Abstract
Human T-cell leukemia virus type I (HTLV-I) is an etiologic agent of adult T-cell leukemia (ATL). The viral protein Tax induces the activation and nuclear translocalization of transcription factor NF-κB, which is proposed to play a crucial role in the transformation of T cells by HTLV-I. However, the HTLV-I genes including Tax are not expressed significantly in primary leukemic cells from ATL patients. In this study, we examined the basis for NF-κB activation in freshly isolated leukemic cells from ATL patients. We found that leukemic cells from ATL patients, like HTLV-I–infected T-cell lines, display constitutive NF-κB DNA binding activity and increased degradation of IκB (an inhibitor of NF-κB). Whereas the NF-κB binding activity in Tax-expressing T-cell lines consisted mostly of p50/c-Rel, fresh ATL samples contained p50/p50 and p50/p65 heterodimers. One T-cell line derived from ATL leukemic cells, TL-Om1, displayed constitutive NF-κB activity, as well as enhanced degradation of IκB, despite the lack of detectable Tax expression. Interestingly, the NF-κB in TL-Om1 consists of p50/p50 and p50/p65 like that in fresh primary leukemic cells. Our results suggest that activation of NF-κB occurs through a Tax-independent mechanism in leukemic cells of ATL patients, possibly due to differential NF-κB subunit activation.
ADULT T-CELL leukemia (ATL) is a highly aggressive T-cell malignancy etiologically linked to a retrovirus, human T-cell leukemia virus type I (HTLV-I).1,2 As the viral genome does not encode a known oncogene3 or promote cis-activation of endogenous proto-oncogenes by site-specific integration,4 the events leading to the occurrence and progression of this hematologic disease remain to be elucidated. A 40-kD nuclear oncoprotein, termed Tax, appears to be responsible for the transforming features of HTLV-I. HTLV-I Tax is a potent transcriptional activator of the HTLV-I long terminal repeat,5,6 as well as numerous cellular genes involved in T-cell activation and growth, such as those encoding interleukin-2 (IL-2)7,8 and the α chain of IL-2 receptor (IL-2Rα).8-10 Because Tax does not bind DNA directly, Tax appears to induce cellular target genes indirectly via host cellular pathways,11 including activation of the NF-κB/Rel family of transcription factors.12-14
In resting T cells, NF-κB/Rel proteins are sequestered in the cytoplasm as latent precursors by their inhibitor, IκBα.15 Subsequent to cellular activation and through proteolytic degradation of IκBα, NF-κB is released and translocates to the nucleus where it transactivates several genes, including IκBα itself.16 Recent studies have shown that Tax induces the degradation of IκBα, which may contribute to constitutive activation of NF-κB in HTLV-I–infected T-cell lines.17-21
Although NF-κB is known to be constitutively activated in Tax-expressing and HTLV-I–infected T-cell lines, the activation status of NF-κB in leukemic cells from ATL patients remains unclear. Some of the genes that can be transactivated by Tax are constitutively expressed in leukemic cells from ATL patients. On isolation, ATL cells spontaneously display surface IL-2Rα22 and express mRNA for cytokines including IL-1α,23,24IL-1β,23-25 IL-6,26,27 IL-8,28IL-9,29 IL-10,30 tumor necrosis factor β,31 transforming growth factor β,32,33 and parathyroid hormone-related protein.34,35 Among them, genes for IL-2Rα,12-14 IL-1α,36IL-6,27 IL-8,28 and tumor necrosis factor β37 harbor the κB enhancer element and Tax activates transcription of these cellular genes through NF-κB binding site. However, primary leukemic cells isolated from ATL patients typically express Tax mRNA and protein at low levels, if at all.38 39These findings imply that there is another mechanism independent of Tax underlying the overexpression of several cellular genes in fresh ATL cells.
In this study, we examined the activation of NF-κB and IκBα in leukemic cells isolated from ATL patients and in control cells from healthy subjects. Tax expression in the primary cells isolated from these patients was hard to estimate quantitatively with Northern and Western blot analyses. We found evidence for constitutive degradation of IκBα protein, increased nuclear expression of NF-κB, and increased expression of IκBα mRNA in ATL cells. Also, TL-Om1, which does not express Tax at all, still exhibits constitutive NF-κB binding activity, composed of p50 and p65, as seen in ATL cells. These observations suggest Tax-independent mechanisms operating for constitutive NF-κB activation in leukemic cells from ATL patients.
MATERIALS AND METHODS
Cells.
MT-2, HUT-102, SLB-1, C5/MJ, and TL-Om1 are human T-cell lines infected with HTLV-I. Jurkat, MOLT-4, and H-9 are human T-cell lines. These T-cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, glutamine, and antibiotics. JPX-9 is a derivative of Jurkat cells carrying the transfected Tax gene under the control of metallothionein promoter.40 Expression of Tax was induced by adding 20 μmol/L CdCl2 into the medium.
Peripheral blood mononuclear cells (PBMC) from 7 patients, 5 with acute-type ATL (patients 3 to 7) and 2 with chronic type ATL (patients 1 and 2),41 were analyzed. The diagnosis of ATL was based on clinical features, hematologic characteristics, the presence of serum antibodies to ATL-associated antigens, and the presence of the HTLV-I proviral genome in DNA from leukemic cells. PBMC were isolated by Ficoll/Hypaque (Pharmacia LKB, Uppsala, Sweden) by density gradient centrifugation. Each patient had more than 90% leukemic cells in the blood at the time of analysis.
Detection of IL-2Rα–positive cells.
The cells were washed once with phosphate-buffered saline (PBS) containing 0.1% NaN3 and mixed with fluorescein isothiocyanate-conjugated murine monoclonal antibody to the α chain of the human IL-2R. After incubation for 30 minutes at 4°C, they were washed with PBS containing 0.1% NaN3 and then analyzed using a FACScan (Becton Dickinson, San Jose, CA).
Northern blot analysis.
Total RNA was extracted from cells using the Trizol method as described by the manufacturer (GIBCO-BRL, Gaithersburg, MD). Twenty micrograms of RNA was electrophoresed through a formaldehyde-agarose gel and transferred to a nylon membrane. Membranes were prehybridized (0.5 mol/L sodium phosphate, 0.1% bovine serum albumin, 7% sodium dodecyl sulfate [SDS], 100 μg/mL salmon testis DNA, and 100 μg/mL yeast RNA) for 2 hours at 65°C and then hybridized overnight with the following [α-32P] radiolabeled probes: cDNA of human IκBα,42 IL-2Rα (kindly provided by Dr T. Taniguchi, Tokyo University, Tokyo, Japan),43glyceraldehyde-3-phosphate dehydrogenase (GAPDH),44 and HTLV-I Tax.45 Radiolabeled probes were generated by using a Megaprime DNA Labeling system (Amersham, Arlington Heights, IL).
Western blotting.
The antibody used in these experiments was rabbit polyclonal antibody to IκBα, C-21 (Santa Cruz Biotechnology, Santa Cruz, CA). Cells were lysed by incubation in radio-immuno-protein-assay (RIPA) buffer (0.5% sodiumdeoxycholate, 1% Nonidet P-40, 0.1% SDS, 66 μg/mL aprotinin, 100 μg/mL phenylmethylsulfonyl fluoride, and 1 mmol/L sodium orthovanadate) for 30 minutes at 4°C. Equal amounts (50 μg) of protein from cell lysates were electrophoresed on 10% SDS-polyacrylamide gel electrophoresis gels and transferred to polyvinylidine difluoride membranes. The membranes were washed with Tris-buffered saline-Tween (0.05%) with 3% nonfat dried milk overnight at 4°C and then blotted for 45 minutes with the antibody. The membranes were washed with Tris-buffered saline-Tween and incubated with a 1:5,000 dilution of horseradish peroxidase-conjugated antirabbit immunoglobulin (Amersham). Thereafter, membranes were developed with enhanced chemiluminescence reagents (Amersham) and exposed to film.
Oligonucleotides.
The sequence of the oligonucleotide corresponding to the κB element from IL-2Rα gene was 5′-gatcCGGCAGGGGAATCTCCCTCTC-3′.46Underlined sequences represent the κB motif. The oligonucleotide, 5′-gatcTGTCGAATGCAAATCACTAGAA-3′, containing the consensus sequence of the octamer binding motif (underlined) was used to identify specific binding of the transcription factor Oct-1. This transcription factor regulates transcription of a number of so-called housekeeping genes. For competition studies, oligonucleotides containing mutant κB and two κB motifs derived from the human immunodeficiency virus type 1 long terminal repeat (LTR) were used, and the sequences are 5′-gatcCGGCAGatctATCTCCCTCTC-3′ and 5′-gatcACAAGGGACTTTCCGCTGGGGACTTTCCAG-3′, respectively. For the preparation of a probe in electrophoretic mobility shift assay (EMSA), a radiolabeled double-stranded oligonucleotide was prepared by annealing and filling in the overhang with the Klenow fragment of DNA polymerase I in the presence of [α-32P]deoxycytidine triphosphate (dCTP) and [α-32P]deoxyadenosine triphosphate (dATP).
Preparation of nuclear extracts.
Nuclear extracts were prepared as described by Antalis et al47 with modifications. Cells (107) were washed twice with cold PBS and the cell pellet was suspended in 400 μL of hypotonic buffer A (10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol [DTT], 2 mmol/L aminoethyl-benzenesulfonyl fluoride [AEBSF], and 0.2% Nonidet P-40) for 10 minutes at 4°C. Nuclei were prepared by microcentrifugation for 5 minutes at 4°C. The nuclear pellet was suspended in 75 μL of buffer C (20 mmol/L HEPES, pH 7.9, 0.4 mol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, 2 mmol/L AEBSF, 33 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL E-64, and 10 μg/mL pepstatin A) and incubated for 30 minutes at 4°C with brief mixing. The mixture was microcentrifuged (15,000 cpm) for 15 minutes at 4°C. Protein concentration was measured by using the Bradford assay (Bio-Rad, Richmond, CA).
EMSA.
As previously described,36 nuclear extracts (5 μg of protein) were preincubated in a 20-μL total reaction volume containing 10 mmol/L Tris-HCl, pH 7.5, 50 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L DTT, 5% glycerol, and 1 μg of polydeoxyinosinic-deoxycytidylic acid (Pharmacia, Piscataway, NJ) for 15 minutes at room temperature. The reaction mixture was then incubated with the radiolabeled oligonucleotide (50,000 cpm) for 15 minutes at room temperature. The samples were analyzed by electrophoresis in a 4% nondenaturing polyacrylamide gel with 0.25 × TBE buffer (22.3 mmol/L Tris, 22.2 mmol/L boric acid, and 0.5 mmol/L EDTA). The gels were dried and analyzed by autoradiography.
Plasmids and transfection.
A reporter plasmid κB-LUC (a kind gift from Dr J. Fujisawa, Kansai Medical University, Osaka, Japan) was constructed by inserting five repeats of the NF-κB binding sequence of the IL-2Rα gene fused to an enhancerless promoter of HTLV-I into pGL2-Basic containing the luciferase gene, dN-LUC.21 The NF-κB expression plasmids, human p50, p65, and c-Rel, were the generous gifts of Dr K. Yamamoto (Kanazawa University, Kanazawa, Japan). Transfections were performed by electroporation.36 In all cases, the reference plasmid pRL-CMV, which contains the Renilla luciferase gene under the control of the cytomegalovirus immediate early enhancer/promoter, was cotransfected to correct for transfection efficiency. For luciferase assay, the transfected cells were lysed in a lysis reagent (Toyo Ink Co, Tokyo, Japan), and luciferase activities were measured according to the manufacturer’s procedures. Each assay was independently repeated at least three times.
RESULTS
HTLV-I–infected T-cell lines constitutively express a factor mediating activation of NF-κB element.
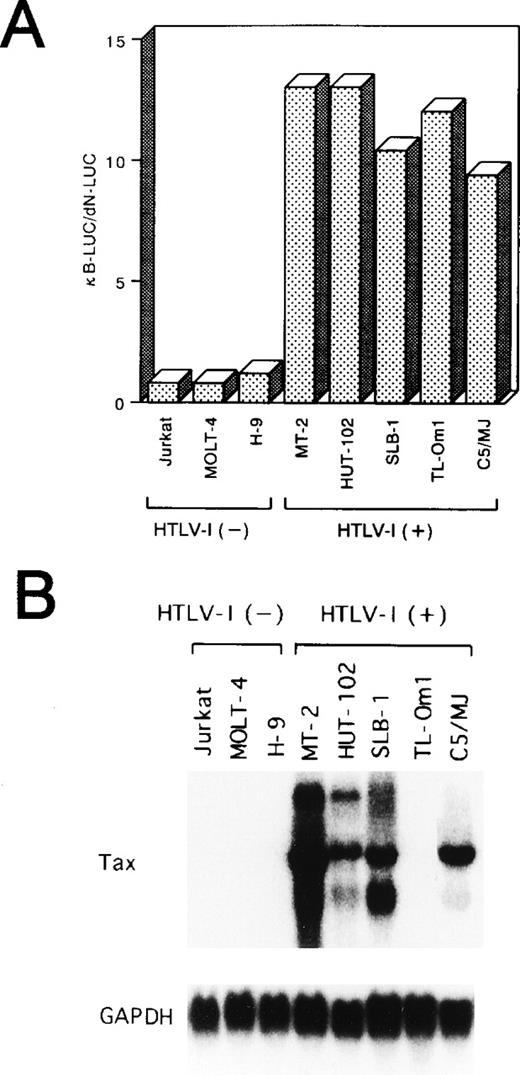
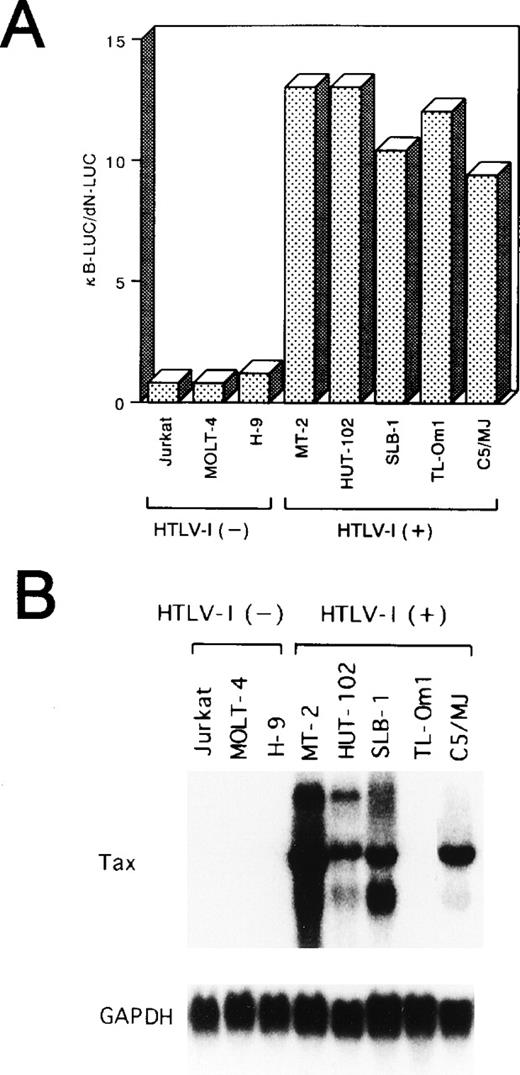
As an initial test of the activity of NF-κB in HTLV-I–infected T-cell lines, a transfection analysis was performed using a reporter construct, κB-LUC, containing a luciferase gene under the control of the NF-κB binding site from the IL-2Rα gene. The level of luciferase activity detected in uninfected cells transfected with κB-LUC was similar to that of cells transfected with dN-LUC (Fig 1A). On the other hand, in all of the five HTLV-I–infected cell lines, the κB-LUC reporter gene was 9 to 13 times more active than dN-LUC, indicating enhanced NF-κB activity in HTLV-I–infected cell lines.
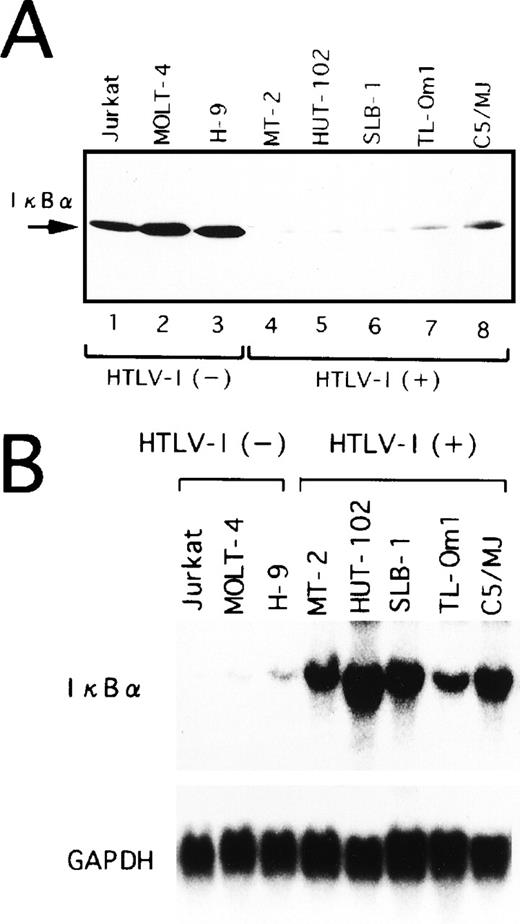
(A) HTLV-I–infected T-cell lines express a constitutive NF-κB–related activity. Various cell lines were transfected with 10 μg of a reporter plasmid containing the luciferase gene fused to five repeats of the κB motif of the IL-2R gene and enhancerless promoter of HTLV-I (κB-LUC). After 24 hours of incubation, lysates were prepared and assayed for luciferase activity. Ratio of luciferase activity in extracts of κB-LUC–transfected cells is expressed to that for dN-LUC–transfected cells. The results represent the mean of three experiments. (B) Northern blot analysis of Tax in various cell lines. Total RNA (20 μg of each) was isolated from various cell lines. Jurkat, MOLT-4, and H-9 are HTLV-I–uninfected T-cell lines. MT-2, HUT-102, SLB-1, TL-Om1, and C5/MJ are HTLV-I–infected T-cell lines. Blots were sequentially hybridized, exposed, stripped, and rehybridized with Tax and GAPDH probes.
(A) HTLV-I–infected T-cell lines express a constitutive NF-κB–related activity. Various cell lines were transfected with 10 μg of a reporter plasmid containing the luciferase gene fused to five repeats of the κB motif of the IL-2R gene and enhancerless promoter of HTLV-I (κB-LUC). After 24 hours of incubation, lysates were prepared and assayed for luciferase activity. Ratio of luciferase activity in extracts of κB-LUC–transfected cells is expressed to that for dN-LUC–transfected cells. The results represent the mean of three experiments. (B) Northern blot analysis of Tax in various cell lines. Total RNA (20 μg of each) was isolated from various cell lines. Jurkat, MOLT-4, and H-9 are HTLV-I–uninfected T-cell lines. MT-2, HUT-102, SLB-1, TL-Om1, and C5/MJ are HTLV-I–infected T-cell lines. Blots were sequentially hybridized, exposed, stripped, and rehybridized with Tax and GAPDH probes.
Among five HTLV-I–infected cell lines, four (MT-2, HUT-102, SLB-1, C5/MJ) expressed high levels of HTLV-I Tax transcript. In contrast, the TL-Om1 cell line48 failed to express significant amounts of Tax at the mRNA level (Fig 1B). TL-Om1 did not express Tax mRNA at all by a highly sensitive polymerase chain reaction method coupled with reverse transcription.49 These results suggest that Tax is not required to maintain NF-κB–dependent transcription in HTLV-I–infected T-cell lines.
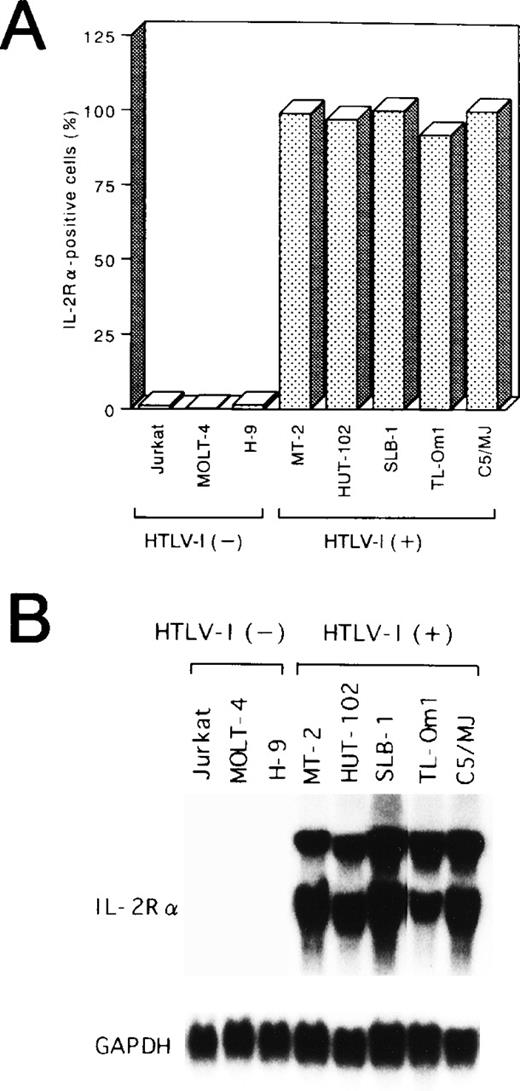
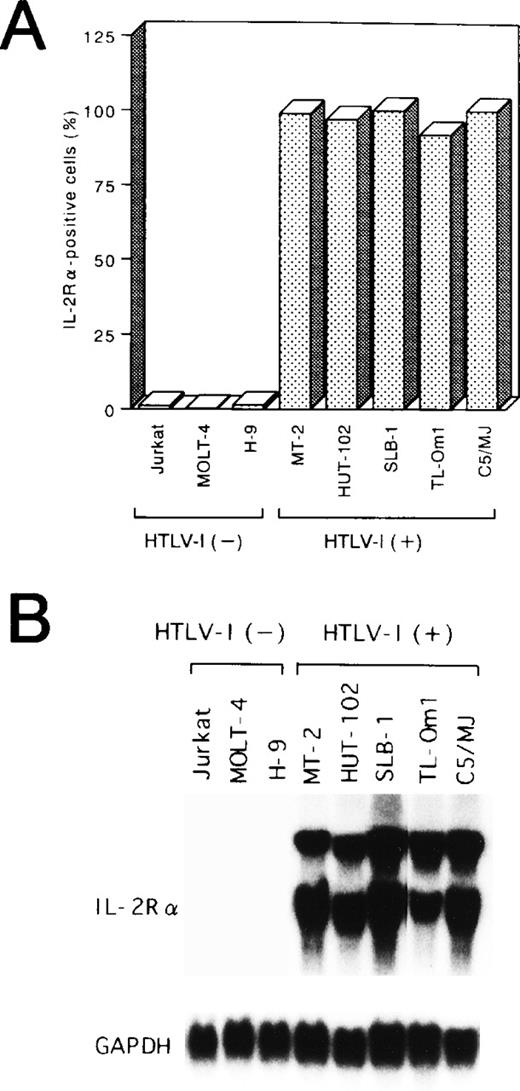
Potential alterations in cellular gene activation associated with the Tax-independent activation of NF-κB in TL-Om1 were next studied by measuring expression of the IL-2Rα protein and its mRNA. Like the other four Tax-expressing cell lines, TL-Om1 expressed IL-2Rα on its surface (Fig 2A). In addition, constitutive expression of the 25S and 16S forms of IL-2Rα mRNA50 was detected in TL-Om1, as well as the other four HTLV-I–infected cell lines, whereas it was not in uninfected cell lines (Fig 2B). These results showed that TL-Om1 has a Tax-independent constitutive expression of the IL-2Rα gene, possibly due to the activation of NF-κB.
Expression of IL-2R at protein (A) and mRNA (B) levels in various cell lines. (A) The percentages of positive cells for IL-2R are shown. (B) Total RNA (20 μg of each) was isolated from various cell lines and assessed for IL-2R or GAPDH mRNA expression.
Expression of IL-2R at protein (A) and mRNA (B) levels in various cell lines. (A) The percentages of positive cells for IL-2R are shown. (B) Total RNA (20 μg of each) was isolated from various cell lines and assessed for IL-2R or GAPDH mRNA expression.
Lower protein levels of IκBα in HTLV-I–infected T-cell lines.
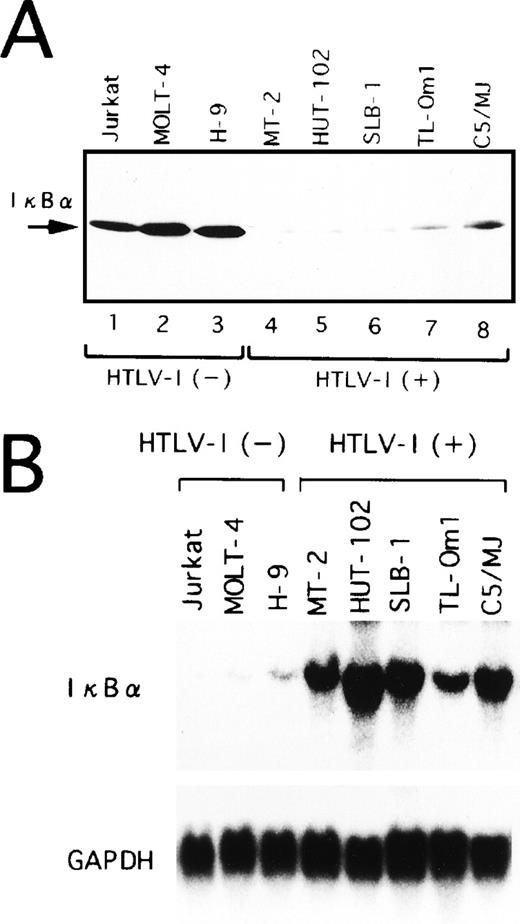
In a variety of cell lines, NF-κB is localized in the cytoplasm through binding to specific cytoplasmic molecules called IκBα. After cellular stimulation by multiple inducers, activation of signal transduction cascades leads to the degradation of IκBα. In agreement with prior studies,21 the protein level of IκBα was significantly lower in HTLV-I–infected cell lines (Fig 3A). More importantly, TL-Om1 cells, which do not express Tax, have reduced IκBα protein at a similar level to HTLV-I–infected cell lines expressing Tax. These results suggest that IκBα protein expression is downregulated in TL-Om1 by a Tax-independent mechanism.
Expression of IκB at protein (A) and mRNA (B) levels in various cell lines. (A) Whole-cell extracts were isolated from the cells and analyzed by Western blotting using anti-IκB antibody. (B) Total RNA (20 μg of each) was isolated from various cell lines and assessed for IκB or GAPDH mRNA expression.
Expression of IκB at protein (A) and mRNA (B) levels in various cell lines. (A) Whole-cell extracts were isolated from the cells and analyzed by Western blotting using anti-IκB antibody. (B) Total RNA (20 μg of each) was isolated from various cell lines and assessed for IκB or GAPDH mRNA expression.
Overexpression of IκBα mRNA in HTLV-I–infected T-cell lines.
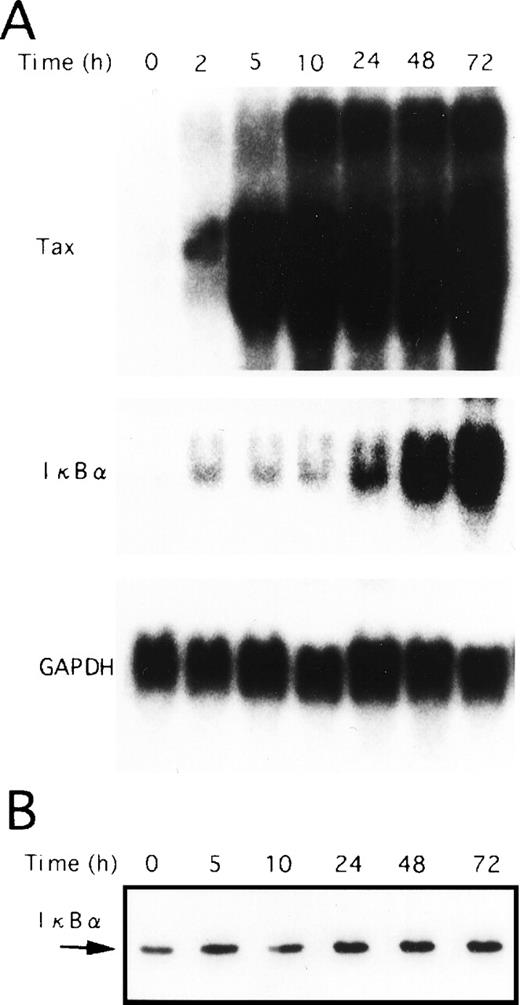
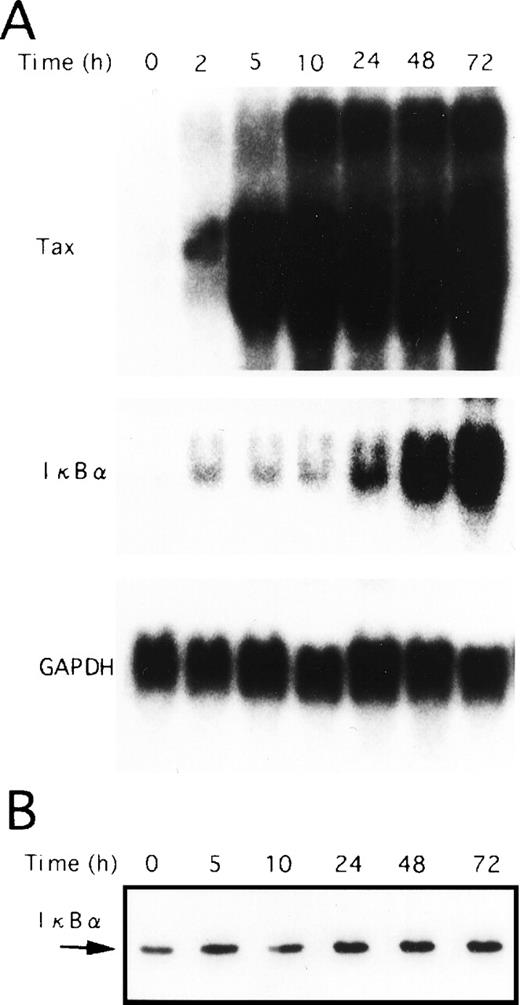
Next, we performed Northern blot analysis of IκBα mRNA in the T-cell lines characterized above. As shown in Fig 3B, the expression of IκBα mRNA in TL-Om1, as well as Tax-expressing cell lines, was strikingly higher than in uninfected ones. Thus, decreased IκBα protein level detected in HTLV-I–infected cell lines, including TL-Om1, is not due to impaired IκBα gene activity, but due to the rapid turnover of IκBα. It has been reported previously that Tax disrupts feedback regulation of NF-κB through destabilization of IκBα,17-21 thus establishing constitutive activation of NF-κB. JPX-9 cells are derived from a human T-cell line Jurkat, and they are permanently transfected with a metallothionein promoter-driven Tax expression vector.40 As a result of the expression of Tax in JPX-9 cells by CdCl2 treatment, IκBα mRNA expression was markedly upregulated (Fig4A). However, the total protein levels for IκBα remained constant (Fig 4B), possibly indicative of a more rapid turnover of IκBα. Taken together, these results show that Tax-mediated destabilization of IκBα is the mechanism for reduction of IκBα protein in HTLV-I–infected cell lines expressing Tax. These data also suggest that rapid degradation of IκBα is maintained by mechanisms other than Tax in TL-Om1 cells.
Tax-induced proteolytic degradation of IκB. JPX-9 cells were incubated with CdCl2 (20 μmol/L) for the indicated time periods and then collected for preparation of either total cellular RNA or whole-cell extracts. (A) Total RNA isolated from either nontreated or CdCl2-treated JPX-9 cells was subjected to Northern blot analysis with Tax, IκB, and GAPDH probes. (B) Whole-cell extracts isolated from CdCl2-treated JPX-9 cells were subjected to Western blotting using anti-IκB antibody.
Tax-induced proteolytic degradation of IκB. JPX-9 cells were incubated with CdCl2 (20 μmol/L) for the indicated time periods and then collected for preparation of either total cellular RNA or whole-cell extracts. (A) Total RNA isolated from either nontreated or CdCl2-treated JPX-9 cells was subjected to Northern blot analysis with Tax, IκB, and GAPDH probes. (B) Whole-cell extracts isolated from CdCl2-treated JPX-9 cells were subjected to Western blotting using anti-IκB antibody.
Enhanced IκBα degradation in primary ATL cells.
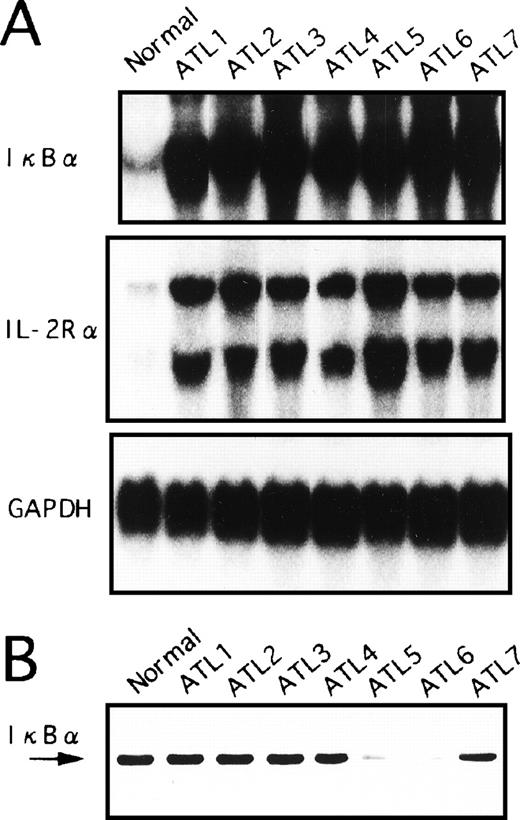
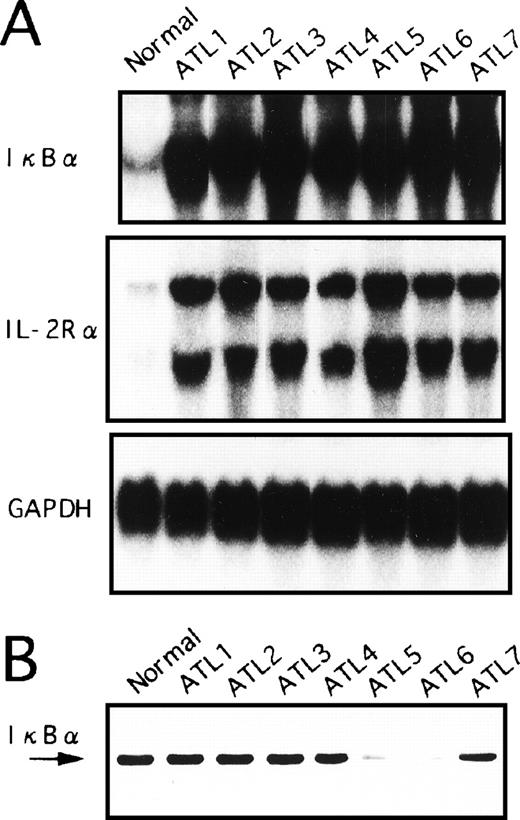
We had postulated that the IκBα/NF-κB pathways may already be activated in ATL leukemic cells in vivo. Therefore, we examined whether the expression of IκBα in freshly obtained PBMC of ATL patients is upregulated. PBMC were isolated from peripheral blood of seven ATL patients and RNA was prepared for Northern blot analysis. As shown in Fig 5A, IκBα mRNA levels in freshly isolated PBMC of ATL patients were much higher than those in PBMC from healthy control subjects. Furthermore, IL-2Rα expression was stronger in primary ATL cells than in normal PBMC (Fig 5A). These results are similar to the high expression of IκBα and IL-2Rα mRNA observed in HTLV-I–infected T-cell lines. In addition, Tax expression in the primary cells isolated from these patients was hard to estimate quantitatively with Northern and Western blot analyses (data not shown).
Expression of IκB at mRNA (A) and protein (B) levels in primary leukemic cells from ATL patients and normal healthy subjects. (A) Total RNA was extracted from PBMC and assessed for IκB, IL-2R, or GAPDH mRNA expression. A representative Northern blot is shown from patients with ATL and healthy subjects (normal). (B) PBMC obtained from patients with ATL or healthy subjects (normal) were assessed for IκB by Western blot analysis.
Expression of IκB at mRNA (A) and protein (B) levels in primary leukemic cells from ATL patients and normal healthy subjects. (A) Total RNA was extracted from PBMC and assessed for IκB, IL-2R, or GAPDH mRNA expression. A representative Northern blot is shown from patients with ATL and healthy subjects (normal). (B) PBMC obtained from patients with ATL or healthy subjects (normal) were assessed for IκB by Western blot analysis.
We next determined the protein levels of IκBα in blood mononuclear cells from patients with ATL and from healthy subjects. Whole-cell extracts were prepared from PBMC and subjected to Western blot analysis (Fig 5B). The IκBα reactivity band was strongly visible in PBMC from healthy subjects. In contrast, IκBα reactivity was weak in PBMC derived from two cases (ATL patients 5 and 6). The remaining five samples did not show apparent reduction of the bands. However, these results were not influenced by the contents of leukemic cells in these samples. While IκBα mRNA levels were dramatically upregulated in ATL cells, the total protein levels did not increase, possibly indicative of protein degradation.
Constitutive NF-κB binding activity in HTLV-I–infected T-cell lines and primary ATL cells.
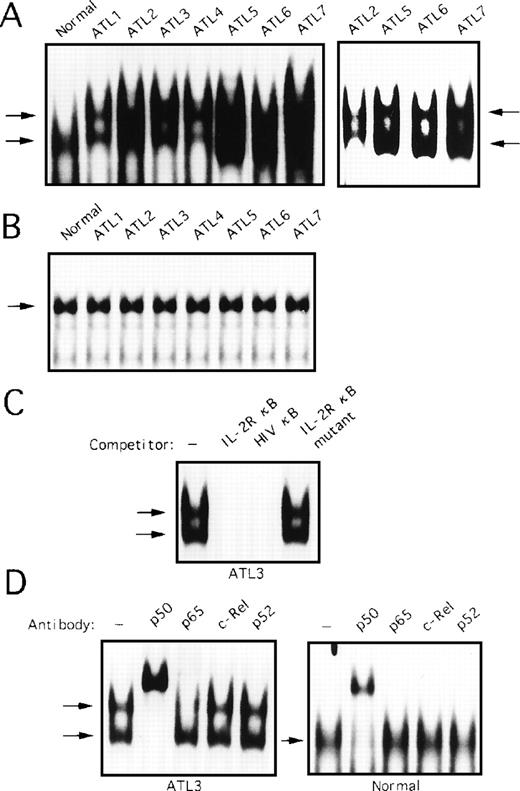
To determine whether degradation of IκBα protein in HTLV-I–infected T-cell lines was associated with NF-κB activation, nuclear extracts of cells were subjected to EMSA. In uninfected T-cell lines, no NF-κB–specific protein DNA complexes were detected in nuclear extracts by EMSA (Fig 6A, lanes 1 through 3). In contrast, enhanced NF-κB activity was present in each of the HTLV-I–infected T-cell lines tested (Fig 6A, lanes 4 through 8). The specificity of the binding activities was shown by competition with excess wild-type and mutant oligonucleotides (data not shown). Constitutive NF-κB binding activity in MT-2 cells was composed predominantly of c-Rel and p50, as antibodies against c-Rel and p50 partially inhibited formation of the NF-κB DNA complex and also produced a shifted complex (Fig 6B, left panel). Similar analyses showed that c-Rel and p50 subunits represented the main DNA binding components in HUT-102, SLB-1, and C5/MJ cells (data not shown). In TL-Om1 cells, two forms of complexes were detected (Fig 6A). Incubation of the nuclear extracts with various antibodies represented that the upper band contains p50 and p65, and the lower band contains only the p50 (Fig 6B, right panel).
NF-κB binding activity in various cell lines. Nuclear extracts were prepared from cells. Nuclear extracts (5 μg) were incubated with a [32P]-radiolabeled oligonucleotide that corresponds to the κB sequence of the IL-2R gene and analyzed by EMSA. (B) In super-shift assays, antibody specific for each NF-κB subunit (indicated above the lanes) was incubated with MT-2 and TL-Om1 extracts before probe addition.
NF-κB binding activity in various cell lines. Nuclear extracts were prepared from cells. Nuclear extracts (5 μg) were incubated with a [32P]-radiolabeled oligonucleotide that corresponds to the κB sequence of the IL-2R gene and analyzed by EMSA. (B) In super-shift assays, antibody specific for each NF-κB subunit (indicated above the lanes) was incubated with MT-2 and TL-Om1 extracts before probe addition.
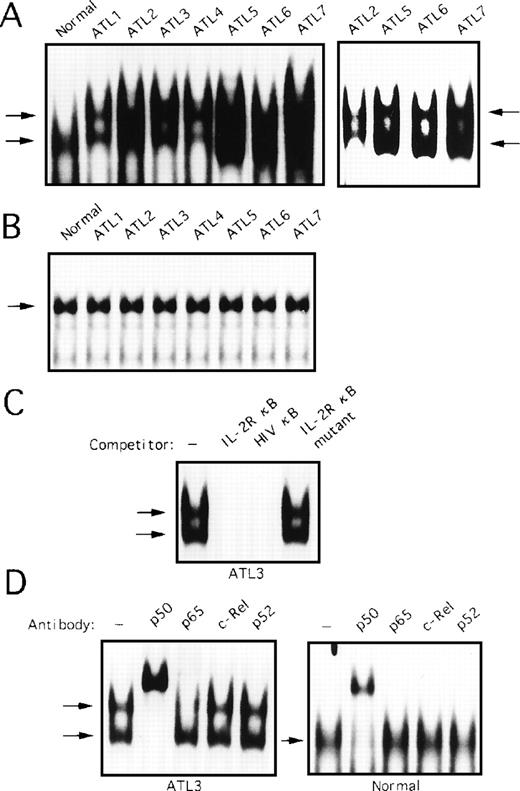
We determined whether degradation of IκBα protein in primary ATL cells involved abnormal NF-κB–specific DNA binding activity. To address this question, nuclear extracts of PBMC obtained from seven patients with ATL and healthy subjects, the same samples as analyzed in Fig 5, were subjected to EMSA. In extracts from healthy subjects, NF-κB–specific complex was detected (Fig7A, left panel). This protein DNA complex was composed primarily of p50; interaction with anti-p65, c-Rel, and p52 antibodies was negligible (Fig 7D, right panel). On the other hand, two forms of complexes were detected, an upper band and a lower band in extracts from primary ATL cells (Fig 7A, left panel). Although the observed complexes formed broad bands in patients 2, 5, 6, and 7, lighter exposure of the gel showed two bands in these cases (Fig 7A, right panel). The specificity of these complexes was verified by competition with an excess of wild-type and mutant oligonucleotides (Fig 7C). Incubation of the protein DNA complex with anti-p50 resulted in almost complete supershift of the upper and lower migrating bands (Fig 7D, left panel). Incubation with anti-p65 reduced only the upper band completely. Further, anti-c-Rel and p52 antibodies failed to shift any bands (Fig 7D, left panel). This result suggests that the upper band contains p50 and p65, and the lower band contains the p50 homodimers. No differences between ATL patients and healthy subjects in binding to the octamer motif on DNA were found (Fig 7B).
Activation of NF-κB in primary ATL cells. (A) Nuclear extracts (5 μg) from PBMC from patients with ATL or from healthy subjects (normal) were assessed for activation of NF-κB by EMSA. The right panel represents a lighter exposure of patients 2, 5, 6, and 7. (B) Nuclear extracts from patients with ATL or from healthy subjects (normal) were analyzed for protein binding to the octamer consensus sequence by EMSA. (C) Nuclear extracts from patient 3 sample were coincubated with 100-fold excess of unlabeled oligonucleotides and assessed in parallel. (D) Nuclear extracts were preincubated with NF-κB subunit-specific antibody, as indicated above each lane, before the addition of radiolabeled probe (as in Fig 6).
Activation of NF-κB in primary ATL cells. (A) Nuclear extracts (5 μg) from PBMC from patients with ATL or from healthy subjects (normal) were assessed for activation of NF-κB by EMSA. The right panel represents a lighter exposure of patients 2, 5, 6, and 7. (B) Nuclear extracts from patients with ATL or from healthy subjects (normal) were analyzed for protein binding to the octamer consensus sequence by EMSA. (C) Nuclear extracts from patient 3 sample were coincubated with 100-fold excess of unlabeled oligonucleotides and assessed in parallel. (D) Nuclear extracts were preincubated with NF-κB subunit-specific antibody, as indicated above each lane, before the addition of radiolabeled probe (as in Fig 6).
Transactivational activities of different members of the NF-κB/Rel family at the IL-2Rα κB site.
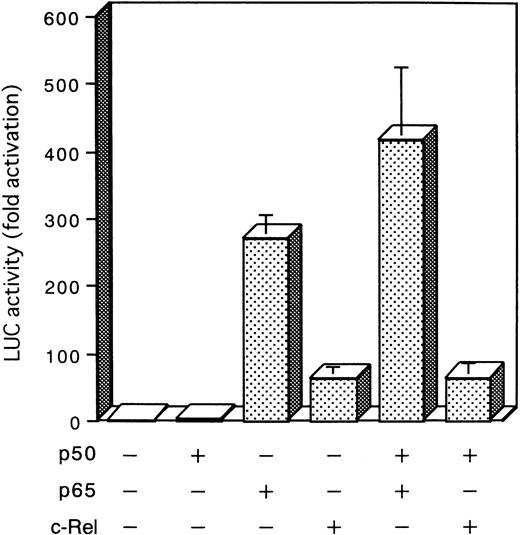
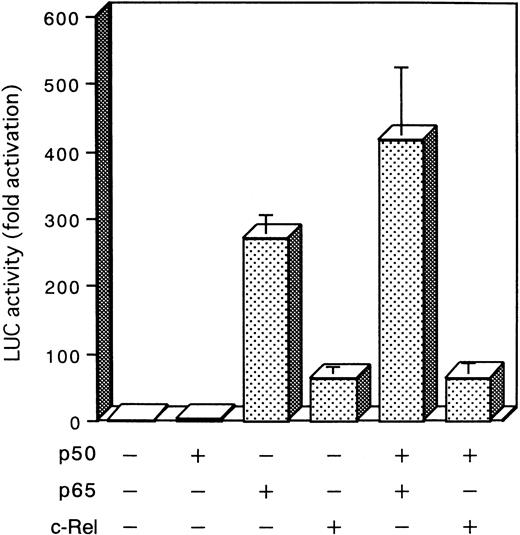
To determine the transcription activity of different subunit members of the NF-κB/Rel family, expression plasmids for three κB-related proteins, p50, p65, and c-Rel, were cotransfected in Jurkat cells with a reporter plasmid, κB-LUC. As shown in Fig 8, expression of p65 and c-Rel, by themselves or in combination with p50, led to an increase in luciferase activity, while expression of p50 was devoid of effect on the reporter gene transcription. These results recapitulate the observed EMSA and IL-2Rα expression data, indicating that p65 or c-Rel binding can activate NF-κB–dependent transcription. However, p50 homodimers can bind to the κB element in the IL-2Rα, but are unable to support transcriptional activation.
Effect of NF-κB subunit on NF-κB transcriptional activity. Jurkat cells were transfected by electroporation with a reporter plasmid (5 μg) containing the luciferase gene fused to five repeats of the κB motif of the IL-2R gene and enhancerless promoter of HTLV-I (κB-LUC) along with combinations of the cDNA expression plasmids encoding p50 (5 μg), p65 (5 μg), and c-Rel (5 μg) indicated at the bottom of the panel. After 24 hours of growth, cells were harvested and assayed for luciferase activity. Increase of the luciferase activity in cell extracts is shown relative to that after parental plasmid transfection. The mean ± standard error of mean (SEM) for three independent assays is presented.
Effect of NF-κB subunit on NF-κB transcriptional activity. Jurkat cells were transfected by electroporation with a reporter plasmid (5 μg) containing the luciferase gene fused to five repeats of the κB motif of the IL-2R gene and enhancerless promoter of HTLV-I (κB-LUC) along with combinations of the cDNA expression plasmids encoding p50 (5 μg), p65 (5 μg), and c-Rel (5 μg) indicated at the bottom of the panel. After 24 hours of growth, cells were harvested and assayed for luciferase activity. Increase of the luciferase activity in cell extracts is shown relative to that after parental plasmid transfection. The mean ± standard error of mean (SEM) for three independent assays is presented.
DISCUSSION
We analyzed for the first time the functional status of the NF-κB/IκBα pathway in primary leukemic cells from ATL patients. In all patients, NF-κB protein’s DNA binding activity was detected by EMSA in the leukemic cell extracts. Furthermore, this study shows that in contrast to healthy subjects, IκBα in freshly isolated leukemic cells from patients with ATL has already undergone proteolytic degradation. IκBα mRNA was upregulated in ATL cells of patients. However, IκBα protein levels did not increase in concert with the highly elevated expression of the mRNA. Furthermore, IκBα protein levels were significantly lower in two samples. Thus, increased turnover of the IκBα protein in leukemic cell extracts of patients with ATL was associated with activation of NF-κB. These results were similar to those observed in HTLV-I–infected and Tax-expressing T-cell lines. Recently, like IκBα, Tax-mediated breakdown of IκBβ was reported.51 52 In preliminary experiments, little or no detectable amount of IκBβ was found in primary ATL cells. Together, these results suggest that the degradation of IκBα and IκBβ may play a role in the constitutive activation of NF-κB in ATL.
Importantly, the identity of the shifted bands was confirmed by supershifting with NF-κB–specific antibodies. We observed that the dynamic alterations in NF-κB binding activity occurred in ATL patients. In all patients’ extracts, the DNA protein complex consists of p50/p65 heterodimers and p50 homodimers. In contrast, NF-κB binding activity in healthy subjects was composed of p50 homodimers alone. Interestingly, cotransfection with various NF-κB subunits indicated that p65 and c-Rel, but not p50, was able to activate transcription at the IL-2Rα κB site. The observation that constitutive binding of heterodimeric p50/p65 correlated with expression of IL-2Rα in ATL patients supports the notion that in vivo p50/p65 heterodimers and p50 homodimers can bind to the κB element in the IL-2Rα promoter, but only p50/p65 heterodimers can activate transcription.
HTLV-I Tax has been shown to induce a degradation of IκBα, which may contribute to induce the nuclear expression of a number of NF-κB/Rel species.17-21 NF-κB is known to mediate the Tax-dependent transactivation of IL-2Rα,12-14IL-1α,36 IL-6,27 IL-8,28 and tumor necrosis factor β genes.37 Considering the previously reported enhanced production of these inflammatory cytokines23,24,26-28,31 and the increased expression of surface molecules such as IL-2Rα22 in leukemic cells of ATL patients, it is not surprising that activated NF-κB/IκBα pathway was seen in ATL cells. However, consistent with previous studies,38,39 primary leukemic cells isolated from ATL patients did not express Tax at a significant level. Because viral expression in vivo differs significantly from that observed in cell lines in vitro, it is of interest to understand the differences of NF-κB activation between leukemic cells in vivo and cell lines established in vitro. Tax induces predominantly the c-Rel–containing complexes17,53 and transcriptionally activates the c-Rel gene.53 Consistent with these studies, in HTLV-I–infected T cells that constitutively express high levels of Tax, p50 and c-Rel were the major DNA binding components; similar c-Rel containing complexes were also observed previously,19,53 54 suggesting Tax-independent mechanisms by which primary ATL cells exhibit persistent nuclear expression of p50/p65 heterodimers.
In this study, we have shown that the ATL-derived TL-Om1 cell line fails to express detectable levels of Tax. However, TL-Om1 was shown to carry full-length proviral genome of HTLV-I by Southern blot analysis.48 It was also found that the HTLV-I LTR function was not enhanced in TL-Om1, as the LTR luciferase activity in TL-Om1 was one fourteenth of that in MT-2. Furthermore, Tax induced transcription from the LTR in TL-Om1 (data not shown). We have also shown the rapid degradation of IκBα in TL-Om1 cells, which may be directly involved in constitutive NF-κB activation. This line still expresses IL-2Rα mRNA and protein at high levels. In addition, TL-Om1 cells express chemokine genes such as IP-10 and I309, and intercellular adhesion molecule 1 at the same levels as those in Tax-expressing T-cell lines infected with HTLV-I.49 55These results suggest that Tax is not the only mechanism for constitutive expression of certain cellular genes in HTLV-I–infected T cells. We believe in the possibility of Tax-independent mechanisms operating for constitutive NF-κB activation in leukemic cells of ATL patients. TL-Om1 should help to identify new targets of NF-κB by comparing gene expression between this cell line and other Tax-expressing cell lines.
ACKNOWLEDGMENT
We thank Drs T. Taniguchi, J. Fujisawa, and K. Yamamoto for providing plasmids, Dr M. Nakamura for providing JPX-9, and Fujisaki Cell Center, Hayashibara Biochemical Laboratories Inc (Okayama, Japan) for providing Jurkat, HUT-102, and C5/MJ. We also thank Dr Y. Yamasaki for providing blood of ATL patients and M. Yamamoto and M. Sasaki for excellent technical assistance.
Supported in part by a Grant in Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan, and a Cooperative Research Grant No. 1998-10-A-14 from the Institute of Tropical Medicine, Nagasaki University.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Naoki Mori, MD, Department of Preventive Medicine and AIDS Research, Research Field of Pathogenesis and Clinical Sciences, Institute of Tropical Medicine, Nagasaki University, 1-12-4 Sakamoto, Nagasaki 852-8523, Japan.

![Fig. 6. NF-κB binding activity in various cell lines. Nuclear extracts were prepared from cells. Nuclear extracts (5 μg) were incubated with a [32P]-radiolabeled oligonucleotide that corresponds to the κB sequence of the IL-2R gene and analyzed by EMSA. (B) In super-shift assays, antibody specific for each NF-κB subunit (indicated above the lanes) was incubated with MT-2 and TL-Om1 extracts before probe addition.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2360/5/m_blod4071006abw.jpeg?Expires=1769220214&Signature=pLVnn7uSJxUl5rgZVGqk4kBjbx2MKTYxkFYJDSD0I2dm7U8JGArbusd2SraMls~20qStLLzZUWepgw1gABhqBNC-yYEHI0t~RbiEttmGlhxP3KSyske9yU0znoN4GhZO7E5CX~unblQPu6iLay89pAJ82syGS8ueu3MUJHwZsb8xdKBxhVuwAbalokW08WSY8JkXZaUzjVRnIYoZpJW8XSdhr3shpWLOaezvY8uP7dyFj19BMFUHSerykjV1vTtZcUqRgmaiw0ph0~gpEWKMnJxtYbh8~Sw8uu1dE237M65BJAV4fsWqXBhYwI2pnmlt7KLpda~REX0Rv0guCaWsIg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 6. NF-κB binding activity in various cell lines. Nuclear extracts were prepared from cells. Nuclear extracts (5 μg) were incubated with a [32P]-radiolabeled oligonucleotide that corresponds to the κB sequence of the IL-2R gene and analyzed by EMSA. (B) In super-shift assays, antibody specific for each NF-κB subunit (indicated above the lanes) was incubated with MT-2 and TL-Om1 extracts before probe addition.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2360/5/m_blod4071006abw.jpeg?Expires=1769817968&Signature=XeBm85xGIN9h6hj0yZ5sVsDN4Q7q3kyh0BwbKK8eDhNUM8YblVtUeHyzOV1dVIT7XBR79RSE5OIeKPeYBPWjkDKsG9UKMi5AW5S~FQ72bXYDPuQQ8qDD1zbKh-jPmkUuFugPcKK0ftif5M0SinNdCSAZTUO76uTXEgBhfNpWvZcFmJo6oaa69-VZZR1r0rGFbWCESLRhDQyNXuXFJLPaqWDc5DGFfF0~3Yvto7sOaN-49Qxb52PLsgy7JA29hk4bmtGLqMiEJBjryJA4RtFU37L5Bmz3aHqT5w3td9fkrHitv9sVR~HLHY0uwsoU-fInVyOBCh94MNXBuvug750Qjg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)