained in continuous cytogenetic and hematologic remission >623 days after receiving the third dose of CMA-676.
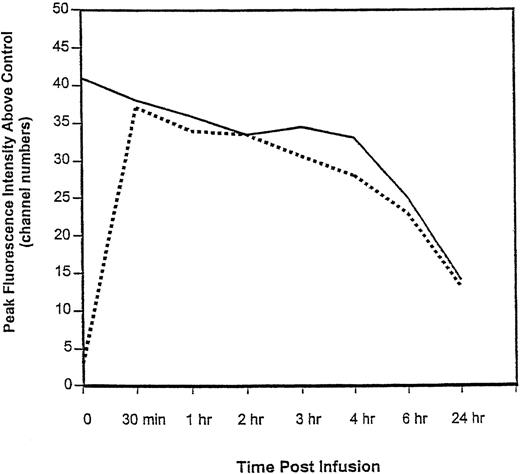
Pharmacokinetics and saturation of peripheral CD33 sites.
Detectable plasma levels of CMA-676 were identified in all patients immediately after intravenous administration and the half-life of CMA-676 was estimated to be 38 ± 21 hours for patients receiving the 9 mg/m2 dose. Figure 2shows the relationship between the total number of cell surface CD33 sites and CD33 sites bound with CMA-676 in peripheral blood cells for a characteristic patient over time. Thirty minutes after infusion, CD33 sites were almost completely saturated with CMA-676. Twenty-four hours after administration, fewer CD33 sites and bound drug conjugate were evident, suggesting internalization of the CD33-immunoconjugate complex. In the patients who received the maximum dose (9 mg/m2), a median of 92.2% (range, 79.2% to 100%) of CD33 binding sites on peripheral blood blast-sized cells were bound by the drug conjugate 30 minutes after infusion of a dose. Peak CD33 saturation levels in relation to treatment response are shown in Fig 3. These results show that saturation of sites is not, by itself, sufficient to insure treatment response.
Relationship between total number and CMA-676–bound CD33 sites on peripheral blood cells in a characteristic patient over time. The solid line represents the number of CD33 sites available for binding to CMA-676 as estimated by the maximal fluorescence intensity obtained by incubating an aliquot of cells in vitro with excess CMA-676. The dashed line represents the fluorescence intensity of bound CMA-676 to cell surfaces. Near-complete saturation is seen 30 minutes after the start of the infusion.
Relationship between total number and CMA-676–bound CD33 sites on peripheral blood cells in a characteristic patient over time. The solid line represents the number of CD33 sites available for binding to CMA-676 as estimated by the maximal fluorescence intensity obtained by incubating an aliquot of cells in vitro with excess CMA-676. The dashed line represents the fluorescence intensity of bound CMA-676 to cell surfaces. Near-complete saturation is seen 30 minutes after the start of the infusion.
Relation of leukemic blast cell dye efflux and maximum CMA-676 saturation of CD33 sites on peripheral blood blast-sized cells with treatment response (N = 36). (▴) Denotes patients who had <5% leukemic blasts by morphologic examination of bone marrow aspirate and biopsy specimens after treatment. (•) Denotes patients whose leukemia did not disappear. Peripheral blood samples from four patients were unavailable for analysis. The efflux from the dominant population is represented in the five instances in which efflux profiles were bimodal. *Leukemic cell specimens from patients FH-023 and FH-024 were 2 and 3 days old, respectively, at the time of efflux measurement. Because blast-sized cells from each showed uncharacteristically low DiOC2 loading, obtained efflux values may underestimate true efflux.
Relation of leukemic blast cell dye efflux and maximum CMA-676 saturation of CD33 sites on peripheral blood blast-sized cells with treatment response (N = 36). (▴) Denotes patients who had <5% leukemic blasts by morphologic examination of bone marrow aspirate and biopsy specimens after treatment. (•) Denotes patients whose leukemia did not disappear. Peripheral blood samples from four patients were unavailable for analysis. The efflux from the dominant population is represented in the five instances in which efflux profiles were bimodal. *Leukemic cell specimens from patients FH-023 and FH-024 were 2 and 3 days old, respectively, at the time of efflux measurement. Because blast-sized cells from each showed uncharacteristically low DiOC2 loading, obtained efflux values may underestimate true efflux.
Response correlation with drug efflux.
We evaluated the efflux of DiOC2 from pretreatment leukemic blast cells as a measure of functional drug resistance. Figure 3 shows the relationship between measured leukemic blast cell drug efflux levels and saturation of CD33 sites with response. Elimination of leukemia appeared to be correlated with a low capacity by leukemic blast cells to extrude DiOC2. For example, of the 30 patients evaluated with the assay in whom doses of CMA-676 saturated >75% of available CD33 sites on peripheral blood blast cells, 8 of 17 patients with leukemic blast cells that showed ≤40 channel numbers of DiOC2 efflux had <5% blasts in the bone marrow after treatment. In contrast, none of the 13 patients with leukemic blast cells expressing >40 channel numbers of DiOC2 efflux entered remission.
Immune responses.
No humoral responses to anti-hP67.6 antibody were detected. A humoral response to the calicheamicin-linker complex was documented in one patient after receiving a third dose of CMA-676 and in a second patient during retreatment with CMA-676.
DISCUSSION
This study describes the use of an antibody-drug conjugate capable of targeting and safely eliminating target leukemic cells in vivo. Disappearance of AML cells from the bone marrow and peripheral blood of eight patients with complete restoration of normal hematopoiesis in three patients was observed after treatment with antibody-targeted chemotherapy. These results show that eradication of CD33+AML cells can allow restoration of normal hematopoiesis by remaining CD33− precursors. For some patients, we hypothesize that the CD33− precursors are predominantly or completely nonmalignant. This is based on findings that, in some cases of AML, the clonal abnormality originated in either a committed progenitor or an early multipotent cell whose proliferative expression is mainly restricted to the granulocyte/monocyte lineage.12Because selection of CD33− precursors from some of these leukemias allowed normal hematopoietic growth in culture, the malignant clone may involve few, if any, CD33−precursors.5 6
In other cases of AML, particularly in older patients, the clonal abnormality has been found in both the erythroid and myeloid lineages, showing malignant involvement of multipotent precursors.12In addition, clonal karyotypic abnormalities have been found in primitive precursor cells from some AML patients.13,14 It is conceivable that normal hematopoiesis could also be restored in these patients even if a substantial portion of CD33−precursors were malignant because normal precursors express at least a short-term proliferative advantage. While this explanation appears inconsistent with the observed leukemic growth in a immunodeficient mouse model after infusion of isolated primitive (CD34+CD38−) precursors from human leukemia specimens,15 it is consistent with the report that a patient undergoing allogeneic transplantation who was inadvertently given an infusion of donor AML cells initially recovered with normal donor hematopoiesis.16
Each patient whose blood counts returned completely to normal had experienced relapse after an allogeneic bone marrow transplant (data not shown). Therefore, it is possible that a graft-versus-leukemia effect eliminated the remaining CD33− leukemic cells after elimination of the bulk of AML cells. However, in a recently initiated phase II study of CMA-676, normal hematopoiesis was restored in some patients who had not received a transplant.17Molecular remissions have been observed in a portion of patients with acute promyelocytic leukemia who received unconjugated anti-CD33 antibody (HuM195).18 Although an AML patient with 8% blasts in his bone marrow achieved a CR with HuM195 given at a supersaturating dose, complete clinical responses have not been reported in patients with large tumor burdens.19 Hence, the observed elimination of leukemia after treatment with CMA-676 was not likely due to antibody-mediated effects alone.
Myelosuppression was the most clinically significant adverse event. The severe marrow hypoplasia observed in two patients who received 9 mg/m2 is consistent with depletion of CD33+hematopoietic progenitor cells and with the time required for CD33− cells to restore hematopoietic function.20 To prevent prolonged neutropenia, two (instead of three) scheduled doses of CMA-676 at 9 mg/m2 are generally administered in the phase II study. In that study, a third dose can only be administered if bone marrow cellularity is >15% after two doses of CMA-676. The 9-mg/m2 dose level was selected for the phase II study because consistently >75% of CD33 sites were saturated at this dose and nonhematologic toxicity was not considered to be dose-limiting in the phase I study.
Modest and reversible hepatic transaminase elevations and hyperbilirubinemia were observed in some patients who received CMA-676 at high dose levels. Only two patients developed a positive immune response to the calicheamicin-linker complex after receipt of several doses of CMA-676, in contrast with findings in other studies in which frequent immune responses occurred after administration of immunoconjugates containing either murine-derived monoclonal antibodies or naturally occurring toxins.7
Only patients with relapsed or refractory disease were eligible for this clinical trial. In this setting, the leukemia cells in many patients expressed functional drug resistance that may have prevented killing by the drug conjugate (a known substrate of p-glycoprotein, data not shown). This was suggested by the correlation between clinical response and low levels of dye efflux by leukemic blast cells. This observation raises the possibility that the complete response rate might be improved by using agents that specifically block p-glycoprotein activity (eg, cyclosporine) in combination with CMA-676.
In summary, we found that CMA-676 selectively ablated malignant hematopoiesis in some patients with refractory or relapsed AML and that therapy was associated with few toxic effects. Studies are currently underway to evaluate this agent in pediatric and elderly populations. It is anticipated that CMA-676 will be evaluated in the setting of newly diagnosed and minimal residual disease. Because CMA-676 was associated with few nonhematologic toxic effects, it potentially could be studied as a replacement for anthracycline in combination chemotherapy regimens and combined with existing conditioning regimens for hematopoietic stem cell transplant.
ACKNOWLEDGMENT
We are indebted to Drs Dan Schochat, Dennis Parenti, Geoff Yarrangton, Phillip Hamann, and Lois Hinman for efforts in initiating and supporting this work; Dr Irene Georgieff, Melissa Auen, and Rosalane Dacanay for excellent technical assistance; and Penny Hoeltzel for providing superb editorial comments.
Supported by Wyeth-Ayerst Research. E.L.S. is supported by an American Cancer Society Clinical Oncology Career Development Award. I.D.B. is an American Cancer Society Clinical Research Professor.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to E.L. Sievers, MD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-280, PO Box 19024, Seattle, WA 98109-1024; e-mail: esievers@fhcrc.org.