Abstract
We have recently demonstrated that CD11b−/dullCD11c+ and CD11b+hiCD11c+ dendritic cell (DC) precursor subsets represent two distinct DC differentiation pathways from murine bone marrow lineage-phenotype negative (Lin−)c-kit+ hematopoietic progenitor cells (HPCs) stimulated with granulocyte-macrophage colony-stimulating factor (GM-CSF) + stem cell factor (SCF) + tumor necrosis factor (TNF). We show here that transforming growth factor-β1 (TGF-β1) significantly inhibits the generation of these CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors. Phenotypically, this inhibitory effect was accompanied by markedly suppressed expression of Ia and CD86 antigens as well as major histocompatibility complex (MHC) class II transactivator (CIITA) and CC-chemokine receptor 7 (CCR7) mRNAs in Lin−c-kit+ HPC cultures stimulated with GM-CSF + SCF + TNF at day 6. TGF-β1 could also suppress mature DC differentiation from CD11b+hiCD11c+ DC precursors, but not the differentiation from CD11b−/dullCD11c+ DC precursors. In the absence of TNF, TGF-β1 markedly suppressed the expression of CIITA and CCR7 mRNAs in GM-CSF + SCF-stimulated Lin−c-kit+ HPCs at either day 6 or day 12 and induced the differentiation solely into monocytes/macrophages as evident in morphology, active phagocytic, and endocytic activities. These cells expressed high levels of F4/80 and E-cadherin antigens, but low or undetectable levels of Ia, CD86, and CD40 molecules. However, upon the stimulation with TNF + GM-CSF, these cells could further differentiate into mature DCs expressing high levels of Ia and E-cadherin, characteristics for Langerhans cells (LCs), and gained the capacity of enhancing allogenic MLR. Taken together, all of these findings suggest that TGF-β1 polarizes murine HPCs to generate LC-like DCs through a monocyte/macrophage differentiation pathway.
DENDRITIC CELL (DC) development from hematopoietic progenitor cells (HPCs) has been classified into four stages: proliferating DC progenitors, nonproliferating DC precursors, immature antigen capturing DCs, and mature DCs with T-cell stimulatory function.1 Accordingly, heterogeneous DC subpopulations in different tissues may originate from distinct DC progenitors/precursors and/or from the same progenitors/precursors induced by differential sets of cytokines in situ.1-9 Several cytokines have been shown to regulate the growth, differentiation, and survival of DCs.4-16 Both stem cell factor (SCF) and Flt3 ligand sustain the growth of DC progenitors.4-7,14 Administration of Flt3 ligand stimulates outgrowth of at least three DC subsets, such as CD11b−CD11c+, CD11bdullCD11c+, and CD11b+hiCD11c+ DC subsets in mice.6,7 Granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3) enhance DC differentiation into intermediate stage, whereas tumor necrosis factor α (TNFα) and CD40 ligand (CD40L) stimulate final maturation of DCs.4,5,9-16However, mature DCs in vivo are small leukocyte populations.1 2 It remains unclear how growth and differentiation of DC progenitor or precursor cells are regulated and which cytokines may account for the generation of heterogeneous DC subpopulations with different tissue distributions and functions.
Transforming growth factor-β1 (TGF-β1) is a pleiotropic cytokine produced by many types of cells.17,18 Accumulating evidence indicates that TGF-β1 plays an essential role for the generation of Langerhans cells (LCs) in vivo and in vitro.10,19,20 LCs are DCs that exist in the epidermis.21 Disruption of the TGF-β1 gene results in a profoundly developmental deficiency of LCs in mice.20 Furthermore, in concert with GM-CSF and IL-4, TGF-β1 promotes LC differentiation from human peripheral blood monocytes,10 suggesting that TGF-β1 is an essential cytokine for LC differentiation. However, the cellular and molecular mechanisms for TGF-β1 to regulate LC differentiation from early HPCs remain to be elucidated. It has been demonstrated that TGF-β1 requires collaboration with GM-CSF and TNFα to induce DC differentiation from HPCs,19,22 whereas, in the absence of TNFα, TGF-β1 completely suppressed mature DC generation from GM-CSF–induced murine bone marrow progenitor cells.22Moreover, targeted disruption of TGF-β1 gene in mice shows a prominent feature with enhanced expression of major histocompatibility complex (MHC) class II molecules, which results in the progressive multifocal inflammatory processes and autoimmune diseases.23,24 These data suggest that TGF-β1 may function as a natural suppressor of the inflammatory process through regulating the expression and function of MHC class II antigen.23,24 Because MHC class II antigen is highly expressed in DCs that play a central role in controlling immune responses,1 2 these observations suggest that the bipotent role of TGF-β1 in regulating the generation of DCs is dependent on the differentiation state of HPCs, DC precursors, and the supplemented cytokines.
We have recently demonstrated in vitro that CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors are two distinct nonproliferating DC precursors generated from murine Lin−c-kit+ HPCs in the presence of GM-CSF + SCF + TNFα.9 Using this DC differentiation model,9 25 we investigated here the effect of TGF-β1 on DC differentiation from murine Lin−c-kit+HPCs. We describe that TGF-β1 could markedly inhibit the generation of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursor subsets by polarizing HPC differentiation into monocytes/macrophages with the capacity to differentiate into LC-like DCs in vitro.
MATERIALS AND METHODS
Cytokines and antibodies.
Recombinant murine SCF and GM-CSF were kindly provided by Dr T. Sudo (Basic Research Institute of Toray Co, Kanagawa, Japan) and by Kirin Brewery Co (Tokyo, Japan). Human TGF-β1 was purchased from R&D System (Minneapolis, MN). Mouse TNFα was produced as described previously.26 These cytokines were used at the optimal concentrations as previously described.9,25 An anti–c-kit antibody (ACK-2) was kindly provided by Dr T. Sudo and conjugated with biotin by using a NHS-Biotin kit (Pharmacia-Biotech, Uppsala, Sweden) according to the manufacturer’s instructions.27 A rat monoclonal antibody (MoAb) to murine DCs, DEC-205 (NLDC145), was a generous gift of Dr R.M. Steinman (Rockefeller University, New York, NY).28 29 The MoAb to mouse E-cadherin was purchased from Dainipon Pharmaceutical Co (Osaka, Japan). Other MoAbs and reagents used for immunostaining were obtained from PharMingen (San Diego, CA), unless otherwise indicated.
Mice.
C57BL/6 and Balb/c mice were obtained from Clea Animal Co (Tokyo, Japan) and maintained under pathogen-free conditions in the Animal Facility of Department of Molecular Preventive Medicine, School of Medicine, the University of Tokyo (Tokyo, Japan). All animal experiments complied with the standards set out in the Guideline for Care and Use of Laboratory Animals of the University of Tokyo.
Suspension culture of Lin−c-kit+HPCs.
Bone marrow cells were obtained by aspirating femurs and tibiae of 8- to 10-week-old female mice. Lin−c-kit+HPCs were isolated from nonadherent bone marrow mononuclear cells (MNCs) using an EPICS ELITE cell sorter (Coulter Electronics, Hialeah, FL) as previously described.9 25 In brief, nonadherent MNCs were stained with an indirect staining composed of a biotin-conjugated anti–c-kit MoAb and phycoerythrin (PE)-labeled streptavidin followed by a set of fluorescein isothiocyanate (FITC)-labeled MoAbs to CD3 (145-2C11), CD4 (H129.19), CD8 (53-6.7), B220 (RA3-6B2), Gr-1 (Ly-6G), CD11a (2D7), and CD11b (M1/70). The contamination by other types of cells in these preparations was consistently less than 0.5%, as shown by an immunofluorescence analysis.
Purified Lin−c-kit+ HPCs were incubated as previously described at a cell concentration of 1 to 3 × 104 cells/mL in Iscove’s modified Dulbecco medium (IMDM; GIBCO, Rockville, MD) supplemented with 10% fetal bovine serum (FBS), 5 × 10−5 mol/L 2-mercaptoethanol, penicillin G (100 U/mL) and streptomycin (100 μg/mL) in the presence of GM-CSF + SCF + TNFα.10 26 TGF-β1 was added in the cultures in a various combination as indicated. Optimal conditions were maintained by splitting these cultures at day 4 and exchanged the medium containing fresh cytokines every 3 to 4 days.
In some experiments, CD11b-/dullCD11c+ and CD11b+hiCD11c+ DC precursor subsets were sorted at day 6 from Lin−c-kit+ HPC cultures stimulated with GM-CSF, SCF, and TNFα as previously described.9 In some experiments, TGF-β1–induced Lin−c-kit+ HPC cultures stimulated with GM-CSF + SCF were collected at day 12, washed twice, and recultured in the presence of GM-CSF + TNFα for an additional 3 to 5 days. All of the staining and sorting procedures were performed in the presence of 1 mmol/L EDTA to avoid cell aggregation. Reanalysis of the sorted populations showed a purity greater than 98%.
Immunofluorescence analysis.
Immunofluorescence analyses were performed as previously described.9 25 In three-color analyses, 4 × 105 cells were incubated with biotinylated hamster anti-CD11c MoAbs and rat anti-CD11b MoAbs, followed by staining with Cy-Chrome (CyC)-labeled streptavidin and PE-conjugated goat antirat IgG(Fab′)2 antibodies. The cells were then stained with FITC-conjugated various MoAbs. In some experiments, the cells were first stained with a rat anti–E-cadherin MoAb, followed by staining with FITC-conjugated goat antirat IgG(Fab′)2antibodies and a PE-labeled anti-Ia MoAb. In other experiments, the cells were first stained with biotinylated antibodies and shown by CyC-conjugated streptavidin, followed by staining with PE-labeled anti-CD11c and FITC-conjugated anti-CD11b antibodies. The instrument compensation was set in each experiment using single-color and/or two-color stained samples.
Reverse transcription-polymerase chain reaction (RT-PCR).
Total RNAs were extracted from 2 × 105 indicated cells using RNAzol B (Biotex Laboratories Inc, Houston, TX) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 200 ng of total RNAs in 25-μL reaction volume using an RT-PCR kit (Takara Shuzo, Kyoto, Japan) with random primers. Thereafter, cDNA was amplified for 30 cycles consisting of 94°C for 30 seconds, 57°C for 1 minute, and 72°C for 1.5 minutes with the CIITA oligonucleotide primers (5′-CCAGAACTGGTTGTAGAGCC-3′ and 5′-CAGCTTGCTAGGCTCCAATT-3′), which specifically result in a 500-bp cDNA encoding CIITA gene.30,31 CCR7 mRNA was examined by using oligonucleotide primers (5′-CATCAGCATTGACCGCTACGT-3′ and 5′-GGTACGGATGATAATGAGGTAGCA-3′), which are specific for murine CCR7.32 As a control, mouse β-actin transcript was amplified in parallel as previously described.9 25 The PCR products were fractionated on 1.5% agarose gel or 5% polyacrylamid gel and visualized by ethidium bromide staining.
Endocytosis and phagocytosis.
The endocytosis experiments were performed as previously reported.33 In brief, the cells were incubated with 0.1 mg/mL FITC-Dextran (FITC-DX; 4,000 Daltons; Sigma Chemical Co, St Louis, MO) at 37°C or 0°C for 60 minutes. Uptake was stopped by adding ice-cold phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA) and 0.02% sodium azide. The phagocytosis experiments were performed as reported.34Briefly, the cells were harvested from the cultures and resuspended at approximately 4 to 5 × 105/mL in complete IMDM. Eight microliters of FITC-latex beads of 0.75 μm diameters (Carboxylate Microspheres, Wako, Japan) were added to the cell suspension and mixed well. The cells were incubated for 0 or 30 minutes at 37°C. The incubation was extinguished by adding ice-cold 5% BSA-0.02% azide-PBS and washed three times with 2.5% fetal calf serum (FCS)-0.02% azide-PBS. To confirm the endocytic and phagocytic activity of DC precursors, these cells were stained again with a rat-antimouse CD11b MoAb and a biotinylated hamster antimouse CD11c MoAb, followed by staining with PE-conjugated goat antirat IgG(Fab′)2antibodies and CyC-conjugated streptavidin. Finally, the percentage and density of FITC-positive cells were examined based on tri-color analysis by gating on the CD11b−/dullCD11c+/dull cell population using a cell sorter as described.9
MLR.
Splenic MNCs were prepared as described previously from allogenic mice (Balb/c).9 The adherent cells were first removed by incubating them at 37°C for 60 minutes in IMDM medium containing 10% FCS. To obtain highly purified T cells, the nonadherent splenic MNCs were incubated with rat anti-B220 and mouse anti-Ia MoAbs followed by staining with antirat IgG and antimouse IgG-conjugated magnetic beads to deplete B220+ and Ia+ cells using Dynal-beads (Dynal, Oslo, Norway). After treatment with mitomycin C (MMC; 15 μg/mL),35 the indicated stimulator cells (from 100 to 3 × 104 cells) were added to T cells (3 × 105) in each well of 96-well round-bottomed microtest tissue-culture plates (Nunc, Roskilde, Denmark). After incubating at 37°C for 4 to 5 days, cell proliferation was determined using 3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT; Sigma Chemical). In brief, 15 μL of MTT (5 mg/mL in PBS) was added into each well and the plates were incubated at 37°C for an additional 4 hours. The resultant absorbance at 550 nm was read using a microplate immunoreader.
Nonspecific esterase (NSE) staining.
Cells were cytocentrifuged for 5 minutes at 500 rpm on a microscope slide and used for NSE staining (α-naphthyl acetate esterase staining kit; Sigma) according the instructions of the manufacturer.
Statistical analysis.
Differences were evaluated using the Student′s t-test.P values of less than .05 were considered to be statistically significant.
RESULTS
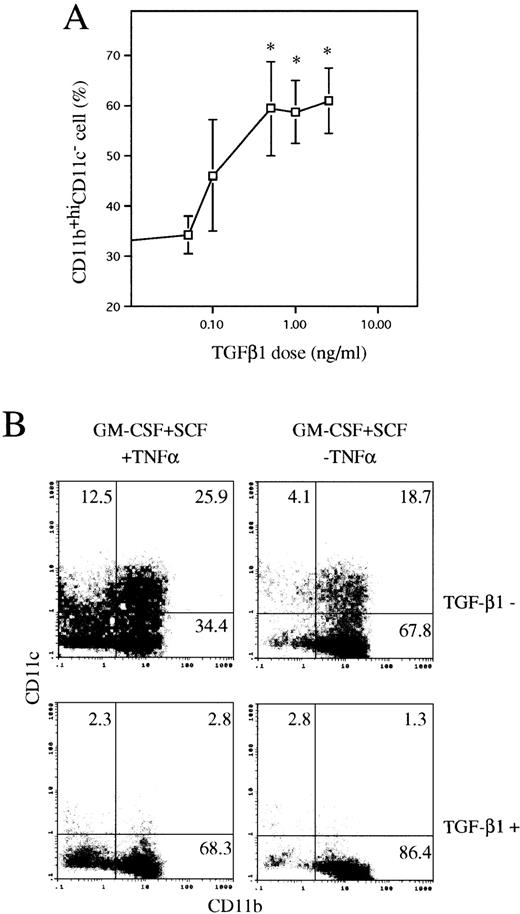
TGF-β1 inhibits the generation of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors from Lin−c-kit+ HPCs at day 6.
To elucidate the role of TGF-β1 in DC generation, Lin−c-kit+ HPCs were stimulated with GM-CSF + SCF + TNFα in the presence of various doses of TGF-β1 ranging from 0 to 2.5 ng/mL, as indicated. Cells were then first analyzed at day 6, when two distinct CD11b−/dullCD11c+ (10% ± 3.5%) and CD11b+hiCD11c+ (26% ± 5.6%) DC precursor subsets could be clearly identified. Addition of TGF-β1 dose-dependently decreased the generation of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursor subsets to 2.0% ± 1.5% and 5% ± 3.5%, respectively (Fig 1A). Moreover, the absolute numbers of the two DC precursor cells were also markedly reduced more than fourfold and threefold, respectively (Fig 1B). The maximal inhibitory effect of TGF-β1 was reached at a concentration of 2.5 ng/mL; therefore, this dose was used in the following experiments, unless otherwise indicated. In contrast, TGF-β1 markedly increased the CD11b+hiCD11c− cell fraction in GM-CSF + SCF + TNFα-stimulated Lin−c-kit+cultures (Fig 2A and B) and most of these CD11b+hiCD11c− cells were Gr-1 negative (data not shown), suggesting that TGF-β1 might potentiate the differentiation of HPCs into monocytes/macrophages. Interestingly, in the absence of TNFα, GM-CSF + SCF could induce the generation of CD11b+hiCD11c+ DC precursors rather than CD11b-/dullCD11c+ ones, which was completely blocked by the addition of TGF-β1 (Fig 2A and B).
TGF-β1 dose-dependently inhibited the generation of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors at day 6 from murine Lin−c-kit+ HPCs stimulated with GM-CSF + SCF + TNF. (A) The percentage of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors. (B) The absolute numbers of CD11b−/dullCD11c+ and CD11b+hiCD11c+DC precursors. The data represent the mean value ± SD of the percentage and numbers of the two DC precursor subpopulations observed in more than five experiments. *P < .05 significance as compared with those cultures in the absence of TGF-β1.
TGF-β1 dose-dependently inhibited the generation of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors at day 6 from murine Lin−c-kit+ HPCs stimulated with GM-CSF + SCF + TNF. (A) The percentage of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors. (B) The absolute numbers of CD11b−/dullCD11c+ and CD11b+hiCD11c+DC precursors. The data represent the mean value ± SD of the percentage and numbers of the two DC precursor subpopulations observed in more than five experiments. *P < .05 significance as compared with those cultures in the absence of TGF-β1.
TGF-β1 enhanced differentiation of CD11b+hiCD11c− cells from murine Lin−c-kit+ HPCs in the presence of GM-CSF + SCF with or without TNF at day 6. (A) Dose-dependent induction of CD11b+hiCD11c− cells by TGF-β1 in the cultures stimulated with GM-CSF + SCF + TNF. The data represent the mean value ± SD of the percentage of CD11b+hiCD11c− cells observed in more than five experiments. *P < .05 significance as compared with the cultures in the absence of TGF-β1. (B) Induction of CD11b−/dullCD11c+, CD11b+hiCD11c+, and CD11b+hiCD11c− cells by TGF-β1 in Lin−c-kit+ HPC cultures stimulated by various combination of cytokines as indicated. Quads were set up on the isotype-matched control dot plot, and the results are representative of more than five experiments.
TGF-β1 enhanced differentiation of CD11b+hiCD11c− cells from murine Lin−c-kit+ HPCs in the presence of GM-CSF + SCF with or without TNF at day 6. (A) Dose-dependent induction of CD11b+hiCD11c− cells by TGF-β1 in the cultures stimulated with GM-CSF + SCF + TNF. The data represent the mean value ± SD of the percentage of CD11b+hiCD11c− cells observed in more than five experiments. *P < .05 significance as compared with the cultures in the absence of TGF-β1. (B) Induction of CD11b−/dullCD11c+, CD11b+hiCD11c+, and CD11b+hiCD11c− cells by TGF-β1 in Lin−c-kit+ HPC cultures stimulated by various combination of cytokines as indicated. Quads were set up on the isotype-matched control dot plot, and the results are representative of more than five experiments.
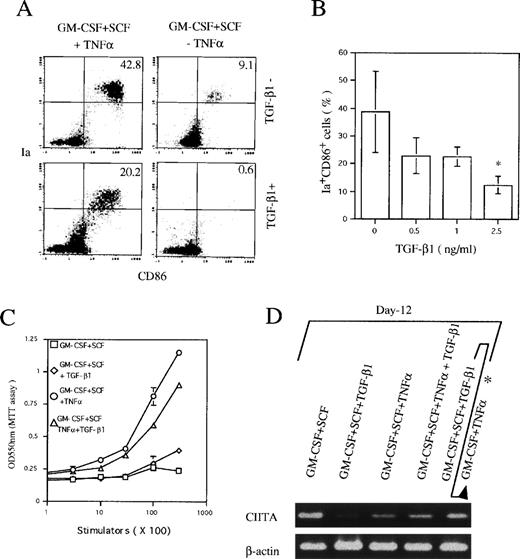
To negate the possibility that TGF-β1 might simply downregulate the expression of CD11c, we also examined at day 6 the expression of Ia and CD86 antigens that have been demonstrated to be highly expressed on CD11b−/dullCD11c+ DC precursor cells, but moderately on CD11b+hiCD11c+ DC precursor cells. As shown in Fig 3A and B, TGF-β1 significantly decreased the generation of Ia+CD86+ cells in Lin−c-kit+ HPC cultures stimulated with GM-CSF + SCF + TNFα, supporting the notion that TGF-β1 could indeed inhibit the generation of two DC precursor cells.
TGF-β1 inhibited the expression of Ia and CD86 antigens and CIITA mRNA in Lin−c-kit+ HPC cultures in the presence of GM-CSF + SCF with or without TNF at day 6. (A) Dose-dependent inhibition on the generation of Ia+CD86+ cells from murine Lin−c-kit+ HPCs stimulated with GM-CSF + SCF + TNF at day 6. The data represent the mean value ± SD of the percentage and absolute numbers of Ia+CD86+ cells observed in more than five experiments. *P < .05 significance as compared with the cultures without addition of TGF-β1. (B) Ia+CD86+ cells in Lin−c-kit+ HPC cultures stimulated with the indicated various combinations of cytokines. Quads were set up on the isotype-matched control dot plot. (C) The expression of CIITA mRNA in Lin−c-kit+ HPC cultures stimulated with indicated various combinations of cytokines. The results are representative of more than three experiments.
TGF-β1 inhibited the expression of Ia and CD86 antigens and CIITA mRNA in Lin−c-kit+ HPC cultures in the presence of GM-CSF + SCF with or without TNF at day 6. (A) Dose-dependent inhibition on the generation of Ia+CD86+ cells from murine Lin−c-kit+ HPCs stimulated with GM-CSF + SCF + TNF at day 6. The data represent the mean value ± SD of the percentage and absolute numbers of Ia+CD86+ cells observed in more than five experiments. *P < .05 significance as compared with the cultures without addition of TGF-β1. (B) Ia+CD86+ cells in Lin−c-kit+ HPC cultures stimulated with the indicated various combinations of cytokines. Quads were set up on the isotype-matched control dot plot. (C) The expression of CIITA mRNA in Lin−c-kit+ HPC cultures stimulated with indicated various combinations of cytokines. The results are representative of more than three experiments.
The expression of MHC class II gene is strictly controlled by a transcription activator CIITA.30 31 In RT-PCR analysis, TGF-β1 potently suppressed CIITA mRNA expression in Lin−c-kit+ HPCs stimulated with GM-CSF + SCF at day 6 irrespective of TNFα addition (Fig 3C), suggesting the correlation of the inhibitory effect of TGF-β1 on DC precursor differentiation from Lin−c-kit+ HPC with suppressed expression of CIITA mRNA.
The inhibitory effect of TGF-β1 on mature DC generation is compromised at day 12 in GM-CSF + SCF + TNFα-stimulated Lin−c-kit+HPC cultures.
In the presence of GM-CSF + SCF + TNFα, Lin−c-kit+ HPCs differentiate into mature DCs at day 12 to 14.9 25 The addition of TGF-β1 significantly reduced the appearance of Ia+CD86+ mature DCs from 36.1% ± 8.7% to 14.4% ± 4.1% in GM-CSF + SCF + TNFα-stimulated cultures at day 12 (Fig 4A and B), accompanied with reduced capacity to enhance allogenic MLR (P< .05 significance; Fig 4C). In contrast to the inhibitory effect of TGF-β1 on the generation of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors at day 6 (Fig 2A), TGF-β1 did not affect the development of CD11b−/dullCD11c+ cells at day 12 (Fig 5) and failed to suppress the transcription of CIITA gene (Fig 4D) in GM-CSF + SCF + TNFα-stimulated Lin−c-kit+ HPC cultures. However, the expression of Ia and CD86 antigens was markedly suppressed on these CD11b−/dullCD11c+cells in the presence of TGF-β1 (Fig 5). These results suggest that the mechanism for TGF-β1–mediated inhibitory effect on the generation of nonproliferating CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors at day 6 may be distinct from that on the generation of Ia+CD86+ mature DCs at day 12 in the presence of TNFα. Moreover, these CD11b−/dullCD11c+ cells could finally differentiate into mature DCs in a prolonged cultures (data not shown), indicating that an alternative DC differentiation pathway, which differs from CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursor cell-mediated ones, might exist in TGF-β1–supplemented cultures.
The effect of TGF-β1 on the generation of mature DCs and the expression of CIITA mRNA in Lin−c-kit+ HPC cultures stimulated with various combinations of cytokines at day 12. (A) The histogram quads, which were set up on the isotype-matched control dot plot, and (B) the percentage of Ia+CD86+ cells generated from GM-CSF + SCF + TNF-stimulated Lin−c-kit+ HPCs. The data represent the mean value ± SD of the percentage of Ia+CD86+ cells. *P < .05 significance as compared with the cultures without the addition of TGF-β1. The results are representative of more than five experiments. (C) Allogenic MLR induced by Lin−c-kit+ HPC cultures in the presence of the indicated combination of cytokines at day 12. Results are expressed as the mean ± 1 SD of triplicate cultures and are representative of three independent experiments. (D) The expression of CIITA mRNA in various combination of cytokine-stimulated Lin−c-kit+ HPCs as indicated at day 12, which represents three independent experiments. (*Lin−c-kit+ HPCs were cultured in the presence of GM-CSF + SCF + TGF-β1 for 12 days and then recultured in the presence of GM-CSF + TNF for an additional 3 to 5 days.)
The effect of TGF-β1 on the generation of mature DCs and the expression of CIITA mRNA in Lin−c-kit+ HPC cultures stimulated with various combinations of cytokines at day 12. (A) The histogram quads, which were set up on the isotype-matched control dot plot, and (B) the percentage of Ia+CD86+ cells generated from GM-CSF + SCF + TNF-stimulated Lin−c-kit+ HPCs. The data represent the mean value ± SD of the percentage of Ia+CD86+ cells. *P < .05 significance as compared with the cultures without the addition of TGF-β1. The results are representative of more than five experiments. (C) Allogenic MLR induced by Lin−c-kit+ HPC cultures in the presence of the indicated combination of cytokines at day 12. Results are expressed as the mean ± 1 SD of triplicate cultures and are representative of three independent experiments. (D) The expression of CIITA mRNA in various combination of cytokine-stimulated Lin−c-kit+ HPCs as indicated at day 12, which represents three independent experiments. (*Lin−c-kit+ HPCs were cultured in the presence of GM-CSF + SCF + TGF-β1 for 12 days and then recultured in the presence of GM-CSF + TNF for an additional 3 to 5 days.)
TGF-β1 inhibited at day 12 the expression of Ia and CD86 antigens on CD11b−/dullCD11c+ cells derived from cultured Lin−c-kit+ HPCs stimulated with the indicated various combinations of cytokines. Histograms shown in the figures were gated on CD11b−/dullCD11c+ cells. Solid and dotted lines indicate the immunofluoresecence intensity of cells stained with a control and the test antibodies, respectively. Representative results from three or more independent experiments are shown.
TGF-β1 inhibited at day 12 the expression of Ia and CD86 antigens on CD11b−/dullCD11c+ cells derived from cultured Lin−c-kit+ HPCs stimulated with the indicated various combinations of cytokines. Histograms shown in the figures were gated on CD11b−/dullCD11c+ cells. Solid and dotted lines indicate the immunofluoresecence intensity of cells stained with a control and the test antibodies, respectively. Representative results from three or more independent experiments are shown.
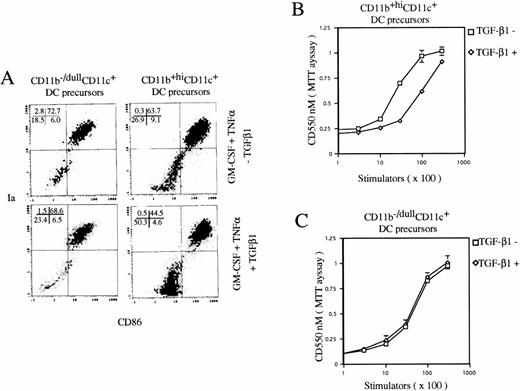
TGF-β1 inhibits differentiation of CD11b+hiCD11c+, but not CD11b−/dullCD11c+ DC precursors into mature DCs.
To examine the effect of TGF-β1 on final maturation of DC from CD11b+hiCD11c+ and CD11b−/dullCD11c+ DC precursors, they were sorted at day 6, as previously described,9 and then recultured in the presence of GM-CSF + TNFα with or without TGF-β1 for an additional 5 days. TGF-β1 could significantly inhibit the generation of Ia+CD86+ mature DCs from CD11b+hiCD11c+, but not CD11b−/dullCD11c+ DC precursors (Fig 6) without changing the expression of CIITA mRNA (data not shown). Consistently, TGF-β1 also decreased the capacity of CD11b+hiCD11c+ DC precursor-derived cells to stimulate allogenic MLR, but did not affect that of the offspring of CD11b−/dullCD11c+ DC precursor cells (Fig 6).
TGF-β1 inhibited DC maturation from CD11b+hiCD11c+, but not CD11b−/dullCD11c+ DC precursors in the presence of GM-CSF + TNF. (A) Histograms of quad of Ia+CD86+ mature DCs were set up on the isotype-matched control dot plot. (B and C) The capacity of cultured cells to stimulate allogenic MLR. The experiments are representative of more than three independent experiments.
TGF-β1 inhibited DC maturation from CD11b+hiCD11c+, but not CD11b−/dullCD11c+ DC precursors in the presence of GM-CSF + TNF. (A) Histograms of quad of Ia+CD86+ mature DCs were set up on the isotype-matched control dot plot. (B and C) The capacity of cultured cells to stimulate allogenic MLR. The experiments are representative of more than three independent experiments.
TGF-β1 potentiates Lin−c-kit+HPCs to differentiate into macrophages with the capacity to differentiate into LCs.
To better understand the cellular mechanism for the differential effect of TGF-β1 on the generation of nonproliferating DC precursors and differentiation of mature DCs, Lin−c-kit+HPCs were then cultured in the presence of GM-CSF + SCF without the addition of TNFα for 12 days. At day 12, GM-CSF + SCF induced a small number of Ia+CD86+ mature DCs from Lin−c-kit+ HPCs (Figs 4 and 5), whereas the addition of TGF-β1 completely blocked the induction of Ia+CD86+ mature DCs (Figs 4A and 5) accompanied with inhibited expression of CIITA mRNA in the same cultures (Fig 4D).
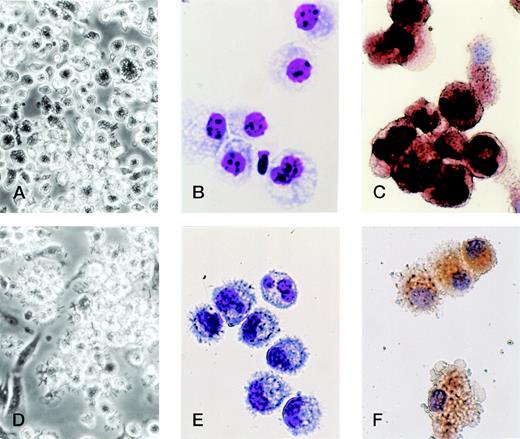
Morphological analyses showed that TGF-β1 in combination with GM-CSF + SCF induced differentiation of Lin−c-kit+ HPCs solely into monocyte/macrophage-like cells with large size, many vacuoles in the cytoplasm, and positive NSE staining, whereas the cell processes and projections were not observed (Fig 7A, B, and C). Phenotypically, they expressed high levels of F4/80, CD16/32, DEC-205, and E-cadherin and low or undetectable levels of Ia, CD86, and CD40 antigens, complying with monocyte/macrophage phenotype (Fig 8A). Although these cells expressed low levels of CD11c and showed the phenotype of CD11b−/dullCD11cdull (Fig 5), they were active in endocytosis (Fig 9A) and phagocytosis (Fig 9C), but incapable of enhancing allogenic MLR (Fig9E). Taken together, all of these results suggest that TGF-β1 can potentiate the differentiation of Lin−c-kit+ HPCs into monocytes/macrophages, but completely blocked the generation of mature DCs in collaboration with GM-CSF + SCF.
TGF-β1 polarized the differentiation of murine Lin−c-kit+ HPCs into DCs through the monocyte/macrophage differentiation pathway. Murine Lin−c-kit+ HPCs were first cultured in the presence of GM-CSF + SCF for 12 days with the addition of TGF-β1 (A, B, and C). The cultured cells were then washed twice and recultured in the presence of GM-CSF + TNF for an additional 3 to 5 days (D, E, and F). (A and D) Observation by phase-contrast inverted microscope; (B and E) Giemsa-Wright staining; (C and F) NSE staining. Original magnifications: for (A) and (D) × 200; for (B), (C), (E), and (F) × 400.
TGF-β1 polarized the differentiation of murine Lin−c-kit+ HPCs into DCs through the monocyte/macrophage differentiation pathway. Murine Lin−c-kit+ HPCs were first cultured in the presence of GM-CSF + SCF for 12 days with the addition of TGF-β1 (A, B, and C). The cultured cells were then washed twice and recultured in the presence of GM-CSF + TNF for an additional 3 to 5 days (D, E, and F). (A and D) Observation by phase-contrast inverted microscope; (B and E) Giemsa-Wright staining; (C and F) NSE staining. Original magnifications: for (A) and (D) × 200; for (B), (C), (E), and (F) × 400.
The phenotype of TGF-β1–induced monocytes/macrophages and their offspring of mature DCs. Murine Lin−c-kit+ HPCs were first cultured in the presence of GM-CSF + SCF for 12 days with the addition of TGF-β1 (A). These cultured cells were then washed twice and recultured in the presence of GM-CSF + TNF for an additional 3 to 5 days (B). Cells were processed for two-color immunofluorescence analyses. Gr-1 and CD40 antigens were examined by FITC-conjugated anti–Gr-1 and CD40 MoAbs. CD86, F4/80, CD16/32, E-cadherin, and DEC-205 antigens were stained with uncoupled rat-antimouse MoAbs, followed by staining with FITC-conjugated antirat IgG. The second color was shown by a PE-conjugated Ia MoAb. Quads were set up on the isotype-matched control dot plot. Representative results from three independent experiments are shown.
The phenotype of TGF-β1–induced monocytes/macrophages and their offspring of mature DCs. Murine Lin−c-kit+ HPCs were first cultured in the presence of GM-CSF + SCF for 12 days with the addition of TGF-β1 (A). These cultured cells were then washed twice and recultured in the presence of GM-CSF + TNF for an additional 3 to 5 days (B). Cells were processed for two-color immunofluorescence analyses. Gr-1 and CD40 antigens were examined by FITC-conjugated anti–Gr-1 and CD40 MoAbs. CD86, F4/80, CD16/32, E-cadherin, and DEC-205 antigens were stained with uncoupled rat-antimouse MoAbs, followed by staining with FITC-conjugated antirat IgG. The second color was shown by a PE-conjugated Ia MoAb. Quads were set up on the isotype-matched control dot plot. Representative results from three independent experiments are shown.
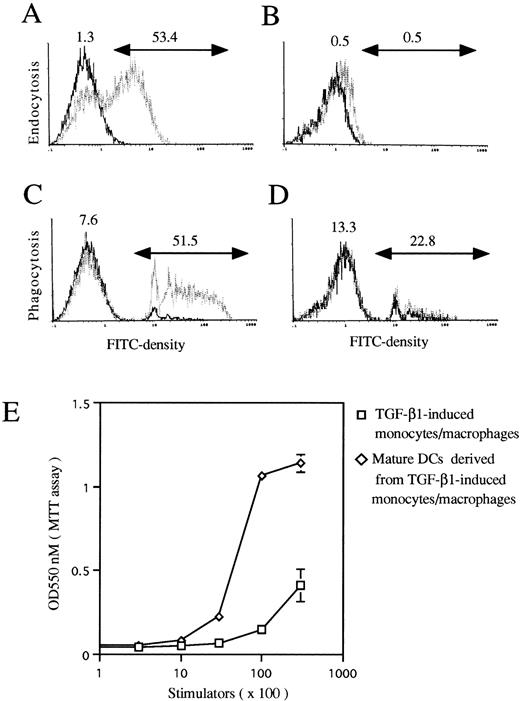
The functional maturation of TGF-β1–induced monocytes/macrophages into mature DC-like cells. Murine Lin−c-kit+ HPCs were first cultured in the presence of GM-CSF + SCF for 12 days with the addition of TGF-β1 (A and C). These cultured cells were then washed twice and recultured in the presence of GM-CSF + TNF for an additional 3 to 5 days (B and D). A three-color immunofluorescence analysis was performed to show the capacity of FITC-DX and FITC-Latex uptake by gating on CD11b−/dullCD11c+ cells as described in Materials and Methods. Solid and dotted lines indicate the FITC intensity of cells as a control and the test of FITC-DX or FITC-latex uptake, respectively. (E) TGF-β1–induced monocytes/macrophages (□) matured into DC-like cells (◊) with the capacity to potently stimulate allogenic MLR. The results are representative of three experiments.
The functional maturation of TGF-β1–induced monocytes/macrophages into mature DC-like cells. Murine Lin−c-kit+ HPCs were first cultured in the presence of GM-CSF + SCF for 12 days with the addition of TGF-β1 (A and C). These cultured cells were then washed twice and recultured in the presence of GM-CSF + TNF for an additional 3 to 5 days (B and D). A three-color immunofluorescence analysis was performed to show the capacity of FITC-DX and FITC-Latex uptake by gating on CD11b−/dullCD11c+ cells as described in Materials and Methods. Solid and dotted lines indicate the FITC intensity of cells as a control and the test of FITC-DX or FITC-latex uptake, respectively. (E) TGF-β1–induced monocytes/macrophages (□) matured into DC-like cells (◊) with the capacity to potently stimulate allogenic MLR. The results are representative of three experiments.
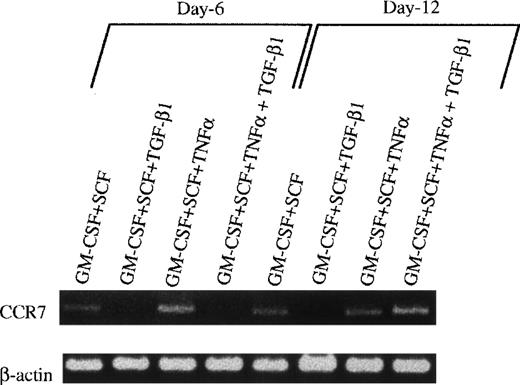
However, when these cells were collected at day 12, washed twice, and exposed to GM-CSF + TNFα for an additional 3 to 5 days, typical mature DCs developed, as evident with DC aggregates and morphology in the secondary cultures (Fig 7D and E) and the phenotype expressing high levels of Ia, CD86, and DEC205 molecules (Fig 8B). Interestingly, all of these mature DCs expressed high levels of E-cadherin antigen, a discernible marker for LC,1,10,35 36 indicating that they are phenotypically LCs (Fig 8B). Functionally, their endocytic (Fig 9B) and phagocytic activities (Fig 9D) were decreased, whereas the capacity of stimulating allogenic MLR was significantly enhanced (Fig 9E). Moreover, most of the mature DCs derived from TGF-β1 + GM-CSF + SCF-induced monocytes/macrophages expressed higher levels of E-cadherin compared with those derived from GM-CSF + SCF-stimulated cells or GM-CSF + SCF + TNFα-induced cells (data not shown), suggesting that TGF-β1 may drive Lin−c-kit+ HPCs to differentiate into LC precursors. Interestingly, phenotypic examination also showed that TGF-β1 significantly suppressed the expression of CCR7 mRNA at day 6 in GM-CSF + SCF-stimulated Lin−c-kit+ HPCs irrespective of TNFα addition (Fig 10). Furthermore, TGF-β1 inhibited at day 12 the expression of CCR7 mRNA in GM-CSF + SCF-stimulated Lin−c-kit+ HPCs in the absence of TNFα, indicating that TGF-β1 may also play an important role in the regulation of the localization of LC precursors in situ by modulating the expression of a chemokine receptor.
The effect of TGF-β1 on the expression of E-cadherin antigen and CCR7 mRNA in cultured Lin−c-kit+ HPCs. (A) Lin−c-kit+ HPCs were cultured in the presence of various combinations of cytokines as indicated for 12 days, followed by washing twice, and were recultured in the presence of GM-CSF + TNF for an additional 3 to 5 days. These recultured cells were processed for two-color analyses by staining with a rat-antimouse E-cadherin MoAb and a PE-labeled mouse antimouse Ia and finally shown by FITC-conjugated goat-antirat IgG(Fab′)2. Histograms shown in the figures were gated on Ia+hicells. Solid and dotted lines indicate the immunofluoresecence intensity of cells stained with a control MoAb and anti–E-cadherin antigen, respectively. Representative results from three experiments are shown. (B) Examination by RT-PCR of CCR7 mRNA in Lin−c-kit+ HPCs stimulated with indicated various combinations of cytokines at day 6 or 12.
The effect of TGF-β1 on the expression of E-cadherin antigen and CCR7 mRNA in cultured Lin−c-kit+ HPCs. (A) Lin−c-kit+ HPCs were cultured in the presence of various combinations of cytokines as indicated for 12 days, followed by washing twice, and were recultured in the presence of GM-CSF + TNF for an additional 3 to 5 days. These recultured cells were processed for two-color analyses by staining with a rat-antimouse E-cadherin MoAb and a PE-labeled mouse antimouse Ia and finally shown by FITC-conjugated goat-antirat IgG(Fab′)2. Histograms shown in the figures were gated on Ia+hicells. Solid and dotted lines indicate the immunofluoresecence intensity of cells stained with a control MoAb and anti–E-cadherin antigen, respectively. Representative results from three experiments are shown. (B) Examination by RT-PCR of CCR7 mRNA in Lin−c-kit+ HPCs stimulated with indicated various combinations of cytokines at day 6 or 12.
DISCUSSION
We have recently showed that murine Lin−c-kit+ HPCs can differentiate into mature DCs through bifurcated differentiation pathways of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors in response to GM-CSF + SCF + TNFα stimulation.9 In this report, we first investigated the regulatory effect of TGF-β1 on DC generation in vitro from Lin−c-kit+ HPCs in the presence of GM-CSF + SCF + TNFα. The results presented here showed that TGF-β1 could potently inhibit the generation of CD11b−/dullCD11c+ DC and CD11b+hiCD11c+ DC precursors from Lin−c-kit+ HPCs stimulated with GM-CSF + SCF + TNFα in vitro. These results indicate that TGF-β1 is a negative regulator for the generation of nonproliferating DC precursor subsets from proliferating DC progenitors, in concordance with its inhibitory effect on early HPCs.24,37 38
TGF-β1 could also suppress DC’s maturation from CD11b+hiCD11c+ DC precursors and Lin−c-kit+ HPCs stimulated with GM-CSF + SCF + TNFα at late stage (for example, at day 12) based on the suppressed expression of MHC class II and CD86 molecules and the reduced capacity of enhancing allogenic MLR. This is substantially supported by the fact that gene-targeted disruption of TGF-β1 results in markedly enhanced expression of MHC class II antigen and various autoimmune diseases in mice.23,24 However, the mechanism for TGF-β1–mediated immunosuppression is still an open question. Several previous reports showed that administration of TGF-β1, gene transfer of TGF-β1, or TGF-β1–induced “DCs” in vitro could prolong murine cardiac allograft survival by inhibiting cellular immunity.39-41 It has recently been demonstrated that many tumor cells secrete TGF-β1 and can activate endogenously produced latent TGF-β1 to bioactive form.42-45 DCs that infiltrate in colon, basal-cell skin cancers,46 and the progressing melanoma metastases47 lack CD86 and therefore have reduced T-cell stimulatory activity.46,47 Moreover, the fact that tumor peptide-pulsed DCs can effectively stimulate the host immune responses to eradicate the melanoma cells48 alternatively suggests that endogenously functional disorder of DCs may partially contribute to the tumorigenicity. Several soluble factors have been implicated in defective DC maturation in cancer, including vascular endothelial growth factor (VEGF)1,49 and IL-10.1 48 Our findings further suggest that TGF-β1 may also play an important role in negatively regulating immune responses in vivo by modulating DC’s development and functions.
Phenotypic examination also demonstrated that murine Lin−c-kit+ HPC-derived DCs and DC precursors mainly expressed CCR1 and CCR7 (data not shown). Unexpectedly, TGF-β1 suppressed the expression of CCR7 mRNA in Lin−c-kit+ HPCs stimulated with GM-CSF + SCF + TNFα at day 6 and in GM-CSF + SCF-stimulated Lin−c-kit+ HPCs either at day 6 or 12, including LC precursors. Because migration of DCs is thought to be regulated by the interaction of chemokine and chemokine receptor.50,51 Disconnecting such migration of DCs may prevent antigen-specific immune responses.1 It may prove to be valuable to elucidate whether TGF-β1 might regulate the migration of DC and DC precursors in vivo through modulating the expression of chemokine receptors.
Our results appear in contrast to previous investigations that TGF-β1 enhances the generation of mature DCs from human CD34+ HPCs in serum-free cultures stimulated with GM-CSF + TNFα.19 A distinct culture system and HPC species may simply account for the discrepancy. However, DCs are heterogeneous populations originating from distinct differentiation pathways,1-9,52-55 and the maturation of DCs can be divided into at least three stages based on the expression of MHC class II and other costimulatory molecules.56,57 Moreover, many other reports demonstrate that TGF-β1 shows different effects on proliferation and differentiation of HPCs, depending on the differentiation state of HPCs and supplemented cytokines.24,37,38 58 We presume that the apparent discrepancy might be related to the distinct effect of TGF-β1 on the generation of DC precursors from HPCs and differentiation of DC precursors into mature DCs under different culture conditions.
Accumulating evidence suggests that monocytes/macrophages can differentiate into LCs.1,10,59 Decreased numbers of LCs and monocytes/macrophages were previously documented in the op/opmouse, a null mutant of the M-CSF gene.59 When combined with GM-CSF + IL-4, TGF-β1 can drive human peripheral monocytes to differentiate into LCs,10 further supporting the notion of the tight connection of monocytes/macrophages with the ontogeny of LCs. We observed that TGF-β1 could not directly induce the differentiation and maturation of LC from Lin−c-kit+ HPCs stimulated with GM-CSF + SCF in the absence of TNFα, but indeed induced the generation of monocytes/macrophages at day 12 to 14, as evident in morphology and phenotype such as the expression of high levels of F4/80, but low or undetectable levels of Ia, CD86, and CD40 molecules. Interestingly, these cells expressed high levels of DEC-205 and E-cadherin antigens (Fig 8A). They were further able to differentiate into mature LCs expressing high levels of E-cadherin and other mature DC markers in response to GM-CSF and TNFα, consistent with previous described role of endogenous TGF-β1 in LC development.20 It is likely that the TGF-β1–induced monocytes/macrophages represent LC precursors. Therefore, we hypothesize that TGF-β1 may potentiate HPCs to differentiate into monocytes/macrophages that will be anchored by increasing the expression of E-cadherin protein in situ in the epidermis, where they differentiate into mature LCs in response to stimuli, such as GM-CSF, TNFα, and IL-4, in inflammatory reactions. Accordingly, locally produced TGF-β1 itself may maintain the DC precursors at immature stage by inhibiting the expression of MHC class II and costimulatory molecules, which plays critical actions in regulating antigen processing and maintaining immune responses.
It is believed that various DC subsets may play distinct roles in immune responses.1,2,5-8,60 Human mature DCs derived from CD1a+CD14− and CD1a−CD14+ DC precursors display distinct role in stimulating humoral immune responses and regulating the secretion of Igs, respectively.5,60 Based on the phenotype and differentiation capacity, murine CD11b+hiCD11c+ DC precursors may correspond to human CD1a−CD14+ DC precursors, whereas CD11b−/dullCD11c+ DC precursor may correspond to human CD1a+CD14− DC precursors.4,5,9 TGF-β1 inhibited the generation of CD11b−/dullCD11c+ DC and CD11b+hiCD11c+ DC precursors from Lin−c-kit+ HPCs and mature DCs from CD11b+hiCD11c+ DC precursors. In contrast, TGF-β1 polarizes the development of LC-like DCs expressing high levels of E-cadherin, c-fms (data not shown), and NSE activity from Lin−c-kit+ HPCs through the monocyte/macrophage differentiation pathway, which obviously differ from the phenotype of CD11b−/dullCD11c+DC and CD11b+hiCD11c+ DC precursor-derived mature DCs9 (Fig11). Such polarization effect of TGF-β1 on DC generation may play important pathophysiological roles in regulating various immune responses.
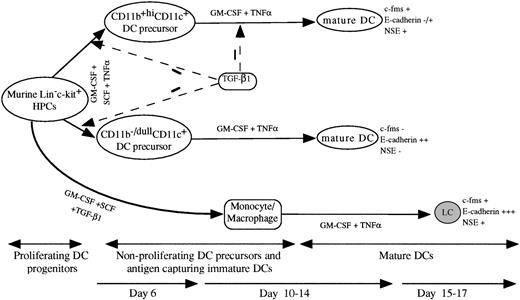
A schematic DC differentiation model in vitro from Lin−c-kit+ HPCs and the regulating role of TGF-β1. HPCs develop into mature DCs through four stages: proliferating DC progenitor cells, nonproliferating DC precursors, antigen capturing immature DCs, and mature DCs. The cytokine combination of GM-CSF + SCF + TNF can induce the generation of mature DCs from murine Lin−c-kit+ HPCs through two unrelated differentiation pathways: CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors that can be clearly identified at day 6 of culture. In response to GM-CSF + TNF, both the DC precursor subsets can independently differentiate at day 10 to 14 into mature DCs with distinct phenotype based on the expression of c-fms mRNA, NSE activity, and E-cadherin. TGF-β1 significantly inhibited the generation of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors from GM-CSF + SCF + TNF-stimulated Lin−c-kit+HPCs at day 6 of culture. TGF-β1 could also suppress DC maturation from CD11b+hiCD11c+, but not CD11b−/dullCD11c+ DC precursors at day 12 to 14. In collaboration with GM-CSF + SCF, TGF-β1 induced Lin−c-kit+ HPCs to differentiate solely into monocytes/macrophages. These cells could further differentiate at day 15 to 17 of culture into LC-like DCs expressing high levels of E-caderin, abundant c-fms, and NSE activity, which obviously differs from CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursor-derived mature DC subsets.
A schematic DC differentiation model in vitro from Lin−c-kit+ HPCs and the regulating role of TGF-β1. HPCs develop into mature DCs through four stages: proliferating DC progenitor cells, nonproliferating DC precursors, antigen capturing immature DCs, and mature DCs. The cytokine combination of GM-CSF + SCF + TNF can induce the generation of mature DCs from murine Lin−c-kit+ HPCs through two unrelated differentiation pathways: CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors that can be clearly identified at day 6 of culture. In response to GM-CSF + TNF, both the DC precursor subsets can independently differentiate at day 10 to 14 into mature DCs with distinct phenotype based on the expression of c-fms mRNA, NSE activity, and E-cadherin. TGF-β1 significantly inhibited the generation of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors from GM-CSF + SCF + TNF-stimulated Lin−c-kit+HPCs at day 6 of culture. TGF-β1 could also suppress DC maturation from CD11b+hiCD11c+, but not CD11b−/dullCD11c+ DC precursors at day 12 to 14. In collaboration with GM-CSF + SCF, TGF-β1 induced Lin−c-kit+ HPCs to differentiate solely into monocytes/macrophages. These cells could further differentiate at day 15 to 17 of culture into LC-like DCs expressing high levels of E-caderin, abundant c-fms, and NSE activity, which obviously differs from CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursor-derived mature DC subsets.
The molecular mechanisms for the polarization effect of TGF-β1 on DC differentiation remain elusive. TGF-β1 can inhibit the expression of CIITA in several types of cells61,62 by suppression of the basal promoter.62 TGF-β1 significantly inhibited the expression of CIITA mRNA in GM-CSF + SCF-treated Lin−c-kit+ HPCs at day 6, irrespective of addition of TNFα or at day 12 in the absence of TNFα, in parallel to its suppressing effect on the generation of nonproliferating CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors in the same cultures. Moreover, gene-targeted disruption of CIITA in mice impairs the expression of MHC class II molecules on DCs and results in functional incapacity of DCs to stimulate allogenic MLR.31These data suggest that the expression of CIITA is tightly related to the differentiation of DC precursors and functionally mature DCs from HPCs. Presumably, the suppressed expression of CIITA may be partially responsible for TGF-β1–induced inhibitory effect on the generation of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors. However, TGF-β1 failed to inhibit the transcription of CIITA gene at day 12 in GM-CSF + SCF-treated Lin−c-kit+ HPCs in the presence of TNFα and in CD11b+hiCD11c+ DC precursors. But it suppressed DC maturation in these cultures by decreasing the expression of Ia and CD86 antigens. Because the expression of MHC class II molecule is not only strictly regulated by CIITA at the transcriptional level, but also regulated at the posttranscriptional level, including translation, synthesis, translocation, and recycling of MHC class II molecules,30,31,56 57 it is conceivable that TGF-β1 may also confer its inhibitory effect on DC maturation at the posttranscriptional level. This may help us to gain insight into the molecular mechanisms for development of DC from early HPCs.
ACKNOWLEDGMENT
The authors express our sincere gratitude to Dr R.M. Steinman for his kind gift of MoAbs to DEC-205 (NLDC145) and 33D1 and to Dr T. Sudo for his generous gift of an anti–c-kit MoAb, GM-CSF, and SCF. We also greatly appreciate Dr C. Vestergaard for helpful discussion.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kouji Matsushima, MD, PhD, Department of Molecular Preventive Medicine, School of Medicine, The University of Tokyo, 7-3-1, Hongo, Bunkyoku, Tokyo 113, Japan; e-mail:koujim@m.u-tokyo.ac.jp.