Abstract
Certain red blood cell (RBC) disorders, including thalassemia, have been associated with an innate protection against malaria infection. However, many in vitro correlative studies have been inconclusive. To better understand the relationship between human RBCs with thalassemia hemoglobinopathies and susceptibility to in vitro infection, we used an in vitro coculture system that involved biotin labeling and flow cytometry to study the ability of normal and variant RBC populations in supporting the growth of Plasmodium falciparum malaria parasites. Results showed that both normal and thalassemic RBCs were susceptible to P falciparum invasion, but the parasite multiplication rates were significantly reduced in the thalassemic RBC populations. The growth inhibition was especially marked in RBCs from -thalassemia patients (both -thalassemia1/-thalassemia2 and -thalassemia1 heterozygote). Our observations support the contention that thalassemia confers protection against malaria and may explain why it is more prevalent in malaria endemic areas.
THE HIGH FREQUENCIES of genetically abnormal red blood cells (RBCs) in persons from endemic areas ofPlasmodium falciparum malaria are thought to have evolved through balanced polymorphisms resulting from protection of RBC variants against this parasite infection.1,2 Various forms of thalassemia, an inherited autosomal recessive hemolytic anemia associated with diminished or absent expression of either the α- or β-globin genes, are common throughout tropical countries, including Thailand.3-5 Several clinical and epidemiologic studies argue that thalassemia genes may confer protection against malaria.6-8 The in vitro culture ofP falciparum in RBCs developed by Trager and Jensen9 has been adapted to describe the resistance of thalassemic RBCs to P falciparum infection.2,10,11However, some studies were unable to show inhibited parasite growth in thalassemic RBCs.12,13 This limitation could be due to subtle variations in culture conditions or the requisite manipulation of RBC parasite cultures because relative rates of parasite growth were measured with normal and abnormal RBCs in separate dishes or wells. To overcome this obstacle, we have recently developed a culture system in which parasites are simultaneously cultured in two different RBC populations, one of which is biotinylated.14 This method offers a direct simultaneous comparison of parasite growth over time in two RBC populations and minimizes the inherent variability among dish-to-dish or well-to-well comparisons. We used this novel coculture system to compare the level of in vitro growth of the P falciparum parasite in RBCs from persons with various forms of α- and β-thalassemia syndromes with normal RBCs.
MATERIALS AND METHODS
Blood samples.
After informed consent, 5 mL of venous blood was obtained from each volunteer, preserved in sterile citrate dextrose solution, and used within 1 week. Blood samples were collected from healthy control volunteers (n = 35) and persons with abnormal RBCs (n = 139), consisting of 28 classical HbH disease (α-thalassemia1/α-thalassemia2;αοα+; −−/−α), 17 HbH with Constant Spring (α-thalassemia1/CS; −−/αCSα), 15 heterozygous α-thalassemia1, 28 nonsplenectomized βο-thalassemia with HbE disease (β-thalassemia/HbE), 30 splenectomized β-thalassemia/HbE, 11 heterozygous β-thalassemia, and 10 HbE heterozygotes. A diagnosis of Hb types for all subjects was made by standard hematologic techniques and gel electrophoresis.15 All thalassemic subjects had normal G6PD levels, no evidence of concurrent infection, and none had received a blood transfusion for at least 3 months. RBCs from a group of 10 frequent blood donors known to support robust-malaria parasite growth were used as a reference standard.
Parasite culture.
A P falciparum strain (TM267TR) from Thailand was maintained in normal group O RBC suspensions at 37°C, 5% CO2atmosphere in RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 10% heat-inactivated AB-positive serum with 2 mmol/L L-glutamine (Flow Laboratories, Herts, UK), and 25 mmol/L HEPES buffer (Calbiochem, San Diego, CA). A cyanmethemoglobin method for measuring hemoglobin leakage indicated that this culture medium was nontoxic for normal and thalassemic RBCs. The medium was changed daily to maintain optimal pH and nutrient levels. The sorbitol lysis method was used to synchronize parasite growth.16
Culture of parasite in two different RBC populations.
We used a modified coculture system in which parasites were simultaneously grown in a mixture of two distinct RBC populations.14 Briefly, a synchronous collection of parasites at 90% ring (young) stage in either normal control RBCs or thalassemic RBCs was used for initiating culture. The parasitemias at the initiation of incubation were nearly equal in all experimental and control cultures. An aliquot containing an equal number of infected RBCs of either group were mixed and added into 1 mL of a coculture containing 200 × 106 RBCs each of normal and abnormal RBCs. One of the two RBC populations was prelabeled with biotin (sulfosuccinimidyl 6-biotinamido hexanoate) (Pierce & Warriner, Ltd, Rockford, UK) at a concentration of 0.3 pg/cell, an amount that does not affect the growth rates or intraerythrocytic development of parasites in either normal or thalassemic RBCs.14 Three replicates of 200 μL each of RBC coculture were transferred into 96-well coster flat-bottomed microtiter plates. Aliquots of 5 × 106 cultured RBCs were taken from the cocultures at the end of the first or second schizogonic cycles and incubated with 10 μL of titrated streptavidin fluorescein isothiocyanate (FITC) (Amersham, Arlington Heights, IL) for 30 minutes at 4°C in the dark. The cells were washed twice in cold phosphate-buffered saline (PBS), and the cell pellet was mixed with the vital stain hydroethidine (Polysciences, Inc, Warrington, PA) at a concentration of 5 μg/mL in PBS for at least 30 minutes at 37°C before flow cytometric analysis. Parallel control experiments were conducted simultaneously by culturing normal control and thalassemic RBCs alone with malaria parasites.
Flow cytometric analysis.
Analysis of the RBCs for biotin/streptavidin-FITC and parasite DNA content was performed by using a FACScan flow cytometer (Becton Dickinson, San Jose, CA) equipped with a 15-mW argon ion laser tuned at 488 nm. Logarithmic green and red fluorescences of FITC and ethidine were measured through 530/30 and 585/42 band pass filters, respectively. RBCs were gated on the basis of their logarithmic amplification of the forward scatter and 90° light scatter signals. Instrument fluorescence calibration and sensitivity were calibrated using Calibrite beads (Becton Dickinson). A total of 30,000 RBCs in replicate wells were analyzed for each sample.
Data were analyzed with CellQuest software (Becton Dickinson). Results were expressed as percent parasitemia of both unbiotinylated and biotinylated RBCs containing parasite DNA by using a two-parameter cytogram analysis. The multiplication rate data from each paired experiment were presented as a relative percent parasitemia ratio between thalassemic and normal RBCs. In addition, all experimental results were compared with a reference standard established from 10 frequent blood donors. Parasite morphology and the number of parasites in each culture were also determined from Giemsa blood smears.
Statistical analysis.
The statistical significance of difference between results was determined by the Mann-Whitney U-test. P values of .05 or less were considered significant.
RESULTS
The use of biotin/streptavidin-FITC and the DNA fluorochrome hydroethidine enabled simultaneous flow cytometric analysis of the two different RBC populations and the parasitemias.
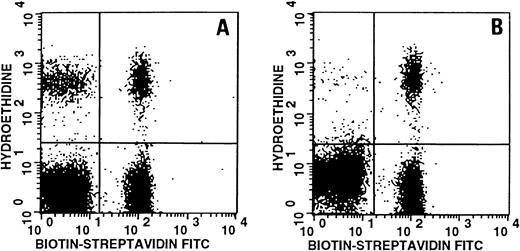
First, to verify that biotinylation would not affect the invasion or growth of malaria parasites, P falciparum–infected biotinylated and unbiotinylated RBCs stained for both surface biotin and intraerythrocytic parasite DNA were compared. Figure1, a two-parameter dot plot of the unbiotinylated normal and biotinylated normal RBCs, indicates that growth rates were similar (Fig 1A). Similar levels of parasitemias and growth rates were also observed in unbiotinylated and biotinylated thalassemic RBCs.
Representative two-parameter dot plot of P falciparum–infected unbiotinylated red blood cells (RBCs) and biotinylated RBCs stained with biotin (x-axis) and hydroethidine (y-axis). (A) Unbiotinylated normal RBCs and biotinylated normal control RBCs; (B) unbiotinylated thalassemia RBCs and biotinylated normal control RBCs. Upper left quadrant shows unbiotinylated RBCs with stained parasite DNA, upper right quadrant shows double staining of both biotinylated RBCs and parasite DNA. Lower left quadrant represents noninfected unbiotinylated RBCs and lower right quadrant shows noninfected biotinylated RBCs. Percent parasitemia in unbiotinylated and biotinylated normal control RBCs are 17.3 and 17.9, respectively (A); percent parasitemia in unbiotinylated thalassemic RBCs and biotinylated normal control RBCs are 1 and 17.7, respectively (B).
Representative two-parameter dot plot of P falciparum–infected unbiotinylated red blood cells (RBCs) and biotinylated RBCs stained with biotin (x-axis) and hydroethidine (y-axis). (A) Unbiotinylated normal RBCs and biotinylated normal control RBCs; (B) unbiotinylated thalassemia RBCs and biotinylated normal control RBCs. Upper left quadrant shows unbiotinylated RBCs with stained parasite DNA, upper right quadrant shows double staining of both biotinylated RBCs and parasite DNA. Lower left quadrant represents noninfected unbiotinylated RBCs and lower right quadrant shows noninfected biotinylated RBCs. Percent parasitemia in unbiotinylated and biotinylated normal control RBCs are 17.3 and 17.9, respectively (A); percent parasitemia in unbiotinylated thalassemic RBCs and biotinylated normal control RBCs are 1 and 17.7, respectively (B).
Then, thalassemic RBCs were cocultured with normal RBCs. The relative multiplication rates of parasites cultured in all thalassemic genotypes tested were significantly lower than that of normal RBCs (P < .0001). Figure 1B shows a comparison of unbiotinylated HbH RBCs containing a lower parasitemia than the biotinylated normal RBCs. A similar pattern was also seen for HbH/CS (α-thalassemia1/CS) and other thalassemias of both nonsplenectomized and splenectomized β-thalassemia/HbE subjects.
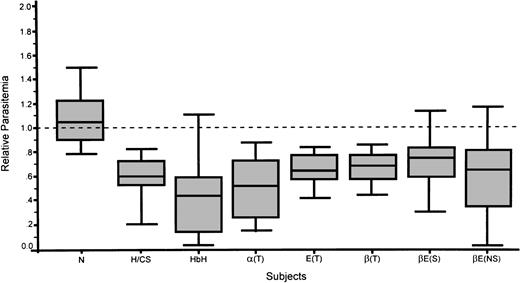
RBCs from nonsplenectomized β-thalassemia/HbE supported a parasite multiplication rate that was 0.61 ± 0.32 of normal control and 0.72 ± 0.26 for splenectomized β-thalassemia/HbE (Fig2). No significant difference in multiplication rate between nonsplenectomized- and splenectomized- β-thalassemia/HbE was found (P = .17). For β-thalassemia trait and HbE trait, the multiplication rates were 0.68 ± 0.16 and 0.66 ± 0.14 of control, respectively. A similar decrease in the multiplication level was also seen in α-thalassemia RBCs. HbH was least able to support parasite growth. The multiplication rates when compared to normal control were 0.44 ± 0.29, 0.58 ± 0.20, and 0.50 ± 0.22 for HbH, HbH/CS, and α-thalassemia1 trait, respectively. There was a significantly lower multiplication rate in α-thalassemia when compared with splenectomized β-thalassemia/HbE, particularly α-thalassemia1 trait and HbH RBCs (P < .004 and .002, respectively). For nonsplectomized β-thalassemia/HbE (P < .05 and P = .28 for HbH and α-thalassemia1 trait). There was no significant difference between the parasite multiplication rate in normal control RBCs and the reference standard group.
P falciparum–multiplication rates in normal control and thalassemic red blood cells expressed as a relative parasitemia in normal controls. Dashed line represents a reference standard established from frequent blood donors.
P falciparum–multiplication rates in normal control and thalassemic red blood cells expressed as a relative parasitemia in normal controls. Dashed line represents a reference standard established from frequent blood donors.
DISCUSSION
To better understand the “malaria hypothesis” in which hemoglobinopathies may confer protection against infection,1 we have recently developed a novel technique in which malaria parasites are simultaneously cultured in two RBC populations.14 This is achieved by the biotinylation of one RBC population that is then mixed with another unbiotinylated RBC population together with P falciparum parasites. By using this coculture system, we found that RBCs from normal and thalassemic subjects were equally susceptible to merozoite invasion as indicated by a measurable parasitemia after the first growth cycle (schizogony). However, in subsequent growth cycles, thalassemia RBCs were significantly less supportive of parasite growth than were normal RBCs (Fig 2). These in vitro findings indicate that parasite growth in thalassemia RBCs is significantly diminished, consistent with recent in vitro findings that poor re-invasion rates are noted in the second and third cycles of parasites in thalassemic RBCs.17 These data are also consistent with clinical observations that describe fewer or milder P falciparum malaria infections in people with thalassemia.6-8
In comparison with parasite growth in the cocultured normal RBCs and thalassemic RBCs, the level of inhibition of growth support of P falciparum among the abnormal RBCs varied (Fig 2). However, the mean multiplication rate in each type of thalassemic RBCs was lower than that obtained in normal RBCs. Variability in RBCs from the same type of thalassemia suggested that the severity of each individual’s disease (anemia/Hb content) may be involved. Moreover, other factors such as RBC age,17 RBC deformability, as well as individual membrane properties may affect growth rates of P falciparum.18,19 A protective role of relatively less surface area of microcytic RBCs available for parasite invasion was also suggested.20,21 Accumulation of unmatched α- and β-globin chains in the cell and in the membrane cytoskeleton could also lead to abnormal linkage Hbs resistant to parasite protease,22 associated with membrane damage by increased generation of free oxygen radical.23 24
Interestingly, our in vitro study showed that parasite growth was especially low in α-thalassemia RBCs, especially from HbH RBCs and in α-thalassemia1 trait, indicating that differences in thalassemic genotypes may confer different levels of protection against malaria. The α-thalassemia RBCs were more resistant to parasite growth than β-thalassemia RBCs, a finding that may relate to inclusion bodies, known to accumulate in vivo from excess β-globin chain.4 However, in vitro demonstration of this phenomenon would require addition of redox dyes or elevation of temperature,4,25,26 manipulations that would severely alter the established culture conditions and potentially lead to aberrant parasite growth. Overall, that less severe disease among persons with α-thalassemia is associated with a selective advantage against malaria infection may account for the relatively high prevalence of α-thalassemia in comparison with β-thalassemia in Southeast Asia.3 5
In summary, the combination of biotin/streptavidin-FITC enabled simultaneous flow cytometric analysis and parasite growth rate of the two distinct RBC populations. With this approach, the inhibitory effect of several different forms of thalassemia has been shown in vitro. The mechanism of this protection is unclear but may be because of the interaction between thalassemia phenotype, modifications of the RBC membrane, and abnormal intracellular environment. The biotin-labeled RBC coculture method may be useful in defining these mechanism(s).
Supported in part by Siriraj-China Medical Board, Grant No. 75-348-221 and by the Malaria Program of the US Army Medical Research and Materiel Command, Ft Detrick, MD.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kovit Pattanapanyasat, PhD, Office for Research and Development, Faculty of Medicine, Siriraj Hospital, Bangkok 10700, Thailand.