Abstract
Allogeneic bone marrow transplantation (allo-BMT) is associated with both graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) effect. In the present study, we examined the contribution of cytotoxic effector mechanisms, which are mediated by tumor necrosis factor- (TNF-), Fas ligand (FasL), or perforin, to GVHD and GVL effect in a murine BMT model. Bone marrow cells plus spleen cells (BMS) from wild-type, FasL-defective, or perforin-deficient donors were transferred into lethally irradiated recipients in the parent (C57BL/6) to F1 (C57BL/6 × DBA/2) BMT model with or without prior inoculation of DBA/2 leukemia L1210 or P815 mast cytoma cells. The effect of anti–TNF- antibody administration was also examined. Whereas the defect or blockade of each cytotoxic pathway could ameliorate lethal acute GVHD, the GVL effect was differentially affected. The wild-type BMS recipients died of acute GVHD within 50 days without residual leukemia cells. The FasL-defective BMS recipients showed 60%< survival over 80 days without acute GVHD or residual leukemia cells. Administration of anti–TNF- antibody resulted in early leukemia relapse and the recipients died within 25 days with massive leukemia infiltration in the liver. The perforin-deficient BMS recipients died within 60 days with residual leukemia cells. These results suggest that blockade of the Fas/FasL pathway could be used for ameliorating GVHD without impairing GVL effect in allo-BMT.
ALLOGENEIC bone marrow transplantation (allo-BMT) has been a potentially curative therapy for patients with a variety of diseases, including hematologic malignancies.1,2However, the major obstacle in allo-BMT is acute graft-versus-host disease (GVHD), which is caused by T cells present in the donor marrow graft.3-6 Although the incidence and the severity of acute GVHD can be dramatically improved by T-cell depletion or the combination of immunosuppressive agents such as cyclosporin, corticosteroid, and methotrexate, the risk of leukemia relapse could be increased in turn possibly due to the lack of antileukemia effect of allogeneic T cells infused.7
Molecular mechanisms involved in the pathogenesis of GVHD have been well studied in the past decades.8 Three cytotoxic pathways are reported to be involved in the pathogenesis of GVHD; tumor necrosis factor (TNF)/TNF receptor (TNFR) system, Fas (CD95)/Fas ligand (FasL) system, and perforin/granzyme system.9-17 Administration of anti–TNF-α antibody ameliorated acute GVHD in a mouse model,9 and TNFR p55-deficient recipient mice showed a reduced mortality after allo-BMT.10 Allo-BMT using FasL-defective gld (generalized lymphoproliferative disease) mice as a donor did not cause hepatic and cutaneous GVHD.11Recently, we demonstrated that a metalloproteinase inhibitor, which inhibits TNF-α and FasL release, prevented lethal acute GVHD in the parent to F1 spleen cell transfer model.12 It has been also reported that acute GVHD induced by transfer of spleen cells from mice doubly deficient in perforin and FasL, but not FasL alone, to major histocompatibility complex (MHC)-mismatched recipients was significantly ameliorated.13 Similarly, the onset of lethal GVHD was delayed in allo-BMT from perforin-deficient mice.11 These findings implicated all three of these cytotoxic pathways in the pathogenesis of GVHD.
The antileukemic effect of allo-BMT, so called graft-versus-leukemia (GVL) effect, was first reported by Barnes and Loutit in 1956.18 GVL effect is substantiated by clinical evidence that the risk of leukemia relapse is increased in patients who received T-cell–depleted allo-BMT or BMT from an identical twin and is considered to be caused by immunocompetent cells in the grafts.19-22 Although T and natural killer (NK) cells in the donor grafts are thought to play an important role in the GVL effect,19 23-32 its effector mechanisms are less clear than GVHD. Thus, it remains to be determined whether the same effector mechanisms are involved in GVHD and the GVL effect. If GVHD and the GVL effect are mediated by different mechanisms, it may be possible to spare the GVL effect while avoiding GVHD. Ideally, the GVL effect without GVHD is most beneficial for the effective treatment of leukemia patients after allo-BMT.
In the present study, to examine whether the effector mechanisms leading to the GVL effect and GVHD can be differentiated by their cytotoxic pathways, we performed a series of experiments using a murine GVL model. (C57BL/6 × DBA/2) F1 mice, which received a prior inoculation of L1210 leukemia or P815 mastcytoma cells, were lethally irradiated and transplanted with bone marrow (BM) cells and spleen cells from wild-type, gld, or perforin-deficient C57BL/6 mice or were administered with anti–TNF-α monoclonal antibody (MoAb). This GVL model is similar to human allo-BMT for the patients with leukemias, because the GVL effect was mediated by donor-derived immunocompetent cells and early leukemia relapse was observed in the recipients of T-cell–depleted BM or syngeneic BM. Our present results indicated that, whereas TNF, FasL, and perforin are all involved in the pathogenesis of GVHD, blockade of the Fas/FasL pathway can markedly ameliorate lethal acute GVHD without impairing the GVL effect in this model system. This suggests that selective amelioration of acute GVHD while preserving the GVL effect can be achieved on the basis of differential contribution of cytotoxic effector mechanisms to GVHD and GVL.
MATERIALS AND METHODS
Mice.
Six- to 8-week-old female C57BL/6 (B6, H-2b) and (C57BL/6 × DBA/2) F1 (BDF1, H-2b/d) mice were purchased from Japan SLC (Shizuoka, Japan) and maintained in our animal facilities. B6 gld/gld (gld) mice were purchased from Jackson Laboratory (Bar Harbor, ME). B6 perforin knock-out (PKO) mice were purchased from IBL (Gunma, Japan).
MoAbs.
Antimouse TNF-α (MP-6 XT22) and antimouse Thy1.2 (30H-12) MoAbs were obtained from PharMingen (San Diego, CA).
Cells.
L1210 murine T-cell leukemia (DBA/2 origin, H-2d), P815 murine mastcytoma cells (DBA/2 origin, H-2d), L929 murine fibrosarcoma (C3H/He origin, H-2k), A20 murine B-cell lymphoma (BALB/c origin, H-2d), and L5178Y murine T-cell lymphoma cells (DBA/2 origin, H-2d) were obtained from American Type Culture Collection (Rockville, MD). Cells were cultured in RPMI1640 medium supplemented with 10% fetal calf serum, antibiotics, 2-mercaptoethanol, and L-glutamine. Mouse FasL (mFasL) transfectant (mFasL/L5178Y) was generated by transfection of mFasL cDNA into L5178Y cells by electroporation as previously described.33
BMT.
Recipient BDF1 mice were lethally irradiated (12 Gy) using a60Co irradiator (MBR 1505 R2; Hitachi, Tokyo, Japan). BM cells were flushed from the shafts of femurs and tibias of donor mice and single-cell suspensions were prepared. In some experiments, BM cells were further treated with anti-Thy1.2 MoAb and rabbit complement and used as T-cell–depleted BM cells (TCD-BM). Single-cell suspensions of spleen cells from donor mice were used as the source of GVHD- and GVL-effector cells. Recipients received 2 × 107 BM cells with or without 2.5 × 107 spleen cells from wild-type, gld, or PKO B6 mice in 0.5 mL phosphate-buffered saline (PBS) via the tail vein. The day of BMT was designed as day 0. For anti–TNF-α treatment, 1 mg of MoAb was administered intraperitoneally (IP) on days −1 and 0 and then 0.5 mg was administered IP every other day until day 28. All recipients were maintained on sterilized water containing antibiotics (2 mg/mL neomycin sulfate) from day −2 to day 14 post-BMT. Recipient mice were monitored for clinical signs of GVHD, including survival, weight loss, diarrhea, alopecia, and hantched posture. A minimum of 5 recipient mice was used in each experimental group. Representative mice were killed at various time points post-BMT to harvest tissues for histological examination.
GVL model.
To evaluate GVL effect after BMT, mice were inoculated with 1 × 104 of L1210 or 1 × 105 of P815 cells via the tail vein 2 days before BMT. Representative mice were killed at various time points post-BMT to detect residual tumor cells by histological examination.
Histopathology.
Liver, spleen, and BM were harvested from recipients at various time points after BMT. Tissues were fixed in 10% buffered formalin and paraffin embedded. Sections were stained with hematoxylin and eosin and examined under microscopy. Peripheral blood was also obtained from recipients at various time points after BMT. Smeared specimens were stained with May-Giemsa and examined under microscopy.
Cytotoxic assay.
To analyze the sensitivity to TNF-α–mediated cytotoxicity, L1210, P815, and L929 cells (2 × 105/well) were cultured in 96-well flat-bottom microtiter plates in the presence or absence of serially diluted recombinant mouse TNF-α (PharMingen, San Diego, CA) for 24 hours at 37°C in a 5% CO2 humidified atmosphere. Cell viability was then measured by alamar Blue method according to the manufacturer’s instructions (Alamar Bioscience, Inc, Sacramento, CA). To analyze the sensitivity to Fas-mediated cytotoxicity, 51Cr-labeled L1210, P815, and A20 cells were cultured with mFasL/L5178Y at the indicated E/T ratios. Cytotoxicity was measured by the 6-hour 51Cr release assay as described previously.34
T-cell proliferation assay.
Responder spleen cells (2 × 105/well) from wild-type,gld, or PKO B6 mice were cultured with 100-Gy irradiated stimulator L1210 or P815 cells (2 × 104/well) in 96-well round-bottom microtiter plates at 37°C in a 5% CO2 humidified atmosphere. After 5 days, the cultures were pulsed for 18 hours with 0.5 μCi/well of [3H]thymidine (3H-TdR) and harvested using a Micro 96 Harvester (Skartron, Lier, Norway). Incorporated radioactivity was measured in a β counter (Micro Beta Plus; Wallac, Turku, Finland).
Cytotoxic T lymphocyte (CTL) assay.
To examine the generation of L1210- or P815-specific CTLs, spleen cells (1 × 106/mL) from wild-type, gld, or PKO B6 mice were cocultured with 100-Gy irradiated L1210 or P815 cells (1 × 105/mL) for 5 days and then restimulated with irradiated L1210 or P815 cells in the presence of 10 U/mL interleukin-2 (IL-2; Shionogi, Osaka, Japan) for 5 days. CTL activity was tested against 51Cr-labeled L1210 or P815 cells (5 × 103) at the indicated E/T ratios by the 6-hour51Cr release assay as described previously.34
RESULTS
Contribution of TNF-α, FasL, and perforin to lethal acute GVHD.
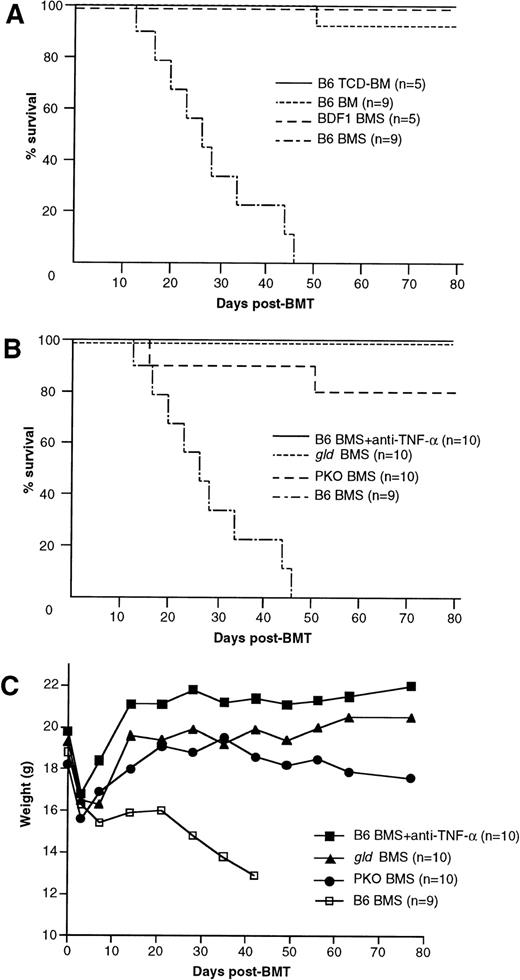
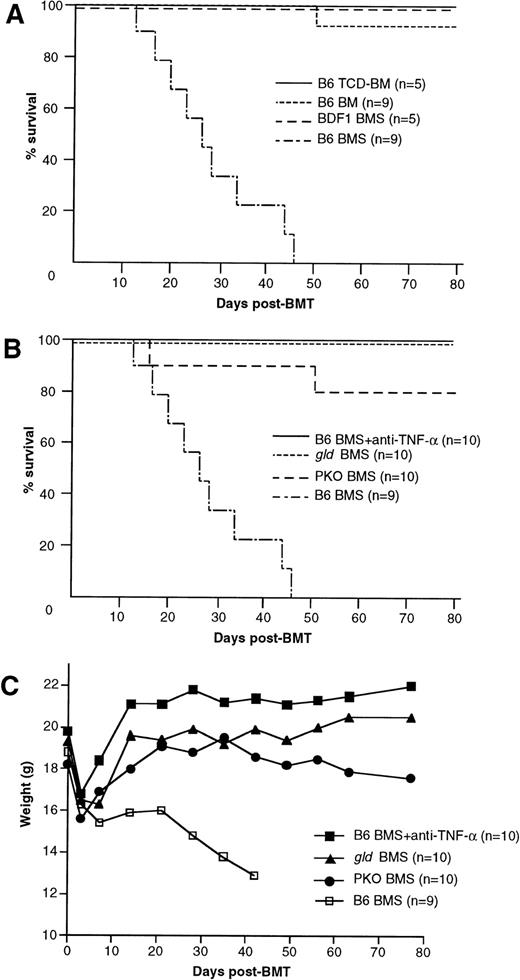
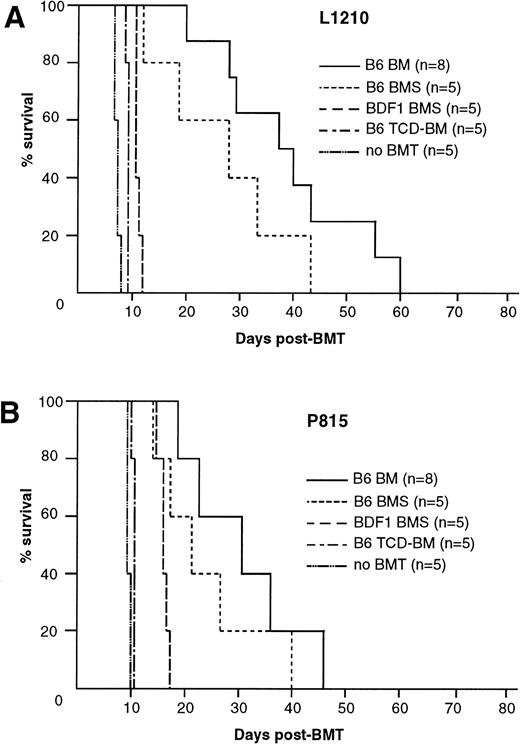
Our GVL model is based on the acute GVHD model in C57BL/6 (B6) parent to (C57BL/6 × DBA/2) F1 (BDF1) BMT. Thus, before we examine the involvement of TNF/TNFR, Fas/FasL, and perforin/granzyme pathways in the GVL effect, we verified their contribution to acute GVHD in the present system. The recipients that received B6 BM cells plus 2.5 × 107 spleen cells (B6 BMS) showed typical manifestations of acute GVHD, such as mortality within 50 days, progressive body weight loss, hantched posture, patchy alopecia, and diarrhea, whereas the recipients of BM cells alone (B6 BM), T-cell–depleted BM cells (B6 TCD-BM), or syngeneic BDF1 BM cells plus 2.5 × 107 spleen cells (BDF1 BMS) regained normal body weight rapidly after transplantation and showed no manifestation of acute GVHD. Survival rates of these recipients are shown in Fig 1A. When anti–TNF-α MoAb was administered to the recipients of wild-type B6 BMS, it successfully prevented the development of acute GVHD with rapid body weight recovery and survival over 80 days in all of the recipients (Fig 1B and C). There was no apparent manifestation of acute GVHD in these recipients, but liver sections obtained on day 28 showed slight infiltration of mononuclear cells (data not shown). The recipients of FasL-defectivegld BMS also exhibited long survival (>80 days) without hantched posture, alopecia, or diarrhea, but recovery of body weight was slightly impaired as compared with the anti–TNF-α group. The recipients of perforin-deficient PKO BMS exhibited a survival rate of 80% on day 80 with slightly impaired recovery of body weight and patchy alopecia in the skin, but did not manifest hantched posture or diarrhea. Liver sections harvested from the recipients of gldBMS or PKO BMS on day 28 exhibited moderate infiltration of mononuclear cells around bile ducts (data not shown). Because these recipients survived over 100 days, this histological manifestation is not considered to be the cause of mortality. These results indicated that the TNF-α, FasL, and perforin systems all play crucial roles in this model of lethal acute GVHD.
Mortality and body weight during acute GVHD after allo-BMT. (A) Lethal GVHD was induced by transfer of B6 BM cells plus 2.5 × 107 spleen cells (B6 BMS) into lethally irradiated BDF1 mice. Recipients of B6 BM cells (B6 BM), B6 T-cell–depleted BM cells (B6 TCD-BM), or syngeneic BDF1 BM cells plus spleen cells (BDF1 BMS) manifested no signs of GVHD and almost all recipients survived more than 80 days, except for 1 recipient of B6 BM. (B and C) Contribution of TNF--, FasL-, and perforin-mediated cytotoxic pathways to lethal acute GVHD. Lethally irradiated BDF1 mice were transplanted with BM cells plus 2.5 × 107 spleen cells from wild-type B6 (B6 BMS), B6-gld (gld BMS), or perforin-deficient (PKO BMS) mice. In another group, B6 BMS recipients were administered with anti–TNF- MoAb (B6 BMS + anti–TNF-). Mortality (B) and body weight (C) were monitored at the indicated days after BMT. Administration of anti–TNF- MoAb to the B6 BMS almost completely ameliorated the mortality and the body weight loss resulted from acute GVHD. Survival of the gld BMS and PKO BMS recipients was 100% and 80%, respectively, on day 80 after BMT. The data represent the results of three similar experiments.
Mortality and body weight during acute GVHD after allo-BMT. (A) Lethal GVHD was induced by transfer of B6 BM cells plus 2.5 × 107 spleen cells (B6 BMS) into lethally irradiated BDF1 mice. Recipients of B6 BM cells (B6 BM), B6 T-cell–depleted BM cells (B6 TCD-BM), or syngeneic BDF1 BM cells plus spleen cells (BDF1 BMS) manifested no signs of GVHD and almost all recipients survived more than 80 days, except for 1 recipient of B6 BM. (B and C) Contribution of TNF--, FasL-, and perforin-mediated cytotoxic pathways to lethal acute GVHD. Lethally irradiated BDF1 mice were transplanted with BM cells plus 2.5 × 107 spleen cells from wild-type B6 (B6 BMS), B6-gld (gld BMS), or perforin-deficient (PKO BMS) mice. In another group, B6 BMS recipients were administered with anti–TNF- MoAb (B6 BMS + anti–TNF-). Mortality (B) and body weight (C) were monitored at the indicated days after BMT. Administration of anti–TNF- MoAb to the B6 BMS almost completely ameliorated the mortality and the body weight loss resulted from acute GVHD. Survival of the gld BMS and PKO BMS recipients was 100% and 80%, respectively, on day 80 after BMT. The data represent the results of three similar experiments.
Characterization of L1210 leukemia and P815 mastcytoma cells.
We used L1210 T-cell leukemia and P815 mastcytoma cells in our GVL model. Both L1210 and P815 are derived from a DBA/2 (H-2d) mouse. Both tumor cells showed moderate expression of Fas and low expression of TNF-RI and TNF-RII detected by flow cytometry (data not shown). Intravenous (IV) inoculation of 1 × 104 L1210 and 1 × 105 P815 cells into BDF1 (H-2b/d) mice resulted in 100% death of recipients within 8 and 12 days, respectively, with marked hepatosplenomegaly. B6 (H-2b) mice completely rejected these cells (data not shown).
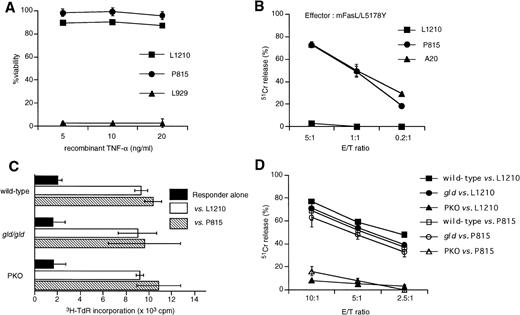
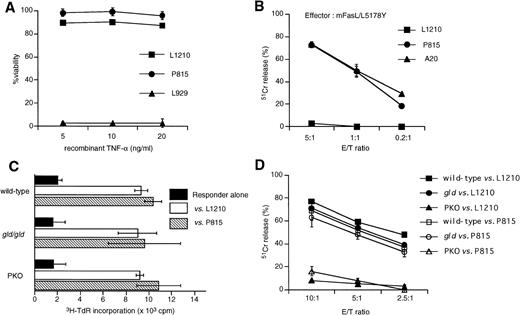
To determine how L1210 and P815 cells could be eliminated in vivo in the GVL model, we first examined the susceptibility of L1210 and P815 cells to TNFR- or Fas-mediated cytotoxicity in vitro. L1210 and P815 cells were cultured in the presence of recombinant mouse TNF-α or cultured with mouse FasL transfectant (mFasL/L5178Y) cells. Neither L1210 nor P815 was susceptible to TNF-α in vitro, whereas L929 fibrosarcoma showed high susceptibility to TNF-α (Fig 2A). Although both L1210 and P815 cells express Fas, their in vitro susceptibilities to Fas-mediated cytotoxicity were different. As shown in Fig 2B, P815 cells were susceptible to Fas-mediated cytotoxicity, whereas L1210 were not. We next examined whether spleen cells from wild-type, gld, or PKO B6 mice can equally respond to L1210 or P815 cells. Proliferative responses of the gld or PKO spleen cells to irradiated L1210 or P815 cells were comparable with that of wild-type B6 spleen cells (Fig2C). We also tested CTL activity after the in vitro stimulation of wild-type, gld, or PKO spleen cells with irradiated target tumor cells (Fig 2D). PKO-derived CTL exhibited almost no or little killing activity against both L1210 and P815 cells, whereas killing activity of CTL from gld mice was almost comparable to that from wild-type B6 (Fig 2D). These in vitro results suggested that L1210 cells would be killed by predominantly by the perforin pathway but not by Fas or TNF-α pathway and that P815 cells would be killed by CTLs predominantly by the perforin pathway despite their susceptibility to FasL transfectants.
Characterization of L1210 murine leukemia and P815 murine mastcytoma cells. (A) L1210 and P815 cells were cultured in the presence of recombinant TNF- for 24 hours and then cell viability was analyzed using the alamar Blue method. Both L1210 and P815 were resistant to TNF-, whereas L929 was susceptible to TNF-. (B)51Cr-labeled L1210 or P815 cells were cocultured with murine FasL transfectant (mFasL/L5178Y) cells at the indicated E/T ratios for 6 hours and then cytotoxicity was measured by51Cr-release assay. (C) Proliferative response of spleen cells obtained from wild-type, gld, or PKO B6 mice against allogeneic L1210 or P815 cells. Spleen cells (2 × 105) were cultured with irradiated L1210 or P815 cells (2 × 104) for 5 days and pulsed with 3H-TdR during the last 16 hours. Spleen cells were prepared from one mouse of each strain and were used immediately after preparation. Data are represented as the mean ± SD of triplicated samples. Spleen cells obtained from each mouse responded equally to L1210 or P815 cells. (D) Killing of L1210 cells by CTL derived from wild-type, gld, and PKO B6 mice. Spleen cells were collected after 5 days of coculture with irradiated L1210 cells as described in (B) and restimulated with irradiated L1210 cells in the presence of 10 U/mL IL-2 for 5 days. CTL activity was tested against 51Cr-labeled L1210 or P815 target cells at the indicated E/T ratios. Although killing activity of CTL derived from gld mice was only slightly diminished as compared with that from wild-type mice, PKO-derived CTL exhibited no significant cytotoxicity against L1210 cells. Data are represented as the mean ± SD of triplicated samples. A representative of three experiments is shown.
Characterization of L1210 murine leukemia and P815 murine mastcytoma cells. (A) L1210 and P815 cells were cultured in the presence of recombinant TNF- for 24 hours and then cell viability was analyzed using the alamar Blue method. Both L1210 and P815 were resistant to TNF-, whereas L929 was susceptible to TNF-. (B)51Cr-labeled L1210 or P815 cells were cocultured with murine FasL transfectant (mFasL/L5178Y) cells at the indicated E/T ratios for 6 hours and then cytotoxicity was measured by51Cr-release assay. (C) Proliferative response of spleen cells obtained from wild-type, gld, or PKO B6 mice against allogeneic L1210 or P815 cells. Spleen cells (2 × 105) were cultured with irradiated L1210 or P815 cells (2 × 104) for 5 days and pulsed with 3H-TdR during the last 16 hours. Spleen cells were prepared from one mouse of each strain and were used immediately after preparation. Data are represented as the mean ± SD of triplicated samples. Spleen cells obtained from each mouse responded equally to L1210 or P815 cells. (D) Killing of L1210 cells by CTL derived from wild-type, gld, and PKO B6 mice. Spleen cells were collected after 5 days of coculture with irradiated L1210 cells as described in (B) and restimulated with irradiated L1210 cells in the presence of 10 U/mL IL-2 for 5 days. CTL activity was tested against 51Cr-labeled L1210 or P815 target cells at the indicated E/T ratios. Although killing activity of CTL derived from gld mice was only slightly diminished as compared with that from wild-type mice, PKO-derived CTL exhibited no significant cytotoxicity against L1210 cells. Data are represented as the mean ± SD of triplicated samples. A representative of three experiments is shown.
Establishment of the GVL model.
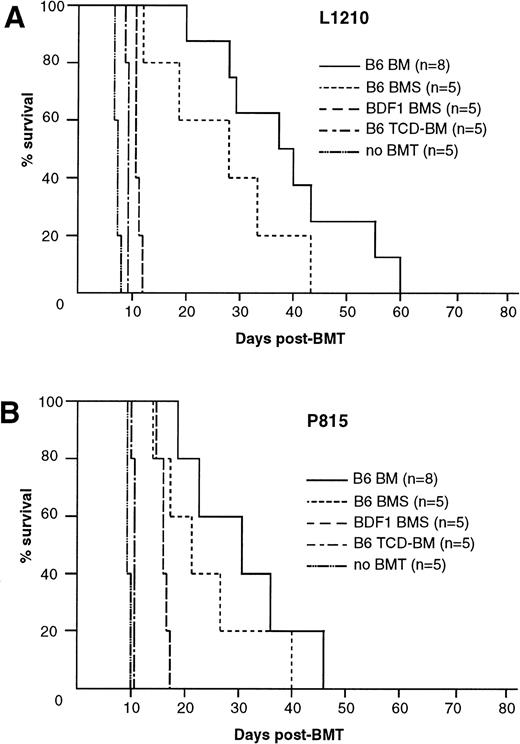
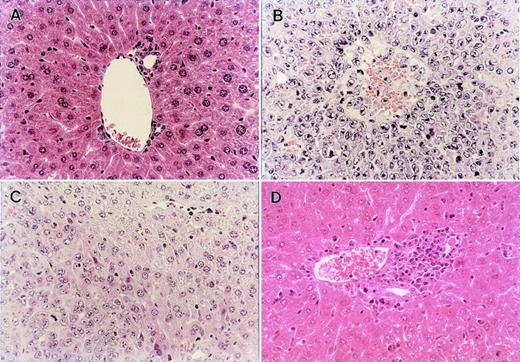
IV inoculation of 1 × 104 L1210 leukemia cells into BDF1 mice resulted in 100% mortality within 8 days with marked hepatosplenomegaly when the recipients received only irradiation 2 days after inoculation without BMT (Fig 3A). When BMT was performed 2 days after leukemia inoculation, all recipients of B6 TCD-BM and BDF1 BMS died within day 12 and 14, respectively, with marked hepatosplenomegaly. Liver sections obtained from these recipients showed massive leukemia infiltration (Fig4B). Thus, these two groups of recipients exhibited poor GVL effect. The recipients of B6-BM survived longer than these two groups and did not manifest apparent hepatosplenomegaly. However, these recipient mice finally died within 60 days, probably due to the leukemia, because their liver sections obtained on day 28 showed residual leukemia cells (Fig 4C) with only mild infiltration of mononuclear cells around the bile ducts and almost all recipients of B6 BM without leukemia inoculation survived more than 80 days with no apparent manifestation of GVHD (Fig 1A). In contrast, the recipients of B6 BMS exhibited a similar survival to the recipients without inoculation of leukemia cells and sections of the liver obtained on day 28 showed manifestations of severe acute GVHD, but no residual leukemia cell (Fig 4D). Similar results were observed by using another tumor P815 cells (Fig 3B). These results indicated that the transfer of B6 BMS exerted both GVL effect and lethal acute GVHD in this system.
GVL effect after allo-BMT. GVL effect was evaluated in BDF1 mice that were inoculated IV with 1 × 104 L1210 cells (A) or 1 × 105 P815 cells (B) 2 days before the BMT as described in Fig 1A. (A) Mice inoculated with L1210 and P815 cells died within 8 and 10 days, respectively, when they received irradiation alone without BMT (no BMT). Transfer of B6 TCD-BM or syngeneic BDF1 BMS resulted in early leukemia relapse. Recipients of B6 BM or BMS survived longer than the above-noted three groups, but all mice died within 60 days. Data represent the results of three similar experiments.
GVL effect after allo-BMT. GVL effect was evaluated in BDF1 mice that were inoculated IV with 1 × 104 L1210 cells (A) or 1 × 105 P815 cells (B) 2 days before the BMT as described in Fig 1A. (A) Mice inoculated with L1210 and P815 cells died within 8 and 10 days, respectively, when they received irradiation alone without BMT (no BMT). Transfer of B6 TCD-BM or syngeneic BDF1 BMS resulted in early leukemia relapse. Recipients of B6 BM or BMS survived longer than the above-noted three groups, but all mice died within 60 days. Data represent the results of three similar experiments.
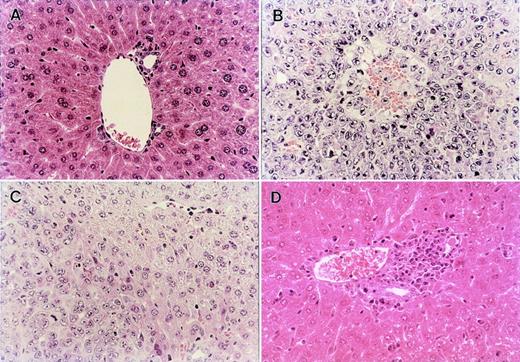
Histopathological examination of the liver obtained from normal BDF1 and the recipients of B6 TCD-BM, B6 BM, or B6 BMS in the GVL experiment shown in Fig 3A. (A) Liver section from an age-matched normal BDF1 mouse. (B) Massive leukemia cell infiltration was observed in the liver section from the recipients of B6 TCD-BM at the time of death. (C) Liver section obtained from the recipients of B6 BM on day 28 after BMT showed residual leukemia cells among normal liver tissue. (D) Liver section from the recipients of B6 BMS on day 28 after BMT showed severe mononuclear cell infiltration, but there was no residual leukemia cell in any fields of the section. Each figure shows a representative of three similar experiments. Similar results were also observed by using another tumor P815 cells. Original magnification × 400.
Histopathological examination of the liver obtained from normal BDF1 and the recipients of B6 TCD-BM, B6 BM, or B6 BMS in the GVL experiment shown in Fig 3A. (A) Liver section from an age-matched normal BDF1 mouse. (B) Massive leukemia cell infiltration was observed in the liver section from the recipients of B6 TCD-BM at the time of death. (C) Liver section obtained from the recipients of B6 BM on day 28 after BMT showed residual leukemia cells among normal liver tissue. (D) Liver section from the recipients of B6 BMS on day 28 after BMT showed severe mononuclear cell infiltration, but there was no residual leukemia cell in any fields of the section. Each figure shows a representative of three similar experiments. Similar results were also observed by using another tumor P815 cells. Original magnification × 400.
Contribution of TNF-α, FasL, and perforin to the GVL effect.
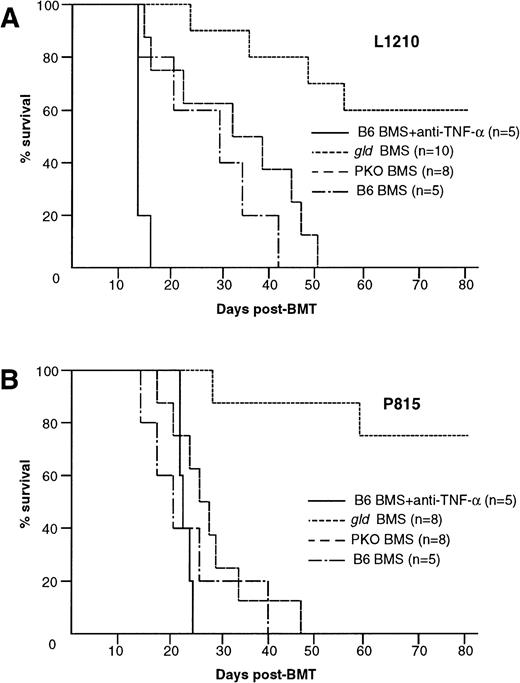
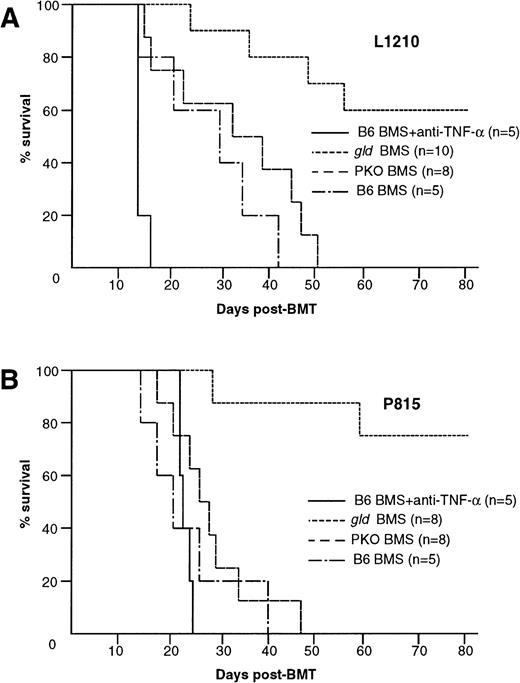
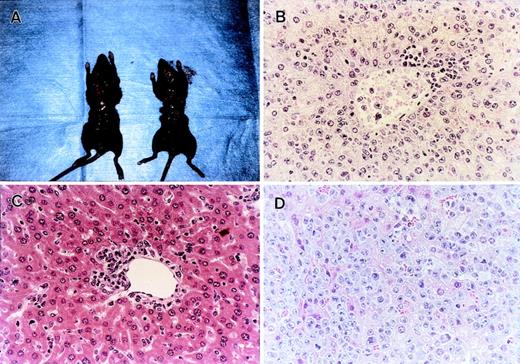
We showed that lethal acute GVHD could be ameliorated by modulation of either TNF-α, FasL, or perforin system (Fig 1B). Considering clinical application, it is important to examine how these modulations affect the GVL effect. We then used gld or PKO B6 mice as the BMS donors and anti–TNF-α MoAb in our GVL model (Fig 5A and B). Interestingly, after inoculation of L1210 cells, all B6 BMS recipients that received anti–TNF-α MoAb died within 12 days with marked hepatosplenomegaly (Figs 5A and 6A), and the liver sections obtained from these mice showed massive infiltration of leukemia cells (Fig 6B). On the other hand, the recipients ofgld BMS exhibited survival rates of 100% on day 28 and 60% on day 80 (Fig 5A). No residual leukemia cell was observed in liver, spleen, BM, and peripheral blood in representative mice killed on day 28 or mice that died on days 24, 35, 48, and 55 (not all data shown). Liver sections obtained from representative mice on day 28 showed focal infiltration of mononuclear cells in portal areas, but there was no leukemia infiltration (Fig 6C). Although the cause of death of these 4 mice that died within 80 days was not clear, cachexia and/or infection were suspected because these mice showed a slight body weight loss and some mice showed manifestation of infection when they died. In fact, serum TNF-α levels on day 7 were relatively higher in recipients inoculated with tumor cells before BMT than recipients without tumor cells (data not shown). All recipients of PKO BMS died within 60 days and their liver sections on day 28 showed residual leukemia cells (Fig6D). These residual leukemia cells might contribute to the mortality, because the recipients of PKO BMS could survive more than 80 days without leukemia inoculation (Fig 1B). All recipients of gld or PKO BMS received anti–TNF-α MoAb died within day 12 (data not shown). Similar results were obtained by using another tumor P815 cells, which are sensitive to in vitro Fas-mediated cytotoxicity, in contrast to L1210 (Fig 5B). These results indicated that TNF-α and to a lesser extent perforin play critical roles in the GVL effect in the present system and that the blockade of FasL could spare the GVL effect while preventing lethal acute GVHD.
Contribution of TNF-–, FasL-, or perforin-mediated cytotoxicity to the GVL effect. GVL effect was evaluated in BDF1 mice that were inoculated IV with 1 × 104 L1210 or 1 × 105 P815 cells 2 days before the BMT as described in Fig1B. (A) Administration of anti–TNF- MoAb to the recipients of B6 BMS resulted in early death within 16 days. Six of 10 gld BMS recipients survived more than 80 days, whereas all B6 BMS or PKO BMS recipients died within 51 days. (B) Similar results were also observed by using another tumor P815 cells. Administration of anti–TNF- MoAb resulted in early death within 24 days. Six of 8 gld BMS recipients survived more than 80 days, whereas all B6 BMS or PKO BMS recipients died within 47 days. Data represent the results of three similar experiments.
Contribution of TNF-–, FasL-, or perforin-mediated cytotoxicity to the GVL effect. GVL effect was evaluated in BDF1 mice that were inoculated IV with 1 × 104 L1210 or 1 × 105 P815 cells 2 days before the BMT as described in Fig1B. (A) Administration of anti–TNF- MoAb to the recipients of B6 BMS resulted in early death within 16 days. Six of 10 gld BMS recipients survived more than 80 days, whereas all B6 BMS or PKO BMS recipients died within 51 days. (B) Similar results were also observed by using another tumor P815 cells. Administration of anti–TNF- MoAb resulted in early death within 24 days. Six of 8 gld BMS recipients survived more than 80 days, whereas all B6 BMS or PKO BMS recipients died within 47 days. Data represent the results of three similar experiments.
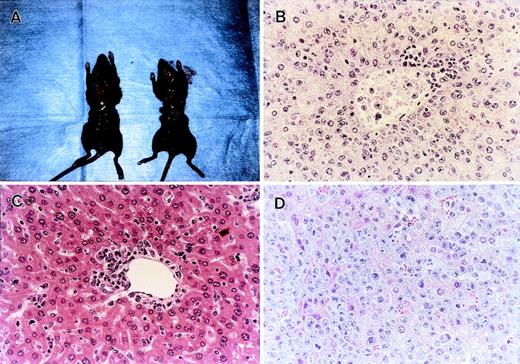
Histopathological examination of the GVL experiments shown in Fig 5A. (A) Administration of anti–TNF- MoAb resulted in early death with marked hepatosplenomegaly (left) compared with an age-matched normal mouse (right). The liver section obtained at the time of death showed massive leukemia infiltration (B). (C) Liver section obtained from gld BMS recipients on day 28 showed focal mononuclear infiltration around portal area, but there was no residual leukemia cells in any fields of the section. Residual leukemia cell was not detected in spleen, BM, or peripheral blood. (D) Residual leukemia cells were observed in small areas of the liver section from the PKO BMS recipients. Each figure shows a representative of three similar experiments. Similar results were also observed by using another tumor P815 cells. Original magnification × 400.
Histopathological examination of the GVL experiments shown in Fig 5A. (A) Administration of anti–TNF- MoAb resulted in early death with marked hepatosplenomegaly (left) compared with an age-matched normal mouse (right). The liver section obtained at the time of death showed massive leukemia infiltration (B). (C) Liver section obtained from gld BMS recipients on day 28 showed focal mononuclear infiltration around portal area, but there was no residual leukemia cells in any fields of the section. Residual leukemia cell was not detected in spleen, BM, or peripheral blood. (D) Residual leukemia cells were observed in small areas of the liver section from the PKO BMS recipients. Each figure shows a representative of three similar experiments. Similar results were also observed by using another tumor P815 cells. Original magnification × 400.
DISCUSSION
It is now established that mature T cells in the marrow graft are responsible for both initiating acute GVHD and exerting GVL effect.3-6,19,22 A dramatic decrease in the incidence of GVHD after clinical allo-BMT could be achieved by employing various marrow manipulation protocols designed to eliminate T cells from the donor graft.35-39 However, one potential disadvantage of this approach is that the transfer of T-cell–depleted marrow has been associated with a higher incidence of leukemic relapse.7,22,40-42 Although it has been reported that both T and NK cells were responsible for the GVL effect,23-32 it remains still unclear whether GVHD and the GVL effect are mediated by distinct lymphocyte subpopulations and can be separately mediated. In a human in vitro study, ricin-conjugated anti-CD25 MoAb selectively depleted response of donor lymphocytes to recipient lymphoblasts while conserving response against leukemia cells.23 It has been shown that IL-2 could be administered to recipient mice to enhance the antitumor effect of NK cells without exacerbation of GVHD and that NK cells rather suppressed GVHD at least in part through the production of transforming growth factor-β (TGF-β).24 In contrast to these previous reports describing the cellular mechanisms of the GVL effect, our present study is the first demonstration that GVHD and the GVL effect can be differentiated by their molecular mechanisms of the cytotoxic pathway at the effector phase. In our GVL model, blockade of the Fas/FasL interaction, using FasL-defectivegld mice as a donor, efficiently prevented lethal acute GVHD without impairing the GVL effect. Blockade of the perforin pathway, using PKO mice as a donor, could ameliorate acute GVHD but failed to exert sufficient GVL effect. Thus, these results suggest that blockade of the Fas/FasL pathway could prevent lethal acute GVHD while preserving sufficient GVL effect in patients with certain leukemias.
In the present parent to F1 BMT system, all the TNF-α, FasL, and perforin pathways were shown to contribute to the mortality of acute GVHD. These results are almost consistent with previous reports but different in some points.9-11,13-17 In contrast to the report by Baker et al,11 almost all recipients ofgld or PKO BMS showed long survival (>80 days) and did not manifest severe cachexia (Fig 1B and C). Liver sections from thegld BMS recipients on day 28 after BMT showed moderate mononuclear cell infiltration around bile ducts (data not shown), whereas minimal inflammatory change was observed in the recipients ofgld T cells in their report.11 These differences may result from different BMT systems (B6 to BDF1 BMT v B6 to LP/J BMT).
Recently, two predominant molecular mechanisms of lymphocyte-mediated cytotoxicity have been shown.43-47 The perforin/granzyme pathway is the dominant one for CD8+ CTL and NK cells that play a classical defense role against infected or transformed host cells.43,45 On the other hand, the Fas pathway needs Fas receptor expression on target cells and may be principally involved in regulating immune responses such as deletion of activated or self-reactive T cells.43,44 It has been reported that perforin is a key molecule for tumor surveillance by CD8+ T cells and NK cells in vivo, whereas the Fas system may play only a minor role even if tumor cells were transfected with Fas receptor.48,49 In our present study, L1210 cells are resistant to Fas-mediated cytotoxicity and the GVL effect was thought to be predominantly mediated by the perforin pathway. In this regard, most human tumor cells have been shown to be resistant to Fas-mediated apoptosis even if Fas receptor is expressed on these cells.50-55 Dirks et al54 showed that most myeloid cell lines were resistant to anti-Fas antibody-induced apoptosis. Karawajew et al55 reported that Fas receptor was expressed by most of leukemic blasts obtained from patients with T- and B-lineage acute lymphoblastic leukemia, but that cross-linking of these receptors by anti-Fas antibody was either not able to initiate apoptosis or induced low rates of apoptosis. In addition, our hypothesis that blockade of the Fas/FasL interaction prevent lethal acute GVHD without impairing the GVL effect was also confirmed by using Fas-sensitive P815 cells in our GVL model. These results suggest that the Fas/FasL pathway seems not to be a major pathway when CTL kills leukemia cells. Therefore, it is considered that the perforin pathway may play an essential role in elimination of residual leukemia cells after allo-BMT and thus the perforin pathway should be preserved for exerting the GVL effect. It is also advantageous to spare the perforin pathway for preventing infectious disease, which is one of major complications after allo-BMT, because infected host cells are eliminated predominantly by this pathway. Because the Fas/FasL system makes only a minor contribution to the GVL effect, it should be impaired to reduce acute GVHD.
Our present results also showed a marked impairment of the GVL effect in the recipients receiving anti–TNF-α MoAb. The L1210 cells used were not susceptible to recombinant TNF-α in vitro (Fig 2A). It has been reported that the soluble form (sTNF) and membrane-bound form (mTNF) of TNF-α exhibit different cytotoxicity.56-58mTNF, but not sTNF, can induce target cell apoptosis via TNFR p75, whereas both can induce apoptosis via TNFR p55. Because flow cytometric analysis showed the expression of both TNFR p55 and p75 on the surface of L1210 cells (data not shown), L1210 might be killed by mTNF expressed on CD8+ CTL and/or activated macrophages. Alternatively, it is possible that TNF-α serves as an immunoregulatory factor. Because TNF-α is thought to be involved in both induction and effector phases of GVHD,59 60administration of anti–TNF-α MoAb might diminish not only direct cytotoxic activity of TNF-α, but also T-cell activation responsible for the GVL effect, resulting in the abrogation of both GVHD and the GVL effect.
In summary, we have established a murine GVL model that enabled us to demonstrate the differential contribution of cytotoxic effector mechanisms to the GVL effect and GVHD. Although only two murine tumor cell lines were tested in the present study, this model suggested that GVHD and the GVL effect can be separately modulated. In our present system, blockade of the Fas pathway is thought to be most suitable for preventing lethal acute GVHD without impairing the GVL effect. Further studies with various tumors are needed to generalize this notion. It is also necessary to investigate the contribution of Fas/FasL interactions to the GVL effect other than the direct cytotoxicity such as by promoting the expansion and maturation of donor T cells as previously described by Via et al.14 A better understanding of the molecular mechanisms involved in GVHD and the GVL effect would allow the development of new strategies to prevent GVHD while enhancing the beneficial GVL effect, which will decrease the incidence of leukemic relapse after allo-BMT. We hope our present results provide a new prospect in the field of clinical BMT.
ACKNOWLEDGMENT
The authors thank K. Saito, K. Seino, K. Takeda, T. Kodama, and K. Kayagaki for technical assistance and helpful suggestions.
Supported by grants from the Ministry of Education, Science and Culture and the Ministry of Health, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ko Okumura, MD, The Department of Immunology, Juntendo University School of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; e-mail:kokumura@med.juntendo.ac.jp.