Abstract
A novel DNA virus designated TT virus (TTV) has been reported to be involved in the development of posttransfusion non–A-C hepatitis. We evaluated the frequency and natural course of TTV infection in a cohort of transfusion-dependent thalassemic patients in a 3-year follow-up study. Ninety-three serum hepatitis C virus (HCV) antibody-negative patients (median age of 8 years; range, 0 to 25) from eight centers were studied. Of them, 34 (37%) had an abnormal alanine-aminotransferase (ALT) baseline pattern, and the other 12 (13%) showed ALT flare-ups during the follow-up. TTV DNA in patient sera collected at the time of enrollment and at the end of follow-up was determined by polymerase chain reaction (PCR). In parallel, serum samples from 100 healthy blood donors were also tested. At baseline, 87 patient sera (93.5%) tested positive for the TTV DNA. Of these TTV DNA-positive patients, 84 (96.5%) remained viremic at the end of the study period. Of the 6 TTV DNA-negative patients, 3 acquired TTV infection during follow-up. However, no definite relation was observed between the results of TTV DNA determination and ALT patterns. TTV viremia was also detectable in 22% of blood donors. In conclusion, TTV infection is frequent and persistent among Italian transfusion-dependent patients. The high rate of viremia observed in healthy donors indicates that the parenteral route is not the only mode of TTV spread.
THE INCIDENCE of transfusion-associated hepatitis has been substantially reduced after the implementation of screening for antibodies to hepatitis C virus (HCV) in blood donors.1-6 Nevertheless, chronic transfusion recipients, such as patients affected with homozygous β-thalassemia, still have a high frequency of liver disease due to transfusion-related iron overload and infection with bloodborne agents, either known or undiscovered.6 Hepatitis G virus (HGV), a member of theFlaviviridae family, was initially suggested as a causative agent of transfusion-associated non–A-C hepatitis,7,8 but this was not confirmed in further studies.9-12 Hence, the existence of other agent(s) capable of inducing non–A-C hepatitis has been hypothesized. A novel DNA virus designated TT virus (TTV) has been recently identified by representational difference analysis of sera from a patient with transfusion-associated hepatitis of unknown etiology.13,14 Preliminary data from the United Kingdom and Japan indicate that TTV sequences are detectable in 25% to 45% of patients with fulminant or chronic hepatitis of unknown origin, in 27% to 68% of hemophiliacs, and in 1.9% to 12% of apparently healthy blood donors.14-16 However, more epidemiological and clinical information from both blood donors and recipients is required to establish if TTV is transfusion-transmissible and to clarify its role in the pathogenesis of non–A-C hepatitis. This study provides data on the prevalence of TTV in Italian thalassemic patients. These patients were enrolled in the multicenter Cooleycare program for improving the quality of treatment for β-thalassemia.17As part of the Cooleycare effort, a survey program has been specifically designed for estimating the transfusion risk and describing the natural history of bloodborne infections among chronic transfusion recipients.6,12 18-20 This cohort of transfusion-dependent patients was free of known hepatotropic infections and they were followed for 3 years for clinical signs of liver diseases. The primary aims of this study were (1) to assess the frequency of TTV infection, (2) to investigate the possible relation of viremia with hepatic dysfunction, and (3) to describe the natural course of TTV infection.
SUBJECTS AND METHODS
Thalassemic patients.
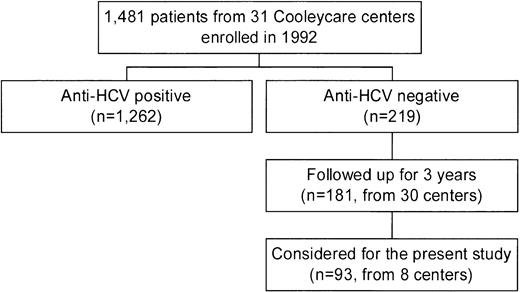
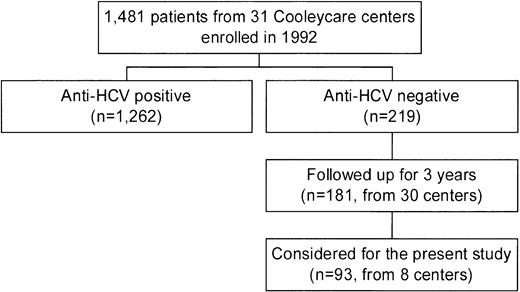
In 1992, the centers of the Cooleycare program were invited to participate in a longitudinal study on the incidence of liver disease in patients with β-thalassemia, as a part of an ongoing survey on transfusion transmitted infections.6,12,18-20 Thirty-one centers agreed to participate, and the 1,481 homozygous β-thalassemia patients enrolled (760 male and 721 female patients with a median age of 17 years; range, 0 to 45 years) received regular blood transfusions at these centers. For each patient, a baseline serum sample collected in December 1992 to March 1993 was sent to the reference laboratory of the Cooleycare group in Milan for anti-HCV determination. Two hundred nineteen patients (14.8%) were found to be anti-HCV− and 1,262 (85.2%) were anti-HCV+. Additional serum samples of the anti-HCV− patients were requested from the participating centers after 3 years, together with a record reporting the results of alanine-aminotransferase (ALT) measurements determined at each transfusion event and the number of red blood cell units transfused. Informative records on 181 subjects (82.6%, 95 males and 86 females with a median age at enrollment of 7 years; range, 0 to 28 years) were obtained from 30 centers. The biochemical, clinical, and virological features of this cohort of patients during the 3 years of follow-up have been very recently described by the Cooleycare group.6 For the purpose of this investigation, we decided to study the 93 anti-HCV− patients (48 males and 45 females, median age at enrollment of 8 years; range, 0 to 25 years) attending 8 of the 30 centers which have completed the program (Fig1). The participating centers were chosen according to the following criteria: (1) being in charge of at least seven anti-HCV− patients each; (2) being representative of the different regions of the country (at least one center from Northern, Central-Insular, and Southern Italy). All the patients received deferoxamine therapy according to current protocols. Hepatitis B surface antigen (HBsAg) determination was negative in all cases. Median levels of serum ferritin were 1,790 ng/mL (range, 330 to 7,110 ng/mL) at baseline, and 1,840 ng/mL (range, 340 to 6,480) at the end of the follow-up. Analysis of the follow-up samples showed that 2 of the 93 patients seroconverted to HCV during the study period. In regard to liver function, 34 patients (37%) had an abnormal ALT pattern at the baseline. ALT elevation was moderate in 27 subjects (79%), mild in 5 (15%), and minimal in 2 (6%). Another 12 anti-HCV− patients (13%) showed biochemical signs of liver dysfunction during the subsequent follow-up period. Of them, 3 (25%) had a moderate ALT increase, and were negative for HCV RNA, HGV RNA, and antibodies to hepatitis B core antigen (anti-HBc) determinations. During follow-up, 1 of the 3 patients developed chronic liver dysfunction, while the ALT levels of the remaining 2 patients returned to baseline levels.
Blood donors.
The presence of TTV DNA was also determined in 100 Italian blood donors. This group included 73 men and 27 women with a median age of 36 years (range, 21 to 64 years) and a median number of five blood donations. No abnormality was detected in these donors at physical examination and no sign or symptom of liver disease was reported in the medical history, collected at each blood donation. HBsAg, anti-HCV, and anti-HIV determinations were negative and serum ALT levels were normal.
Virological and biochemical laboratory tests.
The following assays, except for ALT determination in thalassemic patients, were performed at the reference laboratory of the Cooleycare group. The anti-HCV status was determined using second- and third-generation assays (Ortho Diagnostic Systems, Raritan, NJ). Qualitative serum HCV RNA determination was performed using the Amplicor HCV kit (Roche Molecular Systems, Basel, Switzerland). HBsAg and anti-HBc were determined by enzyme immunoassays (EIAs) (Murex, Dartford, UK; and Abbott Laboratories, Chicago, IL). HGV RNA was performed by reverse transcriptase-polymerase chain reaction (RT-PCR), as previously described.12
Serum ferritin was determined on baseline and follow-up samples by IMx Ferritina (Abbott Divisione Diagnostici, Roma, Italy). ALT measurements were determined at each transfusion event in the Cooleycare centers using standard methods. The upper reference limit (URL) for ALT was 40 U/L in males and 30 U/L in females. The ALT pattern was classified as normal if the enzyme levels were persistently below the URL and as abnormal if ALT values were persistently or intermittently above the URL. As previously described elsewhere,6 a baseline ALT pattern (based on the values observed during the first 6 months of the study) and a follow-up pattern (based on the values reported during the remaining study period) were defined for each patient. Liver dysfunction was classified as minimal (for peak value of ALT less than twice the URL), mild (between 2 and 3 times the URL), or moderate (above 3 times the URL).
Determination of TTV DNA by PCR.
Serum TTV DNA was detected by a seminested PCR. Specifically, serum DNA purified from an equivalent of 7 μL of serum was amplified by PCR in a 9600 thermal cycler (Perkin-Elmer, Emeryville, CA), using the following protocol: 1 cycle at 95°C for 9 minutes; 35 cycles at 94°C for 30 seconds, 58°C for 30 seconds, 72°C for 45 seconds; 1 cycle at 72°C for 7 minutes. The reaction conditions were: 30 pmol of each primer (sense NG059 5′-ACA GAC AGA GGA GAA GGC AAC ATG-3′, antisense NG063 5′-CTG GCA TTT TAC CAT TTC CAA AGT-3′14) and 2.5 U of AmpliTaq Gold (Perkin-Elmer) in 50 μL reaction volume. Five microliters of the product of amplification was submitted to a second round of PCR using a semi-nested primer set (sense NG061 5′ GGC AAC ATG TTA TGG ATA GAC TGG 3′, antisense NG063) under the same conditions described above. Multiple positive and negative controls were included in each PCR assay. PCR products were analyzed in a 2% agarose gel electrophoresis with ethidium bromide staining. Results of all samples testing positive for TTV DNA in the PCR were confirmed in separate assays and sequences of PCR products confirmed by automated sequencing on an ABI 373 sequencer (Perkin-Elmer, Foster City, CA).
RESULTS
Thalassemic patients.
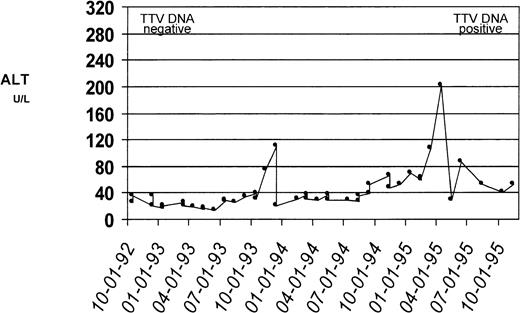
The results on PCR detection of TTV DNA in patient sera collected at time of enrollment into the Cooleycare program (1992-1993) and at the end of the 3-year follow-up period (1995-1996) are reported in Table1. Of the 93 patient sera collected in 1992-1993, 87 (93.5%) tested positive for TTV DNA. Of these patients with TTV viremia at baseline, 84 (96.5%) were still positive for TTV DNA at the end of the study period, while viremia became undetectable in the remaining 3 (3.5%) patients. Of the 6 patients with baseline negative TTV DNA, 3 (ages at enrollment were 1, 3, and 8 years) acquired TTV infection during the 3-year follow-up, as documented by conversion to viremia in the sample collected in 1995-1996. Two of the three patients had normal ALT levels throughout the follow-up period while the remaining patient had biochemical evidence of hepatitis (ALT peak, 196 U/L) (Fig 2). There was no relation between the presence of TTV viremia and patients’ age, gender, serum ferritin levels, and pattern of ALT levels.
Serum ALT profiles of a patient who acquired TTV infection. Biochemical evidence of liver disease in this patient during the study period was reflected by changes in the ALT level. The ALT levels (U/L, dotted line) were plotted against the date of determination; the upper reference limit for ALT was 40 U/L. Serum from this patient collected at the time of enrollment tested negative for TTV DNA by PCR. The serum samples collected at the conclusion of the 3-year follow-up tested positive for TTV DNA.
Serum ALT profiles of a patient who acquired TTV infection. Biochemical evidence of liver disease in this patient during the study period was reflected by changes in the ALT level. The ALT levels (U/L, dotted line) were plotted against the date of determination; the upper reference limit for ALT was 40 U/L. Serum from this patient collected at the time of enrollment tested negative for TTV DNA by PCR. The serum samples collected at the conclusion of the 3-year follow-up tested positive for TTV DNA.
Blood donors.
Of the sera from 100 healthy blood donors, 22 were found to be TTV+, representing a prevalence rate of 22%. TTV infection was more frequent in male (20 of 73, 27.4%) than in female donors (2 of 27, 7.4%), representing a difference of 20% (95% confidence interval [CI], 5.7 to 34.2). The age distribution was not statistically different in TTV DNA+ and DNA−donors (40 years ± 11 v 38 years ± 11).
DISCUSSION
Specific TTV sequences were detected in a vast majority of transfusion-dependent thalassemic patients in Italy, supporting the hypothesis that TTV is a transfusion-transmissible agent. For the patients evaluated in this study, the rate of TTV infection at the baseline evaluation (93.5%) was higher than that previously found for HCV and HGV in the same population of β-thalassemic patients (85% and 33% respectively).6 12 In addition, we found that 3 of the 6 patients who were TTV DNA− at the baseline evaluation acquired the infection during the following 3 years of observation. Although it seems reasonable to assume that these cases of primary TTV infections resulted from the blood transfusion therapy, a community-acquired origin could not be excluded. In this regard, a longitudinal study comparing the incidence of TTV infection in transfused versus nontransfused young patients would be informative.
TTV DNA was found in more than one fifth of Italian repeat blood donors, which are selected on the basis of being at low risk for bloodborne infections. By comparison, in the same population of donors, the prevalences of HCV and HGV infections were 1.1% and 6.5%, respectively.21-23 On the basis of these observations, it is likely that the parenteral route may not be the only mode of spread of TTV in Italy. In this regard, recent data showed that TTV DNA is present in feces, which would suggest that the fecal-oral route of viral transmission is possible.24 Some of the epidemiological features identified in the present investigation, ie, the higher frequency of TTV infection in Italian donors (22%) compared with those in Japan and the United Kingdom (12% and 1.9%),14 15 and the higher prevalence of viremia in males than in females (27.4% v 7.4%), may be helpful to clarify the routes of infection, and will be the subject of future studies.
Only a small proportion (3.5%) of the patients who had baseline infection have undergone spontaneous clearance of serum TTV DNA during the 3-year study period. The disappearance of detectable TTV DNA in these cases, however, does not necessarily indicate a complete recovery from infection, and may merely represent a latent phase of ongoing infection. By comparison, we recently showed that more than 25% of the thalassemic patients with transfusion-associated HGV recovered from infection within a few years.12 It remains to be elucidated if the persistence of TTV is due to chronic infection or to frequent reinfection of the patients with different strains of TTV.
Based on the data reported here, some considerations on the clinical significance of TTV infection may be appropriate. The high prevalence of TTV in thalassemic patients, who frequently have hepatic dysfunction despite the absence of known hepatotropic infections,6together with the observation that one of the three patients who acquired TTV infection during the study period had a clinical course suggestive of posttransfusion hepatitis, would support the original hypothesis that this agent might cause liver disease. On the other hand, it should be taken into consideration that transfusion-dependent patients can theoretically be infected by a number of unidentified bloodborne pathogens other than TTV, and are also at risk of developing iron-induced hepatic damage.6,25 Taken together, the observations that approximately one half of the patients with persistent viral replication had normal liver enzymes throughout the period of follow-up and that TTV viremia is also very common in apparently healthy individuals, would suggest that the course of TTV infection is indolent in most cases. Our hypothesis is also supported by the data of Naoumov et al,16 who found that the prevalence of TTV infection is not any higher in patients with chronic liver disease than in controls. However, a role of this agent in inducing clinical disease in some individuals cannot be excluded. Preliminary data seem to indicate that TTV has structural similarities to parvoviruses,14 which may sometimes induce life-threatening complications in blood recipients.26 27 In addition, the DNA sequencing studies performed so far indicate that a certain degree of genetic diversity exists between different isolates of TTV. Thus, particular viral strains may have different pathogenetic effects in inducing transfusion-associated disease.
In conclusion, this prospective survey on transfusion-associated TTV infection adds new data to the growing literature indicating that hitherto unidentified bloodborne agents continue to cause chronic infections among transfusion recipients. Future efforts on the identification and characterization of these viruses, as well as the description of their effects in infected individuals, may be useful in the management of patients and to further reduce the risk of blood transfusion.
ACKNOWLEDGMENT
The authors thank Dr R. Bohenzky and the sequencing-informatics team at Sentinel Biosciences, Inc for their expert technical support.
Cooleycare members who have provided samples and data for this study: M. Alessi (Catania); C. Artaz (Aosta); M.G. Batzella (S. Gavino Monreale); P. Bellavita (Bergamo); G. Bertrand (Sassari); F. Betto (Rho); A. Biolchini (Iglesias); C. Borgna (Verona); S. Calò(Magenta); A. Cambosu, A. Carta (Oristano); E. Cichella (Rovigo); V. Cilla (Matera); E. Corvaglia (Casarano); D. Costantino (Locri); C. De Rosa (Napoli); F. Di Gregorio (Catania); P. Di Paola (Palermo); D. Gallisai (Sassari); G. Girelli (Roma); M. Lendini (Olbia); R. Longhi (Como); C. Magnano (Catania); L. Luongo (Agrigento); A. Mangiagli (Siracusa); A. Meo (Messina); S. Strada (Monza); S. Montin (Monselice); G. Forni (Genova); P. Rizzone Favacchio (Ragusa); F. Schettini (Bari); G. Sciorelli (Monza).
Cooleycare members providing samples and data for this study are reported in the .
Supported in part by a grant from the Italian National Institute of Health (“Progetto Sangue,” Istituto Superiore di Sanità).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Daniele Prati, MD, Centro Trasfusionale e di Immunologia dei Trapianti, IRCCS Ospedale Maggiore, Via Francesco Sforza, 35, 20122 Milano, Italy.