Before de novo synthesized von Willebrand factor (vWF) leaves the endothelial cell, it undergoes endoproteolytic cleavage of its propeptide (vW antigen II). The processed vWF and propeptide are either released constitutively or, following activation of the endothelium, released through the regulated pathway. In a recent study (Borchiellini et al, Blood 88:2951, 1996), we showed that the half-life of mature vWF and of its propeptide differ fourfold to fivefold. We postulated that the molar ratio of the propeptide to mature vWF could serve as a tool to assess the extent of endothelial cell activation under physiologic and clinical conditions. To test this hypothesis, we measured mature vWF and propeptide in patients with documented acute and chronic vascular disease, including patients with thrombotic thrombocytopenic purpura (TTP), acute septicemia, and diabetes mellitus. These data were compared with experimental conditions in healthy subjects in which perturbation of the endothelium was simulated by physical exercise or by administration of 1-deamino-8-D-arginine vasopressin (DDAVP) or endotoxin. In all individuals of the latter study group, both vWF and propeptide levels were elevated during the acute phase of the experimentally induced vascular perturbation; at later time points after stimulation, only vWF levels remained elevated. In patients with sepsis and TTP, both vWF and propeptide were elevated several-fold. Thus, this pattern can readily be explained in terms of acute perturbation of the endothelium. In contrast, in patients with diabetes mellitus propeptide levels were only slightly elevated, whereas vWF levels were elevated twofold to threefold. This pattern is a typical feature of chronic, low-grade activation of the endothelium. These observations support our hypothesis that measurement of both propeptide and vWF levels allows to discriminate between chronic and acute phases of endothelial cell activation in vivo. Measurement of only vWF is less indicative in this respect.
VON WILLEBRAND FACTOR (vWF) is a large adhesive glycoprotein that mediates the adhesion of platelets at sites of vascular damage and also functions as a stabilizing carrier protein of coagulation factor VIII. It is one of the circulating blood proteins that is produced and released by vascular endothelial cells1 and is frequently used as an indicator of endothelial cell dysfunction in vascular disorders.2-6Before de novo synthesized vWF leaves the endothelial cell, it undergoes endoproteolytic cleavage of its propeptide (also known as vW antigen II7) and, together with the propeptide, is released through both the constitutive pathway and by stimulus-induced exocytosis of specialized secretory vesicles (Weibel-Palade bodies). Both in cultured, resting endothelial cells and in stimulated endothelial cells, the stoichiometry of the released propeptide to the released mature vWF is essentially equimolar.8 However, in normal plasma the molar concentration of the propeptide is about one tenth of the concentration of mature vWF.9,10 Because the propeptide disappears four to five times faster from the circulation than mature vWF, it seems reasonable to assume that the observed differences in steady-state concentration are due to differences in half-life of the respective polypeptides. Upon perturbation of the endothelium, for instance elicited by experimental disseminated intravascular coagulation (DIC) or administration of 1-deamino-8-D-arginine vasopressin (desmopressin, DDAVP), both mature vWF and propeptide concentrations rapidly increase. Because of its rapid turnover, the propeptide concentration returns to its baseline value much faster after termination of the vascular challenge than the vWF levels.9,10 On the basis of these observations, it was postulated that measurement of both propeptide and mature vWF levels could provide a means to assess the extent and time course of endothelial cell activation under clinical conditions.9 10 For instance, if both vWF and propeptide levels are elevated, this would be indicative of acute vascular perturbation, whereas conditions in which only vWF is elevated, this would rather reflect chronic endothelial cell activation. In the present cross-sectional study, we have tested this hypothesis. We measured mature vWF and propeptide in patients with thrombotic thrombocytopenic purpura (TTP), sepsis, and diabetes mellitus. Healthy individuals, in whom an acute increase of vWF and propeptide levels were provoked by a standardized exercise test or injection of a low dose of endotoxin or DDAVP, served as a control in this study.
MATERIALS AND METHODS
Patient Selection
TTP.
Thirteen patients with TTP were studied (mean age, 36 years; range, 23 to 45). Four patients had TTP after allogeneic or autologous bone marrow transplantation (BMT). All patients had microangiopathic hemolytic anemia, thrombocytopenia (mean platelet count, 28 × 109/L; range, 12 to 48), and impaired renal function. Mean hemoglobin level was 5.1 mmol/L (range, 2.9 to 6.4); mean lactate dehydrogenase (LDH) level was 2,311 U/L (range, 658 to 3,841; normal value, <640 U/L). The mean values of these parameters were not different in patients with classic TTP and post-BMT TTP. Samples were obtained before treatment was started.
Sepsis.
Fourteen consecutive patients were recruited into the study from a primary medical-surgical intensive care unit. The entry criteria for patients with suspected sepsis syndrome were (American College of Chest Physicians/Society of Critical Care Medicine consensus conference11) the presence of a temperature (>38.3°C or <35°C) and low blood pressure. The sepsis syndrome was verified by positive blood cultures. Seven patients had a pulmonary and seven an abdominal focus for the sepsis. All patients had signs of DIC: all had a prolonged activated partial thromboplastin time (aPTT; 66 ± 8 seconds; range, 53 to 74 seconds; normal value, <36 seconds) and prolonged prothrombin time (PT; 24.6 ± 6.1 seconds; range, 18 to 38 seconds; normal value, <15.4 seconds). Fibrin degradation products (FnDPs) were elevated in 11 patients (4.9 ± 4.1 μg/mL; normal value, <1.0 μg/mL) and antithrombin levels were decreased in 13 patients (50.6% ± 21.3%; range, 22% to 97%). Platelet count was decreased in 11 patients (mean, 130 × 109/L; range, 18 to 372).
Diabetes mellitus.
Twenty-two patients with type 1 (n = 7) and type 2 (n = 15) diabetes mellitus were studied. The mean age was 60 years (range, 19 to 83). Ten patients suffered from retinopathy, 10 from nephropathy, and eight from neuropathy. The mean HbA1c was 7.3% ± 1.8%. There was no difference in HbA1C levels between type 1 and type 2 diabetes. Six patients were insulin-dependent; all other patients used oral antidiabetic drugs. Samples were taken when patients were relatively stable and no acute metabolic complications or infections occurred.
Propeptide and vWF Levels and Platelet Count
To examine a possible relationship between vWF and propeptide levels and platelet count, patients with either low or elevated platelet count were also included in this study.
Bone marrow aplasia.
Five patients with thrombocytopenia due to chemotherapy for acute leukemia, two patients with primary aplastic anemia, and two patients with congenital thrombocythemia were studied. Their mean age was 40 years (range, 19 to 59). Mean platelet count was 23 × 109/L (range, 6 to 45). Patients with signs of infection or thromboembolic complications were excluded.
Essential thrombocythemia.
Seven patients with high platelet count due to essential thrombocythemia (ET) were studied (mean age, 53 years; range, 28 to 76). Mean platelet count was 910 × 109/L (range, 494 to 1,800). All other causes for thrombocytosis were excluded by clinical features and laboratory investigations. Bone marrow examination by cytogenetic analysis was performed to exclude chronic myeloid leukemia. All ET patients were studied during treatment with aspirin.
Controls.
Controls were subjects referred to the hospital, but who upon serial clinical and laboratory investigations were shown to have no vascular disease, infections, malignancies, diabetes, or other diseases that could affect vWF and propeptide levels. Eighteen individuals were recruited as a control for this study (mean age, 48 years; range, 19 to 77).
Healthy Subjects
Experimental endotoxemia.
This study was designed as described previously.9 In the present, more extended study, eight healthy male volunteers were treated with endotoxin, administered as a 4-ng/kg injection intravenously in 1 minute. At different time points after the injection of endotoxin, blood samples were collected from the antecubital vein.
DDAVP.
This study group consisted of nine healthy volunteers (five males and four females; mean age, 31 years) previously studied to assess the half-life of vWF after administration of DDAVP.12 They received 0.4 μg DDAVP/kg body weight.
Exercise.
Five healthy males (mean age, 40 years; range, 35 to 45) were subjected to a standardized exercise test (cycle ergometer) as described previously.13 Blood samples were collected immediately before the exercise test and at 15 minutes after maximal performance.
Collection of Blood and Assays
Blood, after collection in vacutainer tubes containing 3.2% buffered citrate solution (1:9 vol/vol), was immediately placed on ice. After centrifugation at 3,000g for 20 minutes at 4°C, plasma was aliquoted and stored at −70°C until batchwise assessment. Propeptide and mature vWF concentrations were measured by enzyme-linked immunosorbent assay (ELISA) as described previously.9Normal plasma from a pool of 30 donors served as standard. This plasma pool contains 6.3 nmol/L propeptide, as assessed by calibration against purified recombinant propeptide,9 and 50 nmol/L of vWF (in half homo-dimers, estimated concentration14). Soluble P-selectin (sP-selectin), a specific marker of platelet activation,15-17 was measured by ELISA as described previously.16 Platelets were counted with a Coulter counter. All samples were obtained after informed consent.
Statistical Analysis
All data are presented as the mean ± SEM. The means in vWF and propeptide levels were compared by Student’s t-test with vWF and propeptide levels found in the respective control samples. The Pearson correlation coefficient was used as a measure of linear association between two variables.
RESULTS
Effect of Experimental Endotoxemia, Administration of DDAVP, and Exercise on vWF and Propeptide Levels in Healthy Subjects
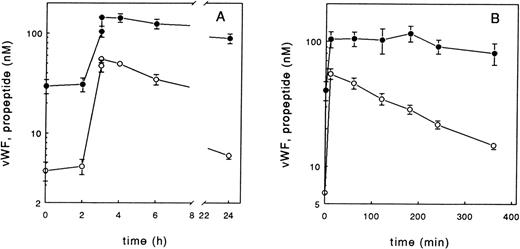
As previously shown,9 combined elevations of vWF and propeptide levels is a typical feature of experimental endotoxemia and DDAVP-induced vascular perturbation. To document this picture in more detail, eight subjects received endotoxin and nine received DDAVP. Subsequently, vWF and propeptide levels were measured at different time points after injection. In all subjects studied, administration of low-dose endotoxin led to a distinct increase of both vWF and propeptide levels after a lag phase of 1 to 2 hours (Fig1A). The rise of vWF and propeptide concentration was followed by a decline of propeptide levels, whereas the vWF concentration remained elevated for at least 20 hours. The half-life of vWF and propeptide after administration of endotoxin, calculated from the disappearance curves, was approximately 12 and 3 hours, respectively. This observation clearly documents that under conditions in which acute perturbation of the endothelium is induced, the clearance of circulating propeptide is much faster than that of vWF. Similarly, administration of DDAVP resulted in a prompt increase of both propeptide and vWF levels (Fig 1B). The propeptide level returned close to baseline values after about 6 hours, whereas at this time point the vWF level was still twice as high as the vWF concentration before injection of DDAVP. The estimated half-lives of propeptide and vWF differed about threefold to fourfold. To facilitate comparison with clinical data (see later), data of peak levels of vWF and propeptide and vWF and propeptide concentration measured at later time points after endothelial stimulation are summarized (Table1). We also tested the effect of physical exercise on the plasma concentrations of propeptide and vWF in healthy volunteers. Similar to DDAVP and endotoxin, exercise enhances vWF release twofold to threefold and the propeptide level fivefold to eightfold (Table 1).
Effect of administration of endotoxin (A) or DDAVP (B) on vWF (•) and propeptide levels (○) in healthy individuals. Endotoxin (4 ng/kg body weight) or DDAVP (0.4 μg/kg body weight) were administered at time 0. The endotoxin study group consisted of 8 subjects, the DDAVP study group of 9 subjects. Data points represent the mean and bars the SEM.
Effect of administration of endotoxin (A) or DDAVP (B) on vWF (•) and propeptide levels (○) in healthy individuals. Endotoxin (4 ng/kg body weight) or DDAVP (0.4 μg/kg body weight) were administered at time 0. The endotoxin study group consisted of 8 subjects, the DDAVP study group of 9 subjects. Data points represent the mean and bars the SEM.
Propeptide and vWF Levels in Patients With Chronic and Acute Vascular Disease
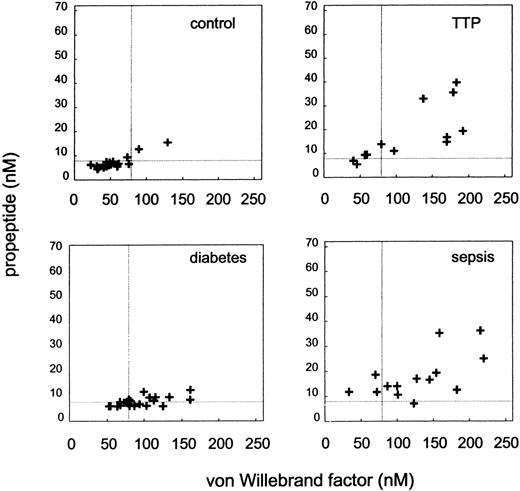
A total of 49 patients was studied with different signs of vascular pathology. Patients with diabetes mellitus suffered from chronic vascular dysfunction, whereas patients with TTP and sepsis were admitted to the hospital with acute symptoms of vascular pathophysiology. Eighteen patients, referred to the hospital for underlying disorders other than vascular disease, served as a control group in this study. In the latter study group, the mean vWF and propeptide level was 54.2 ± 6.0 and 7.1 ± 0.7 nmol/L, respectively. The mean level of mature vWF was significantly increased in all patient groups studied (P < .001, Table2). These values were about twice as high as the mean plasma levels of patients without vascular pathology. In patients with diabetes mellitus, the mean propeptide level was normal (8.0 ± 0.4 nmol/L). However, there was a significant correlation between propeptide and vWF levels (r = .61, P < .01). There was no difference in glycosylated hemoglobin levels and vWF or propeptide levels between type 1 and type 2 diabetes. The individual data for each of the patients and the control group are shown in Fig2. In patients with TTP, both the mean vWF level (112.7 ± 16.6 nmol/L) and the mean propeptide level (17.3 ± 3.3 nmol/L) were substantially elevated (P < .001 andP < .01, respectively). Similarly, in septic patients, both vWF and propeptide concentrations were significantly elevated (127.8 ± 14.7 and 17.8 ± 2.3 nmol/L, respectively; P < .001). The data, on an individual basis, are shown in Fig 2. In the majority of these patients, both vWF and propeptide were elevated. In patients with TTP, a significant correlation between the LDH and propeptide levels was observed (r = .82, P < .001). The correlation between LDH and vWF levels was not significant (r = .51, P = .09). In patients with sepsis, a significant correlation between FnDPs and propeptide was found (r = .58, P < .03). The correlation between vWF levels and FnDPs was not significant (r = .47, P = .07).
Relation between plasma vWF and propeptide levels in patients with TTP (n = 13), diabetes (n = 22), sepsis (n = 14), and control group (patients referred to the hospital, but without vascular disease, n = 18). Dotted lines represent the upper limit of the 95% confidence interval of, respectively, vWF and propeptide levels of the control group.
Relation between plasma vWF and propeptide levels in patients with TTP (n = 13), diabetes (n = 22), sepsis (n = 14), and control group (patients referred to the hospital, but without vascular disease, n = 18). Dotted lines represent the upper limit of the 95% confidence interval of, respectively, vWF and propeptide levels of the control group.
Propeptide and vWF Levels in Patients With Thrombocytopenia and Thrombocythemia
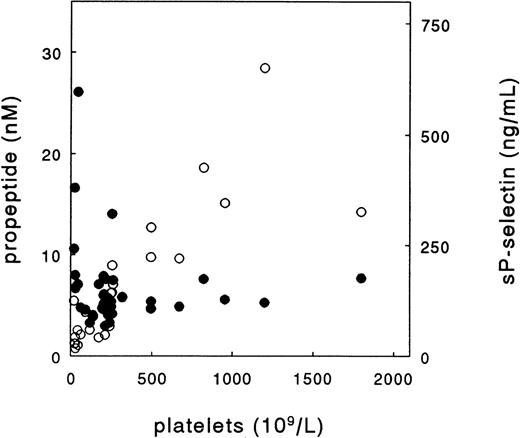
As both vWF and its propeptide may not only originate from endothelial cells but also from platelets, vWF and propeptide levels were measured in plasma from healthy individuals and patients with low or high platelet counts. Figure 3 shows the relationship between propeptide concentration and platelet count in patients with bone-marrow aplasia, essential thrombocythemia and healthy controls. These parameters did not correlate (r = .2, difference not significant [NS]). Similarly, vWF levels did not correlate with platelet number (not shown). In contrast, there was a significant relationship (r = .7, P < .001) between platelet number and the concentration of plasma sP-selectin (Fig 3), a specific marker of platelet activation.16 17 Also in patients with TTP, sepsis, or diabetes, there was no correlation between platelet count and propeptide or vWF concentration (not shown). These observations suggest that in these individuals both propeptide and mature vWF originate from the endothelium, rather than from platelets.
Relation between platelet count with plasma propeptide (•) and sP-selectin (○) concentration in patients with bone-marrow aplasia (n = 5), patients with essential thrombocythemia (n = 7), and healthy subjects (n = 19).
Relation between platelet count with plasma propeptide (•) and sP-selectin (○) concentration in patients with bone-marrow aplasia (n = 5), patients with essential thrombocythemia (n = 7), and healthy subjects (n = 19).
DISCUSSION
The primary purpose of this study was to determine the potential value of measurement of plasma concentrations of both vWF and its propeptide as a means to discriminate between acute and chronic vascular disease. This concept is illustrated by a number of control experiments in healthy subjects in which perturbation of the endothelium was provoked by administration of endotoxin or DDAVP, both agents known for their ability to elicit increases of plasma vWF and propeptide levels. DDAVP induces immediate release of vWF and propeptide, whereas lipopolysaccharide (LPS)-induced secretion is preceded by the release of one or more second messengers, which most likely mediate vWF and propeptide secretion through the regulated pathway.9,18,19 In all subjects studied, the concomitant rise of vWF and propeptide levels was followed by a rapid decline of propeptide levels, whereas vWF concentrations only slowly normalized (Table 1 and Fig 1). Similarly, physical exercise induced a rapid increase of both vWF and propeptide. Thus, during the acute phase of vascular perturbation, both vWF and propeptide were elevated. However, at later time points (eg, 6 or 24 hours after stimulation), only vWF levels were still elevated. Clearly, the vWF-propeptide is more rapidly cleared from the circulation than mature vWF.9 10 Thus, there is an experimentally based rationale for the hypothesis that elevated propeptide (together with elevated vWF) is a marker of acute but transient endothelial cell activation.
Indeed, an increase of both vWF and propeptide levels was seen in patients with symptoms of frank, acute vascular disease, including TTP and septicemia. We decided to concentrate on these disorders because it is well documented that endothelial injury and the concomitant increase of vWF occurs early in endotoxemia and in TTP.20,21 In contrast, in patients with a long history of vascular disease, such as diabetes mellitus, propeptide levels were only slightly elevated, even in cases with high vWF levels (Fig 2). Apparently, marginally increased propeptide concentrations in combination with elevated vWF levels reflect a chronic event or, alternatively, the decay of a preceding acute vascular insult. Although the mean propeptide level in the patients with diabetes mellitus was not significantly different from the mean propeptide level of the control group, the individual propeptide levels clearly correlated with vWF levels. This observation is in agreement with a recent study of Vischer et al, who also found a close correlation between propeptide and vWF levels in diabetes.22 On the basis of these data, it seems reasonable to assume that in the majority of patients studied with TTP and sepsis, vWF elevations are due to acute perturbation of the endothelium, whereas in patients with diabetes, elevated vWF levels rather reflect chronic stimulation of the endothelium. This view is in accordance with the clinical picture of these disorders. Thus, a new aspect of this study is that measurement of both propeptide and vWF levels in individual patients allows discrimination between acute and chronic stimulation of the endothelium; measurement of vWF concentrations only does not allow firm conclusions in this respect. This study could provide a basis for a more differentiated and detailed analysis of endothelial cell activation that may occur in response to vascular pathology. Although blood samples were collected immediately upon presentation, it seems reasonable to assume that some patients had experienced vascular stimulation for time periods that were long in comparison to the 3-hour half-life of the propeptide. Therefore, in these cases the vWF and propeptide levels not only would reflect increased secretion, but also plasma clearance. On the other hand, in some TTP patients propeptide and vWF levels reached peak levels (≈50 and 150 nmol/L, respectively) that were also observed briefly after exposure of healthy individuals to DDAVP or endotoxin. This suggests that the provoking event in these patients had occurred just before admission.
It should be noted that in this cross-sectional study patients were studied at single time points after admission to the hospital because of acute symptoms or an exacerbation of the disease. Prospective, serial studies should reveal whether indeed measurements of both propeptide and vWF levels have a predictive value in terms of disease activity and are useful in monitoring the degree of vascular involvement as well as the response to therapy. In TTP, there was a strong correlation between propeptide concentrations and LDH (P< .001), a marker of hemolysis and severity of disease, whereas the correlation between vWF and LDH was not significant (P = .09). Similarly, in patients with sepsis, propeptide levels and FnDPs, a marker of DIC, were correlated (P < .03), whereas vWF levels and FnDPs were not (P = .07). These data suggest that propeptide is more reliable than vWF as a marker of vascular disease activity, at least in acute vascular disorders. It is possible that the difference in correlation as revealed by ELISA is related to the rather complex quaternary structure of vWF compared with the structure of its propeptide. These differences in biochemical nature could cause differences in the precision of vWF measurements. It should also be noted that in patients with concomitant organ failure, such as nephropathy or liver disease, these dysfunctions could have affected the metabolism of vWF and its propeptide. This would also complicate the interpretation of data on the plasma levels of these proteins. Similarly, altered patterns of posttranslational modifications (in TTP or diabetic patients) or edema formation (in sepsis) could affect the steady-state level of propeptide and vWF.
It could not be excluded that, as suggested previously,23in the patients studied platelet activation contributed to the elevated propeptide (and vWF) levels. Indeed, some septic patients and all patients with TTP suffered from severe thrombocytopenia, obviously due to platelet activation. However, there was no relationship between platelet number and propeptide levels (not shown). Also in patients with either high or low platelet counts due to underlying disorders other than TTP or sepsis propeptide levels did not correlate with platelet number (Fig 3). On the other hand, plasma levels of sP-selectin, a specific marker of platelet activation,16,17clearly correlated with platelet number in these patients (Fig 3). Further, the amount of propeptide stored in the α-granules of platelets is probably not sufficient to account for the increased propeptide concentrations.9,10 24 It seems more likely therefore that vascular endothelial cells are the major source of circulating propeptide and vWF in patients and healthy subjects examined in this study. Therefore, another interesting feature of our study is that the vWF propeptide is a genuine marker of acute endothelial cell activation.
The question as to the mechanism that induces vWF and propeptide release and pathways through which these proteins are released by the endothelium under pathologic conditions is more difficult to answer. VWF and propeptide can be released through both the regulated and constitutive pathway.1 However, the storage capacity of endothelial cells is limited, and it seems likely that, particularly in cases where the endothelium is exposed to repeated challenges, the vWF/propeptide pool is exhausted. In addition, replenishment of these stores requires about 1 to 2 days after a single exposure to endothelial cell agonists, at least in cultured endothelial cells.25 Further, only a fraction of newly synthesized pro-vWF is directed to the Weibel-Palade bodies.26 On the basis of these data, it is to be expected that only in cases of isolated episodes of endothelial challenge, such as may occur in TTP or an event preceding overt septicemia, released vWF and propeptide primarily originate from the Weibel-Palade bodies. Pertinent to this point is the observation that in some patients with sepsis or TTP, propeptide and vWF levels approximate the peak levels of propeptide and vWF observed under experimental endothelial cell perturbation (≈50 and 150 nmol/L, respectively; Fig 1). Subsequent and persistent activation of the endothelium and concomitant rises of plasma vWF and propeptide most likely reflect enhanced constitutive release. However, the latter condition would require upregulation of de novo pro-vWF synthesis. Indeed, endothelial cell–specific vWF synthesis can be subject to enhanced transcriptional activity.27,28 It seems reasonable to assume, therefore, that elevated propeptide and vWF levels in patients with persistent endothelial cell injury not only reflect regulated secretion but also increased transcriptional activity and subsequent enhanced constitutive release. As unprocessed pro-vWF is primarily released through the constitutive pathway,26measurement of pro-vWF could provide a means to discriminate between constitutive and regulated release.
ACKNOWLEDGMENT
We gratefully thank Drs J. Voorberg, K. Mertens, P.J. Lenting, and W.G. van Aken (CLB, Sanquin Blood Supply Foundation, Amsterdam, The Netherlands) for valuable discussion and comments on the manuscript.
Supported by the Dutch Thrombosis Foundation (Grant No. 96.001).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jan A. van Mourik, PhD, Department of Blood Coagulation, CLB, Sanquin Blood Supply Foundation, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: J_van_Mourik@clb.nl.