The influence of antiplatelet glycoprotein (GP) antibodies on megakaryocytopoiesis in patients with idiopathic or immune thrombocytopenic purpura (ITP) has been well studied. However, the influence of GP antibodies on proplatelet formation is poorly understood. Here we investigated whether in vitro human megakaryocyte colony formation and proplatelet formation are affected by various monoclonal antiplatelet GP antibodies (MoAb). The megakaryocyte colony formation inhibition assay was performed by methylcellulose culture with modifications, using peripheral blood nonadherent mononuclear cells. The proplatelet formation inhibition assay was performed by megakaryocytes derived from CD34+ cells, stimulated with thrombopoietin + stem cell factor, which were then incubated with antiplatelet GP MoAb for 24 or 48 hours. Anti-GP-Ib MoAb (CD42b; HIP1) slightly inhibited megakaryocyte colony formation (P < .05). and strongly inhibited proplatelet formation (after 24 hours incubation, P < .0002; after 48 hours incubation, P < .0007). Anti-GP-IIb MoAb (CD41; 5B12) inhibited only proplatelet formation (only after 24 hours incubation,P < . 03). Anti-integrin vβ3MoAb (CD51/CD61; 23C6) only slightly inhibited colony size (P < .05). However, anti-GP-IIIa MoAb (CD61; Y2/51) did not inhibit either colony formation or proplatelet formation. These results suggest that antiplatelet GP MoAbs have differing effects on in vitro megakaryocyte colony formation and proplatelet formation.
IDIOPATHIC OR IMMUNE thrombocytopenic purpura (ITP) is a disorder characterized by thrombocytopenia, increased levels of platelet-associated IgG (PAIgG), and normal or increased numbers of marrow megakaryocytes.1,2 The thrombocytopenia is caused by IgG antibodies that are directed against platelet glycoprotein (GP)-Ib, -IIb, and -IIIa, as well as the platelet GP-IIb/-IIIa complex.3,4 These antigens exist on both platelets and megakaryocytes. Thus, megakaryocytes are also affected by these antiplatelet GP antibodies.5-7 The influence of antiplatelet antibodies on megakaryocytopoiesis in patients with ITP has been previously studied using cell cultures. De Alarcon et al8 studied children with acute ITP and reported an increase in the number of megakaryocyte colony-forming units (CFU-Meg), and Sugiyama et al9 studied patients with chronic ITP and also observed a significant increase in the number of CFU-Meg. By contrast, Abgrall et al10,11 reported that the number of CFU-Meg was reduced in patients with chronic ITP and was increased in patients with acute ITP. An experimental model of thrombocytopenia in animals, produced by injection of antiplatelet serum, has been used to study thrombocytopenia. Some investigators have previously reported that inhibition of platelet production did not seem to occur in this experimental model.12,13 Levin et al14 and Burstein et al15 showed only a delayed increase in the number of megakaryocyte colony-forming cells after administration of platelet antiserum, and Levin et al14 reported a greater increase in megakaryocyte colony-forming cells in the spleen than in the murine bone marrow.
During megakaryocytopoiesis, mature megakaryocytes extend cytoplasmic processes, termed proplatelets, in vitro and in vivo.16,17The formation of proplatelets by megakaryocytes is believed to be the final stage of megakaryocytopoiesis. In recent years, the understanding of proplatelet formation by megakaryocytes, induced from human peripheral blood progenitors, has been advanced through the use of tissue culture systems that permit this differentiation process to occur in vitro.18-20 Proplatelet formation in patients with ITP may be affected by antiplatelet GP antibodies, because proplatelets contain platelet GP. If proplatelet formation is inhibited by these antibodies, severe thrombocytopenia may develop. The influence of antiplatelet GP antibodies on proplatelet formation, however, is poorly understood.
In this study, we investigated whether in vitro megakaryocyte colony formation and proplatelet formation are affected by monoclonal antibodies (MoAb) raised against various platelet antigens, using human megakaryocytes, derived from peripheral blood progenitors.
MATERIALS AND METHODS
Human Subjects
Peripheral blood was obtained from healthy adult volunteers with their informed consent. Serum and plasma were stored at −80°C until use.
MoAbs for Inhibition Assays
Mouse IgG1 MoAbs, specific for GP-IIb (CD41; clone 5B12), GP-IIIa (CD61; clone Y2/51), and a negative control (clone DAK-GO1), were purchased from Dako (Glostrup, Denmark). An anti-GP-Ibα MoAb (CD42b; IgG1, clone HIP1) was purchased from PharMingen (San Diego, CA), and an anti-integrin αvβ3MoAb (CD51/CD61; IgG1, clone 23C6) was purchased from Southern Biotechnology Associates Inc (Birmingham, AL).
These antibodies contained sodium azide, which we removed by dialysis as described below. After dialysis, the influence of the remaining sodium azide was tested in the megakaryocyte colony formation inhibition assay cell cultures.
Dialysis of antibodies for inhibition assays.
Antibodies were dialyzed against Iscove’s modified Dulbecco’s medium (IMDM; GIBCOBRL, Life Technologies, Inc, Rockville, MD) using a dialysis cassette (#66450, Slide-A-Lyzer; Pierce Chemical Co, Rockford, IL) for 20 hours at 4°C. Dialyzed antibodies were sterilized using an ultracleaning filter (Millex-GV13OS; Millipore Co, Bedford, MA) and were quantified by a sandwich enzyme-linked immunosorbent assay (ELISA).
ELISA.
The ELISA plates (EIA Flat Plate I; Sanko Junyaku, Tokyo, Japan) were coated with 1 μg/mL goat affinity-purified F(ab′)2antimouse IgG (ICN Pharmaceuticals Inc, Costa Mesa, CA) overnight at 4°C. The plates were then washed with phosphate-buffered saline containing 0.05% Tween 20, pH 7.2 (PBS-Tween). Samples were diluted 1:100, 1:500, and 1:2,500 with 1% bovine serum albumin (BSA) in PBS-Tween, and the plates were incubated for 1 hour at room temperature. Mouse antihuman von Willebrand factor MoAb (IgG1, clone F8/86, Dako) at known Ig concentrations was used as the standard. After washing, 0.25 μg/mL alkaline phosphatase-conjugated sheep affinity-purified F(ab′)2antimouse IgG (ICN Pharmaceuticals Inc) was added to the wells, and the wells were incubated for 1 hour at room temperature. Then,p-nitro phenyl phosphate alkaline phosphatase substrate solution (Sigma, St Louis, MO) was added to each well. After 30 minutes, the reaction was stopped by the addition of 50 μL 4 N NaOH, and the absorbance was measured at 410 nm using an ELISA plate reader (SJeia Reader; Sanko Junyaku).
Influence of sodium azide on cell culture.
We tested the effect of the sodium azide remaining in the dialyzed antibody preparations on the numbers of colonies, other than the megakaryocyte colony. We did this by using the megakaryocyte colony formation inhibition assay cell cultures, as described below. This assay showed that there was no statistically significant difference between the numbers of colonies in the presence or absence of the antibody preparations in the culture medium.
Megakaryocyte Colony Formation Inhibition Assay
Separation of human peripheral blood nonadherent mononuclear cells.
Peripheral blood mononuclear cells were separated by centrifugation on Lymphoprep (density = 1.077 g/mL; Nycomed, Oslo, Norway) at 800g for 20 minutes, then suspended in IMDM with 10% fetal calf serum (GIBCO BRL), and incubated for 1 hour in plastic culture dishes (Iwaki, Tokyo, Japan) at 37°C in humidified 5% CO2 in air. The nonadherent cells were collected and used for the cell culture experiments.
Inhibition of megakaryocyte colony formation.
The megakaryocyte colony formation inhibition assay was performed by methylcellulose culture with modifications.21 Peripheral blood nonadherent mononuclear cells (2.5 × 105cells/mL) were cultured in 35-mm plastic dishes containing IMDM supplemented with 10 ng/mL recombinant human thrombopoietin (TPO; Genzyme, Cambridge, MA), 10 ng/mL recombinant human stem cell factor (SCF; Pepro Tech, London, UK),22 1% Insulin-Transferrin-Selenium-X (ITS-X; GIBCO), 5.5 × 10−5 mol/L 2-mercaptoethanol (2-ME; GIBCO), 1% methylcellulose (Dow Chemical Co, Midland, MI), 1% BSA (Alubu MAX I; GIBCO), 10% autologous serum, and 1 μg/mL antibody for 14 days at 37°C in humidified 5% CO2 in air.
The number of colonies and the megakaryocyte colony sizes (cells per colony) were counted using an inverted microscope (Olympus, Tokyo, Japan) in situ. Megakaryocyte colonies were defined as clusters of more than three megakaryocytes and were identified by their morphological characteristics, as described elsewhere.21,23 To assess the accuracy of the in situ identification of megakaryocyte colonies, individual colonies were transferred to tissue culture chamber slides (#4808 Lab-Tek; Miles Scientific, Naperville, IL), air-blown to spread the cells, and fixed with buffered formalin-acetone (pH 6.6) for 30 seconds at 4°C.24 The cells were exposed to the anti-GP-IIb MoAb (5B12) for 30 minutes at room temperature, then stained with biotinylated antimouse Igs and alkaline phosphatase-conjugated streptavidin (LSAB kit; Dako). All of the megakaryocyte colonies identified by in situ observation consisted of GPIIb-positive megakaryocytes.
Colonies of other cell types (ie, granulocyte colonies and macrophage colonies) were defined as clusters of more than 50 cells per colony and were identified by their morphological characteristics, as described elsewhere.21 The number of colonies of other cell types was determined and used to assess the effect of the sodium azide, as described above.
Proplatelet Formation Inhibition Assay
Preparation of megakaryocytes.
Peripheral blood CD34+ cells were used to obtain enriched populations of megakaryocytes for the inhibition assay of proplatelet formation. CD34+ cells were isolated from peripheral blood nonadherent mononuclear cells, using a commercial progenitor cell selection system (Dynal CD34; Dynal, Oslo, Norway) in accordance with the manufacturer’s protocol. The isolated CD34+ cells (cell density 2.6 ± 0.8 × 104 cells/mL [mean ± SEM, n = 6], depending on the individual preparation of cells used) were cultured in 35-mm plastic dishes containing IMDM supplemented with 10 ng/mL TPO, 10 ng/mL SCF, 1% ITS-X, 5.5 × 10−5 mol/L 2-ME, 1% methylcellulose, 1% BSA, and 10% autologous plasma25 at 37°C in humidified 5% CO2 in air. After 9 days, morphologically identified megakaryocyte colonies were collected to 900 μL fresh IMDM supplemented with 1% ITS-X, 5.5 × 10−5 mol/L 2-ME, 1% BSA, and 10% autologous plasma, in a tissue culture chamber slide (#4804 Lab-Tek), using a micropipet. The total number of megakaryocyte colonies collected from dishes was 353.2 ± 43.9 colonies (10.3 ± 0.1 megakaryocytes per colony), and the total volume of collected medium was 400 μL in each experiment. Therefore, the remaining concentration of cytokines (TPO and SCF) in this liquid culture medium was always 3 ng/mL.
Inhibition of proplatelet formation.
The collected megakaryocytes were incubated in the chamber slide for 1 hour at 37°C in humidified 5% CO2 in air. Then, megakaryocytes were aliquoted into the wells of a 96-well tissue culture plate (#430247; Corning Costar, Cambridge, MA) for proplatelet formation. The number of cells evaluated in each well was 360.5 ± 148.7, depending on the individual preparation of cells used. Then, each antibody was added to the wells at a concentration of 10 μg/mL. Megakaryocytes were incubated for 24 or 48 hours at 37°C in humidified 5% CO2 in air. Cells and megakaryocytes with proplatelet formation were counted using an inverted microscope at 100× or 200× magnification. Proplatelets were identified as described previously.26 After the counting was completed, the cells were fixed with buffered formalin-acetone for 30 seconds at 4°C. The cells were exposed to the anti-GP-IIb MoAb for 30 minutes at room temperature, followed by staining with LSAB kit, and the number of GP-IIb-positive cells were counted using an inverted microscope.
Evaluation of inhibition of proplatelet formation.
The percentage of megakaryocytes with proplatelet formation per well (%PPF) was calculated as the number of megakaryocytes with proplatelet formation divided by the number of GP-IIb-positive cells times 100. The proplatelet formation activity is expressed using the following formula: proplatelet formation activity (%) = (sample %PPF/negative control %PPF) × 100.
Statistical Analysis
Statistical significance was determined using the Student’st-test.
RESULTS
Influence of Antiplatelet GP MoAbs on Megakaryocyte Colony Formation
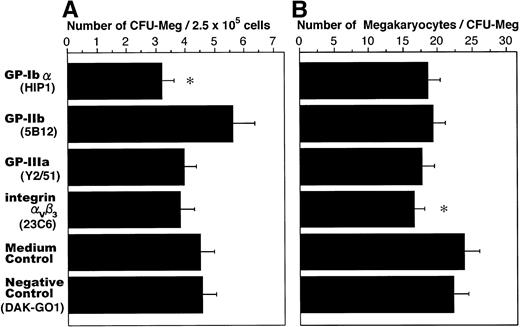
The influence of antiplatelet GP MoAbs on megakaryocyte colony formation is shown in Fig 1. The number of megakaryocyte colonies and the average colony size were not significantly different between the culture medium control and the negative control. Anti-GP-Ibα antibody slightly inhibited colony formation by megakaryocytes (P < .05) compared with the controls, whereas the anti-GP-IIb, anti-integrin αvβ3, and anti-GP-IIIa antibodies did not inhibit colony formation. Only the anti-integrin αvβ3 antibody slightly reduced the average megakaryocyte colony size (P < .05), compared with the controls.
Influence of antiplatelet GP MoAbs on megakaryocyte colony formation. Peripheral blood nonadherent mononuclear cells (2.5 × 105 cells/mL) were cultured in IMDM supplemented with 10 ng/mL TPO, 10 ng/mL SCF, and 1 μg/mL each antiplatelet GP MoAb for 14 days. The number of colonies (A) and colony size (B) were counted using an inverted microscope. The data are the means ± standard error of mean (SEM) from duplicate cultures in two experiments using cells from three donors. *Significantly different compared with the controls (P < .05).
Influence of antiplatelet GP MoAbs on megakaryocyte colony formation. Peripheral blood nonadherent mononuclear cells (2.5 × 105 cells/mL) were cultured in IMDM supplemented with 10 ng/mL TPO, 10 ng/mL SCF, and 1 μg/mL each antiplatelet GP MoAb for 14 days. The number of colonies (A) and colony size (B) were counted using an inverted microscope. The data are the means ± standard error of mean (SEM) from duplicate cultures in two experiments using cells from three donors. *Significantly different compared with the controls (P < .05).
Influence of Antiplatelet GP MoAbs on Proplatelet Formation
We first determined the culture time for the preparation of megakaryocytes. Peripheral blood CD34+ cells (4.5 × 103 cells/mL) were cultured with 10 ng/mL TPO and 10 ng/mL SCF in 35-mm plastic dishes for 14 days. Megakaryocyte colonies that contained megakaryocytes with proplatelet formation were counted daily on days 1 through 14 of the culture period, using an inverted microscope. The appearance of megakaryocytes with spontaneous proplatelet formation occurred 9 days after the beginning of culture (Fig 2). Their number peaked between days 9 and 10 (Fig 3). Therefore, collection of the megakaryocyte colonies from the culture dishes was determined on the 9th day of culture, before maximal proplatelet production.
Spontaneous proplatelet formation by megakaryocytes in methylcellulose culture medium. Peripheral blood CD34+cells were cultured with 10 ng/mL TPO and 10 ng/mL SCF in 35-mm plastic dishes. On day 9 of the culture, megakaryocytes with spontaneous proplatelet formation were observed under an inverted microscope (bar, 50 μm).
Spontaneous proplatelet formation by megakaryocytes in methylcellulose culture medium. Peripheral blood CD34+cells were cultured with 10 ng/mL TPO and 10 ng/mL SCF in 35-mm plastic dishes. On day 9 of the culture, megakaryocytes with spontaneous proplatelet formation were observed under an inverted microscope (bar, 50 μm).
Determination of culture days for the megakaryocyte colony collection. Peripheral blood CD34+ cells (4.5 × 103 cells/mL) were cultured with 10 ng/mL TPO and 10 ng/mL SCF in 35-mm plastic dishes for 14 days. Megakaryocyte colonies that contained megakaryocytes with proplatelet formation were counted daily on days 1 through 14 of the culture period, using an inverted microscope. Their number peaked between days 9 and 10. Therefore, collection of the megakaryocyte colonies from the culture dishes was determined on the 9th day of culture, before maximal proplatelet production. The data are the means ± SEM from duplicate cultures of one experiment.
Determination of culture days for the megakaryocyte colony collection. Peripheral blood CD34+ cells (4.5 × 103 cells/mL) were cultured with 10 ng/mL TPO and 10 ng/mL SCF in 35-mm plastic dishes for 14 days. Megakaryocyte colonies that contained megakaryocytes with proplatelet formation were counted daily on days 1 through 14 of the culture period, using an inverted microscope. Their number peaked between days 9 and 10. Therefore, collection of the megakaryocyte colonies from the culture dishes was determined on the 9th day of culture, before maximal proplatelet production. The data are the means ± SEM from duplicate cultures of one experiment.
We then examined the spontaneous proplatelet formation of the collected megakaryocytes in a 96-well tissue culture plate. Megakaryocyte colonies were derived from peripheral blood CD34+ cells (1.8 ± 0.5 × 104 cells/mL) stimulated with 10 ng/mL TPO and 10 ng/mL SCF, and were collected from culture dishes on the 9th day (343.0 ± 3.0 colonies). The collected megakaryocytes were first incubated in the chamber slide for 1 hour at 37°C in humidified 5% CO2 in air and aliquoted into the wells of a 96-well tissue culture plate in the same manner as for the proplatelet formation inhibition assay (190.9 ± 11.2 cells per well). The plated megakaryocytes developed spontaneous proplatelets in the absence of antibodies (Fig 4). The time 0 corresponded with the 9th day of culture, as shown in Fig 3. The number of proplatelets that developed from megakaryocytes peaked at 24 hours. This peak corresponded with the peak of proplatelet formation shown in Fig 3 (day 10). Thereafter, the total number of proplatelets decreased, fragmenting into smaller platelet-like pieces.
Time course of spontaneous proplatelet formation by collected megakaryocytes. We observed the production of spontaneous proplatelet formation in the same manner as the proplatelet formation inhibition assay in the absence of antibodies. Megakaryocyte colonies were derived from peripheral blood CD34+ cells (1.8 ± 0.5 × 104 cells/mL) stimulated with 10 ng/mL TPO and 10 ng/mL SCF, and they were collected on the 9th day from culture dishes (343.0 ± 3.0 colonies). Then, collected megakaryocytes were aliquoted into the wells of a 96-well tissue culture plate (190.9 ± 11.2 cells per well) and were incubated. The number of megakaryocytes with proplatelet formation was counted after 5, 24, and 48 hours of incubation, respectively, using an inverted microscope. The %PPF calculation is described in detail in the Materials and Methods section. The time 0 corresponds with the 9th day of culture in Fig 3. The purity of the GP-IIb-positive cells was 98.9% ± 0.3% per well, by indirect immunostaining. The data are the means ± SEM from duplicate incubations of two experiments.
Time course of spontaneous proplatelet formation by collected megakaryocytes. We observed the production of spontaneous proplatelet formation in the same manner as the proplatelet formation inhibition assay in the absence of antibodies. Megakaryocyte colonies were derived from peripheral blood CD34+ cells (1.8 ± 0.5 × 104 cells/mL) stimulated with 10 ng/mL TPO and 10 ng/mL SCF, and they were collected on the 9th day from culture dishes (343.0 ± 3.0 colonies). Then, collected megakaryocytes were aliquoted into the wells of a 96-well tissue culture plate (190.9 ± 11.2 cells per well) and were incubated. The number of megakaryocytes with proplatelet formation was counted after 5, 24, and 48 hours of incubation, respectively, using an inverted microscope. The %PPF calculation is described in detail in the Materials and Methods section. The time 0 corresponds with the 9th day of culture in Fig 3. The purity of the GP-IIb-positive cells was 98.9% ± 0.3% per well, by indirect immunostaining. The data are the means ± SEM from duplicate incubations of two experiments.
Inhibition of proplatelet formation by antiplatelet GP MoAbs.
The influence of antiplatelet GP MoAbs on proplatelet formation is shown in Fig 5. Proplatelet formation activity was not significantly different between the culture medium control and the negative control in any of the incubations. At 24 hours of incubation, anti-GP-Ibα or anti-GP-IIb antibodies inhibited platelet formation by megakaryocytes (P < .0002 andP < .03, respectively), compared with the controls. At 48 hours of incubation, anti-GP-Ibα antibody inhibited platelet formation by megakaryocytes (P < .0007), compared with the controls. During this incubation time, the inhibition of proplatelet formation by anti-GP-IIb antibody was not statistically different compared with controls. However, though inhibition by the anti-GP-Ibα antibody decreased a little in comparison with the 24 hours of incubation, this inhibition was still stronger compared with other antibodies. Throughout experimental incubation, formation of morphologically abnormal proplatelets did not occur. The purity of the GP-IIb-positive cells was 98.6% ± 0.6% per well, as measured by indirect immunostaining (Fig6).
Influence of antiplatelet GP MoAbs on proplatelet formation. Megakaryocyte colonies were derived from peripheral blood CD34+ cells (2.6 ± 0.8 × 104cells/mL) stimulated with 10 ng/mL TPO and 10 ng/mL SCF and were collected on the 9th day from culture dishes (353.2 ± 43.9 colonies). The collected megakaryocytes were incubated with each antiplatelet GP MoAb (10 μg/mL) in a 96-well tissue culture plate for 24 or 48 hours. The number of cells evaluated in each well was 360.5 ± 148.7, depending on the individual preparation of cells used. The purity of the GP-IIb-positive cells was 98.6% ± 0.6% per well, by indirect immunostaining. Cells and megakaryocytes with proplatelet formation were counted using an inverted microscope. The proplatelet formation activity calculation is described in detail in the Materials and Methods section. The data are the means ± SEM from duplicate incubations in two experiments using cells from three donors. *Significantly different compared with the controls (P < .0002). **Significantly different compared with the controls (P < .0007). ***Significantly different compared with the controls (P < .03). Incubation time: ▩, 24 hours; ░ 48 hours.
Influence of antiplatelet GP MoAbs on proplatelet formation. Megakaryocyte colonies were derived from peripheral blood CD34+ cells (2.6 ± 0.8 × 104cells/mL) stimulated with 10 ng/mL TPO and 10 ng/mL SCF and were collected on the 9th day from culture dishes (353.2 ± 43.9 colonies). The collected megakaryocytes were incubated with each antiplatelet GP MoAb (10 μg/mL) in a 96-well tissue culture plate for 24 or 48 hours. The number of cells evaluated in each well was 360.5 ± 148.7, depending on the individual preparation of cells used. The purity of the GP-IIb-positive cells was 98.6% ± 0.6% per well, by indirect immunostaining. Cells and megakaryocytes with proplatelet formation were counted using an inverted microscope. The proplatelet formation activity calculation is described in detail in the Materials and Methods section. The data are the means ± SEM from duplicate incubations in two experiments using cells from three donors. *Significantly different compared with the controls (P < .0002). **Significantly different compared with the controls (P < .0007). ***Significantly different compared with the controls (P < .03). Incubation time: ▩, 24 hours; ░ 48 hours.
Indirect immunostaining of human megakaryocytes with proplatelet formation. The collected megakaryocytes that were derived from peripheral blood CD34+ cells stimulated with 10 ng/mL TPO and 10 ng/mL SCF were incubated in the absence of antibodies in a 96-well tissue culture plate for 24 hours. After fixation, megakaryocytes were exposed to the anti-GP-IIb MoAb (5B12), followed by staining with biotinylated antimouse immunoglobulins and alkaline phosphatase-conjugated streptavidin. Megakaryocytes and proplatelets were stained with anti-GP-IIb MoAb (bar, 50 μm).
Indirect immunostaining of human megakaryocytes with proplatelet formation. The collected megakaryocytes that were derived from peripheral blood CD34+ cells stimulated with 10 ng/mL TPO and 10 ng/mL SCF were incubated in the absence of antibodies in a 96-well tissue culture plate for 24 hours. After fixation, megakaryocytes were exposed to the anti-GP-IIb MoAb (5B12), followed by staining with biotinylated antimouse immunoglobulins and alkaline phosphatase-conjugated streptavidin. Megakaryocytes and proplatelets were stained with anti-GP-IIb MoAb (bar, 50 μm).
DISCUSSION
We believe that studying the effects of antiplatelet GP MoAbs on cultured megakaryocytes will provide important information about both megakaryocytopoiesis and the role of antiplatelet autoantibodies in ITP. The present study showed that (1) anti-GP-Ibα MoAb (HIP1) slightly inhibited megakaryocyte colony formation and strongly inhibited proplatelet formation, (2) anti-GP-IIb MoAb (5B12) inhibited only proplatelet formation (only after 24 hours of incubation), (3) anti-integrin αvβ3 MoAb (23C6) slightly inhibited only colony size, and (4) anti-GP-IIIa MoAb (Y2/51) did not inhibit either colony formation or proplatelet formation.
These results raise two important points. First, our megakaryocyte colony formation inhibition assay data imply that the sites recognized by the anti-GP-Ibα and anti-integrin αvβ3antibodies play a significant role in megakaryocytopoiesis. The anti-GP-Ibα antibody seemed to affect immature cells, because it reduced the number of colonies, whereas the anti-integrin αvβ3 antibody seemed to affect cell proliferation, because the average colony size was reduced. The GP-IIb/-IIIa complex is expressed on the megakaryocytes’ membrane before GP-Ib in the early stage of maturation.27Nevertheless, the anti-GP-IIb and anti-GP-IIIa antibodies did not affect megakaryocytopoiesis. These results suggest that inhibition of megakaryocytopoiesis varies according to the specific anti-GP antibodies used. Our findings may explain conflicting reports8-11 about the effects of antiplatelet GP antibodies on in vitro megakaryocyte colony formation in patients with ITP.
Second, we have shown that antiplatelet GP antibodies affect not only megakaryocyte maturation,2 as reported previously, but also proplatelet formation. Furthermore, the same antiplatelet GP MoAb affected megakaryocyte colony formation and proplatelet formation. However, the anti-integrin αvβ3 and anti-GP-IIIa antibodies did not inhibit proplatelet formation. These results suggest that inhibition of proplatelet formation varies according to the specific anti-GP antibodies used. The anti-GP-Ibα and anti-GP-IIb MoAbs had a more dramatic effect on proplatelet formation than the other antibodies. In particular, the former MoAb, anti-GP-Ibα, strongly and persistently inhibited proplatelet formation. In contrast, inhibition by the latter MoAb, anti-GP-IIb, was not persistent. We think that these influences do not delay the kinetics, but the inhibition of proplatelet formation, because proplatelet formation activity did not increase more than the controls after 48 hours of incubation. If this inhibition was to occur in vivo in patients with ITP, platelet production would be inhibited, and these patients might develop severe thrombocytopenia.
In patients with ITP, platelets are frequently bound to antiplatelet antibodies and are then destroyed by the reticuloendothelial system.1 Assays that measure antiplatelet antibodies have been developed, and patients with ITP usually have increased levels of PAIgG. In most cases, there is a significant correlation between the PAIgG level and the platelet count28; however, this correlation does not always hold true29-31: the PAIgG level is low in patients with severe thrombocytopenia and is high in patients with slight thrombocytopenia. To account for this finding, there are three points to consider in relation to the results of our study. First, when antibodies such as HIP1, that slightly inhibit colony formation and strongly inhibit proplatelet formation, are present in the patients’ plasma, severe thrombocytopenia may develop. Second, when antibodies such as 5B12, that only inhibit proplatelet formation, or antibodies such as 23C6, that only slightly inhibit colony size, are present in the patients’ plasma, moderate thrombocytopenia may develop. Finally, when antibodies such as Y2/51, that do not inhibit colony formation or proplatelet formation, are present in the patients’ plasma, slight thrombocytopenia may develop. However, these hypotheses are somewhat inconsistent with previous investigators’ reports, because the proliferation of megakaryocytes is not depressed by antiplatelet antibodies in patients with ITP. In fact, the number of megakaryocytes in the bone marrow of ITP patients does not decrease,1,2 and the number of megakaryocyte colony-forming cells are increased by the injection of platelet antiserum, in vivo.14 15 Therefore, these in vivo studies indicate that the inhibition of colony formation is unlikely to be the leading cause of thrombocytopenia.
As for the platelet production in patients with ITP, there are differences of opinion among specialists. Branehog et al32,33 and Harker et al34 reported platelet production (platelet turnover) to be increased in ITP patients. According to Harker et al, the total megakaryocyte mass increases 2 to 8 times from normal, indicating that thrombopoiesis is effective in ITP.34 In contrast, more recent studies of autologous platelet turnover have shown that platelet production is not increased in all ITP patients. Ballem et al35 have reported that 30% of ITP patients show decreased platelet production, 43% have production rates within the normal range, and 27% have increased platelet production. Similarly, Tomer et al36 have shown that the platelet turnover is less than normal in some ITP patients. Stoll et al37 have shown that the rate of platelet production is not increased in most patients with moderate ITP. Thus, the role of platelet production in the pathogenesis of ITP is controversial. We estimate that if proplatelet formation is inhibited by antiplatelet antibodies, in vivo, then platelet production may be depressed. In particular, the reports by Ballem et al35 and Tomer et al36 are not inconsistent with our data on inhibited proplatelet formation and may explain depressed platelet production in patients with ITP. In other words, the cause of thrombocytopenia may be a result of the inhibition of proplatelet formation of megakaryocytes by the antiplatelet antibodies, as well as of the destruction of the platelets in the reticuloendothelial system. It is necessary, however, that more studies be performed using various approaches, because the inhibition of proplatelet formation in patients with ITP is poorly understood.
The results of this study suggest that the degree of thrombocytopenia may be related to the epitope recognized by the patients’ antibodies, because the antibodies we used were MoAbs. Nagasawa et al38reported that the proliferation of CFU-Meg is suppressed by an anti-GP-IIb/-IIIa MoAb (TM83), whereas the effect of an anti-GP-Ib MoAb (TM60) was negligible. Handagama et al39 made a heterologous antiplatelet antibody and reported that rat megakaryocyte proplatelet formation is inhibited by the antibody. However, which epitope was recognized by the antibody is unknown, because the antibody used was polyclonal. The effects on guinea pig megakaryocyte proplatelet formation by antiplatelet MoAbs have been studied previously, and an anti-integrin αvβ3 MoAb (LM609), an anti-integrin αv MoAb (LM142), and an anti-GP-IIb/-IIIa MoAb (PG2) each inhibited proplatelet formation.40,41 We suspect that the differences between the results of our study and those of other studies are a result of the antibodies used, because they recognize different epitopes on the same GP.42-44 Unlike previous studies, we used the same MoAb to examine both colony formation and proplatelet formation. Thus, we were able to compare inhibition of colony formation and the inhibition of proplatelet formation.
In conclusion, our in vitro study has shown that both human megakaryocyte colony formation and proplatelet formation are inhibited by an anti-GP-Ibα antibody, suggesting that GP-Ibα plays an important role in megakaryocytopoiesis. Several investigators have reported that thrombocytopenia is more severe in patients with anti-GP-Ib autoantibodies, whereas ITP patients with anti-GP-IIb/-IIIa autoantibodies do not develop severe thrombocytopenia.45,46Moreover, Hasegawa et al47 reported that antiplatelet GP-Ib antibody may impair platelet production by megakaryocytes in ITP. These clinical reports strongly support our experimental findings. However, the inhibition of colony formation in vitro did not agree with in vivo studies because the number of megakaryocytes in the bone marrow of ITP patients did not decrease. Also, the megakaryocyte colony-forming cells increased when platelet antiserum was injected. From the above mentioned facts and discussion, the inhibition of proplatelet formation by the antiplatelet antibodies may be at the basis of thrombocytopenia in patients with ITP, in which depressed platelet production35 36 occurs. Our present findings suggest that autoantibodies against different platelet GPs in patients with ITP may have differing effects on proplatelet formation. This subsequently results in the development of different degrees of thrombocytopenia. Further studies are needed to obtain in vitro evidence that proplatelet formation is inhibited by antiplatelet serum, and the antiserum must be analyzed.
ACKNOWLEDGMENT
The authors thank Dr D. B. Douglas for comments on the manuscript. The authors appreciate technical assistance in ELISA from Mr H. Miyazawa.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ryo Takahashi, Department of Clinical Hematology, Kyorin University School of Health Sciences, 476 Miyashita, Hachioji, Tokyo 192-8508 Japan; e-mail: ryo@technologist.com.