Abstract
Although endothelial cells are quiescent and long-lived in vivo, when they are removed from blood vessels and cultured in vitro they die within days to weeks. In studies of the interaction of E1−E4+ replication–deficient adenovirus (Ad) vectors and human endothelium, the cells remained quiescent and were viable for prolonged periods. Evaluation of these cultures showed that E1−E4+ Ad vectors provide an “antiapoptotic” signal that, in association with an increase in the ratio of Bcl2 to Bax levels, induces the endothelial cells to enter a state of “suspended animation,” remaining viable for at least 30 days, even in the absence of serum and growth factors. Although the mechanisms initiating these events are unclear, the antiapoptoic signal requires the presence of E4 genes in the vector genome, suggesting that one or more E4 open reading frames of subgroup C Ad initiate a “pro-life” program that modifies cultured endothelial cells to survive for prolonged periods.
THE VASCULAR SYSTEM is covered by a single layer of endothelium, cells derived from the mesoderm.1,2 Although endothelial cells replicate in response to angiogenic mediators in association with hypoxia, wound healing, inflammation, and tumor growth,2-5 the normal adult endothelium is quiescent and remarkably long-lived, with an average half-life of years.5 Endothelial cells removed from the vasculature and cultured in vitro in the presence of serum and growth factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), proliferate to reach confluence.6-10 Confluent monolayers of endothelial cells are contact inhibited for growth if they are not passaged,11 and, even in the presence of serum and growth factors, the cells detach and die.12 In the absence of serum and growth factors, endothelial cells rapidly undergo apoptosis.12-14
As part of studies focused on adenovirus (Ad)-mediated gene transfer to human endothelium, we noted that endothelial cells infected by E1−E4+ replication–deficient Ad vectors remained viable for prolonged periods, even in the absence of serum and growth factors and independent of the transgene carried by the Ad vector. This study presents evidence that E1−E4+ Ad vectors function to provide an “antiapoptotic” signal that, accompanied by an increase in the ratio of Bcl2 to Bax levels in the cells, puts cultured primary human endothelial cells in growth factor–free and serum-free conditions into a state of “suspended animation,” maintaining cell viability for at least 30 days. Although the mechanisms of induction of this state are unclear, they require the presence of the Ad E4 genes, suggesting that the one or more open reading frames (ORF) of E4 may provide a signal to induce these cells to revert to a more in vivo–like phenotype.
MATERIALS AND METHODS
Endothelial cells.
Primary human endothelial cells were isolated from freshly obtained umbilical cords by collagenase treatment6 and grown in “growth factor medium” (M199 medium, 20% fetal calf serum, 10 ng/mL VEGF [Peprotech, Piscataway, NJ], 5 ng/mL bFGF [Peprotech], and 1 unit/mL heparin sulfate) at 37°. Cells from passage 2 to 5 were used in all the experiments. Where indicated, studies were carried out using “growth factor–free medium” (X-vivo; Bio-Whitaker, Walkersville, MD; contains no serum and no growth factors).
Adenovirus vectors.
The Ad vectors used in this study included: (1) E1−E4+ AdNull (E1−, E3−, E4+, cytomegalovirus early/intermediate promoter/enhancer [CMV], no transgene in the expression cassette)15; (2) E1−E4+ Adβgal (E1−, E3−, E4+; CMV promoter driving the Escherichia coli β-galactosidase [βgal] gene15); (3) E1−E4+ AdGFP (identical to E1−E4+ Adβgal, but with a modified form of the Aeguorea victoria green fluorescent protein cDNA [GFP] in place of βgal)16,17; (4) E1−E4− Adβgal (same as Adβgal, but with a complete deletion of the E4 gene, using the E coliβ-glucuronidase gene as a spacer in the E4 region)18; and (5) AdZ.11E4ORF6 (E1−E3−; expresses only E4ORF6 from the E4 promoter and all other E4ORFs deleted; CMV promoter driving the E coli βgal). Ad vector stocks were purified by cesium chloride centrifugation and dialysis and quantified by plaque forming units (pfu) in 293 cells, as previously described.19 All Ad vectors had a particle/pfu ratio of approximately 100, and all vectors were determined to be free of replication-competent Ad.20 21
Growth curves and cell survival.
To evaluate the growth response of the endothelial cells to Ad vector infection, the cells were cultured in 6 well plates (for quantification of viable cell number) or poly-D-lysine–coated glass coverslips with grids to allow for identification of the specific locations (for studies of growth curves; Brinkman Instruments, Westbury, NY). The cells were washed, and the Ad vectors (50 pfu/cell) were then added in “growth factor–free medium” (X-vivo; Bio-Whitaker) with gentle shaking. Control cells were maintained in growth factor–free medium alone. After 90 minutes, the cells were washed, and for the first 24 hours, the cells were cultured in growth factor medium. Cells were then cultured for up to 30 days in growth factor medium or growth factor–free medium, as indicated. At various times, the total cell number and percentage of viable cells (trypan blue exclusion) were determined.
Endothelial markers.
To insure that endothelial cells infected with Ad vectors maintained their differentiation with respect to an endothelial-specific marker, endothelial cells were infected with E1−E4+AdNull as described above in growth factor medium or growth factor–free medium in 6 well plates. After 30 days in culture, the cells were evaluated for expression of von Willebrand factor using a mouse monoclonal antibody (Dako Corp, Carpenteria, CA). Alkaline phosphatase–labeled goat antimouse antibody (Jackson Immunoresearch Laboratories, Westgrove, PA) was used to identify the primary antibody, and the enzyme reaction product (red) was visualized using a new fuchsin substrate (Dako Corp). Cellular structures were visualized by hematoxylin counter staining. Samples were analyzed by microscopy. Irrelevant isotype-matched monoclonals were used as controls. Expression of the VEGF receptor KDR was evaluated by flow cytometry in the Ad vector–infected endothelial cells maintained as above, using a mouse monoclonal antibody (gift from Imclone Systems, New York, NY) and a flourscine-conjugated secondary goat antimouse IgG (Sigma Immunochemicals, St Louis, MO). Irrelevant isotype-matched monoclonals were used as controls. Expression of E-selectin in endothelial cells was assessed after treatment of interleukin-1β (IL-1β) (10 units/mL; R&D Systems, Minneapolis, MN) for 18 hours and by flow cytometry using a rabbit polyclonal antibody (R&D Systems).
Persistence of the Ad genome.
To evaluate the endothelial cells for the presence of the Ad genome over time, E1−E4+ AdNull vector–infected cells were collected after 0, 24, and 48 hours, 7 and 30 days after infection. Mock-infected cells were used as a control. Total DNA was extracted using overnight digestion of the cell pellet with Proteinase K (100 μg/mL) (Sigma) in digestion buffer (100 mmol/L NaCl, 10 mmol/L Tris-HCl, 25 mmol/L EDTA, pH 8.0, and 0.5% sodium dodecyl sulfate [SDS]) and subsequent phenol/chloroform extraction and ethanol precipitation. Total DNA (500 μg) was used as template for Ad5-specific polymerase chain reaction (PCR) using Ad5 E4 primers (sense: 5′-GTAGAGTCATAATCGTGCATCAGG; antisense: 5′-TTTATATGGTACCGGGAGGTGGTG). As a control, the DNA was also evaluated using E1-specific primers (sense: 5′-GAGACATATTATCTGCCACGGAGG; antisense: 5′-TTGGCATAGAAACCGGACCCAAGG). The cycling parameters were 94°, 30 seconds; 65°, 30 seconds; and 72°, 30 seconds, for a total of 40 cycles. The PCR products were resolved in a 1% agarose gel containing ethidium bromide.
DNA synthesis.
To quantify DNA synthesis in endothelial cells after Ad vector infection, cells cultured (104/well) in a 96-well plate were either mock treated with growth factor–free medium alone or infected with either E1−E4+ Adβgal or E1−E4− βgal (50 pfu/cell) for 90 minutes in growth factor–free medium. The medium was changed to growth factor medium. After 24 hours, fresh growth factor medium or growth factor–free medium containing [3H]thymidine (0.5 μCi/well; 85 mCi/mmol; Amersham Life Science, Arlington Heights, IL) was added, and cells were incubated for an additional 24 hours. Cells were harvested by trypsin and EDTA treatment and collected on glass-fiber filters. Filter-bound radioactivity was measured in a liquid scintillation counter. Each analysis was done in triplicate.
DNA analysis.
To assess the content of the DNA in the endothelial cells after Ad vector infection, adherent cells were harvested together with floating cells, washed with phosphate buffered saline (PBS), pH 7.4, and resuspended in low ionic strength buffer (0.1% sodium citrate, 0.1% Triton-X100, 50 μg/mL propidium iodide [Sigma], and 20 μg/mL DNase free RNaseA [Boeghringer Mannheim, Indianapolis, IN]). After overnight incubation at 4°, the DNA content of the cells was analyzed in a flow cytometer (Elite Profile; Coulter, Miami, FL). The results are presented in graphic format showing cell count on the y-axis and fluorescence intensity on the x-axis. To show the proportion of cells showing <2n complement of DNA (including the apoptosis fraction), the x-axis was displayed in log format.22
DNA fragmentation in apoptotic endothelial cells was also assessed by incorporating fluorescein-12-dUTP at the 3′-OH DNA ends using the enzyme terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling assay.23 Endothelial cells were grown in poly-D-lysine–coated glass coverslips, the subconfluent cells were infected with E1−E4+ or E1−E4− Ad vectors, and the cells were maintained in growth factor medium or growth factor–free medium. Mock-infected cells were used as a control and were maintained as above. Cells maintained in growth factor medium were analyzed on day 10, and cells maintained in growth factor–free medium were evaluated on day 1 postinfection using the Apoptosis Detection System (Promega, Madison, WI), following the manufacturer’s protocol. Samples were analyzed under fluorescence microscope, and the number of green fluorescent nuclei, characteristic of apoptosis, were counted. Propidium iodide counter stain was used to visualize the nucleus and cytoplasm. The percentage of apoptotic cells was calculated from triplicate measurements.
Western analysis.
To evaluate the endothelial cells for levels of Bcl2 and Bax protein, the cells were harvested by trypsin and EDTA treatment, washed once with PBS, and lysed with boiled lysis solution (10 mmol/L Tris-HCl, pH 7.4; 1% SDS; and 1 mmol/L sodium vanadate). The lysate was boiled for an additional 5 minutes and cleared by centrifugation (14,000 rpm, 4°, 10 minutes). Protein content was estimated using Pierce reagents (Rockford, IL). The cellular proteins (50 μg) were resolved in a 12% SDS-polyacrylamide gel and transferred to nitrocellulose membranes by semidry blotting. Rabbit polyclonal or mouse monoclonal primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were used at 1:250 dilutions to detect human Bax and Bcl2, respectively. Rabbit polyclonal antibodies were used at 1:100 dilution to detect human β-actin (Sigma Immunochemicals). Immunoreactive bands were visualized by a secondary antibody conjugated to horseradish peroxidase and enhanced chemiluminescence (Amersham, Little Chalfont, Buckinghamshire, UK). Additional controls (not shown) included nonimmune serum (for Bcl2) and irrelevant isotype-matched monoclonals (for Bax and β-actin).
Statistical analysis.
All data are presented as mean ± standard error of the mean. All comparisons were made using the two-tailed Student’s t-test.
RESULTS
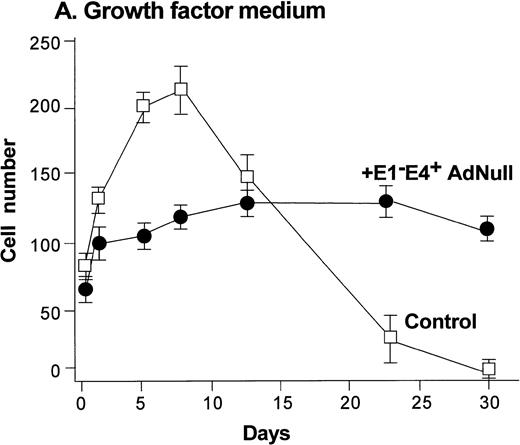
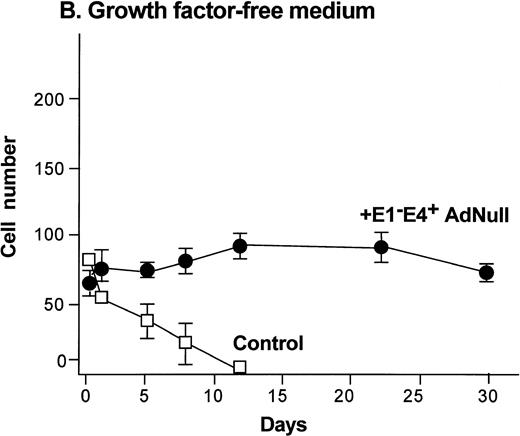
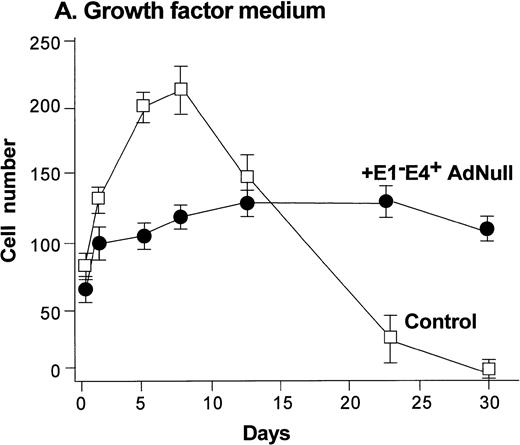
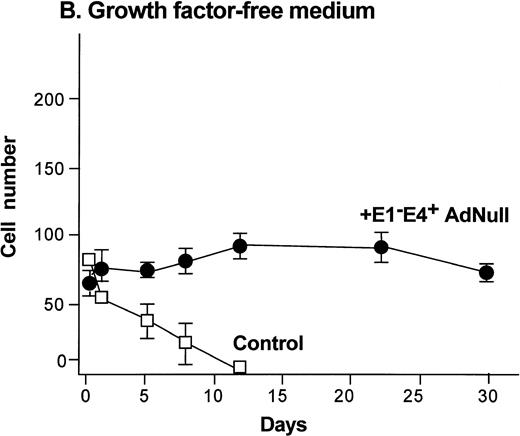
To evaluate the effect of an E1−E4+ Ad vector on the growth and viability of primary human endothelial cells, growth curves and viability of E1−E4+AdNull–infected and control cells cultured in growth factor medium and in growth factor–free medium were compared (Fig1). As is typical for human endothelial cells cultured in the presence of growth factors and serum, control subconfluent cells cultured in growth factor medium became confluent by day 8; thereafter, the number of adherent cells declined progressively, such that by day 30, no cells remained adherent (Fig 1A). In contrast, under the same conditions, AdNull-infected cells were growth inhibited, and the number of adherent cells remained constant for 30 days, the duration of the experiment. Endothelial cells infected with E1−E4+ Adβgal or with E1−E4+ AdGFP under the same conditions with growth factor medium showed similar growth inhibition as with the AdNull vector (not shown). Strikingly, when subconfluent endothelial cells cultured in growth factor–free medium were exposed to the E1−E4+ AdNull vector, the control cells died by day 12, whereas the AdNull-infected endothelial cells remained adherent for at least 30 days (the duration of the study) without a loss of cell number (Fig 1B).
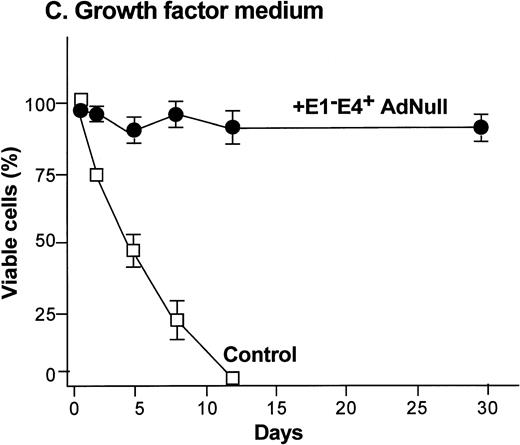
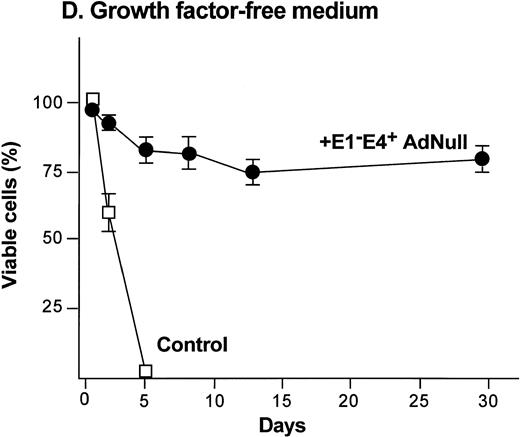
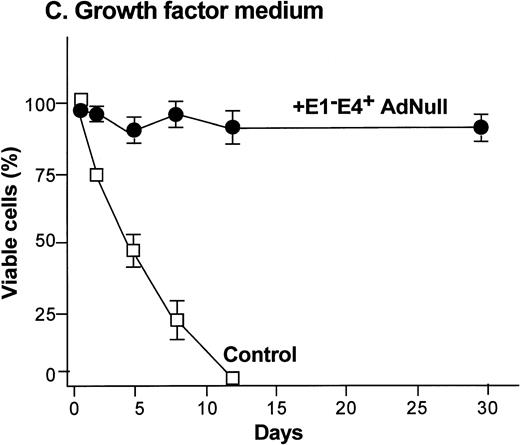
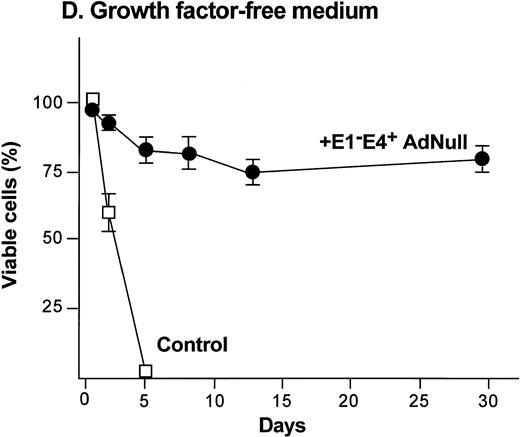
Effect of an E1−E4+replication–deficient Ad vector on the growth and viability of primary human endothelial cells. (Panels A, B) Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips. (Panels C, D) Confluent endothelial cell cultured in 6 well plates. (A) Number of endothelial cells over time cultured in growth factor medium after exposure to the E1−E4+ AdNull vector (50 pfu/cell) or to no vector (“control”). (B) Number of endothelial cells over time cultured in growth factor–free medium after exposure to AdNull compared with uninfected cells (“control”). (C) Viability of confluent endothelial cells over time cultured in growth factor medium after infection with E1−E4+AdNull vector (50 pfu/cell) compared with no vector (“control”). (D) Viability of confluent endothelial cells over time cultured in growth factor–free medium after infection with the AdNull vector compared with control. For panels C and D, viability was assessed by trypan blue dye exclusion. For all panels, the data represents the mean ± standard error of the mean of triplicate measurements.
Effect of an E1−E4+replication–deficient Ad vector on the growth and viability of primary human endothelial cells. (Panels A, B) Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips. (Panels C, D) Confluent endothelial cell cultured in 6 well plates. (A) Number of endothelial cells over time cultured in growth factor medium after exposure to the E1−E4+ AdNull vector (50 pfu/cell) or to no vector (“control”). (B) Number of endothelial cells over time cultured in growth factor–free medium after exposure to AdNull compared with uninfected cells (“control”). (C) Viability of confluent endothelial cells over time cultured in growth factor medium after infection with E1−E4+AdNull vector (50 pfu/cell) compared with no vector (“control”). (D) Viability of confluent endothelial cells over time cultured in growth factor–free medium after infection with the AdNull vector compared with control. For panels C and D, viability was assessed by trypan blue dye exclusion. For all panels, the data represents the mean ± standard error of the mean of triplicate measurements.
To examine the effect of an E1−E4+ Ad vector on the viability of confluent endothelial cultures, confluent endothelial monolayers cultured in growth factor medium or in growth factor–free medium were infected with E1−E4+AdNull vector, and viability was assessed by trypan blue dye exclusion. In growth factor medium, the control cells lost viability progressively and died by day 12 (Fig 1C). In marked contrast, 90% of AdNull-infected cells remained viable for 30 days, the duration of the experiment. In the growth factor–free medium, the control cells lost viability quickly and died by day 5 (Fig 1D). In marked contrast, 80% of the AdNull-infected cells were still viable at day 30. Endothelial cells infected with E1−E4+ Adβgal or with E1−E4+ AdGFP under the same condition with growth factor medium or growth factor–free medium showed maintenance of viability similar to that with the AdNull vector over the 30 days of the experiment (not shown). Assessment of the endothelial cultures 30 days after exposure to the E1−E4+ Ad vector showed that the cells maintained the endothelial cell marker von Willebrand factor and VEGF receptor KDR. E-selectin, another unique endothelial cell marker, was expressed in control as well as E1−E4+ Ad vector–infected cells after IL-1β stimulation. This was observed in cultures maintained in growth factor medium or growth factor–free medium, ie, infection with an E1−E4+ Ad vector did not cause the cells to lose their endothelial cell–specific characteristics (not shown).
Evaluation of the effects of an E1−E4+ Ad vector on cellular DNA synthesis by the cultured endothelium showed that infection with the vector suppressed the synthesis of cellular DNA, independent of the culture conditions. In this context, subconfluent cultures of primary endothelial cells cultured in growth factor medium had a threefold reduction in DNA synthesis when infected with the E1−E4+ AdNull vector (P < .0001; Fig2A). Likewise, subconfluent cultures of endothelial cells cultured in growth factor–free medium had a threefold reduction in DNA synthesis when infected with the E1−E4+ AdNull vector (P < 0.0003).
Evaluation of the status of the DNA in the endothelial cells after exposure to the E1−E4+ AdNull vector or no vector (“control”). (A) Effect of AdNull on DNA synthesis of subconfluent endothelial cells. Cells were exposed to the AdNull vector (50 pfu/cell) or no vector (“control”) and were cultured in 96 well plates in growth factor medium or growth factor–free medium. After 24 hours, [3H]thymidine was added, and the incubation was continued for 24 hours. The data represents the mean ± standard error of the mean of triplicate measurements. (B) Effect of AdNull infection on the status of cellular DNA. Endothelial cells cultured in 6 well plates were exposed to the AdNull vector as per panel A and were evaluated by flow cytometry after propidium iodide staining. Shown are data for the confluent cultures of endothelial cells infected with AdNull in growth factor medium or growth factor–free medium. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with a log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (C) Evaluation of apoptosis in cells infected with E1−E4+AdNull vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4+ AdNull vector as described in the Fig 1 legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.
Evaluation of the status of the DNA in the endothelial cells after exposure to the E1−E4+ AdNull vector or no vector (“control”). (A) Effect of AdNull on DNA synthesis of subconfluent endothelial cells. Cells were exposed to the AdNull vector (50 pfu/cell) or no vector (“control”) and were cultured in 96 well plates in growth factor medium or growth factor–free medium. After 24 hours, [3H]thymidine was added, and the incubation was continued for 24 hours. The data represents the mean ± standard error of the mean of triplicate measurements. (B) Effect of AdNull infection on the status of cellular DNA. Endothelial cells cultured in 6 well plates were exposed to the AdNull vector as per panel A and were evaluated by flow cytometry after propidium iodide staining. Shown are data for the confluent cultures of endothelial cells infected with AdNull in growth factor medium or growth factor–free medium. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with a log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (C) Evaluation of apoptosis in cells infected with E1−E4+AdNull vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4+ AdNull vector as described in the Fig 1 legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.
Flow cytometry analysis using propidium iodide staining showed that the control endothelial cells cultured in growth factor medium showed a sub-G0/G1 DNA peak beginning by day 5, and by day 12, 100% of the cells were in this peak (Fig 2B). Strikingly, E1−E4+ AdNull–infected cells cultured in growth factor medium did not show a sub-G0/G1DNA peak. Likewise, when the confluent endothelial cells were cultured in growth factor–free medium, the control cells showed a sub-G0/G1 DNA peak starting by day 5, and by day 12, 100% of the cells were in this peak (Fig 2B). In contrast, 85% of AdNull-infected cells had a ≥2n complement of DNA at day 12, with only 15% showing a sub-G0/G1 peak. Based on the likelihood that the sub-G0/G1 peaks corresponded to 180 to 200 base pairs of nucleosomal DNA fragments (<2n complement) characteristic of programmed cell death (apoptosis),22 the flow cytometry experiments were repeated with an assay to quantify free DNA 3′-OH ends, a characteristic of apoptosis.23 When cultured in growth factor medium, 15.0 ± 3.0% of control cells were undergoing apoptosis by day 10. In contrast, 5.0 ± 0.5% of the E1−E4+ AdNull vector–infected cells showed free DNA 3′-OH ends (P < 0.05; Fig 2C). Because about 30% of control cells died by day 10 (Fig 1A), it is likely that control cell death in growth factor medium resulted from a combination of apoptosis and possibly necrosis. When cultured in growth factor–free medium, 26.0 ± 0.5% of control cells were undergoing apoptosis by day 1, whereas only 1.0 ± 1.0% of E1−E4+ AdNull vector–infected cells were undergoing apoptosis (P < 0.0001). Together with the growth curve data (Fig 1), this data is consistent with the concept that death of control cells in growth factor–free medium was primarily through apoptotic pathways, and suggests that E1−E4+ AdNull infection protected endothelial cells from apoptosis in the absence or presence of growth factors.
Vector genome–specific PCR assessment of the cultures of E1−E4+ AdNull–infected cells showed that at 30 days, the vector DNA was present in the subconfluent cells cultured in growth factor medium, as well as confluent cells cultured in growth factor–free medium (not shown). As expected, E1A genes were not detected in the DNA of the E1−E4+-infected cells (not shown).
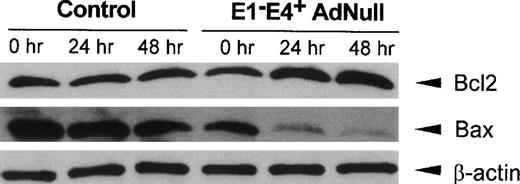
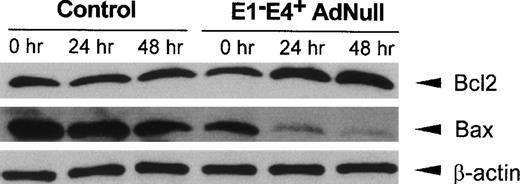
Bcl2, a mediator that promotes cell survival, and Bax, an initiator of cell death, have been implicated in the control of apoptotic pathways initiated by growth factor withdrawal,24-26 with an increase in the relative amounts of Bcl2 compared with Bax protein levels favoring cell survival.26 27 Evaluation of Bcl2 and Bax protein levels in control confluent endothelial cells cultured in growth factor–free medium showed that Bcl2 protein levels did not change significantly, with a small decrease in Bax protein levels observed over time (Fig 3). In contrast, in the E1−E4+ AdNull–infected cells, Bcl2 protein levels increased significantly beginning at 24 hours, whereas Bax protein levels decreased progressively over time. The control β-actin levels remained constant in both control and E1−E4+ AdNull–infected endothelial cells.
Bcl2 and Bax protein levels in primary human endothelial cells after infection with an E1−E4+ Ad vector. Confluent endothelial cells were infected with the E1−E4+ AdNull vector or no vector (“control”), cultured in growth factor–free medium for 0 to 48 hours and assessed by Western analysis for human Bcl2 and Bax using rabbit polyclonal and mouse monoclonal antibodies, respectively. Equal protein loading was confirmed with a control antibody to detect human β-actin detected by a rabbit polyclonal antibody. Blots were developed by enhanced chemiluminescence.
Bcl2 and Bax protein levels in primary human endothelial cells after infection with an E1−E4+ Ad vector. Confluent endothelial cells were infected with the E1−E4+ AdNull vector or no vector (“control”), cultured in growth factor–free medium for 0 to 48 hours and assessed by Western analysis for human Bcl2 and Bax using rabbit polyclonal and mouse monoclonal antibodies, respectively. Equal protein loading was confirmed with a control antibody to detect human β-actin detected by a rabbit polyclonal antibody. Blots were developed by enhanced chemiluminescence.
Based on the knowledge that E1−E4+ Ad vectors express low levels of E4 ORF,28-31 and that several E4+ ORF have functions that may be linked to the cell cycle,32-34 we hypothesized that E4 region gene products may play a role in enhancing endothelial cell survival after infection. To evaluate this hypothesis, cell growth and survival, DNA synthesis, cell cycle analysis, analysis of free DNA 3′-OH ends, and the levels of Bcl2 and Bax were compared in endothelial cells after infection with E1−E4+ or E1−E4−Ad vectors. In contrast to the control cells that became confluent by day 8, E1−E4− Ad–infected subconfluent cells cultured in growth factor medium grew slowly and never became confluent (Fig4A). Like the control uninfected cells, the endothelial cells infected with the E1−E4− vector gradually lost viability, such that by day 18, no cells had survived. In contrast, as observed with the E1−E4+ AdNull vector (Fig 1A), the E1−E4+ Adβgal vector–infected cells did not grow, but the number of viable cells remained constant for the 30-day duration of the experiment. When confluent cells were cultured in growth factor–free medium, both the control and E1−E4− Ad vector–infected cells lost viability quickly, such that by day 5 all the cells had died (Fig 4B). In contrast, the cells infected with E1−E4+Ad vector remained viable throughout the 30-day duration of the experiment.
Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.
Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.
The differences in the effect of E1−E4− and E1−E4+ Ad vectors on endothelial cells were not linked to differences in suppression of DNA synthesis, in that the E1−E4− Ad vector- induced inhibition of endothelial cell DNA synthesis in growth factor medium and in growth factor–free medium in a fashion similar to that of the E1−E4+ vector (Fig 4C; E1−E4− Ad vector compared with control in growth factor medium, P < 0.0001; E1−E4− Ad vector compared with control in growth factor–free medium, P < 0.0001; compare Fig 4C with Fig 2A). Interestingly, the endothelial cells infected with E1−E4− Ad vector showed increasing amounts of fragmented <2n DNA over time in both growth factor–free medium and growth factor medium, similar to that of the control cells (Fig4D). Furthermore, assessment of free DNA 3′-OH ends showed that, similar to control cells, 12.0 ± 2.5% of E1−E4− Ad vector–infected cells exhibited free DNA 3′-OH ends by day 10 in growth factor medium (P > 0.4; Fig 4F). In growth factor–free medium, by day 1, 28.0 ± 4.0% of E1−E4− Ad vector–infected cells was undergoing apoptosis, similar to that observed in control cells (P > 0.6), ie, infection with the E1−E4− Ad vector did not suppress apoptosis. Consistent with this observation, Bcl2 and Bax protein levels did not change in the E1−E4− Ad vector–infected endothelial cells compared with the control cells (Fig4E). Taken together, these results suggest that Ad E4 gene products play a role in the ability of the E1−E4+ Ad vectors to induce the survival of primary endothelial cells in vitro.
To begin to identify the E4ORF(s) that is required for the prolonged survival of endothelial cells, the effect of E4ORF6 protein on the survival of endothelial cells after E1−E4ORF6 Ad vector infection was assessed. We chose to evaluate E4ORF6 because of the known interaction of E4ORF6 protein and P53 that has impact on cell cycle regulation and survival.33 However, when confluent cells were cultured in growth factor–free medium, endothelial cells infected with E1−E4ORF6 Ad vector died by day 5, similar to the control and the E1−E4− Ad vector–infected cells (Fig 4B). These results suggest that AdE4ORF6 protein alone was not sufficient for the prolonged survival of endothelial cells in growth factor–free medium.
DISCUSSION
In vivo, uninjured endothelial cells are quiescent and long-lived, with very low levels of DNA synthesis and proliferation.5,9 When subconfluent endothelial cells are cultured in the presence of growth factors and serum, the cells initially proliferate, but then rapidly lose viability if they are not passaged. When cultured in the absence of growth factors, the cells fail to grow, and they rapidly enter apoptotic pathways and die.13 14 In the present study, we have observed that infection of cultured primary human endothelial cells with E1−E4+ Ad vectors evokes a phenotype characterized by minimal growth, longevity, suppression of DNA synthesis, and suppression of apoptotic pathways, regardless of the presence or absence of growth factors. The E1−E4+ Ad vector seems to freeze the cells in a phenotype that has at least some characteristics shown by uninjured, resting endothelium in vivo. The remarkable sustenance of viability of endothelial cells by E1−E4+ Ad vectors in the absence of growth factors has never been described for primary endothelial cells.
Possible mechanisms of Ad vector–mediated endothelial cell survival.
Although the mechanisms by which the E1−E4+Ad vectors accomplish the prolonged survival of Ad vector–infected endothelial cells in vitro are unknown, they seem to be linked, at least in part, to inducing the Bcl2 and Bax pathways to favor cell survival, and they seem to be initiated by low level expression of E4 gene products in the E1−E4+ Ad vectors. Consistent with this concept, retrovirus-mediated transfer and expression of Bcl2 in cultured endothelial cells have been shown to reduce apoptosis in the absence of bFGF.35 The increased ratio of Bcl2 and Bax, and the requirement of E4 gene products associated with the prolonged survival of Ad vector–infected endothelial cells provide clues to mechanisms that may be involved.
First, wild-type Ad encodes several proteins that promote infected host cell survival to prevent premature cell death.34 The E1A 12S gene product is antiapoptotic and capable of immortalizing various cell types.34,36 The E1B 19-kD protein is analogous to Bcl2 in blocking apoptosis initiated through several cell death pathways.37 38 However, the possibility of E1−E4+ Ad vector–mediated endothelial survival being mediated through E1A or E1B gene products is not possible because the E1−E4+ Ad vectors used in this study are completely E1A deleted and partially E1B deleted, ie, the vectors cannot possibly express the 12S- or 19-kD genes.
Second, the E2 gene encodes the 72-kD single-stranded DNA binding protein, the terminal protein precursor, and the Ad DNA polymerase, all of which are essential for viral DNA replication.39Although a low level expression of E2 gene is possible in the absence of the E1A gene, E2 gene products are not known to be involved in host cell growth and survival functions.28,33 Ad E3 region encodes 19- and 14.7-kD proteins that effectively inhibit cellular immune response to Ad virus infection and Fas ligand–induced apoptosis.40-42 However, the E1−E4+ Ad vectors used in this study have a large deletion in the E3 region that eliminates both the 19- and 14.7-kD genes.19,43 Ad late genes that encode hexon, penton, and fiber are not likely to be involved in enhanced survival of endothelial cells infected with AdNull.34
Third, because the E1−E4+ Ad vectors, but not the E1−E4− vectors, induced the prolonged survival of infected cells, it is logical to conclude that Ad E4 gene products may play a role in the E1−E4− Ad vector–infected endothelial cell survival. The E4 region of Ad contains seven ORFs that encode a variety of regulatory functions.34,44-51 The E4ORF6 and E4ORF3 seem to encode redundant functions involved in host cell shutoff and accumulation of late viral mRNAs.46 The E4ORF6 protein interacts with the tumor suppressor p53 and inhibits p53-mediated transcriptional activation.33 E4ORF6/7 protein binds as a homodimer to the cellular transcription factor E2F to promote E2 promoter activity.45 E4ORF4 protein binds and activates protein phosphatase 2A and may have a role in the regulation of DNA synthesis and AP-1 transcription factor activity.49,50 E4ORF1, E4ORF2, and E4ORF3/4 proteins encoded by Ad9 serotype cooperate with E1A in cell transformation.48,51 Although E4 gene expression is downregulated in the absence of E1A gene,28,51-53 a basal level expression of E4 gene is still detected in the cells infected with E1-deleted Ad vectors,28,30,54,55 and this low level expression of E4 proteins seems to be sufficient to function to modify gene expression from the vector.29-31,55 Any of the E4 products could be linked to the antiapoptotic function of the E1−E4+ Ad vector infection. However, the increase in the ratio of Bcl2 to Bax protein levels in E1−E4+ Ad vector–infected endothelial cells is consistent with a role for E4ORF6 protein in blocking p53-mediated transcriptional activation,33,56 possibly by relieving p53 repression of Bcl233,56 and activation of Bax gene expression.57 58
Finally, it is possible that low level expression of one or more E4ORF promotes cellular gene(s) that play a role in cell survival, and that one relevant to the in vivo phenotype of endothelial cells. It is known that infection of cells with Ad vectors upregulates cell signaling molecules such as MAP kinase,59 as well as cytokines, such as interleukin-1 and interleukin-6.60-62Consistent with this concept, E4ORF6 has been shown to block p53-mediated apoptosis.63 However, E4ORF6 alone was not sufficient for prolonged survival of endothelial cells in the absence of serum and growth factors, ie, it is highly likely that other E4ORFs in combination with E4ORF6 may be required for the antiapoptotic role of AdE4 gene. Ongoing studies are designed to identify those E4ORFs.
ACKNOWLEDGMENT
The authors thank Philip Leopold and Barbara Ferris (Weill Medical College, New York, NY) for assisting in fluorescence microscopy, and N. Mohamed (Weill Medical College, New York, NY) for help in preparing this manuscript.
Supported, in part, by the National Institutes of Health/National Heart, Lung and Blood Institute P01 HL51746, P01 HL59312, the Cystic Fibrosis Foundation, Bethesda, MD; Will Rogers Memorial Fund, Los Angeles, CA; and GenVec, Inc, Rockville, MD (R.R., S.W., and R.G.C.). S.R. is supported by NIH/NHLBI R01 HL58707 and the New York Heart Association Grant-in-Aid, New York, NY.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ronald G. Crystal, MD, Division of Pulmonary and Critical Care Medicine, The New York Hospital-Cornell Medical Center, 520 E 70th St, ST505, New York, NY 10021; e-mail: geneticmedicine@mail.med.cornell.edu.

![Fig. 2. Evaluation of the status of the DNA in the endothelial cells after exposure to the E1−E4+ AdNull vector or no vector (“control”). (A) Effect of AdNull on DNA synthesis of subconfluent endothelial cells. Cells were exposed to the AdNull vector (50 pfu/cell) or no vector (“control”) and were cultured in 96 well plates in growth factor medium or growth factor–free medium. After 24 hours, [3H]thymidine was added, and the incubation was continued for 24 hours. The data represents the mean ± standard error of the mean of triplicate measurements. (B) Effect of AdNull infection on the status of cellular DNA. Endothelial cells cultured in 6 well plates were exposed to the AdNull vector as per panel A and were evaluated by flow cytometry after propidium iodide staining. Shown are data for the confluent cultures of endothelial cells infected with AdNull in growth factor medium or growth factor–free medium. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with a log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (C) Evaluation of apoptosis in cells infected with E1−E4+AdNull vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4+ AdNull vector as described in the Fig 1 legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914002ax.jpeg?Expires=1769263526&Signature=BnqvbXoJu1AObO0-eaL4TujdL~xXO8w2C6EMfSHKJG50WBHoba-xpI3wh8VYhkv7igw6eSboKkSkIc9-JxE4uPeIyx6WUqpY1t2-JFssW4el9X2ooYiPLqhgU8TKnS-Ad7D4~1shtLTk3jAOxSjCs~KFofdrt2rUFUYcZllo9w8BhuxT4AiSiHQQS8dB4i~6qg2OIPp3DzS-Gsfw0mb6Nk56PmHyyVawQAl3GJEOs5152jguqHoCuZV2NlmFRMfbW9r9SFjyhQQi5VB68JTdSFx3jgJRr7X4i9PShzVvYY3CTQWsFl~82Kh80EKng~rJjhcjl2qlFLHY5A6gSNo6zw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Evaluation of the status of the DNA in the endothelial cells after exposure to the E1−E4+ AdNull vector or no vector (“control”). (A) Effect of AdNull on DNA synthesis of subconfluent endothelial cells. Cells were exposed to the AdNull vector (50 pfu/cell) or no vector (“control”) and were cultured in 96 well plates in growth factor medium or growth factor–free medium. After 24 hours, [3H]thymidine was added, and the incubation was continued for 24 hours. The data represents the mean ± standard error of the mean of triplicate measurements. (B) Effect of AdNull infection on the status of cellular DNA. Endothelial cells cultured in 6 well plates were exposed to the AdNull vector as per panel A and were evaluated by flow cytometry after propidium iodide staining. Shown are data for the confluent cultures of endothelial cells infected with AdNull in growth factor medium or growth factor–free medium. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with a log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (C) Evaluation of apoptosis in cells infected with E1−E4+AdNull vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4+ AdNull vector as described in the Fig 1 legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914002bx.jpeg?Expires=1769263526&Signature=MmAoLlouLGW3amGTDrZV6zxFppI-MKTO7MeWNRX8M1M0fXP-jfnBkn1IfJTT-s7lxUz32whb2DeLoHhCA3S8-focXpUYqEG~sgPf9eZ6WTOvi24ASlRxkBgBau2mrUZMdfV9MTxcR05L1~3jEb1NWfzG6b1M2fNZeGbaG4-DCiIyalchMyg8~QBLYJf~BPW66R70XXGwH9og8Uy0JBeUA81FvVwZsd4EcP-2gyBjY9H-xNLWC8tslw9X1w6qn6OyigO~4Fdu6PSI~sJ9XZYvIu~XBA1L~BAo5qfro3T2valtLP4B8kyMAa~wGRGcYKqlkne89UYf4N3skoxdPQW~kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Evaluation of the status of the DNA in the endothelial cells after exposure to the E1−E4+ AdNull vector or no vector (“control”). (A) Effect of AdNull on DNA synthesis of subconfluent endothelial cells. Cells were exposed to the AdNull vector (50 pfu/cell) or no vector (“control”) and were cultured in 96 well plates in growth factor medium or growth factor–free medium. After 24 hours, [3H]thymidine was added, and the incubation was continued for 24 hours. The data represents the mean ± standard error of the mean of triplicate measurements. (B) Effect of AdNull infection on the status of cellular DNA. Endothelial cells cultured in 6 well plates were exposed to the AdNull vector as per panel A and were evaluated by flow cytometry after propidium iodide staining. Shown are data for the confluent cultures of endothelial cells infected with AdNull in growth factor medium or growth factor–free medium. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with a log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (C) Evaluation of apoptosis in cells infected with E1−E4+AdNull vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4+ AdNull vector as described in the Fig 1 legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod409ram02cz.jpeg?Expires=1769263526&Signature=wF7kioAhMEnJ7irpgAJzo3i~G3pBajoA5W4uwHWf9WFOzh~J1OI5Dy5MMGfFC6QTa4XwnzE~JEUb5Jol4WSg85QrghimjuwvhNCuMkmvGQVjBEx3hCGYNT~ZUstIXmlM3jw2~c8EmBOoEGydO0y2lq6QCErKLICdUBfCoaCuwJAzA9EE5431n89ORPJlgyYKVX11aISpGqBUXU~dVWsaJHlmSvwJzMQ-4mlrYWzMxwdrSbEjLeS~z~Ij~aWLIbxQC6Vfnvls7iH5VSPDoj2oHqU5XWa8OzmW~o25~cdkt924oHBXX7Y5JChZSUuKXVIPH4IdxNBOxnkTojiK1akgQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914004ax.jpeg?Expires=1769263526&Signature=oYyX8qkYnNRBMcd7OQEXx7UvdjH1dUR-tnMrRNFuTZh9DNhE7vb2QI~kaFpS~2Mbp2OwRAAzAjVvK03dTtE1F6zt6T~RRgnShhDc~6nuYFUONUn-eRal~B3EFsWxb3XH6PH-KloJ81GLC2KfwQUs3djli~-Ec8CbmXkikBf2g6qpZix3A5yNMTgq1kmdw3DfmRQ1V~3KDVDbRDxPx7~Y0WSYN~3AODQ4WDpRSLLOgbdkFh16JxUwf3ACvawzCrr960cCIFbNa4wzCcGV9vKi1ACT2DEkbd07gnVE9XO3y9a9gyBby9LRgcYT8ha2Q-rfQUGpWnL9LBzlv10WR-sKmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914004bx.jpeg?Expires=1769263526&Signature=Zt7QxspgyvIUV5w88Q5KQ6idAQglFHoVALd7VR2qFzVTGfyId3qUE1H3Z2Kv4H5Px0TuIcZVidNoQhBjVrm~IBNOstjSKAkJmxxP8jSxwtVWawQyzA1yFZwPC04bUoeDXCAXxhwkhsZnLwP0DP0Gd-m8wPSE4FU2lFI9FkaxetPXkBPWzZoxnJqODiGOSKpI31idJSivJWmaLzDctA9cHq1-VcjatzcR3UA1pZqOz2TpaVU0jkwuEnMHEWKxcBDwOx9ATpAmvzazlE26V0E5qymPfAIgt04agyCxpRLfKECc2GmCpudLFPxbR3XO9dFMzJYs3m1GJW~sfeOYJS5BIg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914004cx.jpeg?Expires=1769263526&Signature=AdKsm~NlvSvpuZYTUh9yqIFfTbz3xdzhy~PkXmdvivn3laYaWRHZV3pJivGEoZQBcbAnVAqYu88lOgboUkjYivBRa2fjIQ6-mx~4MSdPy7Uc-AfQqueC2ty5dZhLkkcnp~-wAORdtYFD3-x9OnasEGEQInzHtqp5P5dFID46eMROeNFQMF2mkNwWaxK2rNV4kQwtvzJf9MDo1fD3eRlVmqigAJwfDUR9o~Lbxzyx0WI5kNnPz1ymQK2-0~cHj8auj5pdc26lSZ2Skj1PK07tHa2Ej7K0KUzUTLmy-P7JhNLi6SwJBS7uFuhn-9Doh9rTYSxIAIa~fRoT0fBDGA0POw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914004ew.jpeg?Expires=1769263526&Signature=IBuUdA8uls4xUSaytpZT6Twb6qcvbTnvBHf2mheMMYSH1ZPvSJOYc3kDsWhqFHyCIwpW2suDutneiZK7tLTw7OLq8gAt31iyTErebQiONMCOBozptYpCB5TE2OPBVQBWR56ny2V~GetMvY17wzQ5b580aQbLnD0Gh2S~Ru9rjpLKWJog9wg2KL1Jssr9DPRcSZwL8FQmU9jTU6O7EtUMpIaSDP1wYo3QkpI8sXfOduBVibMHGImwqLEkSvIITBA-ruUELgUBonxz1ZJDz5y8AU5A8Q~B73YlPzWfPxguR5vwNlBpqQ-lq-cbb6wXS1iyNK-URV-9ehUyZiyPiFJ2iA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914004dx.jpeg?Expires=1769263526&Signature=Ui6WEgfwDGW8vt7cgebe~DKwkgOQLNFvBbq~yu0afk9K2DZjej5~IEM9OzUaeWnyUYWNtID14m6iZ8XbwHQBDqYuPMdqKVVCu1aYKrzDd~-8oL0k4LoHl3~5B4a0Iy1IoTqzguJ0QlU7X7BhxoGbY6ARk-VpG2Z00k5h2av9Kug1d5FDZk-wVO7tZl2ASEuqlVsHBZ75S7PpzKeFPm1Awfpzh53BbjyO6nsEY90ksxcJSDyAuFRwSyUCXl~4zhArZWWlKlykATDEZA956mRgkAvVKL5WsfGOEvhODPywtVQ5ERUcOL2dSUi6G7y6y80WSpsUgpEqYHiXROGWw7CEdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod409ram04fz.jpeg?Expires=1769263526&Signature=VNEuQcdNhSrxx-7VVNhu~nYdajOErcK92xCXuZ6L-XOxr3wc8WM9-cTrn5HlS4X8P7iFrVFMEahpvpBUL6ZVFJ7j4tee7nhraPNVRrutapOM2gt5hJICYEjeQJ~y0fIrUEFv1uRMwY9SsLmB16A49qzOHbhEhBbP~vTANdZ9Qz5dypy29Se5zbje9fDkfEUm7v7X-YkQfkeg~FQ6-qrYqhV1qi4imPklyRqYNr9J9e4ktoNvDLbZmNCCmDaRGWtlNyKjEDv7wTh9kC65~rCrtdZnH5TpXNuTdj~7frvcwIOXBfQyWpIXD1-Wufo3ryqMHBG-l0fFTv3BP8-q~JgJ~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Evaluation of the status of the DNA in the endothelial cells after exposure to the E1−E4+ AdNull vector or no vector (“control”). (A) Effect of AdNull on DNA synthesis of subconfluent endothelial cells. Cells were exposed to the AdNull vector (50 pfu/cell) or no vector (“control”) and were cultured in 96 well plates in growth factor medium or growth factor–free medium. After 24 hours, [3H]thymidine was added, and the incubation was continued for 24 hours. The data represents the mean ± standard error of the mean of triplicate measurements. (B) Effect of AdNull infection on the status of cellular DNA. Endothelial cells cultured in 6 well plates were exposed to the AdNull vector as per panel A and were evaluated by flow cytometry after propidium iodide staining. Shown are data for the confluent cultures of endothelial cells infected with AdNull in growth factor medium or growth factor–free medium. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with a log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (C) Evaluation of apoptosis in cells infected with E1−E4+AdNull vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4+ AdNull vector as described in the Fig 1 legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914002ax.jpeg?Expires=1769271928&Signature=GssdtpYoxqVFs0KLbFF0wGPEVi4Wi50SFcY7i3HqFOh3ROLMtrgV~yR2R~xUaT9ppC31bc~3Naj5XzrlgBkFaMHtomWKM8jVzJCT3J9KgLSP2FOSHajBfdsMVpLH25Uf3yD817eumTfEk~xxdYrut9ERD7h3d6Xxzvn5zUoXp0LfcghWL7hf-JUOgPg726vNtjklT8JysK6qXOFf27QxCgO4-P4mgBR5eBDZdVGQNQiWVc66hKZe41kNC6vlFRk0etnVss~3TD5PZ4nzq3E4R4BIgsM7SOOwwFd01uJM-K9MUdNV5adpsbK4A9RnybRqYxtWLzA6gKS3gCbgXBDnOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Evaluation of the status of the DNA in the endothelial cells after exposure to the E1−E4+ AdNull vector or no vector (“control”). (A) Effect of AdNull on DNA synthesis of subconfluent endothelial cells. Cells were exposed to the AdNull vector (50 pfu/cell) or no vector (“control”) and were cultured in 96 well plates in growth factor medium or growth factor–free medium. After 24 hours, [3H]thymidine was added, and the incubation was continued for 24 hours. The data represents the mean ± standard error of the mean of triplicate measurements. (B) Effect of AdNull infection on the status of cellular DNA. Endothelial cells cultured in 6 well plates were exposed to the AdNull vector as per panel A and were evaluated by flow cytometry after propidium iodide staining. Shown are data for the confluent cultures of endothelial cells infected with AdNull in growth factor medium or growth factor–free medium. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with a log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (C) Evaluation of apoptosis in cells infected with E1−E4+AdNull vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4+ AdNull vector as described in the Fig 1 legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914002bx.jpeg?Expires=1769271928&Signature=DMoQ7dDq9ZAq2vGfjrHosSPWNZdNrR9kH09QH0hria08xp6qVWj0A6ILk1j-5YQnkDBeAg4bRvMwoBeELYJ4Gc-sNpdYam1vhh1-zez58BJbaTCgElCj~lfrNRvfcmIM1fT2TpQ9GXFiTw93KTaRcw5h7GSqsK2tIba8WL1fprgt5rSa~OoO9oKX78N-WQ63eEyQzYN79wUu8MpBw8RtCUMSbptJGvuIlH5WttrM3adAc-VysmkT9yudoJ7xMuaOnDsK-wpk45DeDL0oDn80uAHCZCHoKVftbgiSIjOVJ~~RyKletc~z4akC3dLSgb8s0WHVhm09w0t8KsgD~MzTJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Evaluation of the status of the DNA in the endothelial cells after exposure to the E1−E4+ AdNull vector or no vector (“control”). (A) Effect of AdNull on DNA synthesis of subconfluent endothelial cells. Cells were exposed to the AdNull vector (50 pfu/cell) or no vector (“control”) and were cultured in 96 well plates in growth factor medium or growth factor–free medium. After 24 hours, [3H]thymidine was added, and the incubation was continued for 24 hours. The data represents the mean ± standard error of the mean of triplicate measurements. (B) Effect of AdNull infection on the status of cellular DNA. Endothelial cells cultured in 6 well plates were exposed to the AdNull vector as per panel A and were evaluated by flow cytometry after propidium iodide staining. Shown are data for the confluent cultures of endothelial cells infected with AdNull in growth factor medium or growth factor–free medium. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with a log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (C) Evaluation of apoptosis in cells infected with E1−E4+AdNull vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4+ AdNull vector as described in the Fig 1 legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod409ram02cz.jpeg?Expires=1769271928&Signature=VLPYHpfqFuIn0Fa3igu-Ywv2DAZE14jAAB6pivVeouNedRiN4ky~-ZjYPLC4XnamDmT~BQ5SKpbtXRmBbER43ggfppH9LmJQZRbRxK74Bn~dVXYcA5KPgrJT7zaod0DABB8EwQiM-CV5WaLsfPbH9HfI-trhEgDumsSdOCLuYHMgsEqyEld8wDL3ht647Vjaupq7LgReBB9-ZznPyW2SC3mC8PScjoLben5w~7lIrOERXvTZfGAW1mULJxYm768mTXr4dyVomuRs5risf1HOdDLwHDhsjgqkw-fAeaFdWF1GhB~5zSQu4L9Berj8o91AbRSbc39YFuijyARFluSgFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914004ax.jpeg?Expires=1769271928&Signature=47NW2IeHyLAAsBv26SpcMuaSUur5sMwN9miOtUyxsIwyNaTLYHA4f9P-69GzKw4DvQc8EsyuIszyezMcS8Aawq4ulYe9x-1P1fF-E0UspKI6ucpF6az1iS1i4WW5frdTdz4alrga6IeRbdD8y9O4DSiyHl4xNpW~VlaEN~tVGO9bmSiEBftngkFGXst4ykkVxzmOPVTJ5OFY0tVX-IsjUE8uXjRjcUF~x1v7sEvx042jM4IFlX4le7WT8Kq6q~4wgrnUs0cCXSaViHtEPS~WrjlUIOp8HafR1-DBiyE~qz8mZzwIXu1K~E9ISENSoLa4qFNBstHuJA~hiwEjzZ5gqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914004bx.jpeg?Expires=1769271928&Signature=nyYxOkJiZEmn3mSXndi7nCjBJ9NgnF1fM6l6fwXrmanlgTUmH9AoM3udWDY0tzKJH9-Lza~a63frUaBXd2EplUMdm2WCQcHF1tJvZRKDxI9-UzD5TkgLiPfq3w7FLiPwBhDF2Yd5s5VCqGc4l-HLHv2Z0m2h5IuMQmFDK8aqdSZ-hY-TqJm~7pH2HW5pesTIoi-rnFar-gcb8Fn3t1arxCuZqvSGNf5Mq-6dqcwcpqWx9~~rUa~rpziYPIuDyjb9FUJncP4TmARCe6h72RgtlS3wFgiJqmTMaxb0rmSTTlPoNaXFZ2EoavjOtGc1TSVPf~M4PCj-XUME30UHSQCJWg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914004cx.jpeg?Expires=1769271928&Signature=4J77pw-VWH16yZcXWdUF4cMdOczdLOqBCFP~zVQA8kPSezkZ9KEp0WG8l5WJ~bLYamrwlLj~QUOOscIKTQCblv1Or7GMQ-lBhcMAREhm5sW0dEz6kqXYuJ5Re3o1pTCgCXhc03PJloDBLFIVqFZnKufEh~izttxxMRO7g-Jkc3l13XB-G9Zb1uHN6q7yMCra-IwJgcKdX8k96ATWEL4X~coIJ1IDp3hTTbPLfc753DkORMojx17zD6-vaFwxPHwRJxXXhWUrPS2cWd2k~0ggvXK40KIEZKuEuRsfThGFB1brvD6apoe~OTPW6OHHvGXuC9hSBliHYRZEKJC3j4byFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914004ew.jpeg?Expires=1769271928&Signature=Rrk5qNM75bxCNQR9rifDY3GQSTxWLuxMTL2d4ENr5geGrr24n1gURqoetx-4vGaWuHuqhV5duFTHp2OnPMfc9OJZReM593w7N-ECSmk-feX7hoe6~nn8LuL2arMFQ-GU32mM7ny0JpcbbqrTFsJE25PsTPjTksqXoF6Uvk~Rgu2CBPkCXVZwLZ7D1TYC~~VNnwlCn9~n7KoHveqjL1PCA1i7w0-PG7QC1Vn-Gjj9ONC3Qy7u79X6hb2md1UB2MpttlMOV7SHJBOh5cOd9W8UKxFfZnpQlaCVHed8dQwX0JZG92~5kB8vs397gzGpNkMsTozc06Mo9EqFLyu2OORp4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod40914004dx.jpeg?Expires=1769271928&Signature=KysrYMhL8QVBBWR0Ev2v93J8tynw02uxdA08ihplu45th3x~~tNmdSNtgFDxFGDU1-ZEaVMmy9pQMpGuYKymB66I38ZAXEJ1CfhlokjTjTrvEEsMZMHNLNgfQ6l5O3Z8IwvnRQ6Folu8AdI6Ncthetlz9blSX6RIe9~k9ONtZRH-sRnrOqUhYezAQVQY0NTjAp00pFddE2A7dLqtbg0-VYMjBXJTEvv-ByRVSJYR1PW2-Gt24PComlOtEQ1aD4ag5tHX3n2MYqGTwFVCiLjaY40Om8cUfLEuIIIjeSH2EKbRtiKZfN522o2GVQcw7PLO7c6~zMQ0DJ~O06bW3DVicQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of an E1−E4− Ad vector on growth and survival of primary human endothelial cells. (A) Growth of endothelial cells in response to growth factors after exposure to an E1−E4− vector. Subconfluent primary human endothelial cells were cultured in growth factor medium, as in Fig 1A, after exposure to E1−E4− Adβgal (50 pfu/cell) compared with E1−E4+ Adβgal (50 pfu/cell) or no vector (“control”). Shown is the number of viable cells as assessed by trypan blue dye exclusion. (B) Survival of endothelial cells cultured in growth factor–free medium after exposure to an E1−E4− Ad vector and E1−E4− ORF6 Ad vector. Confluent primary human endothelial cells were cultured in growth factor–free medium after exposure to E1−E4− Adβgal, E1−E4− ORF6 Ad vector, E1−E4+ Adβgal, or control, as in panel A. (C) Effect of an E1−E4− Ad vector on cellular DNA synthesis of primary endothelial cells. Cells were exposed to the E1−E4− Adβgal vector or no vector (“control”) and cultured in growth factor medium or growth factor–free medium, with [3H]thymidine uptake quantified as in Fig 2A. The data for panels A through C represent the mean ± standard error of triplicate measurements. (D) Evaluation of the status of the cellular DNA in cultured endothelial cells after exposure to an E1−E4− Ad vector. Cells exposed to the E1−E4− Adβgal vector or no vector (“control”) were cultured in growth factor medium or growth factor–free medium as described in Fig 2B, and evaluated by propidium iodide staining and flow cytometry. The data for day 1 is displayed with a linear abscissa to best show the >2n peaks; the data for day 5 and day 12 is displayed with log scale to best show the <2n fragmented DNA. The <2n, 2n, and 4n DNA peaks are indicated. (E) Levels of Bcl2 and Bax protein in human endothelial cells after exposure to E1−E4− Adβgal vector. Confluent endothelial cells were exposed to the vector or no vector (“control”) and cultured in growth factor–free medium. The analysis was identical to that in Fig 3. (F) (see page 2939) Evaluation of apoptosis in cells infected with E1−E4−Ad vector. Subconfluent endothelial cells cultured in poly-D-lysine–coated glass coverslips were infected with E1−E4− Ad vector as described in the Fig 1legend. After vector infection, cells were maintained in growth factor medium for 10 days or in growth factor–free medium for 1 day, and apoptotic cells were identified by assessment of free DNA 3′-OH ends.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2936/4/m_blod409ram04fz.jpeg?Expires=1769271928&Signature=dF6-UY9huPgi7uvAhk16lYb-j3b~7VkSJkF8aZQXvjaezojMyDxFSUYNL~M-gHtEtaE-PauqyM1kXvujR-X5ru2-~Gd4TIxhJWYBnmI1l-cUGDT38hSAxseG3jHrAG9d6uWO9iuODTSQTZfAF8JnrECEai~4xEDICqwBv7m3YX6G0FPYlS3I-a8aj1g30ZNS9~Pse7G7UaJ2aLaQ0Ex0aass~MB8tHyUbHGqiIiuYp87zN0hDcdZqwu-r5i1LOZ1JWdqjlzWXrjnvBVBhstX-~dDVDogcuF0R78nYuJaqL2SKSYGdiVL8Qy~JekHDHCOOFqt-B3gqWXZm8NR56TGhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)