Although spontaneous remissions may rarely occur in B-cell chronic lymphocytic leukemia (B-CLL), T cells do generally not develop a clinically significant response against B-CLL cells. Because this T-cell anergy against B-CLL cells may be caused by the inability of B-CLL cells to present tumor-antigens efficiently, we examined the possibility of upregulating critical costimulatory (B7-1 and B7-2) and adhesion molecules (ICAM-1 and LFA-3) on B-CLL cells to improve antigen presentation. The stimulation of B-CLL cells via CD40 by culture on CD40L expressing feeder cells induced a strong upregulation of costimulatory and adhesion molecules and turned the B-CLL cells into efficient antigen-presenting cells (APCs). CD40-activated B-CLL (CD40-CLL) cells stimulated the proliferation of both CD4+ and CD8+ T cells. Interestingly, stimulation of allogeneic versus autologous T cells resulted in the expansion of different effector populations. Allogeneic CD40-CLL cells allowed for the expansion of specific CD8+cytolytic T cells (CTL). In marked contrast, autologous CD40-CLL cells did not induce a relevant CTL response, but rather stimulated a CD4+, Th1-like T-cell population that expressed high levels of CD40L and released interferon-γ in response to stimulation by CD40-CLL cells. Together, these results support the view that CD40 activation of B-CLL cells might reverse T-cell anergy against the neoplastic cell clone, although the character of the immune response depends on the major histocompatibility complex (MHC) background on which the CLL or tumor antigens are presented. These findings may have important implications for the design of cellular immunotherapies for B-CLL.
CHRONIC LYMPHOCYTIC leukemia of B-cell origin (B-CLL) is the most common type of leukemia in the western hemisphere. Despite a continued effort to improve the outcome of B-CLL by new chemotherapeutic agents, the disease remains uncurable. Therefore, it seems rewarding to evaluate alternative treatment options such as immunotherapy.
Although some cases of spontaneous remission in CLL have been reported,1 B-CLL cells generally fail to induce a clinically relevant immune response. Whereas the clinical appearance of B-CLL often remains stable for years, the total tumor cell burden tends to expand at variable speed without any apparent reaction of the immune system against the tumor. This may be caused by an impaired T-cell–mediated immune response,2 including a depressed function of natural killer (NK) cells and antibody-mediated cellular cytoxicity (ADCC)3,4; a reduced susceptibility of the leukemic cells towards the effector cells5; or an inability of the neoplastic cells to function efficiently as antigen-presenting cells (APCs), similar to other lymphoid malignancies.6 7
In the specific case of B-CLL, the malignant cells are the neoplastic counterpart of a subpopulation of CD5+ B cells8,9 that might function as professional APCs and effectively present endogenous tumor antigens to T cells. Moreover, the B-CLL specific idiotype provides a unique tumor antigen that might be recognized by the immune system, similar to other lymphoid malignancies in which this strategy has been tested successfully in clinical trials.10-12 However, despite their strong expression of major histocompatibility complex class I (MHC I) and class II (MHC II) molecules, B-CLL cells are generally ineffective stimulator cells in mixed lymphocyte reactions.13
It has been demonstrated that expression of MHC molecules is not sufficient for the induction of a productive immune response and that adequate expression of adhesion and costimulatory molecules is critical to stimulate a potent T-cell response. Failure to receive these signals renders potential effector T cells anerg or tolerant.14 Among the costimulatory molecules, the B7 family appears to be unique, because it has been demonstrated that B7-1 (CD80) and B7-2 (CD86) are both necessary and sufficient to prevent the induction of anergy.15-17 In normal and malignant B cells, activation of CD40 seems to be a major stimulus for the induction of B7-1 and B7-2.7 18
In this study we sought to determine whether B-CLL cells could be turned into efficient APCs and whether this could reverse T-cell anergy against the neoplastic cell clone. Stimulation of CD40 on B-CLL cells in vitro induced a strong upregulation of adhesion and costimulatory molecules. Repeated stimulation of allogeneic versus autologous T cells with CD40-CLL cells resulted in different effector populations. Allogeneic CD40-CLL cells activated CD8+, cytolytic T cells with activity against both native B-CLL and CD40-CLL cells. In marked contrast, autologous CD40-CLL activated predominantly CD4+T cells that had no major cytolytic activity. The results suggest that cellular vaccination studies in B-CLL may use at least two different strategies that depend on the source of T cells: when using allogeneic, peripheral blood T cells from healthy donors, CD40-CLL cells are very potent in expanding CD8+, cytolytic T cells in vitro; this potential might be used for adoptive immune transfer studies. In contrast, autologous T cells respond to CD40-CLL cells primarily by an expansion of CD4+ Th1-like T cells; this might be exploited and further studied in a trial in which CD40-CLL cells are directly applied to the patient.
MATERIALS AND METHODS
Patients.
After informed consent, peripheral blood samples were obtained from patients with B-CLL. The diagnosis of B-CLL was based on standard clinical and laboratory criteria.19 The study included 12 patients (4 women and 8 men; 49 to 82 years of age). Staging was performed according to the Binet classification.20Characteristics of the patients studied are summarized in Table 1.
HeLa/SF cells transfected with CD40L cDNA.
The CD40L coding region was amplified as previously described.21 Briefly, RNA was isolated from activated human T cells. Reverse transcription was followed by a two-step polymerase chain reaction (PCR). The first-step PCR was performed using sense primers coding for the first 20 nucleotides of the CD40L coding sequence and antisense primers coding for the last 23 nucleotides of the CD40L coding sequence. In a second step, the amplified PCR products reamplified using extended primers. The sense primer (5′-GTA GGA ATT CGT CGA CGC CGC CAC CAT GAT CGA AAC ATA CAA CC-3′) containsEcoRI and Sal I sites, a strong translational start site, and the first 20 nucleotides of the CD40L coding sequence. The antisense primer (5′-GAC TAG TGT CGA CGA ATT CAG AGT TTG AGT AAG CCA AAG-3′) contains the last 23 nucleotides of the CD40L coding sequence, including the stop codon and EcoRI, Sal I, and Spe I sites. For each step, 20 cycles were performed (95°C for 1 minute, 48°C for 30 seconds, and 72°C for 1 minute), followed by one cycle at 72°C for 10 minutes. The 0.8-kb PCR product was digested with EcoRI, gel-purified, and ligated into EcoRI-digested pcDNA3.1 vector (purchased from Invitrogen, NV Leek, The Netherlands). Plasmids containing the CD40L insert were transfected via electroporation in HeLa/SF cells. Transfectants were selected by growth in 250 μg/mL G418 and further subcloned. Biologic activity was determined by costimulation of B-cell proliferation and differentiation.
Isolation of B-CLL cells.
Mononuclear cells (MNCs) from peripheral blood samples were isolated by centrifugation on a Ficoll/Hypaque (Seromed, Berlin, Germany) density gradient and depleted from monocytes by overnight adherence to plastic tissue culture flasks. Subsequently, the nonadherent lymphocytes were cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide (DMSO; Sigma, München, Germany). As assessed by flow cytometry, greater than 95% of these cells typically coexpressed CD19 and CD5 surface molecules.
B-CLL cell culture.
For CD40L-induced activation, freshly isolated B-CLL cells were cultured as previously described.14 Briefly, CD40L or mock-transfected NIH3T3 fibroblasts or HeLa/SF cells were γ-irradiated at 200 Gy, plated at 5 × 105cells/well in 6-well plates in media without G418, and incubated overnight at 37°C in a 5% CO2 humidified atmosphere. Before addition of the B-CLL cells, the feeder layers were washed twice with phosphate-buffered saline, and tumor cells were cultured at 2 × 106 cells/mL in Iscove’s medium (Seromed) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin. For further studies, culture was performed in presence or absence of interleukin-2 (IL-2; 20 IU/mL), IL-4 (1 IU/mL), and interferon γ (IFNγ; 20 IU/mL). For performing functional studies, tumor cells were harvested after 24, 72, and 120 hours of culture; purified by ficoll density gradient centrifugation; washed; and analyzed by flow cytometry. For T-cell restimulation, the activated B-CLL cells (CD40-CLL) were aliquoted and stored in liquid nitrogen. The CD40L-transfected NIH3T3 cell line was a generous gift from Dr J. Schultze (Dana-Farber Cancer Insitute, Boston, MA). With respect to their stimulatory capacity, no differences between CD40L-transfected NIH3T3 fibroblasts and CD40L-transfected HeLa/SF cells were detected; therefore, both feeder cell lines are referred to as t-CD40L throughout the manuscript.
Cytokines and cytokine measurements.
Recombinant human IL-2 (rhIL-2), rhIL-4, and rhIFNγ were obtained from Boehringer Mannheim (Mannheim, Germany) and used as indicated. Cytokine measurements were performed using commercial IL-4 and IFNγ enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Wiesbaden-Nordenstadt, Germany) according to the manufacturer’s instructions. The detection limits of the assays were 5 to 2,000 pg/mL.
Immunophenotyping.
Immunophenotyping was performed with the following monoclonal antibodies (MoAbs) conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or phycoerythrin cyanine 5 (PE-Cy5): CD3, CD4, CD5, CD8, CD19, CD20, CD23, CD25, CD28, CD54, CD56, CD58, CD69, CD95, HLA-ABC, HLA-DR, anti-κ, anti-λ (Coulter/Immunotech, Hamburg, Germany), CD40, CD40L, CD80, CD86, and CD95L (PharMingen, Hamburg, Germany). Fluorescence was measured with a Coulter Epics XL-MCL (Coulter Electronics, Miami, FL).
Purification of T cells.
Purification of T cells was performed by negative selection. Briefly, the monocyte-deprived MNCs from healthy donors or B-CLL patients were stained with a cocktail of antibodies (CD11b, CD16, CD19, CD36, and CD56), labeled with goat anti-rat IgG microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), and selected using magnetic separation columns (Miltenyi Biotec). Isolated cells were greater than 95% pure as determined by immunofluorescent flow cytometry analysis (FACS) and appeared viable by exclusion of trypan blue and forward/side scatter analysis.
Generation of effector T cells.
Purified T cells from healthy donors or B-CLL patients were stimulated with γ-irradiated (75 Gy) CD40-CLL cells or with native B-CLL cells at different effector to target (E:T) ratios ranging from 10:1 to 5:1. Stimulation was performed on days 0, 7, 14, and 21. Briefly, T cells were harvested weekly, washed, and recultured at a concentration of 1 × 106 cell/mL with γ-irradiated, autologous, or allogeneic stimulator cells in Iscove’s medium (Seromed) supplemented with 5% human heat-inactivated AB-serum (Serva, Heidelberg, Germany), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. The addition of IL-2 (20 IU/mL) was perfomed 48 hours after (re)stimulation.
Mixed lymphocyte reaction (MLR).
Irradiated (75 Gy) B-CLL cells and CD40-CLL cells were used as stimulators, cocultured at 1 × 104 cells/well in a final volume of 200 μL with allogeneic T cells at 1 × 105 cells/well in 96-well round-bottom plates, and incubated for 3 days at 37°C in a 5% CO2 humidified atmosphere. The culture medium used was Iscove (Seromed) supplemented with 5% heat-inactivated human AB-serum (Serva), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. All microcultures were performed in triplicate. During the last 12 hours of the 72-hour culture period, cells were pulsed with 0.5 μCi [3H] thymidine (Amersham, Braunschweig, Germany). Cells were harvested onto glass fiber filters and dried, and the [3H] thymidine incorporation was measured by scintillation spectrophotometry in a Wallac Microbeta Plus 1450 scintillation counter (Turku, Finland). The stimulation indexes (SI) were calculated for each individual experiment as follows: SI = cpm(T cells + B-CLL cells)/cpm(T cells).
Cytotoxicity assays.
T-cell–mediated cytotoxicity was determined using a standard 4-hour [51Cr] release assay.22 Unstimulated and CD40-stimulated B-CLL cells as well as NK-sensitive K562 cells were used as targets. Target cells were labeled with 100 μCi of [51Cr] (Na51CrO2; Dupont, Bad Homburg, Germany) per 106 cells for 2 hours at 37°C in a water bath. Thereafter, the cells were washed three times in complete medium and seeded in v-bottomed microtiter plates at a concentration of 2.5 × 103 cells/well. Indicated numbers of effector cells were added in triplicate in 200 μL of complete medium. Supernatants were collected, and the released [51Cr] was measured in a γ-counter (LKB-Wallac 1282, Uppsala, Sweden). Spontaneous release was determined by incubation of target cells in medium alone, and maximum release was determined by resuspending the wells with 10% Triton X-100. Specific lysis was determined for each individual experiment as follows: specific lysis (%) = [(experimental [51Cr] release − spontaneous [51Cr] release)/(maximum [51Cr] release − spontaneous [51Cr] release)] × 100.
Statistical analysis.
Differences between experimental groups were analyzed using the χ2 test and the Student’s t-test.
RESULTS
B-CLL cells lack costimulatory molecules.
B7 costimulatory molecules play a crucial role in the induction of a T-cell–mediated immune response. Lack to receive costimulatory signals after antigen presentation renders T cells anergic or tolerant.23 24 This may be a mechanism by which CLL cells escape the immune response. Therefore, we determined the cell surface expression of MHC, adhesion, and costimulatory molecules by immunophenotyping of freshly isolated B-CLL cells obtained from 12 patients. The results are summarized in Table 2. The expression level of the surface molecules on B-CLL cells was classified according to their mean fluorescence intensity (MFI). All patients tested expressed high (MFI 1.5 to 2.5 logarithm) or even very high (MFI >2.5 logarithm) levels of MHC class I and II molecules. The adhesion molecules ICAM-1 (CD54) were undetectable in 3 of 12 patients, were expressed at intermediate (MFI 0.5 to 1.5 logarithm) in 8 of 12 patients, and were expressed at high levels in 1 of 12 patients. LFA-3 (CD58) was undetectable in 3 of 12 patients and was expressed at low to intermediate levels in 9 of 12 patients. The costimulatory molecule B7-1 (CD80) was not detectable in 8 of 12 patients or showed only low expression (MFI >0.2 to 0.5 logarithm) in 4 of 12 patients. B7-2 (CD86) was not expressed in 2 of 12 patients, whereas 10 of 12 patients expressed it at low to intermediate levels. CD40 was detectable in all B-CLLs (12/12) at low or intermediate levels. Together, these experiments indicated that B-CLL cells showed a markedly reduced expression of costimulatory molecules, especially of B7-1 (CD80).
Stimulation of B-CLL cells by t-CD40L in the presence of IL-4 efficiently upregulates costimulatory molecules.
In the next step, we stimulated freshly isolated B-CLL cells by t-CD40L and mock-transfected feeder cells in the presence of IL-4 (1 IU/mL; see Materials and Methods). Stimulation by t-CD40L induced a cluster formation of B-CLL cells caused by adhesion to the stimulator cells and a hairy-cell like morphology in some of the B-CLL cells (Fig 1A and B). No aggregation or morphologic changes were observed by stimulation with mock controls. May-Grünwald-Giemsa staining of cytospin smears showed that the size of CD40-CLL cells increased due to an expansion of both nuclei and cytoplasm. Moreover, CD40-CLL showed a relatively high degree of vacuolization, corresponding to an activated state of B-lymphoid cells (Fig 1C). No plasmacytoid differentiation was seen.
(A through C) Morphology of t-CD40L–stimulated CLL (CD40-CLL) cells after 3 days of culture. (A and B) Typical cluster formation of CD40-CLL cells that surround the CD40L-transfected NIH 3T3 fibroblasts (original magnifications: [A] × 100; [B] × 650). (C) CD40-CLL cells stained by May-Grünwald-Giemsa (original magnification × 650).
(A through C) Morphology of t-CD40L–stimulated CLL (CD40-CLL) cells after 3 days of culture. (A and B) Typical cluster formation of CD40-CLL cells that surround the CD40L-transfected NIH 3T3 fibroblasts (original magnifications: [A] × 100; [B] × 650). (C) CD40-CLL cells stained by May-Grünwald-Giemsa (original magnification × 650).
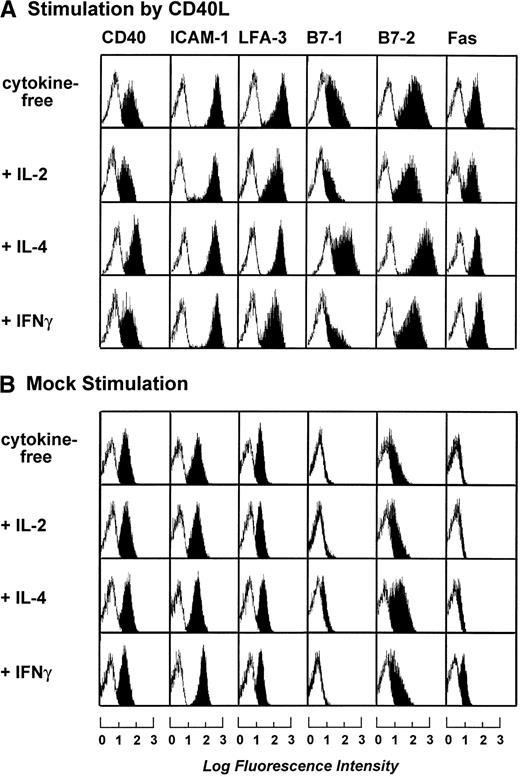
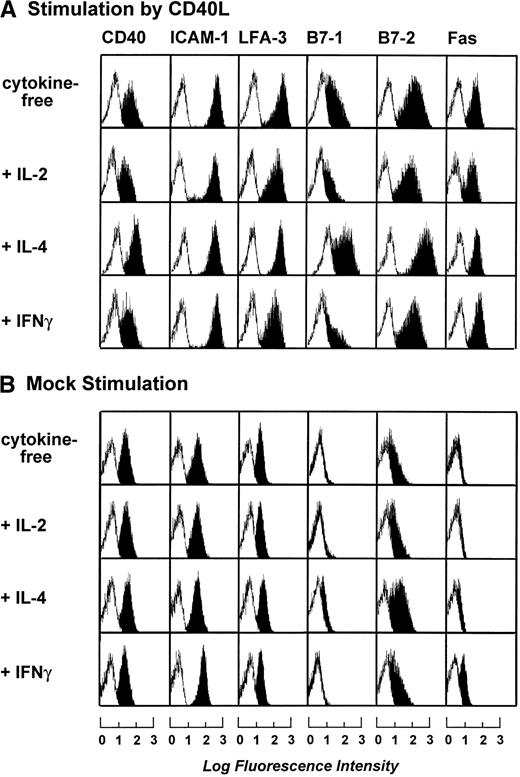
To optimize the cytokine cocktail used for stimulation of B-CLL cells, B-CLL cells were stimulated by t-CD40L or mock-transfected feeder cells for 3 days in the presence of various cytokines such as IL-2 (20 IU/mL), IL-4 (1 IU/mL), and IFNγ (20 IU/mL). Figure 2A and B shows that maximal expression of B7-1 and B7-2 was induced by t-CD40L when combined with IL-4. IL-4 alone or in combination with mock stimulation only mediated a slight increase of B7-2 expression on the B-CLL cell surface (data not shown). Stimulation by t-CD40L in combination with IL-2 and IFNγ even reduced the expression of B7-1 when compared with stimulation without cytokines. The addition of IFNγ induced only an upregulation of Fas (CD95) but had no other effects (data not shown). Taken together, the combination of t-CD40L and IL-4 seemed optimal for enhancing the expression of important costimulatory molecules on B-CLL cells and was therefore used in all subsequent experiments.
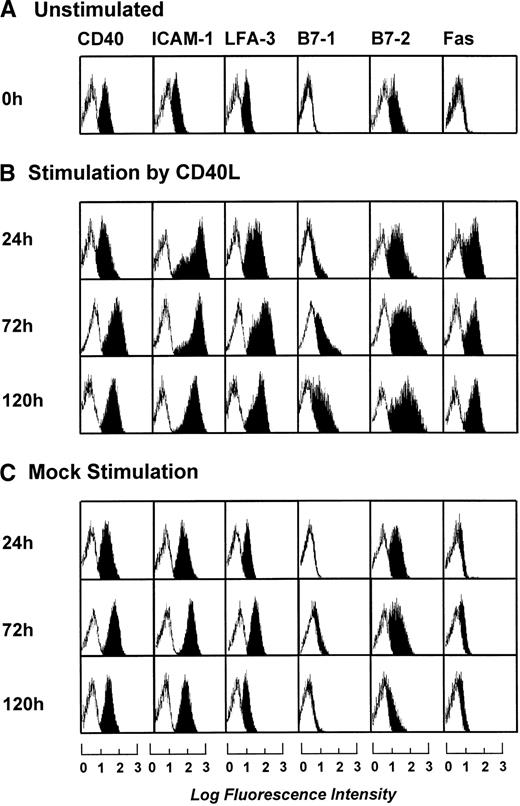
Phenotypic characterization of B-CLL cells after 3 days of stimulation by CD40L or mock-transfected NIH3T3 fibroblasts in the presence of IL-2 (20 IU/mL), IL-4 (1 IU/mL), and IFNγ (20 IU/mL). Blank areas represent the isotype-matched control antibodies and the solid areas represent the fluorescence distribution of the MoAbs tested as assessed by flow cytometric analysis. The results shown are from one experiment and are representative of three independent experiments.
Phenotypic characterization of B-CLL cells after 3 days of stimulation by CD40L or mock-transfected NIH3T3 fibroblasts in the presence of IL-2 (20 IU/mL), IL-4 (1 IU/mL), and IFNγ (20 IU/mL). Blank areas represent the isotype-matched control antibodies and the solid areas represent the fluorescence distribution of the MoAbs tested as assessed by flow cytometric analysis. The results shown are from one experiment and are representative of three independent experiments.
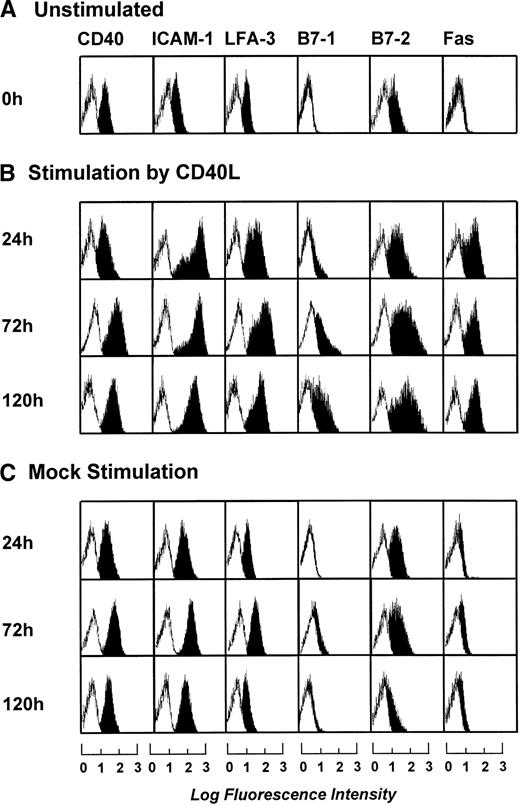
To determine the optimal length of t-CD40L stimulation for full activation of B-CLL cells, time course experiments were performed. B-CLL cells were stimulated with t-CD40L for 24, 72, and 120 hours. As soon as 24 hours after stimulation by t-CD40L, a significant upregulation of ICAM-1 (CD54), LFA-3 (CD58), and Fas (CD95) was detectable (Fig 3B). Expression of costimulatory molecules B7-1 and B7-2 reached its maximum on day 3 of t-CD40L stimulation. Mock stimulation (Fig 3C) in the presence of IL-4 (1 IU/mL) caused a slight increase of the adhesion molecules ICAM-1 and LFA-3, as well as of the costimulatory molecule B7-2. No upregulation was detected for the costimulatory molecules B7-1 and Fas (CD95). Table 3 summarizes the data obtained with leukemic cells from 12 patients after a 3-day t-CD40L stimulation in the presence of IL-4 (1 IU/mL). In all cases, CD40-CLL cells showed an increased expression of B7-1 and B7-2 (Table 3). CD40 expression was further increased in 8 cases. ICAM-1 and LFA-3 were also upregulated in most cases, except if they were already expressed at intermediate levels before t-CD40L stimulation (ICAM-1: patients no. 6, 7, and 10; LFA-3: patients no. 7 through 10). Expression of both MHC class I and II molecules was further increased to high or very high levels in those cases in which the expression was lower before CD40 stimulation.
Phenotypic characterization of unstimulated B-CLL cells and B-CLL cells stimulated in the presence of IL-4 (1 ng/mL) for 24, 72, and 120 hours by either CD40L-transfected or mock-transfected NIH3T3 fibroblasts. The results shown are from one experiment and are representative of three independent experiments.
Phenotypic characterization of unstimulated B-CLL cells and B-CLL cells stimulated in the presence of IL-4 (1 ng/mL) for 24, 72, and 120 hours by either CD40L-transfected or mock-transfected NIH3T3 fibroblasts. The results shown are from one experiment and are representative of three independent experiments.
CD40L-activated B-CLL cells retain the immunophenotypic characteristics of neoplastic cells.
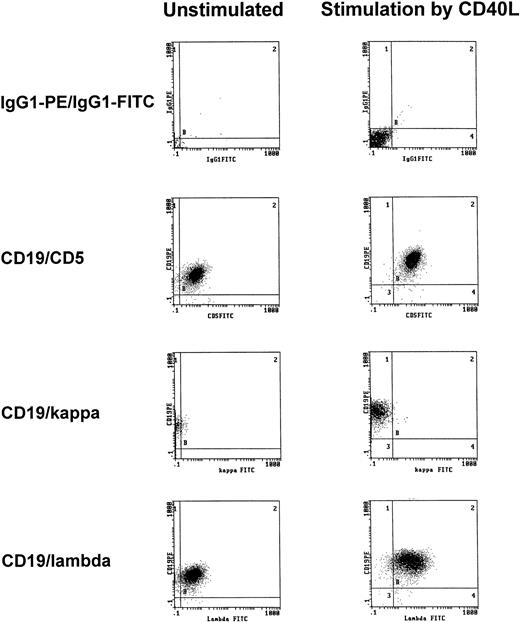
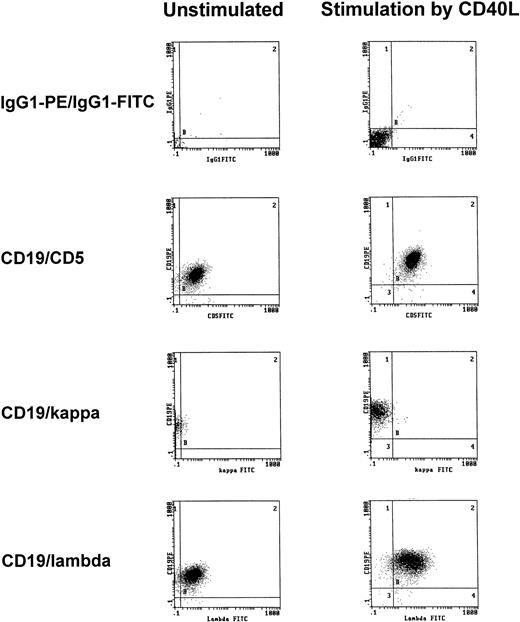
To exclude that treatment with t-CD40L induced the expansion of normal rather than neoplastic B cells, immunophenotypic analyses of light chain restriction and CD5 expression were performed on CD40-CLL cells. As shown in one representative experiment, CD40-CLL cells showed the phenotype of B-CLL cells as demonstrated by coexpression of CD5 and CD19 and by light chain restriction (Fig4).
Representative immunophenotypic characterization of B-CLL cells before and after 3 days of CD40-stimulation in the presence of IL-4 (1 IU/mL) as determined by CD5/CD19 positivity and light chain restriction (patient no. 3). The results shown are from one experiment and are representative of three independent experiments.
Representative immunophenotypic characterization of B-CLL cells before and after 3 days of CD40-stimulation in the presence of IL-4 (1 IU/mL) as determined by CD5/CD19 positivity and light chain restriction (patient no. 3). The results shown are from one experiment and are representative of three independent experiments.
CD40-CLL cells induce a proliferative T-cell response.
We then investigated whether CD40-CLL cells provided a proliferative stimulus to T cells. For this purpose, we used highly (>95%) purified allogeneic T cells and incubated them in the presence or absence of IL-2 (20 IU/mL) with γ-irradiated (75 Gy) native CLL and CD40-CLL cells for 72 hours. CD40-CLL cells were prepared by prestimulation with t-CD40L for 24, 72, and 120 hours. T-cell proliferation was assessed by incorporation of [3H]thymidine added during the last 12 hours of the experiment. The highest stimulatory capacity of the CD40-CLL cells was achieved on day 3 of t-CD40L prestimulation (Fig 5). The experiments shown are representative for 3 different allogeneic T-cell donors and different cases of B-CLL examined.
Proliferative responses of purified allogeneic CD3+ T cells in the absence of IL-2 to γ-irradiated B-CLL cells (patient no. 12) either unstimulated or stimulated for 24, 72, and 120 hours by CD40L-transfected NIH3T3 fibroblasts. [3H] Thymidine incorporation was assessed for the last 12 hours of a 3-day culture. Appropriate controls (CD3+ T cells and B-CLL cells) were always less than 1,500 cpm. The stimulation index was calculated as cpm(T cells + B-CLL cells)/cpm(T cells). Results are representative for three independent experiments and are expressed as the mean ± SD of the stimulation index.
Proliferative responses of purified allogeneic CD3+ T cells in the absence of IL-2 to γ-irradiated B-CLL cells (patient no. 12) either unstimulated or stimulated for 24, 72, and 120 hours by CD40L-transfected NIH3T3 fibroblasts. [3H] Thymidine incorporation was assessed for the last 12 hours of a 3-day culture. Appropriate controls (CD3+ T cells and B-CLL cells) were always less than 1,500 cpm. The stimulation index was calculated as cpm(T cells + B-CLL cells)/cpm(T cells). Results are representative for three independent experiments and are expressed as the mean ± SD of the stimulation index.
In another set of experiments, we analyzed the proliferative response of highly purified allogeneic CD4+ and CD8+ T cells. For this purpose, we used γ-irradiated (75 Gy) native CLL and CD40-CLL cells (day 3) and incubated them for 72 hours with different ratios of highly (>95%) purified CD4+ and CD8+ T-cell subpopulations as well as unpurified peripheral blood mononuclear cells (PBMCs). T-cell proliferation was assessed by [3H]thymidine incorporation during the last 12 hours of the experiment. A representative experiment is shown in Fig 6. CD40-CLL cells but not native CLL cells induced a significant T-cell proliferation, regardless of whether unpurified PBMCs or CD4+ or CD8+ T cells were used. Moreover, CD4+ T cells showed a significant stronger proliferative response than CD8+ T cells. The strongest response was seen with PBMCs; this result is readily explained by the presence of some additional immune effector cells (eg, NK cells) or APCs (eg, dendritic cells) in the unpurified PBMC fraction.
Proliferative response of purified allogeneic CD4+ and/or CD8+ T cells as well as PBMCs not further purified to γ-irradiated native and CD40-stimulated B-CLL cells. Different CD4/CD8 ratios were tested. [3H] Thymidine incorporation was assessed for the last 12 hours of a 3-day culture and determined in cpm ± SD of triplicate determinations. The results shown are from one experiment and are representative for three independent experiments.
Proliferative response of purified allogeneic CD4+ and/or CD8+ T cells as well as PBMCs not further purified to γ-irradiated native and CD40-stimulated B-CLL cells. Different CD4/CD8 ratios were tested. [3H] Thymidine incorporation was assessed for the last 12 hours of a 3-day culture and determined in cpm ± SD of triplicate determinations. The results shown are from one experiment and are representative for three independent experiments.
Different effector cells are induced by subsequent stimulation of allogeneic versus autologous T cells with CD40-CLL cells.
In the next step we determined whether CD40-CLL cells could be used to stimulate T-cell differentiation and expansion. For this purpose, we used highly purified allogeneic and autologous T cells and stimulated them weekly (days 0, 7, 14, and 28) with γ-irradiated (75 Gy) native and CD40-CLL cells at a ratio of 5:1. In both settings, it was possible to expand large numbers of T cells in the presence of CD40-activated B-CLLs and exogenous IL-2 (20 IU/mL). In contrast, the expansion was not possible with native B-CLL cells, even in the presence of IL-2.
When T cells were monitored by flow cytometric analysis, we found that only CD40-activated B-CLLs were able to induce activation markers (CD25 and CD95). A marked difference was seen with regard to the CD4/CD8 ratio. In the allogeneic setting, consecutive stimulations with CD40-CLL cells caused a relative and absolute increase of CD8+ T cells (up to 50% after 3 restimulations) in 4 of 5 cases investigated. In marked contrast, repetitive stimulations in the autologous setting caused an expansion of CD4+ T cells (up to 90% after 3 restimulations) in 6 of 6 cases studied. No increase of CD16+/CD56+ NK cells was observed.
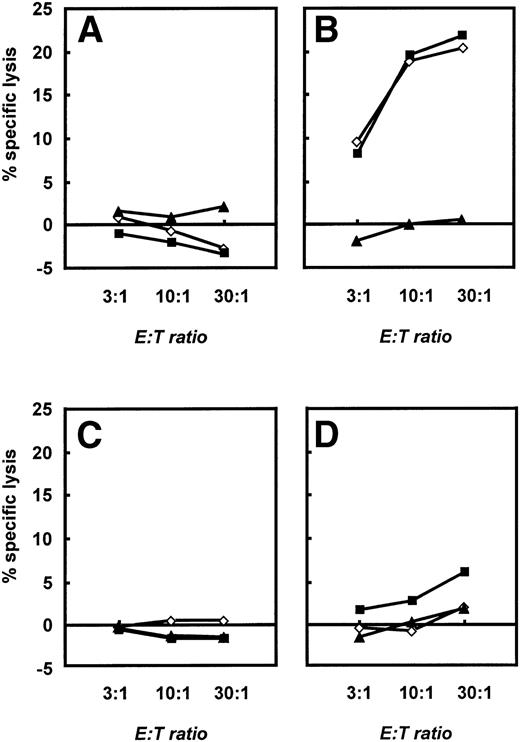
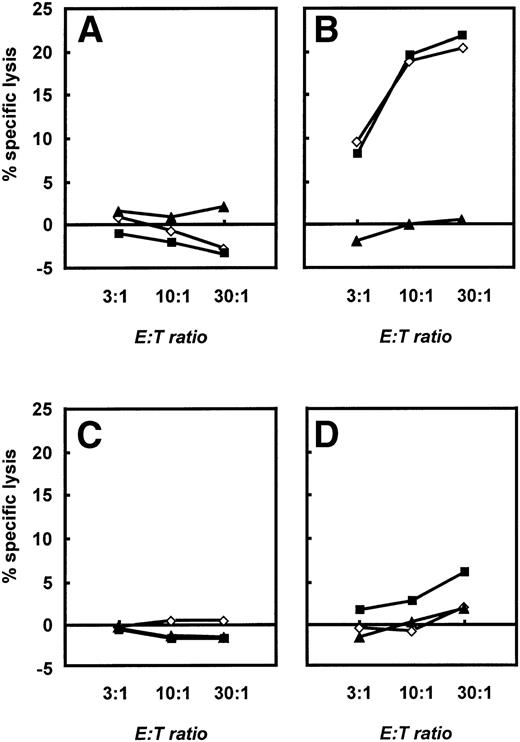
To further characterize the different effector functions of allogeneic versus autologous T cells, we performed 4-hour standard chromium release tests (see Materials and Methods). Native B-CLL cells, CD40-CLL cells, and NK-sensitive K562 cells were used as targets. These different effector cells were expanded until day 28 and then restimulated with CD40-CLL cells and native CLL cells in the presence of IL-2. A significant, cytolytic activity of T cells was only seen if allogeneic T cells from healthy donors were used (Fig 7B). At this time point, allogeneic T cells lysed both native B-CLL cells and CD40-CLL cells. No response against K562 cells was detected. Furthermore, no T-cell response was observed with T cells before stimulation with CD40-CLL (Fig 7A). In marked contrast, autologous T cells expanded by repetitive stimulation with CD40-CLL cells showed only a slight lytic activity against CD40-CLL cells and no lytic activity against native B-CLL cells or the NK-sensitive K562 cell line (Fig 7D). Taken together, the stimulation of allogeneic versus autologous T cells by CD40-CLL cells induced a fundamentally different response in that CD8+ cells with cytolytic activity could only be expanded in the allogeneic system.
Cytolytic response of unstimulated and activated allogeneic and autologous T cells as assessed in a standard 4-hour chromium release assay. A total of 2.5 × 103 B-CLL cells (◊), CD40-CLL cells (▪), and NK-sensitive K562 cells (▴) were placed in 96-well v-bottom plates and T cells were added at E:T ratios of 3:1, 10:1, and 30:1 in a final volume of 200 μL. Cytolytic response was expressed as the percentage specific lysis. (A) Cytolytic response of unstimulated T cells and (B) cytolytic response of T cells restimulated three times with CD40-CLL cells in an allogeneic setting. (C) Cytolytic response of unstimulated T cells and (D) cytolytic response of T cells restimulated three times with CD40-CLL cells in an autologous setting (patient no. 12). The results are representative for three independent experiments in the allogeneic system and three independent experiments in the autologous system.
Cytolytic response of unstimulated and activated allogeneic and autologous T cells as assessed in a standard 4-hour chromium release assay. A total of 2.5 × 103 B-CLL cells (◊), CD40-CLL cells (▪), and NK-sensitive K562 cells (▴) were placed in 96-well v-bottom plates and T cells were added at E:T ratios of 3:1, 10:1, and 30:1 in a final volume of 200 μL. Cytolytic response was expressed as the percentage specific lysis. (A) Cytolytic response of unstimulated T cells and (B) cytolytic response of T cells restimulated three times with CD40-CLL cells in an allogeneic setting. (C) Cytolytic response of unstimulated T cells and (D) cytolytic response of T cells restimulated three times with CD40-CLL cells in an autologous setting (patient no. 12). The results are representative for three independent experiments in the allogeneic system and three independent experiments in the autologous system.
CD40-CLL activated autologous CD4+ T cells show a Th1-type cytokine pattern.
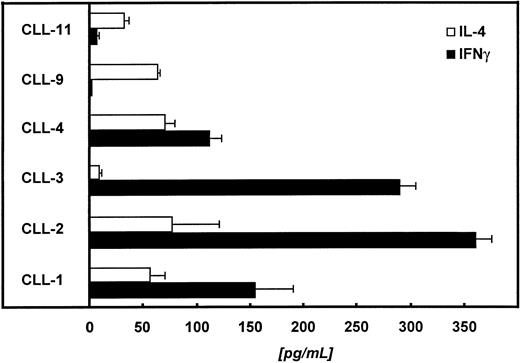
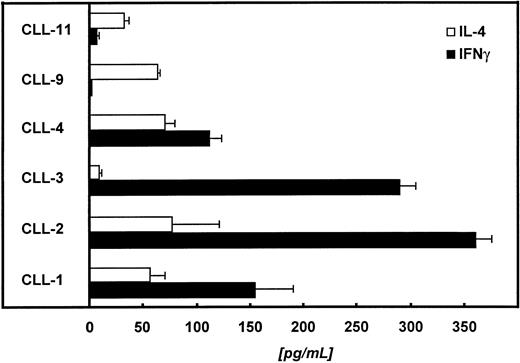
In the next step we tried to characterize the autologous, CD4+ T cells with respect to their cytokine release pattern. For this purpose, we restimulated 1 × 105autologous T cells with 2 × 104paraformaldehyde-fixed (1%) CD40-CLL cells. The supernatant was collected 48 hours later and analyzed for IL-4 and IFNγ. Figure 8 summarizes the data of 6 patients. In 4 of 6 patients tested, we detected predominantly IFNγ, suggesting a Th1-like immune response.
Autologous T cells of 6 different patients were challenged with CD40-CLL cells at an E:T ratio of 5:1. Supernatants were collected 48 hours later and IL-4 and IFNγ were measured by ELISA (detection limit of the IL-4 assay is <5 pg/mL; detection limit of the IFNγ assay is <5 pg/mL).
Autologous T cells of 6 different patients were challenged with CD40-CLL cells at an E:T ratio of 5:1. Supernatants were collected 48 hours later and IL-4 and IFNγ were measured by ELISA (detection limit of the IL-4 assay is <5 pg/mL; detection limit of the IFNγ assay is <5 pg/mL).
DISCUSSION
This report investigates the potential of CD40L-stimulated CLL (CD40-CLL) cells to activate effector T cells for tumor vaccination. The essential finding of this report is that the quality of the autologous T-cell response against CD40-CLL cells differs dramatically from the allogeneic T-cell response against these cells. So far, it was known that CD40-ligation could be used in B-CLL cells to upregulate adhesion and costimulatory molecules.25-27 Our study confirmed these findings by showing that stimulation of B-CLL cells by t-CD40L resulted in a significant upregulation of both adhesion and costimulatory molecules in B-CLL cells in a time-dependent manner, regardless of previous treatment with cytostatic drugs. CD40-CLL cells were able to stimulate both the allogeneic and the autologous T-cell proliferation. The addition of IL-4 further enhanced the expression of both B7-1 and B7-2, whereas IFNγ or IL-2 reduced it compared with t-CD40L alone (Fig 2).
A high expression of B7-1 is critical for the induction of a CTL response.28-30 To induce an effective immune response against B-CLL cells, consecutive T-cell stimulations were performed with CD40-CLL cells. Allogeneic CD40-CLL cells strongly stimulated both CD4+ as well as CD8+ T cells, as previously described.27 In marked contrast, stimulation of autologous CD40-CLL cells strongly favored the outgrowth of CD4+, but not CD8+ T cells. With respect to the effector T-cell function, there were also marked differences. A cytolytic activity was only induced with allogeneic T cells stimulated by CD40-CLL cells. This resulted in a significant alloantigen-specific cytotoxic activity against both CD40-CLL and naive B-CLL cells, similar to previous findings.27 In marked contrast, the stimulation of autologous T cells by CD40-CLL cells induced the expansion of a predominantly CD4+, Th1-like effector cell population without cytolytic activity. Moreover and in contrast to findings in follicular lymphoma and acute lymphoblastic leukemia, CD40-CLL cells did not allow to expand autologous T cells with cytolytic activity.31 32
The inability of CD40-CLL cells to stimulate CD8+ T cells in the autologous system can be explained by several alternative mechanisms. First, CD40 expressed on B-CLL cells may costimulate CD4+ T cells rather than CD8+ T cells.33 Second, autologous peripheral blood T lymphocytes might be less efficient in mounting a cytolytic response against lymphoma cell antigens than T cells derived from the bone marrow or tumor-infiltrating lymphocytes.32 Third, most if not all the anti-idiotype immune responses reported to date have been by CD4+ cells (both Th1- and Th2-type).34-36Fourth, a distinct pattern of costimulatory molecules expressed on CD40-CLL cells may induce the preferential stimulation of Th1-like, CD4+ T cells; for example, it is known that CD8+ T cells require higher densities of B7-1 to attain an equivalent level of activation as CD4+ T cells, and CD40-CLL cells may just not express enough B7-1 and/or B7-2 to activate CD8+ T cells.28 Sixth, the cytokine secretion by stimulated T cells themselves may contribute to the modulation of costimulatory molecules on CD40-CLL cells. These different mechanisms act probably in concert in regulating the threshold by which an ongoing T-cell response is maintained.
However, the exact factors preventing the outgrowth of autologous CD8+ effector T cells with antileukemic activity remain to be elucidated. It seems highly unlikely that autologous, peripheral blood T lymphocytes are intrinsically incapable of mounting a CTL response in B-CLL patients, because the generation of tumor cell-specific CTLs from the peripheral blood of B-CLL patients has been recently demonstrated.37
It will be of interest to learn whether the CD4+ Th1 cells stimulated by CD40-CLL cells are able to induce B-CLL cell death, eg, by triggering a Fas-dependent apoptotic pathway, as shown in human tonsillar and Burkitt’s lymphoma B cells.38-40 Preliminary experiments in our laboratory showed that CLL cells were indeed rapidly eliminated in coculture with these CD4+ T cells (R. Buhmann, unpublished data).
The CD40-CD40L interaction is crucial for the immune response. The ability to trigger and to regulate the induction and expression of CD40L on CD4+ cells appears to be of paramount importance to the generation of the T-cell–dependent antigen response.41,42 An excess of CD40-expressing leukemia cells might interfere with these cognate T-cell responses and provoke an aquired CD40L deficiency syndrome in B-CLL.43 Previous studies and our data suggest that these defects can be restored at least in part by an effective T-cell activation, with lymphoma or leukemia cells expressing costimulatory molecules at sufficient density.6,7,31 Activation of naive T cells can be brought by any APC, as long as sufficient levels of one or more accessory molecules are expressed. Later, during the process of T-cell activation and differentiation, the requirements for full T-cell stimulation decrease, as T cells become more responsive to a particular antigen.42,44 45 Thus, an APC that is only weakly stimulatory for naive T cells can become an efficient stimulator for preactivated T cells. Accordingly, native B-CLL cells might have properties of weakly stimulating APCs that need the concomitant presence of strong APCs (such as CD40-CLL cells or dendritic cells) to fully activate T cells.
Taken together, our results suggest that CD40 activation of B-CLL cells may reverse T-cell anergy against the neoplastic clone. The results might provide new perspectives for the immunotherapy of B-CLL, similar to previous observations in follicular lymphoma7,31 and pre-B acute lymphoblastic leukemia (ALL).6Most importantly, the clinical application to produce tumor vaccines or to generate stimulator cells for adoptive immune transfer strategies in B-CLL by this approach will meet less practical limitations than in other lymphoid malignancies, because B-CLL cells are readily obtained from peripheral blood. Moreover, the identity of CD40-CLL cells can be rapidly tested by measuring κ or λ light chain restriction and CD5/CD19 coexpression on the tumor cell surface by flow cytometry. This will facilitate the practical implementation of these approaches in the adjuvant therapy of B-CLL.
ACKNOWLEDGMENT
The authors are indebted to many individuals for their help in the preparation of this report: Dr Joachim Schultze, Dr Gabriella Pichert, Doris Schmitter, and Dr John Gribben for their instruction in the initial stage of the study; Dr Susanne Danhauser-Riedl for her unconditional assistance in flow cytometry; Dr Heidi Feldmann and Bettina Meier for their excellent expertise in computer aided design of the figures; and, finally, Karin Schulz for her support in molecular biology.
M.H. was supported by the Wilhelm-Sander-Stiftung and the Bayerische Forschungsstiftung. R.B. was supported by a fellowship of the Fritz Thyssen-Stiftung. A.N. was supported by a fellowship of the Deutsche Forschungsgemeinschaft.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Michael Hallek, MD, Genzentrum, Feodor-Lynen-Str. 25, D-81377 München, Germany; e-mail:hallek@lmb.uni-muenchen.de.

![Fig. 1. (A through C) Morphology of t-CD40L–stimulated CLL (CD40-CLL) cells after 3 days of culture. (A and B) Typical cluster formation of CD40-CLL cells that surround the CD40L-transfected NIH 3T3 fibroblasts (original magnifications: [A] × 100; [B] × 650). (C) CD40-CLL cells stained by May-Grünwald-Giemsa (original magnification × 650).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.1992.406k23_1992_2002/5/m_blod406buh01z.jpeg?Expires=1769843876&Signature=CVZorO3S7EJCgKVmB6kkoxQ77yPIY9Kv0zCJVIFdOSPHdUcxOlWD1lZJVQ3yyfOa0ftdw-n6al6pzTyVqg0P5~K2otLj-cjfSl~9oMTkjnKfm-r5gmiHHeLT1nKRmY26AswY6Ah0JlHRdE6DhTySOvSxb1srFwVHxzI7XeQKDILeVIvxXsjpwEeibfMA6CaBWthSKtaOlZpIR4T-XU3WrZrGcLTTu8Ib9psL9x2WPCdOscg13favp6r8Pjd2WZX3LomQa1c2SwsMLvOoG~ca6oTwgOpIq39gZ9f3cQG60RYT7rE4VehVO4VlLpX0RPHyn1nj3I7tJ3F25IcHt9Cbzw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Proliferative responses of purified allogeneic CD3+ T cells in the absence of IL-2 to γ-irradiated B-CLL cells (patient no. 12) either unstimulated or stimulated for 24, 72, and 120 hours by CD40L-transfected NIH3T3 fibroblasts. [3H] Thymidine incorporation was assessed for the last 12 hours of a 3-day culture. Appropriate controls (CD3+ T cells and B-CLL cells) were always less than 1,500 cpm. The stimulation index was calculated as cpm(T cells + B-CLL cells)/cpm(T cells). Results are representative for three independent experiments and are expressed as the mean ± SD of the stimulation index.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.1992.406k23_1992_2002/5/m_blod40623005x.jpeg?Expires=1769843876&Signature=TsyKTLE~HdW80-bHKtPTy5VSLOlcJOOantX6SXJHuujaFeTRSqKQhFwfLkm~ZoWOEf~V5o9VbA7aVk8ejW5kc1tqRtrBdzqMiIJKqCNTlZNmH4rhKL0dff8gU6kN2PwHrsJxRnocAhQXOu20dPlu1Y4e5r2i51imkq9TRtt5HLBU1J0YDMtwmkM5JXjdeDvQ1wnMmFBFCd-uda2FKVzd8UtwWap7c-OSNS6WJUNEOGB7cvmFkG2qyOGC9Aqw95peU2~AR0EM--qfiPEWt~qtv2Bak0Xj-AZVfC3NGEnPNQKzGFWHSPpqksGBDRfBzRYTB2YQqiUkiG8U21ePRatY8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Proliferative response of purified allogeneic CD4+ and/or CD8+ T cells as well as PBMCs not further purified to γ-irradiated native and CD40-stimulated B-CLL cells. Different CD4/CD8 ratios were tested. [3H] Thymidine incorporation was assessed for the last 12 hours of a 3-day culture and determined in cpm ± SD of triplicate determinations. The results shown are from one experiment and are representative for three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.1992.406k23_1992_2002/5/m_blod40623006x.jpeg?Expires=1769843876&Signature=dlCxKGvI7DvPPA0OqVcSk~AQTiKYySqj1Vihi9DR5Wqn95~6n6lHj~CA0GuN1HwyLPJkI~EV1FcYUlnL5cXLRYo51v617qDKwV6-ys-6lKNYuR8TWTK~1e65A43nApTgeovUjH0~UkzYSIe5G7xe02gMmKfFv~u6XUXwKE5G4ZVkxkQRA-ovBZJpyLF4n2rb3VnBj-9yHhbPyJkYPBnXgU9ldDhBmkOQrYAWOY9PzwHNf6cV-HG61korZOWuSe1oFdLd7SlTKszQj9I0QS9fAXqZrD77RbLRZ2LJi6uH05tWJ-xU-sHe3-ns8X0~bO-nRaicfD8u~tlO1kyqKI9zlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. (A through C) Morphology of t-CD40L–stimulated CLL (CD40-CLL) cells after 3 days of culture. (A and B) Typical cluster formation of CD40-CLL cells that surround the CD40L-transfected NIH 3T3 fibroblasts (original magnifications: [A] × 100; [B] × 650). (C) CD40-CLL cells stained by May-Grünwald-Giemsa (original magnification × 650).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.1992.406k23_1992_2002/5/m_blod406buh01z.jpeg?Expires=1769843877&Signature=t35Alxad5jJHQlVNIVvmv5FWUNgMQePLHrgEUAYIaZZ3eLUVtUUnEYouMKfeFrCv4T1vRVYpK82f7no2cZRtK6iBeIoBgGG8TsR2r~Y8CEnL9gsKFHfMKdZpLuqVKs2rEQgi4ImWLDmxmeNwhBEtlJHNrTGynXCUnysS5U3HahQ-hzuzHdUVreTwYB4J0hcZVCgehVZrZBe0T91KuRQSTnFW7OCRiva0iZm~Jd3mckh12FxFmnNaObUSiNKKJWz9O59Jo7Go0J6AFA3QADTpEb-UxsMRzeEgr9WT5xTC9jw2ZvHay~0ohjV7zyzL8cLhK2as-ccDWvKZSASSs0rjUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Proliferative responses of purified allogeneic CD3+ T cells in the absence of IL-2 to γ-irradiated B-CLL cells (patient no. 12) either unstimulated or stimulated for 24, 72, and 120 hours by CD40L-transfected NIH3T3 fibroblasts. [3H] Thymidine incorporation was assessed for the last 12 hours of a 3-day culture. Appropriate controls (CD3+ T cells and B-CLL cells) were always less than 1,500 cpm. The stimulation index was calculated as cpm(T cells + B-CLL cells)/cpm(T cells). Results are representative for three independent experiments and are expressed as the mean ± SD of the stimulation index.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.1992.406k23_1992_2002/5/m_blod40623005x.jpeg?Expires=1769843877&Signature=ZHQOZRmICeNIIWgDzj1agtAXIoCfAYtAutBYuFmStj9eQ7InaVnaMoSiYpXGc7Hwc9Ui4PpylkT-3O9LR5XMxuFvknKo5eO-gB0DzelmPKPPWGnQRf6B~pQhxV~39FPjTLQp3-~0lMMs9vztBlB29I8xp0uvUTA8FeAh8pQddHONf2E2Ro0CWkyeCJr0Tvy7wvmpwT8I0ZC3MY3XdyQ01F3-96Zp3S25LmPUpj7hwH6ipS1WYBe50UnXgD1PJT1VS7RpsfvL9Me1u67itdU4p7BcH9vtL9HipRctNMtKYMM6fXk~wu4fkK1bSEmiq1ReoXyD2YucoIt4LOjTrR1rbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Proliferative response of purified allogeneic CD4+ and/or CD8+ T cells as well as PBMCs not further purified to γ-irradiated native and CD40-stimulated B-CLL cells. Different CD4/CD8 ratios were tested. [3H] Thymidine incorporation was assessed for the last 12 hours of a 3-day culture and determined in cpm ± SD of triplicate determinations. The results shown are from one experiment and are representative for three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.1992.406k23_1992_2002/5/m_blod40623006x.jpeg?Expires=1769843877&Signature=R-DkeSPwATuLpLjzSSgCm0ZPCUPWdJmA0r8pvKU4vOqQJW1a-QfT5BAbIR4ESzf3a8T8wDR-7WwpdNndKsSXy2JdFjnjcOy6gpx2b-EkleF4MtaCW-eEf9CropqbucZO9rIbbeAvRjsLuVEpSSnGKo55OsaW7N~~r9uwsjvxdbN4anWPGv~cK81fMNjYB~g3pajbyp5C8Atq6dvotAlcvAtFuXUJaXggqgZbgeKq3CFfD3VYJEjyqBsT9wFMT5bYehOwIAXMJxQyP8yDC0Rbv9ilhzZlWK3DB4X47b6HsxHDf5C~MF5poi2kIaKVjL-JQbOQFNfqUv7y76UyDkwd2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)