Abstract
The expression of P-glycoprotein (Pgp) is often increased in acute myeloid leukemia (AML). However, little is known of the regulation of Pgp expression by cytotoxics in AML. We examined whether Pgp expression and function in leukemic blasts was altered after a short exposure to cytotoxics. Blasts were isolated from 19 patients with AML (15 patients) or chronic myeloid leukemia in blastic transformation (BT-CML, 4 patients). Pgp expression and function were analyzed by flow cytometric analysis of MRK 16 binding and Rhodamine 123 retention, respectively. At equitoxic concentrations, ex vivo exposure for 16 hours to the anthracyclines epirubicin (EPI), daunomycin (DAU), idarubicin (IDA), or MX2 or the nucleoside analogue cytosine arabinoside (AraC) differentially upregulated MDR1/Pgp expression in Pgp-negative and Pgp-positive blast cells. In Pgp-negative blasts, all four anthracyclines and AraC significantly increased Pgp expression (P = .01) and Pgp function (P = .03). In contrast, MX2, DAU, and AraC were the most potent in inducing Pgp expression and function in Pgp positive blasts (P < .05). A good correlation between increased Pgp expression and function was observed in Pgp-negative (r = .90, P = .0001) and Pgp-positive blasts (r = .77,P = .0002). This increase in Pgp expression and function was inhibited by the addition of 1 μmol/L PSC 833 to blast cells at the time of their exposure to these cytotoxics. In 1 patient with AML, an increase in Pgp levels was observed in vivo at 4 and 16 hours after the administration of standard chemotherapy with DAU/AraC. Upregulation of Pgp expression was also demonstrated ex vivo in blasts harvested from this patient before the commencement of treatment. In 3 other cases (1 patient with AML and 2 with BT-CML) in which blasts were Pgp negative at the time of initial clinical presentation, serial samples at 1 to 5 months after chemotherapy showed the presence of Pgp-positive blasts. All 3 patients had refractory disease. Interestingly, in all 3 cases, upregulation of Pgp by cytotoxics was demonstrated ex vivo in blasts harvested at the time of presentation. These data suggest that upregulation of the MDR1 gene may represent a normal response of leukemic cells to cytotoxic stress and may contribute to clinical drug resistance.
MULTIDRUG RESISTANCE (MDR) is a common obstacle to successful chemotherapy in acute myeloid leukemia (AML).1 Patients often relapse with unresponsive disease after an initial response to treatment with cytotoxic drugs.2 The most common form of drug resistance in relapsed acute leukemia is due to the overexpression of P-glycoprotein (Pgp), a member of the ATP-binding cassette (ABC) superfamily of transporter proteins.3 Pgp encoded by the MDR1 gene4 is believed to function as an energy-dependent, efflux pump resulting in a decrease in intracellular drug concentrations to sublethal levels. There appears to be a direct correlation between expression of the MDR1 gene de novo and outcome in this disease.5 6
Other transport proteins thought to contribute to drug resistance are the multidrug resistance-associated protein (MRP) and lung resistance protein (LRP). Both are also expressed in AML.7 However, the exact role and function of these proteins in AML are still unclear.8,9 A recent study has demonstrated that MDR1/Pgp rather than MRP or LRP expression was of prognostic value in AML and that only MRP function was an independent prognostic factor.10
The regulation of Pgp expression is not well understood. MDR1 expression has been shown to be rapidly inducible in human cell lines in response to a variety of stresses, including heat shock, arsenite, or differentiating agents.11 12 The mechanisms underlying the effect of cytotoxic drugs on MDR1 gene expression and, in turn, the MDR phenotype remain poorly understood.
The acquisition of Pgp-mediated drug resistance during chemotherapy is usually thought to be due to the selection of drug-resistant cells.13,14 However, in previous studies in a human MDR cell line, we demonstrated that a rapid upregulation of the MDR1 gene can occur after the exposure of cells to anthracyclines15and its analogues.16 The rapid increase in MDR1 gene expression after exposure to cytotoxics is strongly supported by earlier reporter gene studies, demonstrating that the MDR1 promoter is activated by anticancer agents in human17 and rodent cell lines.18,19 Chaudhary and Roninson20 also demonstrated small changes in Pgp levels in a number of human cell lines after exposure to a number of cytotoxics. Understanding the underlying mechanisms by which cytotoxics upregulate drug resistance genes is important, especially if this phenomenon can be prevented by cyclosporin A (CyA) and its analogue PSC 833.21
Although studied in cell lines, the effect of cytotoxic agents on Pgp expression in primary human cells after a short exposure to cytotoxic agents has not been determined. The present study was designed to investigate whether the ex vivo exposure of leukemic blasts to the classical anthracyclines, epirubicin (EPI) and daunomycin (DAU), two new lipid soluble anthracycline analogues idarubicin (IDA) and a new morpholino-anthracycline MX2, or cytosine arabinoside (AraC) was able to upregulate Pgp expression and, hence, drug resistance in AML or chronic myeloid leukemia (CML) in blast crisis (BT-CML).
MATERIALS AND METHODS
Materials.
EPI, IDA, doxorubicin (DOX), and verapamil (Vp) were obtained commercially from Pharmacia & Upjohn Pty LTD (New South Wales, Australia). DAU and AraC were purchased from David Bull Laboratories (Melbourne, Australia). MX2 was a gift from Kirin Brewery Co Ltd (Tokyo, Japan). Vp was dissolved in 0.9% saline solution. PSC 833 was obtained from Sandoz Pharma Ltd (Basel, Swizerland) and initially dissolved in absolute alcohol before being diluted in RPMI 1640 to give a stock solution of 0.5 mg/mL (the final ethanol concentration was 35%).
The monoclonal antibody (MoAb) to Pgp (MRK 16) was generously provided by Dr Takashi Tsuruo (Division of Experimental Chemotherapy, Japanese Foundation for Cancer Research, Tokyo, Japan). A fluorescein-labeled F(ab)2 fragment of sheep antimouse IgG was purchased from Silenus Laboratories (Melbourne, Australia). Rhodamine 123 (Rh123) and hydroxystilbamidine methanosulfonate (Fluoro-Gold) were purchased from Molecular Probe (St Louis, MO).
Cell culture.
A variant human T-cell leukemia MDR cell line, CEM/A7R, was used as a model for induction of MDR1 gene expression.15,16 This line was derived from a classical MDR cell line CEM/A7 selected for low level DOX resistance by stepwise selection of the parental line CCRF-CEM cultured in increasing concentrations of DOX.22The resistant line CEM/A7 was maintained in conditioned medium containing 0.07 μg/mL of DOX. The variant line (now stable for more than 5 years) was established by culturing the CEM/A7 cells in the absence of DOX before being subcloned and designated as the CEM/A7R line. This line was not exposed to DOX or other Pgp substrates except in the specific experiments detailed below. At the time of these experiments, all lines were mycoplasma free based on the Mycoplasma T.C Rapid kit (GEN-PROBE, Inc, San Diego, CA).
Clinical sample collection.
Peripheral blood (PB; 16 patients) or bone marrow aspirates (BM; 3 patients) were collected in EDTA or heparinized tubes from 19 patients (15 AML and 4 BT-CML) before treatment and were diluted 1:1 with phosphate-buffered saline (PBS) without Ca2+ or Mg2+. Mononuclear cells were prepared by Ficoll-Hypaque density gradient centrifugation (Pharmacia Biotech AB, Uppsala, Sweden) according to the manufacturer’s recommendations. Interface cells were washed twice with RPMI 1640 medium (GIBCO Labs, Grand Island, NY); resuspended in 10 mL culture medium RPMI 1640 medium; supplemented with 10% fetal calf serum (FCS; Trace Biosciences Pty Ltd, Melbourne, Australia), gentamicin (80 μg/mL), minocycline (1 μg/mL), HEPES (20 mmol/L), sodium bicarbonate (0.21%), glutamine (0.8 mmol/L), 0.1% (1 μmol/L) sodium pyruvate, and 1% nonessential amino acids; and incubated for at least 4 hours before drug treatment. In all cases, cell viability was greater than 95% by trypan blue exclusion and more than 75% of cells were blasts on microscopy of cytospin specimens. When not used immediately, the mononuclear cells were frozen in 40% RPMI 1640, 50% FCS, and 10% dimethyl sulphoxide (DMSO) at −70°C and stored in liquid nitrogen. The viability of frozen cells was always checked using the trypan blue exclusion technique after thawing and was typically greater than 95%.
Drug treatment of isolated blasts and culture conditions.
To measure the effect of drug treatment, leukemic blasts were suspended in 10 mL of culture medium at a concentration 0.5 to 1 × 106/mL. Cells were incubated in the presence or absence of 100 ng/mL IDA, 100 ng/mL MX2, or 200 ng/mL EPI for 4 hours. Alternatively, cells were incubated with or without 20 ng/mL IDA, 50 ng/mL MX2, 100 ng/mL EPI, 80 ng/mL DAU, or 10 ng/mL AraC for 16 hours in the continuous presence or absence of 1 μmol/L PSC 833. The number of viable cells was determined by the use of trypan blue before and after drug treatment. For the patients studied, no statistically significant differences were observed in cell viability in the samples exposed to drug compared with control samples during this short time period. Nonviable cells were excluded from flow cytometric analysis by Fluoro-Gold, a viability stain suitable for multi-color analysis, because it does not interfere with the emission spectrum of Rh123 or fluorescein isothiocyanate (FITC).23
Flow cytometric analysis of Pgp expression.
MRK 16, an MoAb to an external epitope of Pgp, was used in a flow cytometric assay to measure Pgp expression. Cells were collected and washed 3 times in medium containing 10% FCS. MRK 16 (10 μg/mL) was added to cells at room temperature (RT) for 20 minutes. A nonspecific murine MoAb (IgG2a; Becton Dickinson, Sydney, Australia) was used as the isotype control. After an additional 3 washes, cell pellets were resuspended in the same volume of PBS containing 10 μL of a 1:10 dilution of a fluorescein-conjugated F(ab′)2 fragment of sheep antimouse IgG antibody (Silenus Laboratories) for 20 minutes at room temperature in the dark. Cells were washed once again (3×) and fluorescence was analyzed on a FACScan flow cytometer (Becton Dickinson, Sydney, Australia) with the forward scatter (FSC) and side scatter (SSC) gate set around the blast population and using a FSC versus fluorogold dot plot to gate out dead cells. For each sample, 10,000 events were collected. The Lysys II software was used to analyze data. Pgp levels were expressed as the ratio of the arithmetic mean of the fluorescence of MRK 16 versus the IgG2a control (refereed as Pgp ratio) in accordance with the consensus recomendations of Marie et al.24,25 Under the conditions used, the ratio of mean channel fluorescence of MRK16 versus control IgG2afor CCRF-CEM ranged from 0.78 to 1.09 (0.94 ± 0.09). In the positive control CEM/A7R line, Pgp ratios were around 2 (1.92 ± 0.32). Therefore, the threshold for Pgp negative status in blasts isolated from patients with AML or BT-CML was artificially defined as a MCF ratio ≤1.10. Positive Pgp upregulation by cytotoxic treatment was defined as an increase in the Pgp ratio of treated blasts to untreated blasts of ≥10%. In some experiments, the positive MRK 16 staining cells were also analyzed by the Kolmogorov-Smirov (KS) test.10,26 These two methods accurately identify small differences in fluorescence and are useful in the detection of low level protein expression, which frequently occurs in patient samples.26 A strong correlation was observed between the two methods.10
Rh123 accumulation.
Rh123 accumulation was chosen as a sensitive and selective measure of the transport function of Pgp.26,27 The assay was performed as described by Broxterman et al.26 Briefly, blast cells treated with or without cytotoxics in the presence or absence of the modulator PSC 833 (1 μmol/L) were washed three times in RPMI with 10% FCS. Cells were then resuspended in 2 mL RPMI medium at a cell concentration of 5 to 10 × 105 cell/mL and incubated at 37°C for 30 minutes to allow recovery of metabolic activity. Fluorescence was measured after the addition of 200 ng/mL of Rh123 (stock solution, 1 mg/mL in PBS) to the culture medium in the presence or absence of 2 μmol/L PSC 833 or 10 μmol/L Vp. The cells were then incubated in the dark at 37°C for 2 hours and stained with 2 μmol/L Fluoro-Gold to determine cell viability. Quantitation of fluorescence intensity was performed on the Becton Dickinson FACSCAN using Lysis II software. Rh123 fluorescence was measured through a 530 DF 30-nm filter and Fluoro-Gold fluorescence through a 630 DF 32-nm filter. The acquisition gate was set on the FSC versus Fluoro-Gold dot plot for live cell determination. The results are expressed as the ratios of the mean channel fluorescence (MCF) in the presence or absence of modulator (referred to as Rh123 ratio). An increase in Pgp function by cytotoxic treatment was defined as a change in the Rh123 ratio in treated compared with untreated blasts of ≥10%.
Statistical analysis.
Statistical comparisons of Pgp expression and function between drug-treated groups versus controls were performed by analysis of variance followed by Fisher’s multiple comparison test. Correlations of Pgp upregulation and changes in Pgp function by cytotoxics were evaluated by Spearman rank coefficient.
RESULTS
The upregulation of Pgp expression in AML blasts by anthracycline analogues and AraC.
The two lipid soluble anthracyclines IDA and MX2; the classical anthracyclines EPI, DAU, and AraC; and a non-Pgp substrate were used to study the upregulation of Pgp in clinical samples. The relative cytotoxicity of each of these drugs was previously determined in the drug-resistant CEM/A7R and the parental, drug-sensitive line CCRF-CEM in a 3-day growth inhibition assay.16 The concentrations of each cytotoxic used in this study of clinical samples corresponded to the IC50 levels in the CEM/A7R line determined in that assay (20 ng/mL IDA, 50 ng/mL MX2, 100 ng/mL EPI, 80 ng/mL DAU, or 10 ng/mL AraC), although blasts were only exposed to these drugs for a maximum of 16 hours in the current experiments.
To study the upregulation of Pgp and whether it related to the initial Pgp status before drug treatment, leukemic blasts were categorised as Pgp-negative (10 patients) or Pgp-positive (9 patients) based on Pgp expression in the flow cytometric assay (Tables 1 and2, respectively). Initial Pgp ratios in untreated cells ranged from 0.75 to 1.08 (0.98 ± 0.03) for Pgp-negative blasts and 1.17 to 2.22 (1.44 ± 0.09) for Pgp-positive blasts.
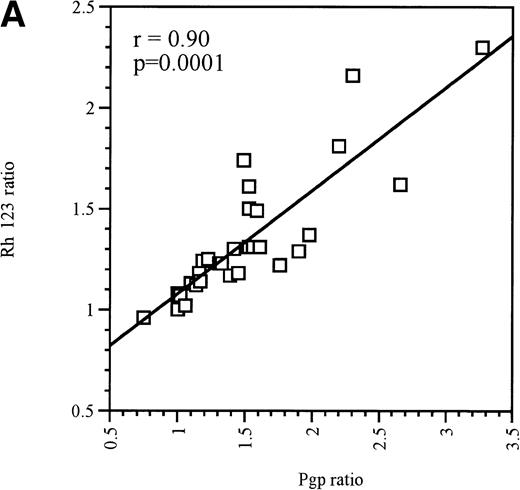
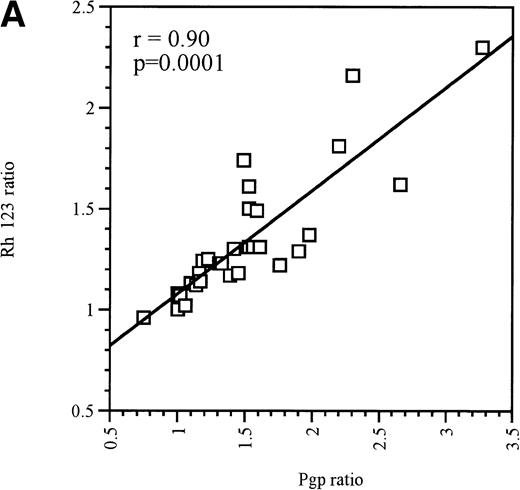
At equitoxic concentrations, ex vivo exposure for 16 hours to the anthracyclines EPI, DAU, IDA, or MX2 or the nucleoside analogue AraC differentially upregulated MDR1/Pgp expression in Pgp-negative and Pgp-positive blast cells. In Pgp-negative blasts, all 4 anthracyclines and AraC significantly increased Pgp expression (P = .01, Table1). Although every patient was not tested with every drug, all Pgp-negative blasts that were treated with MX2, EPI, or DAU displayed a definite upregulation of Pgp levels (Table 1). In contrast, only 80% of blast samples displayed increased Pgp expression after IDA or AraC treatment (Table 1). In each case, the upregulation of Pgp expression was accompanied by a significant increase in Pgp function (P = .03), as measured by a decrease in Rh123 accumulation that was reversible in the presence of 2 μmol/L PSC 833, which was used in this instance as a modulator of Pgp function (ie, only added to cells at the same time as Rh123).26 There was a strong correlation between Pgp upregulation and increased function after exposure to cytotoxics, as shown in Fig 1A (r = .90, P = .0001).
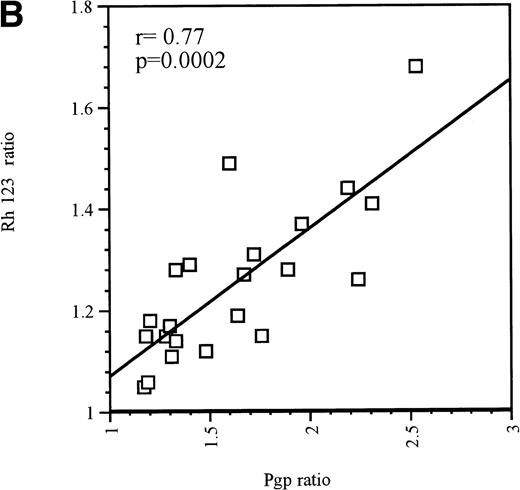
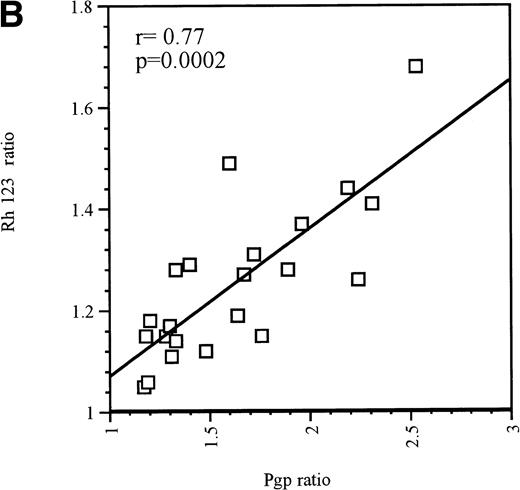
(A) Correlation between Pgp upregulation and increase in Pgp function in Pgp-negative blasts obtained from 10 patients. The data points represent 32 independent drug treatments for which Pgp expression and function measuments were performed as described in Table1. Pgp expression or function was expressed as the ratio of the arithmetic mean of fluorescence (MCF) of MRK 16 versus the IgG2a control or Rh123 fluorescence in the presence or absence of 2 μmol/L PSC 833, respectively, as described in Materials and Methods. (B) Correlation between Pgp upregulation and increase in Pgp function in Pgp-positive blasts obtained from 9 patients. The data points represent 22 independent drug treatments for which Pgp expression and function measuments were performed as described in Table2. Pgp expression or function was expressed as the ratio of the MCF of MRK 16 versus the IgG2a control or Rh123 fluorescence in the presence or absence of 2 μmol/L PSC 833 respectively as described in Materials and Methods. (□) Pgp ratio/Rh 123 ratio.
(A) Correlation between Pgp upregulation and increase in Pgp function in Pgp-negative blasts obtained from 10 patients. The data points represent 32 independent drug treatments for which Pgp expression and function measuments were performed as described in Table1. Pgp expression or function was expressed as the ratio of the arithmetic mean of fluorescence (MCF) of MRK 16 versus the IgG2a control or Rh123 fluorescence in the presence or absence of 2 μmol/L PSC 833, respectively, as described in Materials and Methods. (B) Correlation between Pgp upregulation and increase in Pgp function in Pgp-positive blasts obtained from 9 patients. The data points represent 22 independent drug treatments for which Pgp expression and function measuments were performed as described in Table2. Pgp expression or function was expressed as the ratio of the MCF of MRK 16 versus the IgG2a control or Rh123 fluorescence in the presence or absence of 2 μmol/L PSC 833 respectively as described in Materials and Methods. (□) Pgp ratio/Rh 123 ratio.
Upregulation of Pgp was also observed in the majority of Pgp-positive blast samples. As with Pgp-negative blasts, MX2 upregulated Pgp in 8 of 9 Pgp-positive blasts. Of the 8 Pgp-positive blast samples tested with EPI, 7 responded by upregulating Pgp expression. These 7 blast samples were also exposed to IDA, but only 4 responded by upregulating Pgp expression. The same 4 samples also displayed Pgp upregulation in response to treatment with AraC or DAU. The upregulation of Pgp that resulted from exposure to MX2, DAU, or AraC was significant, as was the associated change in Pgp function (P < .05). From the group of Pgp-positive blast samples, only 1 patient sample did not show Pgp upregulation in response to any of the drugs tested. The strong correlation between increased Pgp expression and function is shown in Fig 1B (r = .77, P = .0002).
Inhibition of Pgp upregulation in leukemic blasts by PSC 833.
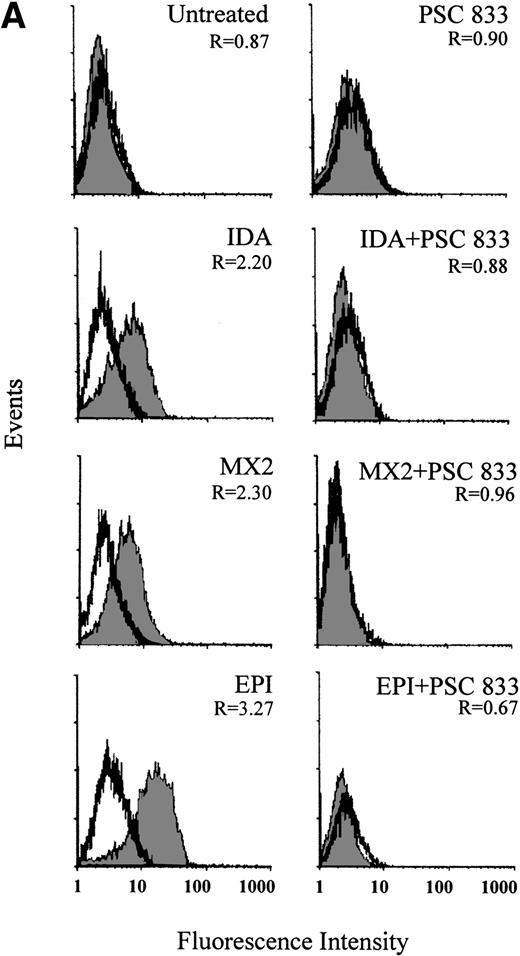
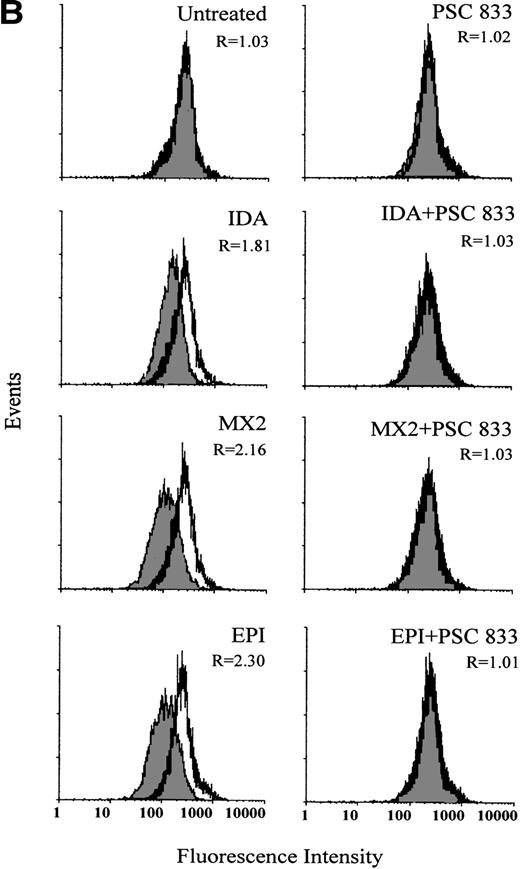
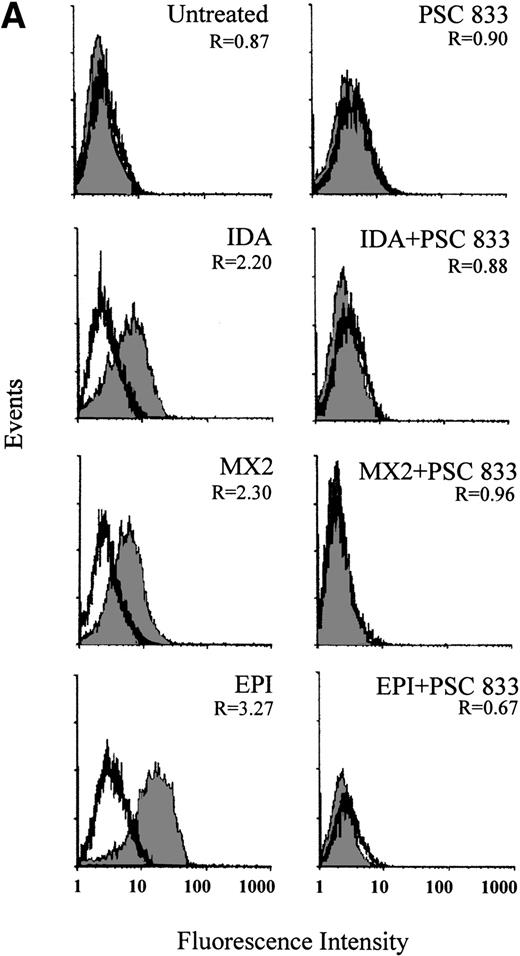
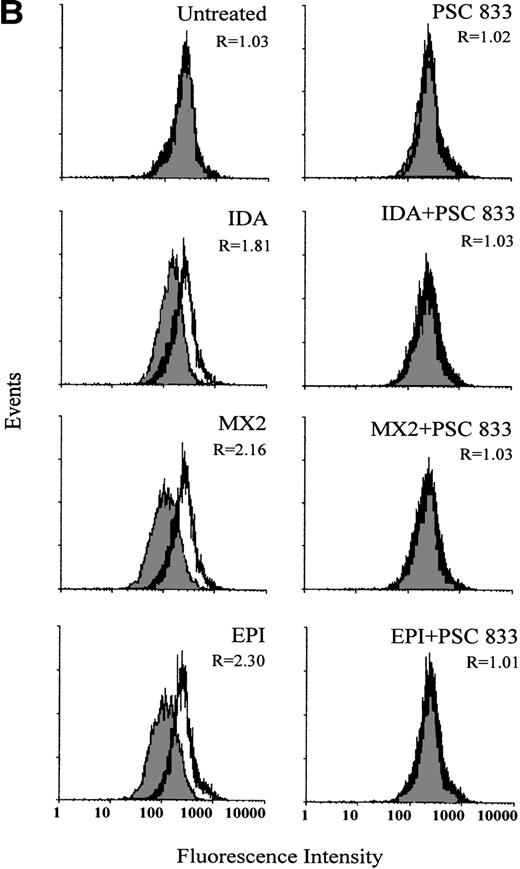
The ability of PSC 833 to inhibit the upregulation Pgp by cytotoxics was also examined in leukemic blasts. These data are summarized in Table 3. Upregulation of Pgp by all 5 cytotoxics was almost totally inhibited by the exposure of cells to 1 μmol/L PSC 833 at the same time as cytotoxics were added to the blast samples (Fig 2A and Table 3).
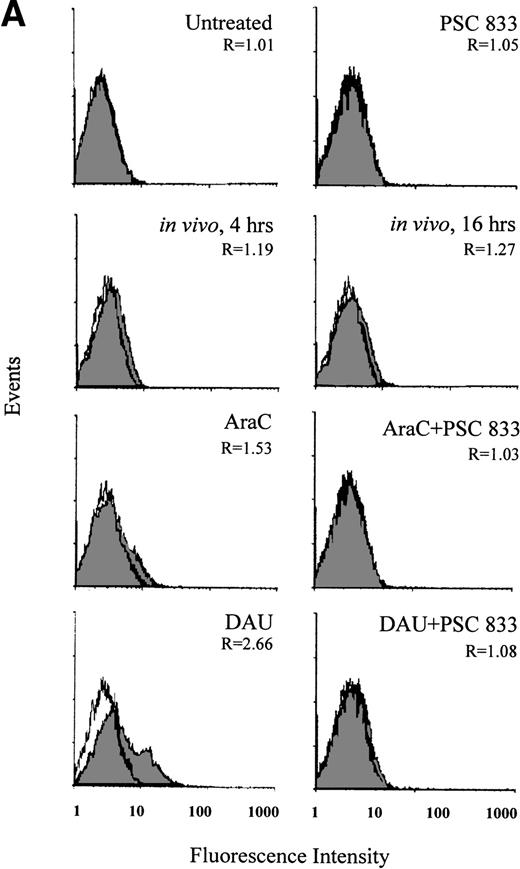
(A) Upregulation of Pgp expression measured by flow cytometric analysis using MRK 16 binding (solid histogram) compared with an IgG2a control (open histogram) in Pgp-negative leukemic blasts obtained from a single patient after 16 hours of treatment with 20 ng/mL IDA, 50 ng/mL MX2, and 100 ng/mL EPI. The blasts were isolated from a patient with BT-CML. The inhibitory effect of 1 μmol/L PSC 833 on the upregulation of Pgp is also shown. Pgp levels were expressed as the ratio of the MCF of MRK 16 versus the IgG2a control as described in Materials and Methods. This ratio (R) is indicated in each case. (B) Flow cytometric analysis of Pgp function based on Rh123 accumulation in the absence (solid histogram) or presence of 2 μmol/L PSC 833 (open histogram) in blasts from the same patient treated as described in (A). Pgp function was expressed as the ratio of MCF in the presence or absence of 2 μmol/L PSC 833 as described in Materials and Methods. This ratio (R) is indicated in each case.
(A) Upregulation of Pgp expression measured by flow cytometric analysis using MRK 16 binding (solid histogram) compared with an IgG2a control (open histogram) in Pgp-negative leukemic blasts obtained from a single patient after 16 hours of treatment with 20 ng/mL IDA, 50 ng/mL MX2, and 100 ng/mL EPI. The blasts were isolated from a patient with BT-CML. The inhibitory effect of 1 μmol/L PSC 833 on the upregulation of Pgp is also shown. Pgp levels were expressed as the ratio of the MCF of MRK 16 versus the IgG2a control as described in Materials and Methods. This ratio (R) is indicated in each case. (B) Flow cytometric analysis of Pgp function based on Rh123 accumulation in the absence (solid histogram) or presence of 2 μmol/L PSC 833 (open histogram) in blasts from the same patient treated as described in (A). Pgp function was expressed as the ratio of MCF in the presence or absence of 2 μmol/L PSC 833 as described in Materials and Methods. This ratio (R) is indicated in each case.
Semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) was used to examine the changes in MDR1 mRNA after drug treatment in the presence or absence of 1 μmol/L of PSC 833 in 3 patient samples. The results confirmed those obtained in the flow cytometry experiments (data not shown).
The inhibition of Pgp upregulation by PSC 833 was accompanied by corresponding changes in Rh123 accumulation (Fig 2B). Pgp was upregulated to different levels after 16 hours of exposure to equitoxic concentrations of each drug, correlating with a dramatic increase in Pgp function. This was seen as a decrease in Rh123 accumulation in drug-treated cells. Not only was upregulation of Pgp by cytotoxics inhibited by the presence of PSC 833, but the change in Rh123 accumulation was also prevented. An example of such an experiment is shown in Fig 2B. Similar results were obtained for other patient samples (data not shown).
The Pgp phenotype of AML blasts changes during induction chemotherapy.
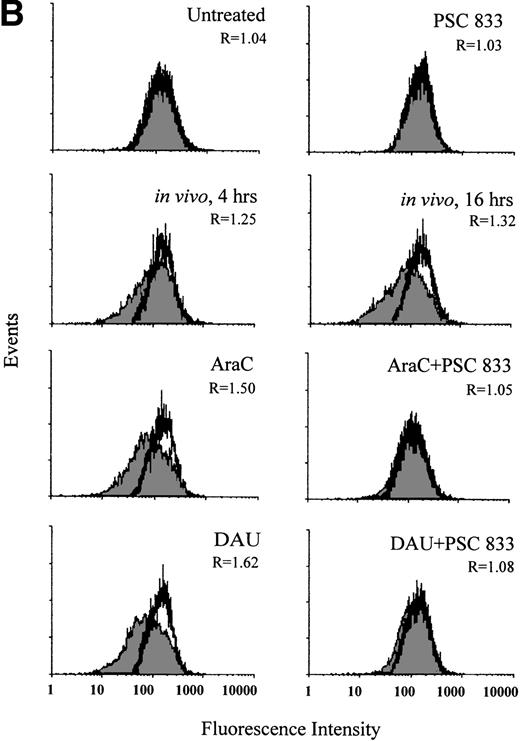
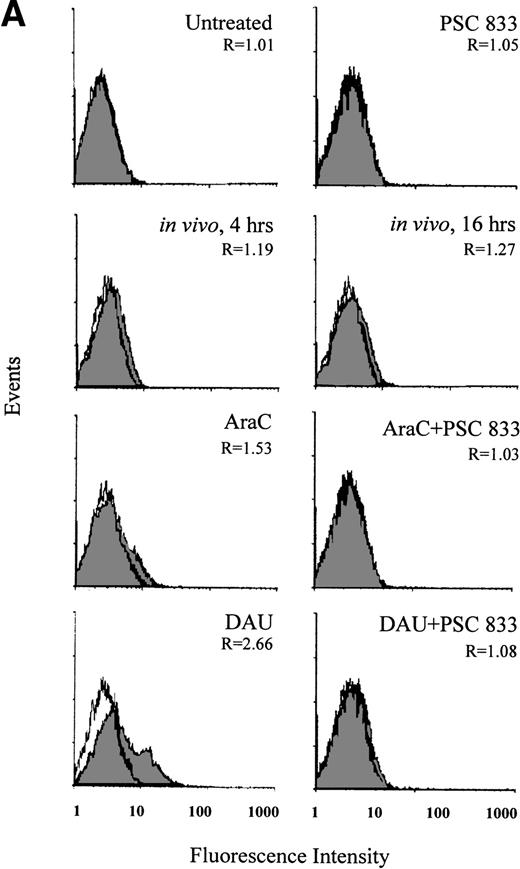
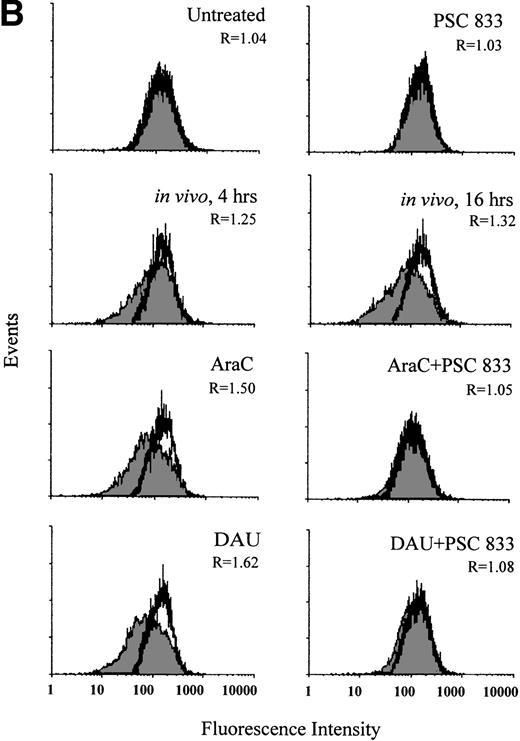
In blasts from a patient with AML, the change in Pgp expression in vivo was monitored during the administration of DAU/AraC induction chemotherapy. Blast cells were isolated from this patient before (0 hour) and at 4 and 16 hours after chemotherapy administration. Pgp expression was significantly increased at 4 hours and further increased at 16 hours after the onset of chemotherapy (P = .006; Fig 3A). The increase in Pgp expression was accompanied by a significant increase in Pgp function at both time points but was more obvious at 16 hours than at 4 hours (P = .003; Fig 3B). As recomended,30 KS analysis was also applied to the interpretation of Pgp upregulation in this case (Fig3A). This confirmed that the number of Pgp-positive cells significantly increased from 1.23% ± 0.3% at 0 hour to 6.8% ± 1.0% at 4 hours and to 11.2% ± 0.8% at 16 hours after chemotherapy (P = .001).
(A) Flow cytometric analysis of Pgp expression using MRK 16 binding (solid histogram) compared with an IgG2a control (open histogram) in Pgp-negative blasts from a patient with AML undergoing chemotherapy (in vivo) and after ex vivo experiments. Pgp expression was expressed as the ratio of the MCF of MRK 16 versus the IgG2a (R) as described in Materials and Methods. The analysis of samples was performed before treatment (0 h), at 4 hours, and at 16 hours of DAU/AraC treatment. In the ex vivo experiments, cells were exposed for 16 hours to medium alone, to 100 ng/mL DAU in the absence or presence of 1 μmol/L PSC 833, or to 10 ng/mL AraC in the absence or presence of 1 μmol/L PSC 833. (B) Flow cytometric analysis of Pgp function based on Rh123 accumulation in blasts from the same patient as shown in (A) at 0, 4, and 16 hours after DAU/AraC combination chemotherapy (in vivo) and in blasts treated with the same drugs ex vivo. Pgp function was determined by flow cytometric analysis using Rh123 accumulation in the absence (solid histogram) or the presence of 2 μmol/L PSC 833 (open histogram). Pgp function was expressed as the ratio of MCF in the presence or absence of 2 μmol/L PSC 833 as described in Materials and Methods. This ratio (R) is indicated in each case.
(A) Flow cytometric analysis of Pgp expression using MRK 16 binding (solid histogram) compared with an IgG2a control (open histogram) in Pgp-negative blasts from a patient with AML undergoing chemotherapy (in vivo) and after ex vivo experiments. Pgp expression was expressed as the ratio of the MCF of MRK 16 versus the IgG2a (R) as described in Materials and Methods. The analysis of samples was performed before treatment (0 h), at 4 hours, and at 16 hours of DAU/AraC treatment. In the ex vivo experiments, cells were exposed for 16 hours to medium alone, to 100 ng/mL DAU in the absence or presence of 1 μmol/L PSC 833, or to 10 ng/mL AraC in the absence or presence of 1 μmol/L PSC 833. (B) Flow cytometric analysis of Pgp function based on Rh123 accumulation in blasts from the same patient as shown in (A) at 0, 4, and 16 hours after DAU/AraC combination chemotherapy (in vivo) and in blasts treated with the same drugs ex vivo. Pgp function was determined by flow cytometric analysis using Rh123 accumulation in the absence (solid histogram) or the presence of 2 μmol/L PSC 833 (open histogram). Pgp function was expressed as the ratio of MCF in the presence or absence of 2 μmol/L PSC 833 as described in Materials and Methods. This ratio (R) is indicated in each case.
Upregulation of Pgp and corresponding changes in Pgp function in blasts (Fig 3A and B) from the same patient were demonstrated ex vivo by 16 hours of exposure of blast cells to 100 ng/mL DAU or 10 ng/mL AraC harvested before the initiation of chemotherapy. The induction of Pgp was also prevented by the presence of 1 μmol/L PSC 833 (Fig 3A).
Sequential change in Pgp status in blasts after chemotherapy.
Sequential samples from three patients with Pgp-negative blasts at the time of clinical presentation (1 AML and 2-BT-CML) were available for the purpose of this analysis (Table 4). Pgp expression was upregulated twofold to fourfold when blasts from these patients were exposed ex vivo for 16 hours to equicytotoxic concentrations of 20 ng/mL IDA, 50 ng/mL MX2, or 100 ng/mL EPI. All 3 patients had Pgp-negative blasts initially (Pgp ratio of 0.75, 0.87, and 1.00; Table 4), but when retested 1, 3, and 5 months after chemotherapy, the blasts were found to be Pgp-positive (Pgp ratio of 1.19, 1.51, and 1.76, respectively; Table 4). When a KS analysis was applied to the initial and subsequent samples, the number of Pgp-positive cells had increased from negative (0% to 2.3%) to 12.8%, 18.87%, and 22.1% in patients no. 1, 7, and 8, respectively. All 3 patients were refractory to treatment and died of progressive disease.
DISCUSSION
Flow cytometry analysis of Pgp expression using the MoAb MRK 16 and changes in the accumulation of Rh123 were used in this study of blast cells from patients with AML or BT-CML to examine Pgp upregulation. As previously recommended,10,24-26 changes in Pgp levels induced by cytotoxics were determined by comparing the ratios of the MCF for the specific antibody MRK 16 over the isotope control; changes in Pgp function were tested by comparing the MCF of Rh123 (in the presence or absence of 2 μmol/L PSC 833).26
Using these methods, we have demonstrated the upregulation of Pgp in leukemic blasts from patients with AML and BT-CML. At equitoxic concentrations, ex vivo exposure to anthracyclines (EPI or DAU), anthracycline analogues (IDA or MX2), and AraC upregulated Pgp expression in both Pgp-negative and Pgp-positive blasts. After 16 hours of exposure, all 4 anthracyclines as well as AraC upregulated Pgp in Pgp-negative blasts, whereas DAU, MX2, and AraC appeared to be the most potent in upregulating Pgp in Pgp-positive blasts (Tables 1 and 2). There was a good correlation between Pgp upregulation and the change in Pgp function as measured by the accumulation of Rh123 in both the Pgp-negative and Pgp-positive blasts (Fig 1A and B).
We had previously demonstrated that upregulation of Pgp by anthracyclines in the CEM/A7R cells was inhibited by the presence of 1 μmol/L PSC 833.21 In leukemic blasts we have now demonstrated that the upregulation of Pgp by anthracyclines and the non-Pgp substrate AraC was also preventable by the addition of 1 μmol/L PSC 833 (Table 3 and Fig 2A). This inhibition of Pgp upregulation correlated with the change in Pgp function (Fig 2B). To date, our study is the first to show that the MDR phenotype of leukemic blasts changes after a short (16 hours) exposure to cytotoxics. Upregulation of MDR1 mRNA by cytotoxic agents in acute leukemia was also confirmed by semiquantitative RT-PCR analysis in blasts from 3 patients with AML (data not shown). The increase in MDR1 mRNA was inhibited by the presence of 1 μmol/L PSC 833.
More direct evidence of the involvement of cytotoxics in the regulation of Pgp expression was observed in the study of leukemic blasts from a patient undergoing chemotherapy. Pgp expression and function were significantly increased in blasts harvested after 4 and 16 hours of exposure to DAU/AraC chemotherapy (Fig 3). Upregulation of Pgp by DAU/AraC was also demonstrated ex vivo in blasts isolated from the same patient before the initiation of chemotherapy (Fig 3). The induction of Pgp by both DAU and AraC ex vivo was inhibited by the addition of 1 μmol/L PSC 833 (Fig 3A). These data suggest that the MDR phenotype may alter within 4 to 16 hours of the onset of chemotherapy. How this finding impacts on clinical drug resistance during tumor progression is not clear. However, the rapid upregulation of Pgp expression provides evidence that the ultimate expression of the MDR phenotype may be a direct consequence of the exposure of cells to chemotherapeutic agents. Our observations are supported by a recent report of a sustained increase in the drug-resistant phenotype after chemotherapy (using a variety of cytotoxics) in neuroblastoma cells.28 The level of drug resistance progressively increased with the intensity of chemotherapy treatment.28
Extensive studies have provided quite good evidence that Pgp overexpression is relatively common in primary, treatment refractory and relapsed AML.5,6 However, only a few studies have reported that Pgp levels increase after chemotherapy.29This was confirmed in this study in 3 patients (1 patient with AML and 2 with BT-CML) in which blasts were Pgp-negative by flow cytometry at the time of initial clinical presentation. Pgp expression was upregulated ex vivo by anthracyclines and AraC in blasts isolated before the initiation of chemotherapy. Serial samples at 1 to 5 months after chemotherapy showed the presence of Pgp-positive blasts (Table4). All 3 patients were refractory to chemotherapy and died of progressive disease.
The mechanism by which the drugs used in this study upregulate Pgp expression is unknown. Currently available evidence suggests that there are at least two levels of control of MDR1 gene transcription. DNA methylation most probably defines the first level of control. This was originally described by Kantharidis et al30 in tumor cell lines and a small number of chronic lymphocytic leukemia (CLL) samples and later confirmed by Nakayama et al31 in a study of AML samples. Both studies described the inverse correlation between methylation of the MDR1 promoter and transcription and hence suggested that methylation may act as an on-off switch for transcription of the MDR1 gene. The second level of control occurs via trans-acting factors that mediate the cellular response to various stimuli and stresses. This level of control is exemplified by the many studies that have demonstrated activation of MDR1 promoter activity by a variety of stressful stimuli,32 including cytotoxics.17
The fact that induction can occur rapidly (4 to 16 hours) in response to drugs that are pumped by Pgp (EPI and DAU) and others that are not (IDA, MX2, and AraC) strongly suggests that upregulation involves a nonspecific increase in the transcription of the MDR1 gene. The proximal promoter of the human MDR1 gene may be directly activated by some cytotoxic drugs17 as well as many other factors.32 However, these findings have been controversial with respect to their relevance in the clinical context, because the endogenous promoter does not always behave in an analogous manner to the transfected promoter.33,34 Both anthracycline analogues IDA and MX2 are thought to be poor substrates for Pgp-mediated transport due to their highly lipophilic properties, diffusing rapidly through the cell membrane to bind to DNA.35,36 The change in Pgp levels that resulted from exposure to these anthracycline analogues (IDA and MX2) and the unrelated cytotoxic AraC suggests that upregulation of the MDR1 gene may represent a normal response of leukemic cells to cytotoxic stress that may, in turn, contribute to clinical drug resistance. Although these cytotoxics can have a number of effects in cells, recent findings have demonstrated that a c-jun NH2-terminal protein kinase (JNK), a member of the mitogen-activated protein kinase family, is activated by a variety of stressful stimuli, including exposure to cytotoxic agents, ultimately resulting in c-jun (a key component of the AP-1 site binding factor) phosphorylation.37 The nuclear translocation and increased binding activity of YB-1 in response to cytotoxics has been shown to affect Pgp expression.38 JNK is known to effect the nuclear translocation of other factors,39 but whether it has the same effect on YB-1 is not known.
Several studies have demonstrated that exposure to cytotoxics results in ceramide generation,40 which, in turn, activates a number of downstream targets, including c-raf kinase,41protein kinase C (PKC),40 JNK,42and the fas signaling pathway.43 Several of these targets are known to effect expression of Pgp. For example, Cornwell and Smith44 demonstrated activation of MDR1 promoter activity by a signal transduction pathway involving c-raf kinase. Kim et al45 also observed increased MDR1 expression in cell lines after transfection with c-raf kinase.
The exact role of PKC on the Pgp-mediated MDR phenotype remains unclear. PKC-mediated Pgp phosphorylation does not appear to correlate with altered Pgp drug transport function.46-48 PKCα isozyme is overexpressed in DOX-selected MDR cell lines.49-51 However, increased PKCη activity correlated with MDR1 and MRP overexpression in AML,52 ovarian carcinoma,53 and breast cancer.54 Uchiumi et al55 demonstrated that the MDR1 promoter is activated by exposure to the cytotoxic 5-fluorourocil and this activation was blocked by the PKC inhibitor H7. Chaudhary and Roninson20also demonstrated that the upregulation of Pgp expression by cytotoxics in a number of cell lines was blocked by the PKC inhibitors H-7 and staurosporine. Differences in expression, substrate specificity and activator requirements suggest that PKC isoenzymes may have distinct roles in different signaling pathways.56 The identification of PKCη cooverexpression with MDR1 in clinical samples indicates that the isozyme PKCη may play a key regulatory role in the upregulation of the MDR1 gene in response to chemotherapy.
AraC has been previously shown to activate PKC and induce both c-iun and c-fos that make up the AP-1 transcription factor.57,58AraC also activates JNK activity in leukemic cells59 and the p44/42 mitogen-activated protein kinase (MAPK) that leads to phosphorylation of serine residues in the amino-terminal transactivation domain of c-jun.60 Thus, the upregulation of Pgp expression by AraC may potentially be due to the combined activation of PKC, MAPK, and JNK in myeloid leukemia blasts via the AP-1 transcription factor.
The mechanism underlying the effect of CyA and PSC 833 on Pgp upregulation is not known. CyA is known to bind to immunophilin A and inhibit calcineurin in T cells and to prevent T-cell receptor translocation to the nucleus,61 ultimately causing modest decreases in the activity of AP-3 and NF-κB and marked decreases in the activity of AP-1 and NFAT.62 CyA prevents the dephosphorylation of NFAT, thereby preventing its translocation to the nucleus.62 This function of CyA is likely to be important in other cell types. JNK has been shown to bind to NFAT4 and phospholate it at two sites, thereby preventing its translocation to the nucleus but it also potentiates the activity of the other NFAT isoforms through AP-1 activation.39 It is of interest that the MDR1 promoter has binding sites for both AP-1 and AP-3, but a role for NFAT in Pgp expression is unknown. Because the JNK pathway enhances AP-1 activity in response to cytotoxic agents, it is possible that CyA neutralizes this activity such that induction of Pgp and other genes is prevented. Which of these pathways and factors are involved in the induction and inhibition response of the MDR1 gene observed in leukemic blasts is the focus of further work in our laboratory.
Supported in part by the Anti-Cancer Council of Victoria, the Sir Edward Dunlop Foundation for Medical Research, and the Department of Veterans Affairs, Canberra.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to John R. Zalcberg, MB, BS, PhD, FRACP, Director, Division of Haematology and Medical Oncology, Peter MacCallum Cancer Institute, Locked Bag 1, A’ Beckett Street, Melbourne, Victoria, Australia, 3000; e-mail:zalcberg@petermac.unimelb.edu.au.