Abstract
Thymic negative selection renders the developing T-cell repertoire tolerant to self-major histocompatability complex (MHC)/peptide ligands. The major mechanism of induction of self-tolerance is thought to be thymic clonal deletion, ie, the induction of apoptotic cell death in thymocytes expressing a self-reactive T-cell receptor. Consistent with this hypothesis, in mice deficient in thymic clonal deletion mediated by cells of hematopoietic origin, a twofold to threefold increased generation of mature thymocytes has been observed. Here we describe the analysis of the specificity of T lymphocytes developing in the absence of clonal deletion mediated by hematopoietic cells. In vitro, targets expressing syngeneic MHC were readily lysed by activated CD8+ T cells from deletion-deficient mice. However, proliferative responses of T cells from these mice on activation with syngeneic antigen presenting cells were rather poor. In vivo, deletion-deficient T cells were incapable of induction of lethal graft-versus-host disease in syngeneic hosts. These data indicate that in the absence of thymic deletion mediated by hematopoietic cells functional T-cell tolerance can be induced by nonhematopoietic cells in the thymus. Moreover, our results emphasize the redundancy in thymic negative selection mechanisms.
DURING THEIR DEVELOPMENT in the thymus, T-cell precursors randomly rearrange their genes for the α and β chains of the clonotypic T-cell receptor (TCR). Thus, an immature thymocyte repertoire is generated that can recognize both self and foreign antigenic peptides presented by major histocompatability complex (MHC) molecules. TCRαβ expressing immature thymocytes are subsequently submitted to a variety of selection processes. Thymic positive selection is thought to select “useful” cells, ie, thymocytes capable of recognition of self-MHC molecules. In the process of negative selection, self-specific cells are inactivated, thus creating a T-cell repertoire tolerant to self-MHC/peptide ligands.1-3 It has been reported that 3% to 5% of precursors that develop in the thymus will fully mature and populate peripheral lymphoid organs.4,5 Thymocytes whose differentiation is aborted either have not successfully rearranged their TCRα or TCRβ genes,6 do not express a TCRαβ suitable for positive selection,7,8 are autoreactive,9-11 or do not receive other unidentified thymic microenvironmental signals required for full development.5 12-14
The quantitative impact of thymic negative selection has been the subject of recent reports. While the majority of thymic deletion is thought to be mediated by radiosensitive antigen-presenting cells of bone marrow origin, thymic positive selection is known to depend on MHC molecules expressed by radioresistant thymic epithelial cells,1,15 although quantitatively minor exceptions to this rule have been reported.16-23 Therefore, bone marrow chimeras can be generated in which positive selection is fully fuctional, while negative selection is defective. In irradiation bone marrow chimeras in which radioresistant cells express MHC class I and II ligands while radiosensitive elements lack expression of these molecules (MHC I°II°→wt), we have observed a twofold to threefold increase in the generation of mature thymocytes as compared with control chimeras.10 These data indicate that half to two thirds of positively selectable thymocytes are deleted during thymic development. Zerrahn et al9 have reported that approximately 5% of the preselection thymocyte repertoire is self-reactive. During normal thymocyte development, these cells are expected to be deleted. Combined with the estimate of 3% to 5% of thymocytes successfully undergoing thymic selection,4,5these data indicate that at least half of positively selectable thymocytes are deleted during development. Using a more sensitive experimental system, Merkenschlager et al8 reported that approximately 20% of immature thymocytes are activated on interaction with MHC molecules. Because only approximately 5% of developing thymocytes successfully undergo thymic selection,4,5 the latter result suggests that around three quarters of MHC reactive immature thymocytes are deleted. Interestingly, the mature T-cell repertoire in mice expressing MHC class II molecules presenting a single peptide has been reported to contain 60% of cells reactive to MHC class II molecules presenting the normal repertoire of self-peptides.11 These cells would be expected to be deleted in mice expressing normal MHC class II molecules on elements of hematopoietic origin.24 Thus, while in some reports significantly lower frequencies of negatively selected thymocytes have been suggested,7 25 most experimental data indicate that approximately half of positively selectable thymocytes are deleted during development.
In contrast to quantitative aspects, little information is available concerning the qualitative impact of thymic clonal deletion. We report here an analysis of the specificity of an otherwise normal T-cell repertoire that develops in the absence of thymic clonal deletion mediated by cells of hematopoietic origin in MHC I°II°→wt chimeras. On activation, these cells lysed targets expressing host type MHC, but only poorly proliferated on stimulation with host type antigen presenting cells (APC). Moreover, MHC I°II°→wt chimera-derived T lymphocytes did not induce lethal graft-versus-host disease (GVHD) in syngeneic animals. Therefore, thymic negative selection mechanisms appear to be redundant.
MATERIALS AND METHODS
Mice.
Wild-type C57BL/6 mice were purchased from Harlan Netherlands, Zeist, The Netherlands. Mice on C57BL/6 genetic background deficient in MHC class I expression (MHC I°) because of a targeted disruption of the β2-microglobulin gene26 were obtained from Dr B.J. Fowlkes, National Institutes of Health (NIH), Bethesda, MD. Mice deficient in MHC class II expression due to an introduced disruption of the I-Aα gene in C57BL/6 (I-Eα−) embryonic stem cells27 (MHC II°) were provided by Dr H. Bluethmann (Roche, Basel, Switzerland). MHC I° and MHC II° animals were interbred in our facilities to obtain MHC I°II° mice.
Hematopoietic chimeras.
Irradiation bone marrow chimeras were prepared essentially as described previously.28 Briefly, anti-NK1.1 antibody-treated hosts (100 μg PK136 intraperitoneal [IP] 29) were lethally irradiated (1,000 rad γ) using a Cs137source and reconstituted with 5 to 20 × 106 bone marrow cells that had been depleted of T cells using anti-Thy1 antibody AT8330 plus complement. Chimeras were kept on antibiotic containing water (0.2% Bactrim; Roche, Basel, Switzerland) for the complete duration of the experiment, usually 6 weeks.
Cytotoxic T-lymphocyte assays.
Unseparated thymocytes or splenocytes derived from DBA/2 mice or chimeras 6 weeks posttransfer were cultured for 6 days in the presence of T-cell–depleted (anti-Thy1 AT8330 plus complement), irradiated (1,000 rad γ) splenocytes in the absence or presence of 30 U/mL interleukin-2 (IL-2) (EL4 supernatant31). For lysis of RMA targets (C57BL/6 origin32), C57BL/6 APC-stimulated effectors were used, while P815 (DBA/2 origin33) lysis assays were performed using T cells stimulated with DBA/2 APC. Targets (2,000 cells per well) were labelled with Cr51, extensively washed and mixed with effector cells in duplicate at effector (viable cell) to target (E/T) ratios indicated. Cr51 release in the supernatant was measured 4 hours later. Specific lysis is Cr51 release above background as a percentage of maximum (as determined by acid lysis of targets). Actual E/T ratios (E/TA) in the P815 and RMA lysis assays were corrected (E/Tcorr) for anti-CD3ε antibody redirected lysis. P815 targets were incubated with cytotoxic T lymphocytes (CTL) in the presence of 1% 145-2C1134 supernatant at titrated E/T ratios and E/T ratio required for half maximal lysis determined (E/T50%). E/Tcorr = E/TA / E/T50%.
Proliferation assays.
A total of 106 thymocytes or 5 × 105splenocytes derived from DBA/2 mice or chimeras 6 weeks postreconstitution were cultured in triplicate in the presence of indicated numbers of T-cell depleted (anti-Thy1[AT83] plus complement), irradiated (1,000 Rad γ) splenocytes, in the presence or absence of 30 U/mL IL-2 (EL4 supernatant31). 3H-Thymidine was added at day 3 or 4 and incorporation measured 16 hours later by direct β counting (Top Count; Packard Instruments, Meriden, CT).
GVHD.
Sublethally irradiated (720 rad γ), anti-NK1.1 antibody PK13629-treated hosts were injected intravenously (IV) with the indicated number of unseparated thymocytes or splenocytes derived from DBA/2 mice or chimeras 6 weeks posttransfer. Alternatively, lethally (1,000 rad γ) irradiated PK136-treated hosts were injected IV with a mixture of T-cell–depleted bone marrow cells plus unseparated thymocytes, as previously described.35 Mice were kept on antibiotic containing water (0.2% Bactrim) for the complete duration of the experiment. Mortality was monitored for 3 months posttransfer.
RESULTS
Targets expressing host type MHC molecules are lysed by activated MHC I °II °→wt chimera derived CD8+T cells.
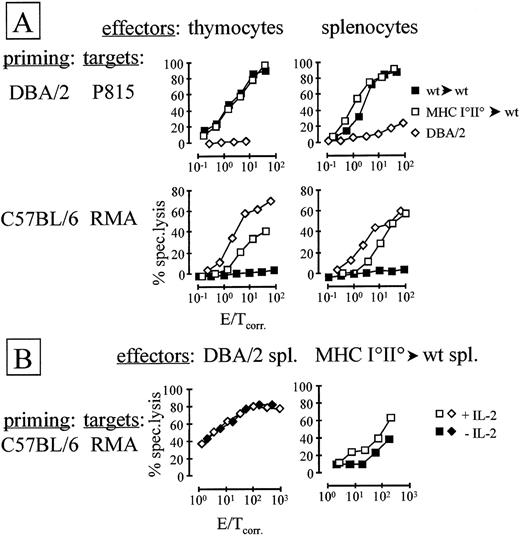
Thymocytes and peripheral T cells from experimental (MHC I°II°→wt) and control (wt→wt) chimeras, as well as from allogeneic controls (DBA/2), were stimulated with irradiated T-cell–depleted splenocytes in the presence of exogenous IL-2 in vitro. After 6 days of stimulation, lysis of targets expressing host type (RMA, H-2b) or allogeneic (P815, H-2d) MHC was analyzed (Fig 1).
Targets expressing host type MHC are lysed by activated T cells derived from deletion-deficient chimeras. (A) Thymocytes or splenocytes of indicated origin were stimulated in vitro with approximately the same number of irradiated DBA/2-derived (top panels) or C57BL/6-derived (bottom panels) T-cell–depleted splenocytes in the presence of exogenous IL-2. After 6 days of stimulation, lysis of indicated targets was assessed. (B) As in (A), but in vitro stimulation of splenocytes was performed with or without added IL-2, as indicated. E/T ratios were corrected for anti-CD3ɛ antibody redirected lysis of P815 targets as described in Materials and Methods.
Targets expressing host type MHC are lysed by activated T cells derived from deletion-deficient chimeras. (A) Thymocytes or splenocytes of indicated origin were stimulated in vitro with approximately the same number of irradiated DBA/2-derived (top panels) or C57BL/6-derived (bottom panels) T-cell–depleted splenocytes in the presence of exogenous IL-2. After 6 days of stimulation, lysis of indicated targets was assessed. (B) As in (A), but in vitro stimulation of splenocytes was performed with or without added IL-2, as indicated. E/T ratios were corrected for anti-CD3ɛ antibody redirected lysis of P815 targets as described in Materials and Methods.
MHC I°II°→wt and wt→wt chimera, but not control DBA/2-derived thymocytes and splenocytes stimulated in vitro with DBA/2 APC efficiently lysed P815 targets (which are of DBA/2 origin33) (Fig 1A). No reproducible difference in the lysis of P815 by wt→wt and MHC I°II°→wt chimera-derived thymocytes and splenocytes was observed (Fig 1A).
When stimulated with T-cell–depleted, irradiated C57BL/6 splenocytes in vitro, DBA/2 and MHC I°II°→wt, but not wt→wt chimera-derived thymocytes and splenocytes readily lysed RMA targets (Fig 1A). These targets are of C57BL/6 origin and therefore express chimera host type MHC molecules.32 RMA targets were invariably significantly better lysed by allogeneic DBA/2 effectors than by MHC I°II°→wt-derived T cells. Moreover, the lysis was completely mediated by CD8-dependent effector cells as evidenced by antibody blocking experiments (data not shown). No significant difference in lysis by thymocytes as compared with splenocytes was observed.
In all experiments described so far, exogenous IL-2 was added to cultures during the activation phase. Interestingly, when exogenous IL-2 was omitted, lysis of RMA targets by MHC I°II°→wt-derived splenocytes was reproducibly (approximately fivefold) reduced as compared with effector cells activated in the presence of IL-2 (Fig 1B). In contrast, lysis of RMA by DBA/2-derived splenocytes was not enhanced by addition of exogenous IL-2 (Fig 1B). These data suggest that sufficient levels of IL-2 are produced in cultures in which DBA/2 splenocytes are stimulated by C57BL/6 APC, while insufficient IL-2 production is obtained with MHC I°II°→wt-derived responder T cells. All thymic effectors required addition of exogenous IL-2 during the activation phase irrespective of the origin of effectors and targets (data not shown).
Poor proliferative response to host type MHC by MHC I °II ° →wt chimera derived T cells.
Thymocytes and splenocytes derived from wt→wt and MHC I°II°→wt chimeras and from control DBA/2 mice were stimulated in vitro with T-cell–depleted, irradiated C57BL/6 or DBA/2 splenocytes in the presence of exogenous IL-2 (Fig 2). While chimera-derived thymocytes and splenocytes proliferated well in response to allogeneic (DBA/2) APC, limited, but significant, proliferation was observed with APC expressing host type (C57BL/6) MHC (Fig 2). In contrast, allogeneic DBA/2-derived thymocytes and splenocytes proliferated well in response to C57BL/6 APC (Fig 2). When exogenous IL-2 was omitted, proliferation of MHC I°II°→wt splenocytes in response to C57BL/6, APC was completely abolished (Fig 2). In contrast to the results obtained in CTL assays, however, proliferative alloresponses were also significantly reduced in the absence of exogenous IL-2 (Fig2).
Deletion-deficient chimera-derived T cells proliferate in response to host type APC. A total of 5 × 105 Splenocytes or 106 thymocytes were stimulated with titrated numbers of T-cell–depleted, irradiated splenocytes. Origin of effectors and APC was as indicated in the figure. Exogenous IL-2 was added to thymocyte and splenocyte cultures unless indicated otherwise.3H-thymidine incorporation was assessed 3 to 4 days later.
Deletion-deficient chimera-derived T cells proliferate in response to host type APC. A total of 5 × 105 Splenocytes or 106 thymocytes were stimulated with titrated numbers of T-cell–depleted, irradiated splenocytes. Origin of effectors and APC was as indicated in the figure. Exogenous IL-2 was added to thymocyte and splenocyte cultures unless indicated otherwise.3H-thymidine incorporation was assessed 3 to 4 days later.
T cells derived from deletion-deficient chimeras fail to induce lethal GVHD in syngeneic hosts.
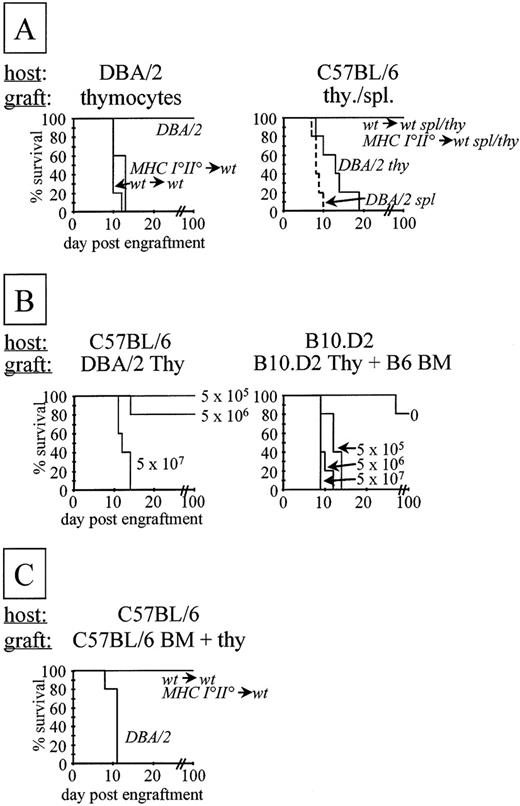
MHC I°II°→wt chimeras survived for prolonged periods of time (over 6 months, data not shown). Activation of naive T lymphocytes, however, requires costimulation delivered by professional antigen presenting cells.36 The latter cells are of bone marrow origin and therefore do not express MHC molecules in MHC I°II°→wt chimeras. To test if MHC I°II°→wt-derived T cells react to host type MHC in vivo, chimera-derived thymocytes and splenocytes were injected IV into sublethally irradiated (720 Rad) syngeneic (C57BL/6) or allogeneic (DBA/2) hosts (Fig 3A). While allogeneic DBA/2 hosts were efficiently killed by MHC I°II°→wt and wt→wt chimera-derived thymocytes, no lethal GVHD was observed in syngeneic hosts up to 100 days posttransfer, at which time the experiment was terminated (Fig 3A). As expected, C57BL/6 hosts were readily killed on injection of control allogeneic (DBA/2) T cells (Fig3A).
T cells derived from deletion-deficient chimeras do not induce lethal GVHD. (A) A total of 1.5 × 108 thymocytes or 1.5 × 107 splenocytes were injected IV into sublethally irradiated, anti-NK1.1 antibody-treated hosts. The origin of the T-cell populations is indicated in italics. Survival of the mice was monitored up to 3 months posttransfer. (B) Comparison of GVHD directed to total host (left) or to cotransferred bone marrow cells only (right). (Left) As in (A), but titrated numbers (indicated) of thymocytes were injected. (Right) Lethally irradiated hosts were injected with a mixture of 107 allogeneic bone marrow plus titrated numbers of syngeneic thymocytes. (C) Lethally irradiated, anti-NK1.1 antibody-treated C57BL/6 hosts were injected IV with a mixture of 5 × 106 C57BL/6 bone marrow cells plus 1.5 × 108 thymocytes of indicated origin.
T cells derived from deletion-deficient chimeras do not induce lethal GVHD. (A) A total of 1.5 × 108 thymocytes or 1.5 × 107 splenocytes were injected IV into sublethally irradiated, anti-NK1.1 antibody-treated hosts. The origin of the T-cell populations is indicated in italics. Survival of the mice was monitored up to 3 months posttransfer. (B) Comparison of GVHD directed to total host (left) or to cotransferred bone marrow cells only (right). (Left) As in (A), but titrated numbers (indicated) of thymocytes were injected. (Right) Lethally irradiated hosts were injected with a mixture of 107 allogeneic bone marrow plus titrated numbers of syngeneic thymocytes. (C) Lethally irradiated, anti-NK1.1 antibody-treated C57BL/6 hosts were injected IV with a mixture of 5 × 106 C57BL/6 bone marrow cells plus 1.5 × 108 thymocytes of indicated origin.
The lack of lethal GVHD mediated by MHC I°II°→wt chimera-derived T cells seems rather unexpected. Bone marrow-derived cells express tissue-specific ligands that in the absence of MHC expression on hematopoietic cells in MHC I°II°→wt chimeras presumably cannot have been encountered during the development of the MHC I°II°→wt chimera-derived T cells. These cells would therefore be expected to respond to such ligands. Therefore, we wished to investigate whether MHC I°II°→wt chimera-derived T lymphocytes are capable of efficient lysis of MHC expressing hematopoietic cells in vivo.
To establish a system in which lysis of bone marrow cells only leads to mortality, we reconstituted lethally irradiated B10.D2 hosts with C57BL/6 bone marrow cells and coinjected titrated numbers of B10.D2 thymocytes. Mortality of hosts was observed with (at least) 100-fold fewer effector cells than that required for a classical H-2d versus H-2b GVHD (Fig 3B).
To investigate if MHC I°II°→wt chimera-derived T lymphocytes can kill host type hematopoietic cells in vivo, we reconstituted lethally irradiated C57BL/6 hosts with syngeneic bone marrow and coinjected large numbers of MHC I°II°→wt or wt→wt chimera or DBA/2-derived thymocytes (Fig 3C). No mortality of C57BL/6 hosts injected with MHC I°II°→wt (or wt→wt) chimera-derived thymocytes was observed up to 100 days posttransfer, at which time the experiment was terminated. As expected, C57BL/6 hosts injected with allogeneic (DBA/2-derived) thymocytes died rapidly (Fig 3C).
DISCUSSION
Thymic clonal deletion is thought to be the major mechanism responsible for the tolerization of the T-cell repertoire.37-39Consistent with this hypothesis, a twofold to threefold increased generation of mature T cells is observed in the absence of clonal deletion by APC of hematopoietic origin in MHC I°II°→wt chimeras as compared with control chimeras.10 Half to two thirds of the mature T lymphocytes developing in these chimeras therefore are presumably self-specific. Consistent with this notion, we find that CD8+ T cells that develop in clonal deletion-deficient animals are capable of lysis of targets expressing host type MHC molecules. Moreover, MHC I°II°→wt-derived T cells proliferate in response to APC expressing host type MHC, although this response is rather poor. In contrast, we have found no evidence for in vivo reactivity of T cells from deletion-deficient chimeras.
Our data relate to earlier work on the tolerogenic capacity of thymic epithelium and peripheral tissues. It has been observed in several systems that expression of MHC molecules by thymic epithelial cells or by peripheral tissues leads to tissue-specific tolerance. Thus, T lymphocytes derived from athymic nude mice or irradiated recipients reconstituted with thymic anlagen or fetal thymi deprived of bone marrow-derived cells are tolerant to donor type MHC expressed by tissues such as heart and skin, but have occasionally been reported to be reactive to cells of hematopoietic origin expressing the same MHC molecules.16,40-47 In irradiation bone marrow (and spleen) chimeras in which F1 hosts were reconstituted with bone marrow derived from one parent (P→F1), a T-cell repertoire has been shown to develop that in vivo is tolerant to MHC of the other parent, while in vitro this is not the case (“split tolerance”).20,48-50 Tolerization of TCR transgenic thymocytes has been shown to occur when the tolerizing ligand is expressed exclusively on thymic epithelial cells.16,18,23 T cells developing in bone marrow chimeric mice expressing mammary tumor virus-encoded superantigens exclusively on thymic epithelial cells are tolerant to the same antigens when expressed on APC.51,52 Finally, transgenic mice expressing MHC molecules exclusively on thymic epithelial cells or on peripheral tissues have been shown to be tolerant to transgene type MHC.17 53-58Thus, thymic epithelial cells and peripheral tissues appear to have tolerogenic capability.
In the thymus medullary, but not cortical, epithelial cells are capable of tolerance induction. It has been reported that in MHC transgenic mice, the T-cell repertoire is (partly) tolerant to ligands exclusively expressed by thymic medullary epithelial cells.17,19,41,58However, T cells from transgenic mice expressing MHC class II molecules exclusively on cortical epithelial cells react vigorously to APC expressing the same MHC molecules, indicating a lack of tolerance induction.25 Moreover T cells derived from relB-deficient mice, which lack thymic medullary epithelium, proliferate in response to normal APC.59 Thus, while positive selection seems to be supported by cortical, but not medullary epithelial cells,60-62 epithelial tolerance induction appears to be limited to the medulla.
Given the tolerogenic capability of thymic epithelium and peripheral tissues, the T-cell repertoire in MHC I°II°→wt chimeras may be expected to be tolerant to tissues expressing MHC molecules in the chimeras, ie, radioresistant cells. In contrast, because MHC molecules were not expressed on radiosensitive tissues in the chimeras, T lymphocytes would be expected to react to hematopoietic cells when they express MHC, eg, in mixed lymphocyte reaction, CTL assays, or when injected into syngeneic hosts.
In vitro, targets expressing host type MHC molecules were efficiently lysed by MHC I°II°→wt, but not control wt→wt chimera-derived thymocytes and splenocytes. The target used, RMA, is a thymoma and therefore of hematopoietic origin.32Proliferative responses on stimulation of MHC I°II°→wt-derived T cells with host type splenic APC were significant, but rather limited. Both responses, however, were well below that observed for allogeneic combinations. Because the frequency of alloreactivity has been estimated to be around 1% to 10% for a given combination,63,64 the percentage of MHC I°II°→wt chimera-derived T cells reactive to host type MHC presumably is less than 1%. Because twofold to threefold more mature T cells developed in clonal deletion-deficient chimeras as compared with control chimeras, the frequency of in vitro reactivity to host type MHC appears unexpectedly low. Alternatively, the low level of reactivity of MHC I°II°→wt chimera-derived T cells to host type MHC molecules could be explained by an unusually low TCR affinity. Both hypotheses can be explained by induction of nondeletional tolerance mediated by thymic epithelium. Moreover, it has been reported that the threshold for activation of mature T lymphocytes is significantly higher (100-fold in one report) than that for thymic clonal deletion.65 Thus, a proportion of mature T cells that in normal mice would have been deleted, but that survived in clonal deletion-deficient chimeras may express TCR with affinity for self ligands that is too low to lead to activation.
Interestingly, in contrast to lysis by allogeneic effectors, lysis of RMA by MHC I°II°→wt-derived T cells partly depends on exogenous IL-2 added to the priming cultures. Moreover, when stimulated with APC expressing host type MHC, the limited proliferative responses by MHC I°II°→wt chimera-derived splenic T-cell populations were practically absent if exogenous IL-2 was omitted. These data are consistent with earlier reports showing that cytotoxicity requires a lower activation threshold than IL-2 production or proliferation.66 Therefore, it appears that only clones expressing TCR with low affinity for self ligands remain functional in the absence of clonal deletion and that other (nondeletional) tolerance mechanisms are responsible for tolerization of clones expressing TCR with higher affinity. A more detailed comparative analysis of cytotoxicity, proliferation, and cytokine production will be required to test this hypothesis.66 67
In vivo, MHC I°II°→wt chimera-derived T lymphocytes did not induce lethal GVHD in sublethally irradiated host type animals. The main immunopathological consequences of GVHD are medullary aplasia, enteropathy and sclerodermia.68 While the intestinal tract has been reported to be targeted in GVHD mediated by MHC class II, but not class I differences, medullary aplasia has been described to result from MHC class I or class II differences.68 Moreover, it has been reported that proliferation is not required to cause lethal GVHD over MHC class I barriers and that CTL or Th1 lymphocyte-mediated lysis of bone marrow cells is sufficient to cause death of host animals.35,68,69 Because in vitro lysis of targets that express host type MHC was observed even in the virtual absence of proliferation, it is surprising that MHC I°II°→wt chimera-derived T cells do not induce mortality due to medullary aplasia in host type animals. To assure that lysis of bone marrow cells will lead to mortality in our experimental system and to increase its sensitivity, we have adapted a previously described GVHD system in which injected T cells react to coinjected bone marrow cells required to reconstitute lethally irradiated hosts.35 Our results indicate that at least 100-fold fewer T effector cells are required to cause mortality in this system than in classical GVHD. While low numbers of allogeneic thymocytes were sufficient to cause mortality in this system, 300-fold more MHC I°II°→wt chimera-derived thymocytes were not. Therefore, while we formally cannot exclude the possibility that the fact that deletion-deficient T lymphocytes are incapable of induction of lethal GVHD is due to quantitative factors, we favor the hypothesis that other mechanisms of nondeletional tolerance are responsible.70
Whatever the explanation for the low level of reactivity to host type MHC by MHC I°II°→wt chimera-derived T lymphocytes in vitro and the absence of lethal GVHD in vivo, it is clear from our data that thymic tolerance mechanisms are largely redundant.
ACKNOWLEDGMENT
We gratefully acknowledge the expert technical help of R. Lees, P. Zaech, and A.-L. Peitrequin. Members of the Ludwig Institute and in particular T. Bianchi, S. Marguerat, and T. Renno are acknowledged for discussions. We thank Drs H. Bluethmann and B.-J. Fowlkes for providing MHC mutant mice.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Joost P.M. van Meerwijk, PhD, Institut National de la Santé et de la Recherche Médicale (INSERM) U395, CHR Purpan, BP 3028, 31024 Toulouse Cedex 3, France.