Abstract
Retrovirus-mediated gene transfer into long-lived human pluripotent hematopoietic stem cells (HSCs) is a widely sought but elusive goal. A major problem is the quiescent nature of most HSCs, with the perceived requirement for ex vivo prestimulation in cytokines to induce stem cell cycling and allow stable gene integration. However, ex vivo culture may impair stem cell function, and could explain the disappointing clinical results in many current gene transfer trials. To address this possibility, we examined the ex vivo survival of nonobese diabetic/severe combined immune-deficient (NOD/SCID) repopulating cells (SRCs) over 3 days. After 1 day of culture, the SRC number and proliferation declined twofold, and was further reduced by day 3; self-renewal was only detectable in noncultured cells. To determine if the period of ex vivo culture could be shortened, we used a vesicular stomatitis virus G protein (VSV-G) pseudotyped retrovirus vector that was concentrated to high titer. The results showed that gene transfer rates were similar without or with 48 hours prestimulation. Thus, the use of high-titer VSV-G pseudotyped retrovirus may minimize the loss of HSCs during culture, because efficient gene transfer can be obtained without the need for extended ex vivo culture.
RETROVIRUS-MEDIATED gene transfer into long-lived pluripotent hematopoietic stem cells (HSCs) could provide permanent correction of hematopoietic gene dysfunction in a variety of genetic diseases. However, the results of clinical gene therapy trials are disappointing, due to the low efficiency of gene transfer into HSCs and/or poor expression in the differentiated progeny of these cells.1-4 Major problems regarding human retrovirus-mediated gene transfer include the poor HSC expression of amphotropic receptors for the retroviral envelope protein5,6 and the quiescent state of the majority of HSCs, which does not favor the integration of retroviral vectors, since cell division is thought to be required.7 Compounding these problems are the difficulties in assaying human HSCs. Protocols for clinical trials are based on results from in vitro clonogenic assays of human progenitors, particularly the long-term culture–initiating cell (LTC-IC) assay. The development of in vivo xenogeneic models, in which human cells are transplanted into severe combined immune-deficient (SCID) mice,8-12 has led to the ability to measure a SCID reconstituting cell (SRC) in murine bone marrow (BM) months after transplantation. Cell fractionation and gene-marking studies provide some evidence that SRCs are more primitive than LTC-ICs.12 13 Although SRCs may still be a heterogeneous population of cells that include long-term repopulating HSCs, this assay provides a better measure of stem cell properties than the LTC-IC assay, especially with regard to the multipotent (ie, myeloid and lymphoid) and self-renewal properties of HSCs.
One approach to the amphotropic receptor problem has been the development of new vectors such as Moloney murine leukemia virus–based retrovirus pseudotyped with the vesicular stomatitis virus G protein (VSV-G).14 VSV-G pseudotyped retroviruses, like conventional retroviruses, can stably integrate into the host genome but have a much broader host range than conventional retroviruses and are more stable, thus permitting concentration by ultracentrifugation to greater than 109 infectious particles per milliliter.15 16 These vectors can efficiently infect mammalian and nonmammalian cells that are resistant to infection by conventional amphotropic retroviruses.
The problem of stem cell quiescence is usually approached by using hematopoietic growth factors (HGFs) ex vivo to stimulate murine17,18 or human19-21 stem and progenitor cells into cycle, and this paradigm has driven much research in animal models and clinical trials. The human studies are based on investigations showing that stimulation with combinations of HGFs such as interleukin-3 (IL-3), IL-6, and Steel factor (SF) can increase the efficiency of gene transfer into LTC-ICs in clinically applicable supernatant infection procedures in the absence of stroma.22,23 While stromal support has been shown to increase the efficiency of gene transfer and may be necessary for optimal stem cell survival,23-25 stroma-based gene transfer methods are impractical. Despite these laboratory advances, HSC gene transfer efficiency in primate26,27 and human1-4 clinical studies has been disappointingly low. Although strategies using combinations of HGFs have increased the efficiency of gene transfer into in vitro clonogenic progenitors, there is little evidence that cytokines increase the frequency of gene transfer into long-term repopulating stem cells as measured in blood cells obtained from patients. Indeed, it is possible that stimulation with HGFs may irreversibly alter HSC properties, and there is now strong murine evidence that sustained HSC self-renewal may not be possible.28-30 If this is also true for human cells, then the potential of HSCs to self-renew may be finite, determined by replicative history.
Thus, one of our major goals is to achieve efficient gene transfer into HSCs after short-term ex vivo culture, to limit progressive cytokine-induced ex vivo proliferation that might lead to stem cell extinction. The current study was undertaken to address whether the poor results of clinical gene therapy trials might reflect poor survival of HSCs and/or poor gene transfer during ex vivo culture, and if the former, whether the period of ex vivo culture could be shortened. To answer these questions, we used the nonobese diabetic (NOD)/SCID model to examine the umbilical cord blood (CB) SRC capacity after different periods of ex vivo culture. We show that the SRC quantity and repopulation capacity declines rapidly during serum-free culture in SF, Flt 3 ligand (FL), and either IL-3 or IL-6/erythropoietin (EPO). However, we also show that prestimulation with HGFs is unnecessary: we found that a high-titer VSV-G pseudotyped retrovirus vector could confer efficient gene transfer into SRCs within 1 day of culture.
MATERIALS AND METHODS
Preparation of CB cells.
Cells were harvested from placentae after delivery by cesarean section. The CB was obtained by needle aspiration of the exposed vessels on the fetal side of the placenta. After diluting the sample (1:6 vol/vol) with IMDM medium (Life Technologies, Grand Island, NY) containing 2% fetal bovine serum ([FBS] Sigma Chemical Co, St Louis, MO) and 0.6% anticoagulant citrate dextrose solution-A ([ACD-A] Cytosol Laboratory, Braintree, MA),31 the CB cells were centrifuged over a layer of Histopaque-1.077 (Sigma) to deplete erythrocytes. The cells were then washed twice in IMDM (2% FBS and 0.6% ACD-A), incubated on ice with ammonium chloride for 20 minutes, and washed once more. The processed cells were either frozen down directly (in IMDM with 50% FBS and 10% dimethyl sulfoxide, Sigma) and further separated on the day of the experiment or further enriched for progenitor cells and then frozen down. Human CD34+CD38−progenitor cells were enriched using a positive selection method for CD34+ cells (Ceprate LC separation system; CellPro, Bothell, WA) or a negative selection method for lineage-negative cells (StemSep; StemCell Technologies, Vancouver, BC, Canada), according to the manufacturer’s instructions. CD34+ cells enriched by the CellPro method were 70% to 90% pure. After negative selection using the StemSep method, the cell suspension contained 31% ± 9% CD34+ cells (n = 6). However, with respect to the purification for CD34+CD38− cells, both methods were comparable: 11% for the CellPro method and 9% for the StemSep method when 10 and seven combined CB samples, respectively, were used for purification of primitive human progenitor cells.
The CB cells enriched for CD34+CD38−progenitor cells (hereafter designated as progenitor cells) were analyzed for phenotype (flow cytometry) and function (CFC and LTC-IC) to determine the day 0 values, ie, the quantity and quality of the cell suspension that was used to initiate cultures for gene transduction.
Infection of hematopoietic cells.
CB progenitor cells were cultured in IMDM medium supplemented with 10% or 20% BIT (BIT 9500, bovine serum albumin, insulin, and transferrin; StemCell Technologies). The following human recombinant cytokines were used: SF (50 ng/mL), FL (100 ng/mL), IL-3 (20 ng/mL), IL-6 (10 ng/mL), and EPO (2 U/mL). FL was provided by Immunex (Seattle, WA) and IL-3 and IL-6 by Genetics Institute (Cambridge, MA), and SF and EPO were purchased from R&D Systems (Minneapolis, MN). Cells were cultured for 24, 48, or 72 hours on non–tissue culture treated plates (Becton Dickinson, San Jose, CA) previously coated with fibronectin (CH-296; a generous gift from Takara Shuzo Co, Otsu, Shiga, Japan). During the last 16 hours of every culture, the cells were exposed to the retrovirus at a multiplicity of infection (MOI) of 130 or 260 and 4 μg/mL Polybrene (Sigma). Then the cells were harvested, washed once, and analyzed for phenotype, function, and gene transfer efficiency.
Construction and purification of the VSV-G pseudotyped retrovirus.
The MMP vector is a derivative of the MFG series of retroviral vectors,32 and pMMP-MDR1 was constructed by inserting the human multidrug resistance 1 (MDR1) gene into the pMMP vector. To prevent a cryptic splicing event that occurs when the MDR1 cDNA is inserted into retroviral vectors,33 two sites were identified and silent mutations were engineered to both of them (J.-S. Lee, manuscript in preparation).
The pMMP-MDR1 plasmid was transfected into the 293GPG packaging cell line34 by the calcium phosphate method. 293GPG cells constitutively express murine leukemia virus gag-pol gene products and produce VSV-G proteins in a tetracycline-inducible fashion. After transfection and selection with 60 ng/mL colchicine, viruses produced from about 60 individual clones were titered by infection on 3T3 cells. The viral supernatants from the clone with the highest titer (up to 1 × 107 infection particles/mL) were concentrated by ultracentrifugation at 50,000g at 4°C for 1.5 hours. The virus pellets were resuspended in small volumes of DME overnight at 4°C. The titer of the concentrated viruses, determined by Southern blot analyses of the infected cells, was normally in the range of 109/mL.
Animals.
NOD/LtSz-scid/scid (NOD/SCID) mice were purchased from Jackson Laboratory (Bar Harbor, ME). They were bred and maintained in the Redstone animal facility of the Dana-Farber Cancer Institute in microisolator cages under well-defined sterile conditions. The mice were used as recipients for human cell transplantation at 8 to 12 weeks of age. Whole-body radiation was given in a single dose (325 to 350 cGy, 110 cGy/min) using a 137Cs source 1 to 4 hours before transplantation. The indicated human cell numbers were injected in a final volume of 0.25 to 0.35 mL phosphate-buffered saline ([PBS] Life Technologies) with 2% FBS. Six to 8 weeks later, the animals were killed by CO2 asphyxiation, after which the BM cells from both the femur and tibia were harvested for further analysis of human cell engraftment.
Analysis of human cell engraftment.
BM cells were harvested by flushing the hindlimb bones with PBS/2% FBS using a 3-mL syringe and a 21-gauge needle. The cell suspension was washed once and then resuspended in PBS/2% FBS. Cells were counted (using trypan blue to exclude dead cells) and assayed by flow cytometry to determine the proportion of human cells. When greater than 0.1% of the cells were of human origin, the samples were further analyzed for the presence of different myeloid and lymphoid lineages by flow cytometry and CFC assays.
Flow cytometric analysis.
For phenotyping the CB cell suspension before and after each culture, cells were labeled with the following antibodies (Abs): CD34-phycoerythrin (PE) (PharMingen, San Diego, CA) and CD38-fluorescein isothiocyanate (FITC) (Immunotech, Westbrook, ME). Irrelevant, isotype-controlled Abs were used in every experiment to determine background staining. For detection of human cells in mouse BM, an anti-CD45 Ab (HLe-1; Becton Dickinson) was used. To further define the different lineages in the total human cell population, BM cells were simultaneously stained with anti–CD45-FITC and an Ab against one of the following lineage markers: CD34-PE, CD33-PE, CD14-PE (all from Becton Dickinson), CD4-PE, CD8-PE, and CD19-PE (all from PharMingen). As part of the analysis of human cell engraftment in mice, BM cells from a naive NOD/SCID mouse were labeled to ensure that the Abs used were specific for human cells. All staining procedures were performed in PBS/2% FBS. Cell labeling was performed on ice (35 minutes), after which the cells were washed twice. Propidium iodide (Sigma) 2 μg/mL was added during the final wash. The flow cytometric analysis was performed on a FACScan (Becton Dickinson).
CFC assay.
To determine the human CFC content of the murine BM after transplantation, marrow cells were plated in IMDM/0.9% methylcellulose media (Methocel MC; Fluka, Buchs, Switzerland) containing 15% defined FBS (Hyclone Laboratories Inc, Logan, UT), 15% human plasma, and the following cytokines: SF (50 ng/mL), IL-3 (20 ng/mL), granulocyte/macrophage colony-stimulating factor ([GM-CSF], 20 ng/mL; a generous gift from Genetics Institute), and EPO (2 U/mL).35 A maximum of 2 × 105 BM cells were plated per dish (Fisher, Pittsburgh, PA). Colonies were scored in situ after 14 to 20 days of incubation at 37°C in a humidified atmosphere of 5% CO2 in air using well-established criteria. Control dishes with 2 × 105 BM cells from nontransplanted NOD/SCID mice were plated to ensure that murine colonies did not form under these culture conditions; apart from an occasional small macrophage colony, these dishes were always empty.
LTC-IC assay.
Test cells, ie, noncultured and cultured CB progenitor cells, were cultured in Myelocult medium (StemCell Technologies) to which 10−6 mol/L freshly dissolved hydrocortisone (Sigma) was added before use. The cultures were initiated by seeding the test cells on an irradiated (3,300 cGy) stroma layer, which was previously established from human BM cells obtained from discarded filters after BM harvests. The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 in air with weekly half-medium changes. After 5 to 6 weeks, the cultures were harvested and assayed for CFCs. The LTC-IC cultures were either set up in limiting dilution, which permits the frequency of LTC-ICs in the test population to be calculated using Poisson statistics,36 or in bulk cultures.
Analysis of gene transfer.
The presence of the gene from the viral construct was determined by polymerase chain reaction (PCR) in human CFCs grown from the harvested mouse BM cells. After scoring the plates, 20 colonies per mouse (or less, if fewer were present) were picked at random from the methylcellulose and directly deposited into Eppendorf tubes containing 500 μL PBS. The colonies were left for 1 hour at room temperature to allow the methylcellulose to dissolve. The cells were then washed twice and resuspended in lysis buffer containing 10 mmol/L Tris hydrochloride (pH 8.3), 2 mmol/L MgCl2, 50 mmol/L KCl, 0.45% Nonidet P40, 0.45% Tween 20, and 1 mg/mL proteinase K, as previously described.25 The samples were left overnight at 37°C or for 2 hours at 56°C. To inactivate the proteinase K, the samples were then heated at 94°C for 10 minutes. DNA was amplified using a DeltacyclerII PCR apparatus (Ericomp Inc, San Diego, CA). The primer set to demonstrate the presence of the transduced gene was chosen from the MMP vector within the proviral construct: 5′-TCTGCTCCCCGAGCTCAATA-3′ and 5′-CCGACTGGTTGTGAGCGAT-3′. GM-CSF or EPO genes were used as internal controls, and fragments were amplified using the primers 5′-TGAAATTTGTCTGCATGAAGGAGT-3′ and 5′-GCATTGTAGATGAAACAGGAGAAA-3′ and 5′-ACGCCTCTTCACCACCCACAA-3′ and 5′-TTCGAGGCCAAAGCAGATGAG-3′, respectively. Samples that failed to show a PCR product of either internal control were not included in the calculation of gene transfer efficiency.
RESULTS
VSV-G pseudotyped retrovirus does not decrease the survival or in vitro function of hematopoietic progenitor cells.
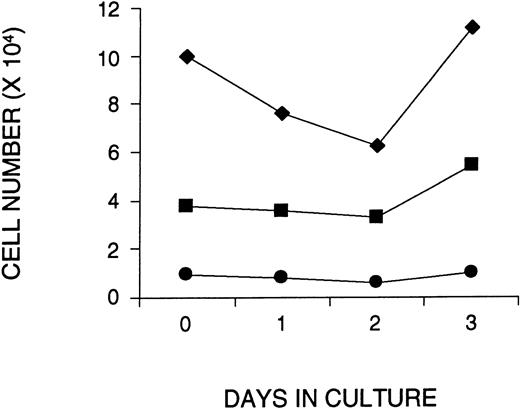
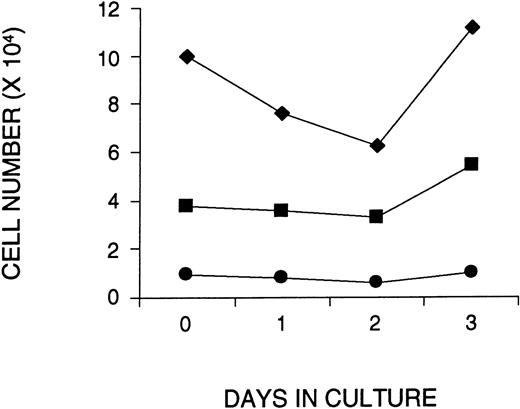
To examine whether ex vivo culture and addition of VSV-G pseudotyped retrovirus affected the survival or in vitro function of primitive hematopoietic cells, CD34+ cells were isolated from approximately 10 combined CBs, and cultured on fibronectin-coated plates in serum-free medium containing SF, FL, and IL-3 for 1, 2, or 3 days. During the last 16 hours of each culture period, the cells were exposed to VSV-G pseudotyped MMP-MDR1 viral supernatant that was produced from a human 293–based packaging cell line and concentrated to very high titer by centrifugation (108 to 109 particles/mL). At the end of each culture period, the cells were analyzed for both phenotype and function and compared with noncultured CB cells. At a MOI of 130 (130 virus particles per cell), the number of total CD34+ and CD34+CD38− cells remained constant during the 3-day culture period, despite a small decrease in total cell number after 2 days of culture (Fig 1). The addition of a twofold higher dose of virus (MOI 260) during the last 16 hours of a 1- or 2-day culture resulted in a slightly greater decrease in cell number (including the most primitive CD34+CD38− cells), although similar or greater recovery was noted by day 3 (data not shown). An in vitro limiting dilution analysis of the number of LTC-ICs before and after culture confirmed this phenotypic analysis (Table 1). In two experiments at an MOI of 130, the number of LTC-ICs was not significantly affected after 3 days of culture. In a third experiment, in which twice the amount of virus was used (MOI 260), the LTC-IC assay was set up in bulk cultures and measured before and after 1 or 3 days of culture. The number of CFCs obtained from noncultured LTC-ICs was similar to the number observed for LTC-ICs established after 1 and 3 days of ex vivo culture (data not shown).
Maintenance of human CD34+CD38− cells in serum-free cultures initiated with CB cells enriched for CD34+ cells. The number of total cells (⧫), CD34+ cells (▪), and CD34+CD38− cells (•) are shown as a function of time in culture. To calculate the number of CD34+ cells or CD34+CD38−cells at each time point, the number of total live cells (counted with a hemocytometer) was multiplied by the percentage of CD34+ cells or CD34+CD38−cells obtained by FACS. Shown is a representative figure of one of three experiments.
Maintenance of human CD34+CD38− cells in serum-free cultures initiated with CB cells enriched for CD34+ cells. The number of total cells (⧫), CD34+ cells (▪), and CD34+CD38− cells (•) are shown as a function of time in culture. To calculate the number of CD34+ cells or CD34+CD38−cells at each time point, the number of total live cells (counted with a hemocytometer) was multiplied by the percentage of CD34+ cells or CD34+CD38−cells obtained by FACS. Shown is a representative figure of one of three experiments.
Ex vivo culture of CB CD34+ cells reduces both the number and quality of SRCs.
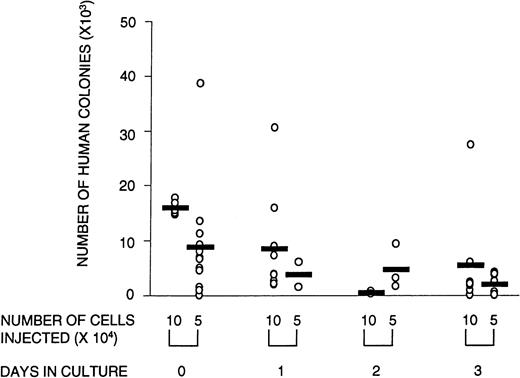
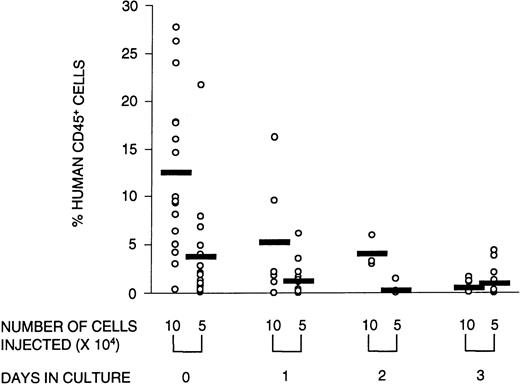
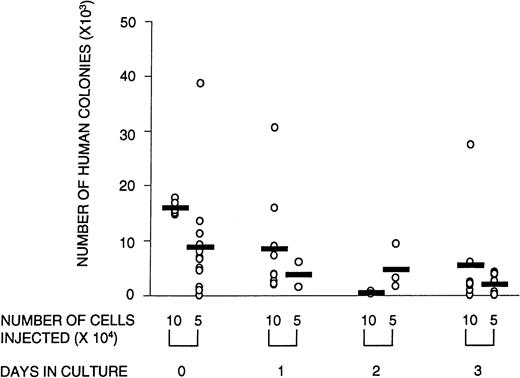
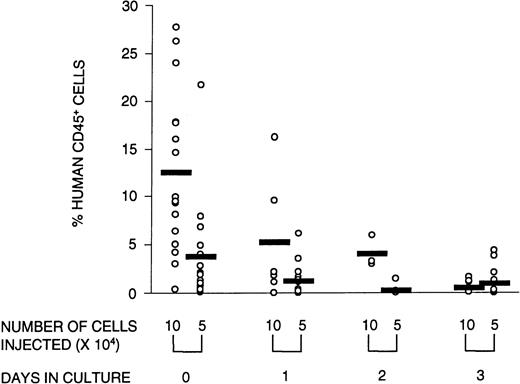
Engraftment in the NOD/SCID mice was defined as the presence of greater than 0.1% human cells in the murine BM with evidence of both myeloid and lymphoid reconstitution, as well as the capacity to form colonies in methylcellulose. To determine whether ex vivo culture affected these SRC parameters, CD34+ cells were cultured for 1, 2, or 3 days and transplanted into NOD/SCID mice. At limiting cell doses, the number of mice with detectable human myeloid and lymphoid cells in the BM (ie, the number of positive mice) was reduced after transplantation with cultured cells. Poisson analysis of the proportion of negative mice shows that the number of SRCs declined over the 3-day culture period (Table 2). This reduction in SRCs was accompanied by a diminished number of human CFCs obtained from mice transplanted with cultured cells (Fig 2). The quality of SRCs was measured by the level of human cell engraftment and by their ability to give rise to SRCs that could reconstitute secondary NOD/SCID recipients. The relative contribution to the different lineages (measured by CD34 [progenitor cells], CD33 and CD14 [myeloid cells], and CD4/CD8 and CD19 [lymphoid cells]) was unaffected by time in culture (data not shown). However, the overall level of human cell engraftment decreased dramatically with time in culture (Fig 3). Whereas the transplantation of 105 noncultured cells resulted, on average, in 13% human cells in the BM of transplanted NOD/SCID mice, after 1 day of culture, this was reduced to less than half, and after 3 days of culture, only 4% of the input level was observed. Thus, the level of engraftment declined 25-fold after 3 days in culture. This could be due in part to a decline in the number of SRCs, although the reduction in SRCs was much less than that, ie, fourfold. The results of the secondary transplantation experiments show that BM cells from a small number of primary recipients of noncultured transplants (14%) could reconstitute secondary recipients (Table3). In contrast, none of the cells harvested from mice transplanted with cultured cells were able to reconstitute secondary recipients. If culture does not affect the self-renewal capacity of SRCs, we would expect 14%, ie, approximately two mice, of all secondary recipients transplanted with cultured cells (16 mice in total) to be reconstituted with human cells. However, no human cell reconstitution was detectable in any of the secondary transplanted mice. It is possible that 1 day in culture may still preserve the self-renewal of SRCs but the number of mice analyzed in this group was too small for detection. Taken together, these results show that culture affects the quality of SRCs in that the proliferative and self-renewal ability diminishes with time in culture.
Number of human CFCs harvested from transplanted animals is lower in animals that received cultured cells versus noncultured cells. Circles represent individual mice that received noncultured cells (day 0) or cells that were cultured for 1, 2, or 3 days in serum-free medium supplemented with SF, FL, and IL-3. The total number of human colonies per femur was calculated by dividing the number of total cells obtained per femur by the number of cells plated per methylcellulose dish (usually 2 × 105 cells), multiplied by the average number of colonies scored in a duplicate set of dishes. There were no significant differences in the relative contribution of the types of colonies between cultured and noncultured cells. Shown are the combined data from three experiments.
Number of human CFCs harvested from transplanted animals is lower in animals that received cultured cells versus noncultured cells. Circles represent individual mice that received noncultured cells (day 0) or cells that were cultured for 1, 2, or 3 days in serum-free medium supplemented with SF, FL, and IL-3. The total number of human colonies per femur was calculated by dividing the number of total cells obtained per femur by the number of cells plated per methylcellulose dish (usually 2 × 105 cells), multiplied by the average number of colonies scored in a duplicate set of dishes. There were no significant differences in the relative contribution of the types of colonies between cultured and noncultured cells. Shown are the combined data from three experiments.
Reconstituting ability of the graft decreases with time in culture. The percentage of human CD45+ cells in the BM of reconstituted recipients transplanted with 105 or 5 × 104 human CB cells that were either not cultured or cultured for 1, 2, or 3 days is illustrated. Open circles represent individual mice; the horizontal bar is the average value of the group. Data from three experiments were combined.
Reconstituting ability of the graft decreases with time in culture. The percentage of human CD45+ cells in the BM of reconstituted recipients transplanted with 105 or 5 × 104 human CB cells that were either not cultured or cultured for 1, 2, or 3 days is illustrated. Open circles represent individual mice; the horizontal bar is the average value of the group. Data from three experiments were combined.
The VSV-G protein is known to be toxic to cells.16 Although the phenotypic analysis and the in vitro functional data do not suggest that this was the case in our experiments, we wished to exclude VSV-G toxicity as the cause of the decline in SRCs. Groups of five mice were transplanted with 105 CD34+ CB cells cultured for 3 days in the same cytokines with or without the addition of virus. All mice were reconstituted, and the level of human reconstitution was comparable in both groups, ie, 0.9% ± 1.2% and 0.4% ± 0.4%, respectively.
Recent reports demonstrate that IL-6 may be an important cytokine for the survival of human progenitor cells,37 and confirm earlier reports of the maintenance of murine HSCs in a combination of SF, IL-6, and EPO.38 Therefore, we sought to investigate whether the quality of SRCs would be better preserved in a cytokine combination that, in addition to SF and FL, contained IL-6 and EPO instead of IL-3. Although the level of human cell engraftment was low in this experiment (Table 4), it was threefold to fourfold higher in recipients of cells cultured in SF, FL, IL-6, and EPO versus SF, FL, and IL-3. However, the number of SRCs after 1 and 3 days of culture in the IL-6 growth factor combination was decreased as much as in the IL-3 combination. Interestingly, when BM cells from four primary recipients that received cells cultured in SF, FL, IL-6, and EPO were transplanted into irradiated secondary recipients (three each), human cell engraftment was observed in two of three secondary recipients from one of the primary recipients (data not shown). These results suggest that SRCs cultured in SF, FL, IL-6, and EPO survive no better than SRCs in SF, FL, and IL-3, while the self-renewal capacity of the surviving SRCs may be better preserved.
Prestimulation of SRCs is unnecessary for efficient infection with VSV-G pseudotyped retrovirus.
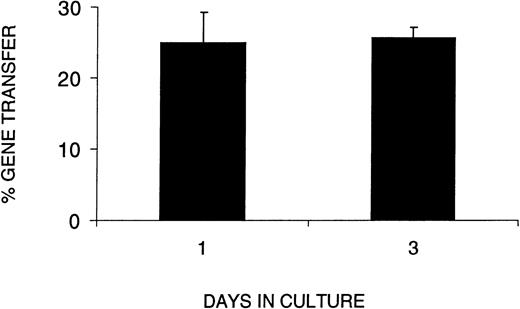
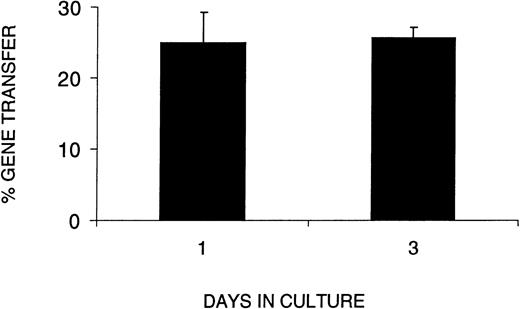
In light of the decrease in SRC number and function during ex vivo culture, we next wished to determine whether the VSV-G pseudotyped retrovirus could infect SRCs without an extended period of prestimulation. We therefore compared gene transfer efficiency in CB cells cultured for 1 or 3 days, ie, no prestimulation or 48 hours prestimulation in serum-free medium with SF, FL, and IL-3. To assess gene transfer efficiency in SRCs using VSV-G pseudotyped retrovirus, we used PCR to detect the transduced retroviral sequence in individual CFCs from the BM of positive mice. Figure 4shows gene transfer efficiency as a function of time in culture (1 or 3 days). The results clearly show that the percentage of CFCs transduced with the retroviral vector after 1 day in culture was not significantly different from that after 3 days in culture. When a similar experiment was performed with SF, FL, IL-6, and EPO, we found that the gene transfer efficiency after 3 days of culture was 2.4-fold higher than after 1 day, ie, 48% versus 20%, respectively.
Gene transfer efficiency with a 1-day infection protocol equals that of a 3-day protocol. The percent gene transfer represents the proportion of methyl cellulose colonies formed by human cells harvested from the BM of reconstituted recipients that were PCR-positive for the MDR gene from the retroviral construct. The days in culture indicate the total number of days human cells were cultured before transplantation into irradiated NOD/SCID recipients. The data represent three combined experiments in which the analysis of day 1 was performed in 2 separate experiments. A MOI of 130 or 260 did not show differences in gene transfer efficiency.
Gene transfer efficiency with a 1-day infection protocol equals that of a 3-day protocol. The percent gene transfer represents the proportion of methyl cellulose colonies formed by human cells harvested from the BM of reconstituted recipients that were PCR-positive for the MDR gene from the retroviral construct. The days in culture indicate the total number of days human cells were cultured before transplantation into irradiated NOD/SCID recipients. The data represent three combined experiments in which the analysis of day 1 was performed in 2 separate experiments. A MOI of 130 or 260 did not show differences in gene transfer efficiency.
DISCUSSION
Initial studies of the effects of culture on SRCs showed a sixfold decrease of BM but no decrease of CB SRCs after 1 week on allogeneic stroma.39 More recent reports suggest that it may be possible to maintain or increase SRC numbers in serum-free cultures supplemented with a cytokine combination of SF, FL, IL-3, IL-6, and G-CSF.37,40 These findings appear to conflict with our data that the SRC number and quality decline rapidly in culture. However, Conneally et al37 report that after culture, the number of CFCs and LTC-ICs generated per SRC is lower than before culture, despite correction for the observed twofold increase in the number of SRCs. Data from Bhatia et al40 suggest a decline in the quality of SRCs, as well. They calculate a fourfold increase in SRCs after 4 days in culture; however, despite this increase, the level of engraftment of 500 CD34+CD38− cells was low (<1%), similar to the level obtained with fourfold fewer day 0 cells.13 This is consistent with their further observation that by day 9 no SRC activity was detectable at all. In summary, both of these studies confirm our own in that they show a decline in SRC function over time.
Previous studies with amphotropic retroviruses have shown that it is possible to obtain efficient gene transfer into primitive hematopoietic progenitors assayed in vitro by CFC and LTC-IC assays.22,24,41 Despite these encouraging data, the results of most clinical studies have been disappointing in that very few HSCs (<1%) appear to be transduced.1-4 Therefore, CFC and LTC-IC data do not predict clinical outcome, and it was of considerable interest to determine the efficiency of retrovirus transduction into SRCs. It was recently shown that the combination of SF, FL, IL-3, IL-6, and G-CSF resulted in a mean gene transfer rate of 32% into SRCs (range, 11% to 43%)42; in this series of experiments, it was necessary to extend the period of prestimulation to 72 hours, with two subsequent 24-hour exposures to virus supernatant, ie, 120 hours ex vivo. Larochelle et al12 reported very poor gene transfer into SRCs using fibronectin-coated dishes and supernatant infection. Better rates of gene transfer could only be obtained when very large numbers of CB cells (3 to 5 × 108) were directly cocultured with retrovirus packaging cells. Dao et al43used the beige/nude/xid mouse model to show that a mean of 5% of SRCs in the CD34+ cell fraction could be transduced in a protocol that requires addition of virus supernatant during 72 hours of culture on a stromal support supplemented with SF, IL-6, and IL-3. Transduction of SRCs in the CD34+CD38−fraction required 7 days of culture and the addition of FL; under these conditions, in which the transferred gene conferred neomycin resistance, low numbers of G418-resistant colonies (2% to 4%) were observed in two of four mice. Gene transfer rates similar to those reported here were observed in one of the above-mentioned studies.42 However, all of these studies required prolonged periods of incubation of the cells in culture, and our data clearly show that this is undesirable.
Although 3 days in SF, FL, IL-6, and EPO resulted in a 2.4-fold increase in gene transfer efficiency compared with 1 day ex vivo, the extended culture was accompanied by a fivefold loss in SRC number and an even greater loss in proliferative potential (Table 4). Thus, the absolute number of transduced SRCs in mice transplanted after 3 days in SF, FL, IL-6, and EPO is lower than in mice transplanted after 1 day, despite the higher gene transfer efficiency. This finding that both the number and quality of SRCs are reduced during ex vivo culture may be an important consideration in the evaluation of results of clinical gene therapy trials. In this respect, it is of particular interest that in the one clinical study with good gene transfer results, BM harvested from children during recovery from chemotherapy was cultured for only 6 hours ex vivo and exposed to retrovirus supernatant in the absence of cytokines; gene transfer rates of 20% were observed in CFCs up to 42 months posttransplant.44 45
Perhaps the most surprising result of this study is that comparable gene transfer rates were observed without or with prestimulation in culture. Cell cycle analysis shows that after isolation the majority (97.5%) of CD34+ CB cells are in G0/G1, and that after 14 to 24 hours in IL-3 and SF approximately 80% remain in this state; by 48 to 72 hours, the proportion of cells remaining in G0/G1 has decreased to about 50%.46 These data suggest that cells that exit G0/G1 would be the preferred targets for retrovirus integration and stable gene transfer. However, recent data from the same group47 show that CD34+mobilized blood progenitor cells stimulated to exit G0 into G1 with IL-3, SF, and FL are severely compromised in their SRC content but cells that remain in G0 retain their SRC activity. The finding of a loss of SRC activity after culture is consistent with our own results. We therefore speculate that the VSV-G pseudotyped particles target many cells, including G0 cells ex vivo, and after transplantation and homing, cell division in vivo in the BM microenvironment leads to integration and stable gene transfer.
In conclusion, our studies show that prolonged ex vivo culture of hematopoietic cells compromises critical aspects of SRC function. We show that the use of a VSV-G pseudotyped retrovirus can obviate the damaging effect of culture, since it allows efficient gene transfer into CB SRCs after only 20 hours ex vivo culture. Whether the same approach will be as successful with BM or mobilized stem/progenitor cells remains to be determined. Since it is impossible to determine in the laboratory whether the SRC assay truly reflects HSC with multipotent and self-renewal capacity, a clinical trial to test the VSV-G pseudotyped virus in a short-term infection protocol is needed.
ACKNOWLEDGMENT
The authors thank Jessica Denham for excellent technical assistance and the personnel of the Redstone animal facility for the care of the animals. We thank Amy Perrault for help in preparing the manuscript.
Supported by Grants No. RO1 HL55709 and 1 P50 HL54785 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Colin A. Sieff, MB, BCh, Dana-Farber Cancer Institute, 44 Binney St, Boston MA 02115.