Loss of response to a gluten-free diet (refractory sprue) and ulcerative jejunitis are complications of celiac disease that may progress to enteropathy-associated T-cell lymphoma (EATL). Both conditions are characterized by the presence of a nonlymphomatous monoclonal T-cell population in the enteropathic mucosa. In EATL, a similar monoclonal population that shows clonal identity with the lymphoma itself is also present in the enteropathic mucosa. In this study we show that in all three circumstances the monoclonal T-cell population is constituted by cytologically normal, noninvasive intraepithelial T lymphocytes that share an identical aberrant immunophenotype with EATL. Patients with refractory sprue and/or ulcerative jejunitis are, therefore, suffering from a neoplastic T-cell disorder for which hematological treatment strategies need to be devised.
ENTEROPATHY-ASSOCIATED T-cell lymphoma (EATL) of the small intestine is a well-documented complication of celiac disease.1 It may occur in patients with a life-long history of celiac disease (gluten sensitive enteropathy) but more often follows a short history of adult celiac disease, the assumption being that these patients have had lifelong, albeit cryptic, gluten sensitivity. In either case EATL is often heralded by a loss of response to a gluten-free diet, a condition sometimes called refractory sprue.2,3 However, not all cases of refractory sprue necessarily progress to EATL. In a related complication of celiac disease known as ulcerative jejunitis,4 5 nonspecific inflammatory mucosal ulcers are present and these patients, too, become resistant to a gluten-free diet. Some patients with ulcerative jejunitis progress to develop EATL and, interestingly, multiple inflammatory mucosal ulcers frequently accompany EATL itself.
The neoplastic cells of EATL are most commonly CD3+, CD4−, CD8− and contain cytotoxic granules recognized by the TIA-1 antibody.6 In a minority of cases the cells may express CD8 and a subtype of the lymphoma has recently been described in which the cells are both CD8+and CD56+.7 These immunophenotypic features approximate those of intra-epithelial T lymphocytes (IEL), which are thought to be the normal cell counterpart of EATL.8Intraepithelial lymphocytes are, however, phenotypically heterogeneous.9,10 Most are cytotoxic T cells that express CD3 and CD8 and have rearranged TCRβ chain genes. There is a minority population of CD4− CD8− IEL with rearranged γδ but not β chain genes. These γδ T cells comprise 10% to 15% of IEL in normal mucosa but may increase in concentration in patients with celiac disease up to a level of 30%.11 Finally, there is a third population of CD56+ cells that accounts for a very small fraction of IEL10 that is virtually undetectable in immunostained paraffin sections of normal or celiac mucosa (unpublished observations, 1998).
Isaacson et al,1 using Southern blotting and DNA extracted from fresh frozen tissue, were the first to report monoclonal rearrangement of TCRβ genes in EATL. Subsequently, several groups have shown monoclonal rearrangement of TCRγ genes in EATL using polymerase chain reaction (PCR).12,13 This method has the advantage that it is applicable to formalin-fixed paraffin-embedded specimens, thus dramatically increasing the number of cases that can be studied. Using PCR followed by sequence analysis, Murray et al13 showed that there was a T-cell population in the “uninvolved” enteropathic small intestinal mucosa adjacent to EATL that shared the same monoclonal TCRγ rearrangement as the lymphoma. Ashton-Key et al5 confirmed this finding and further showed TCRγ monoclonality in the nonspecific “inflammatory” ulcers and intervening enteropathic mucosa in ulcerative jejunitis in the absence of any overt lymphoma. In cases of ulcerative jejunitis where lymphoma subsequently developed, the same clone could be detected in the malignant cells by PCR and sequence analysis. This finding has recently been confirmed by Carbonnel et al,14 who also confirmed the findings of Cellier et al,15 who had shown that in refractory sprue monoclonal populations of T cells were present in the small intestinal mucosa. Cellier et al15 had also shown that this monoclonal population was constituted by phenotypically abnormal CD3− (CD3ε+) CD4−, CD8− IEL.
The studies summarized above raise several questions regarding the significance of the detection of a monoclonal T-cell population in the small intestine. First, where exactly do these cells reside and what is their phenotype? Second, what is the link, if any, between these different complications of celiac disease that are characterized by monoclonal populations of T cells in enteropathic mucosa? Third, is clonality synonymous with neoplasia or even malignancy; and, finally, what are the implications of detecting such a population for patient management?
MATERIALS AND METHODS
Paraffin blocks of duodenal or jejunal biopsy specimens from cases of uncomplicated celiac disease with biopsy evidence of gluten sensitivity (n = 17) and from cases of celiac disease where there had been no response to a gluten-free diet (n = 6) were retrieved from the surgical pathology and consultation files of University College London Hospital. In addition, representative blocks of ulcers or lymphomas and of uninvolved enteropathic mucosa from small intestinal resection specimens from patients with ulcerative jejunitis (n = 5) and EATL (n = 9) were retrieved from the same files. Nine of the biopsy specimens from uncomplicated celiac patients, and tissue from all 5 cases of ulcerative jejunitis and from 5 of the EATL cases had been partially studied and reported previously.5 The clinical features were briefly reviewed and the routine histopathological appearances were also reviewed in each case.
Immunohistochemistry.
Paraffin sections were taken to water and heat-mediated antigen retrieval was performed. Sections for CD3 and CD8 single or double staining and CD56 staining were pressure cooked in citrate buffer (pH 6.0) for 2 minutes at full pressure. Slides for CD4 staining were pressure cooked in EDTA buffer (pH 8.0) for 2 minutes at full pressure.
Sections were hand stained with CD4 (Vector Labs, Peterborough, Lincs, UK) and CD56 (Bradsure Biologicals Ltd, Loughborough, Leicester, UK) using an immunoperoxidase ABC method and counterstained with hematoxylin. Slides were stained with polyclonal CD3 (polyclonal anti-CD3ε; Dako, Ely, Cambridge, UK) and CD8 (Dako) on a Dako TechMate 500 using the Dako ChemMate Labelled Streptavidin Peroxidase/DAB kit (K5001). Sequential double staining for CD8 followed by CD3 was also carried out on a Dako TechMate 500. CD8 was detected using the Peroxidase/DAB kit (brown reaction product) and CD3 was subsequently detected using the Dako ChemMate Alkaline Phosphatase kit (K5005) and visualized using Fast Blue/Naphthol AS-BI (blue reaction product). Because the number of CD4+ IEL is vanishingly small, the percentage of CD4− CD8− IEL can be obtained by counting the number of CD3+ cells (blue) in a total of 100 IEL (brown and blue) in preparations double-stained sequentially for CD8 and CD3.
Molecular genetics.
DNA was extracted from paraffin sections using proteinase K digestion without subsequent organic extraction as previously described.16 Duplicate aliquots of each sample were analyzed for rearrangement of the TCR-γ chain gene using two sets of primers.12 Set 1 consisted of primers directed to the VγI, VγIII-IV, and Jγ1/2 segments and set 2 of primers targeting VγI, VγIII-IV, and JPγ1/2. Forty cycles of PCR of 1 minute at 93°C, 1 minute at 55°C, and 1 minute at 73°C were performed after hotstart by addition of Taq polymerase at 55°C after 5 minutes at 95°C. The final extension time was extended to 6 minutes. DNA extracted from a paraffin block of a T-cell lymphoma was used as a positive control and a reaction without template DNA was run as a negative control in all experiments. Products were run on 10% polyacrylamide gels, stained with ethidium bromide, and viewed under UV light.
RESULTS
Results are summarized in Table 1.
Uncomplicated celiac disease.
There were 15 women and 2 men ranging in age from 19 to 73 years. All patients had presented with malabsorption as adults and had responded both clinically and histologically to a gluten-free diet. The initial biopsy specimen was available for review in each case.
All biopsy specimens showed villous atrophy with crypt hyperplasia and marked increase in IEL. Immunostaining confirmed that the IEL were CD3+ T cells and showed scattered CD3+ T cells in the lamina propria with occasional concentrations just above the muscularis mucosae. The majority of IEL were CD8+ with only rare CD4+ cells and even rarer CD56+ cells. The lamina propria T cells were a mixed population that expressed both CD4 and CD8 in a ratio of approximately 2:1. In double-stained preparations (see Fig 2A), the percentage of CD4−CD8− IEL ranged from 7% to 24%, with a mean of 17%. PCR analysis of TCRγ genes showed a polyclonal ladder or smear in all cases (Fig 1A).
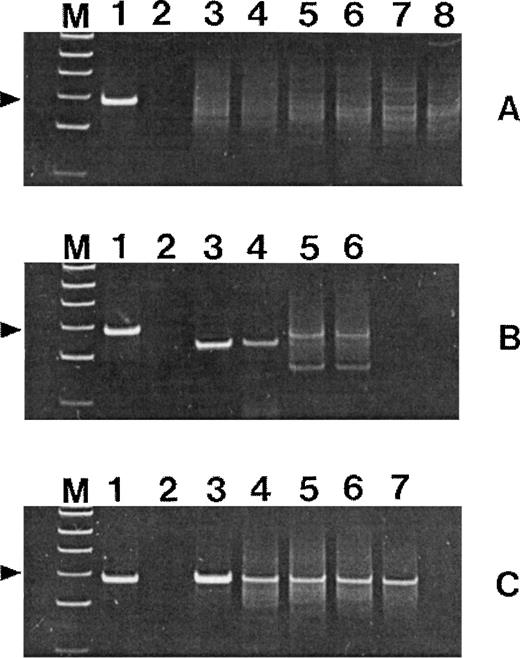
Polyacrylamide gels of TCRγ (set 1) PCR products. Lanes M, ◊XHinfl molecular-weight markers (the 82-bp fragment is indicated); lanes 1, positive control T-cell lymphoma; lanes 2, negative control without template DNA. (A) Lanes 3, 4, and 5, 6, and 7, 8: duplicate amplifications of three cases of uncomplicated celiac disease. (B) Lanes 3, 4 and 5, 6: duplicate amplifications of two cases of nonresponsive celiac disease. (C) Lane 3, EATL tumor mass; lanes 4 through 7, nonlymphomatous mucosa.
Polyacrylamide gels of TCRγ (set 1) PCR products. Lanes M, ◊XHinfl molecular-weight markers (the 82-bp fragment is indicated); lanes 1, positive control T-cell lymphoma; lanes 2, negative control without template DNA. (A) Lanes 3, 4, and 5, 6, and 7, 8: duplicate amplifications of three cases of uncomplicated celiac disease. (B) Lanes 3, 4 and 5, 6: duplicate amplifications of two cases of nonresponsive celiac disease. (C) Lane 3, EATL tumor mass; lanes 4 through 7, nonlymphomatous mucosa.
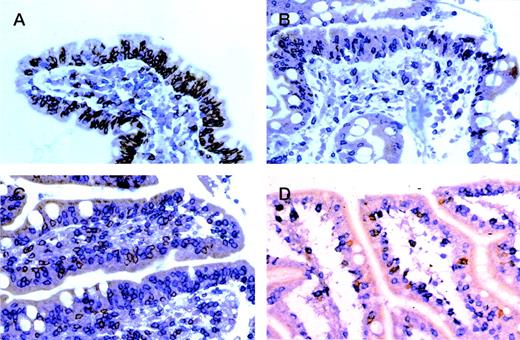
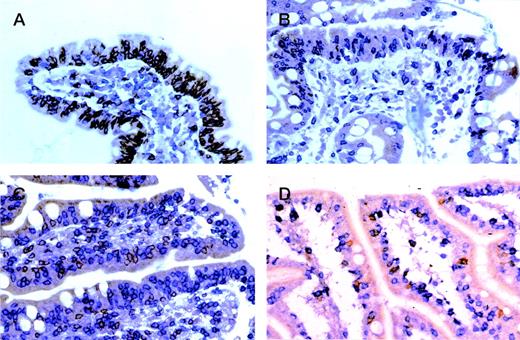
Enteropathic mucosa from cases of (A) gluten-responsive celiac disease, (B) refractory sprue, (C) ulcerative jejunitis, and (D) EATL sequentially immunostained for CD8 (peroxidase-brown) and CD3 (alkaline phosphatase-blue). In (A) IEL are predominantly CD8+ (brown) with only occasional CD3+, CD4/8− (blue) cells. Note predominance of CD3+, CD4+ (blue) cells in the lamina propria. In (B through D), most IEL are CD3+, CD4/8− (blue) with only occasional CD8+(brown) cells.
Enteropathic mucosa from cases of (A) gluten-responsive celiac disease, (B) refractory sprue, (C) ulcerative jejunitis, and (D) EATL sequentially immunostained for CD8 (peroxidase-brown) and CD3 (alkaline phosphatase-blue). In (A) IEL are predominantly CD8+ (brown) with only occasional CD3+, CD4/8− (blue) cells. Note predominance of CD3+, CD4+ (blue) cells in the lamina propria. In (B through D), most IEL are CD3+, CD4/8− (blue) with only occasional CD8+(brown) cells.
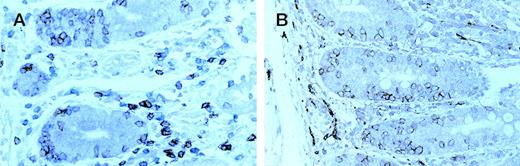
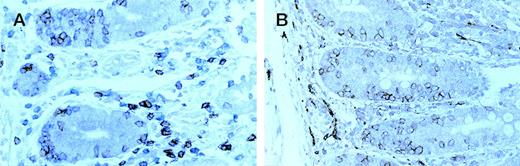
Enteropathic mucosa from a case of type B EATL . (A) sequentially immunostained for CD8 (peroxidase-brown) and CD3 (alkaline phosphatase-blue); (B) immunostained for CD56 (peroxidase-brown). Most IEL are CD8+ (brown in A) and CD56+.
Enteropathic mucosa from a case of type B EATL . (A) sequentially immunostained for CD8 (peroxidase-brown) and CD3 (alkaline phosphatase-blue); (B) immunostained for CD56 (peroxidase-brown). Most IEL are CD8+ (brown in A) and CD56+.
Celiac disease unresponsive to a gluten-free diet.
This group comprised 5 women and 1 man whose ages ranged from 57 to 68 years. In three cases celiac disease had been diagnosed 3, 9, and “many” years previously on the basis of malabsorption with clinical and biopsy evidence of gluten sensitivity; none of these patients had been tested for antibodies to endomysium, but one patient had tested positive for antibodies to α-gliadin. In the three other cases the patients presented with malabsorption and villous atrophy with no prior clinical or biopsy evidence of gluten sensitivity. One of these patients had two sisters in whom celiac disease had been diagnosed and one had tested positive for endomysial antibodies. Currently all six patients have persistent diarrhea and anemia with iron and vitamin B12 deficiency; none has developed lymphoma.
The histological appearances were similar to the biopsy specimens from uncomplicated celiac disease with an increase in cytologically normal IEL. The lamina propria CD3+ T-cell population showed a similar mixture of CD4 and CD8 cells, but there was a marked decrease in the proportion of CD8+ IEL. This was confirmed in CD8/CD3 double-stained preparations where the percentage of CD4−CD8− T cells ranged from 47% to 90%, with a mean of 72.6% (Fig 2B).
PCR analysis of TCRγ genes showed a reproducible dominant band indicative of a monoclonal population in five cases; in one case there were two dominant bands consistent with biallelic monoclonal rearrangement (Fig 1B).
Ulcerative jejunitis.
There were 4 men and 1 woman whose ages ranged from 28 to 67 years. All presented with severe abdominal pain due to an obstructing stricture or perforation after a diagnosis of childhood celiac disease in three cases and malabsorption “several” years and 2 years before presentation, respectively, in two cases. Two patients had developed EATL, 1 and 6 years, respectively, after the onset of ulcerative jejunitis. Surgical resections of the intestine to include both ulcerated and intact mucosa were performed in each case.
The intact mucosa showed the histological features of celiac disease as described above. The immunohistochemical findings were almost identical to those of the cases of unresponsive celiac disease except that the percentage of CD4−CD8− IEL in double-stained sections was much higher, ranging from 73% to 100%, with a mean of 88.6% (Fig 2C). Sections from the ulcer bases contained a mixed CD4+, CD8+ population of T cells without any detectable excess of CD3+, CD4−, CD8− cells in either single- or double-stained preparations.
PCR analysis of TCRγ genes showed a reproducible dominant band indicative of a monoclonal population in blocks taken from both the intact mucosa and the ulcers in each case.
Enteropathy-associated T-cell lymphoma.
There were 3 women and 6 men whose ages ranged from 41 to 75 years. All presented with complications of a small intestinal lymphoma with a long-standing diagnosis of celiac disease in two cases. Surgical resection of the lymphoma and uninvolved mucosa was performed in each case.
In six cases (type A), the lymphoma consisted of pleomorphic medium-sized to large cells that were CD3+, CD4−, CD8−. In three cases (type B) the tumor cells were small and monomorphic and were CD3+, CD4−, CD8+, and CD56+. In all cases the lymphoma cells contained TIA-1+ cytotoxic granules.
In all nine cases the intact mucosa showed the histological features of celiac disease with marked increase in IEL. In the six group A cases the percentage of CD4− CD8− IEL calculated in double-stained sections ranged from 48% to 86% (Fig2D). However, in the three group B cases the percentage of CD4− CD8− IEL ranged from 7% to 18% (Fig 3A) and the great majority of CD3+ IEL expressed both CD8 and CD56 (Fig 3B). In one of these cases small numbers of CD56+ IEL were easily identified in both duodenal and gastric biopsies that had been performed before resection of the lymphoma.
PCR analysis of TCRγ genes showed a reproducible dominant band indicative of a monoclonal population in blocks taken from both the lymphoma and uninvolved mucosa in each case (Fig 2C).
DISCUSSION
The presence of a monoclonal T-cell population in nonlymphomatous enteropathic small intestinal mucosa has been described in EATL, ulcerative jejunitis, and nonresponsive celiac disease.5,12,13-15 Cellier et al,15 in a study of fresh mucosal biopsy specimens, showed that in nonresponsive celiac disease this population resides in the intraepithelial T-cell compartment. They also showed that this population had a markedly aberrant immunophenotype (sCD3ε−, cCD3ε+, CD4−, CD8−, TCRαβ−, and γδ−) in comparison with the major sCD3+, CD8+, TCRαβ+ and minor CD4−, CD8− γδ+ populations of T cells that comprise the increased IEL in uncomplicated celiac disease. Such comprehensive immunophenotyping cannot be achieved in paraffin-embedded material, but the same monoclonal IEL population can nevertheless be demonstrated in paraffin sections of mucosal biopsy specimens from nonresponsive celiacs by sequential double staining for CD8 and CD3ε, which allows an estimation of CD3ε+, CD4−, CD8− T cells. The presence of a similar increase in cCD3ε+, CD4−, CD8− IEL in the “uninvolved” enteropathic mucosa in ulcerative jejunitis and type A EATL, which in both conditions harbors a monoclonal T-cell population,5,13 16strongly suggests that here, too, the clonal population resides in the IEL compartment. Thus, the presence of a monoclonal IEL population links the mucosa in these three conditions. It proved impossible to show these abnormal T cells in the bases of the nonlymphomatous ulcers in ulcerative jejunitis, despite molecular evidence of a monoclonal population of T cells because of the large number of CD4+and CD8+ T cells present as part of the inflammatory process (data not shown).
Most cases of EATL are of the type A variety in which the neoplastic cells express CD3ε but are CD4− and CD8−. Sequence analysis has shown that these tumor cells are clonally identical with a T-cell population in the enteropathic mucosa now identified as the IEL.5,13 This points to a direct link between the monoclonal T-cell population in nonresponsive celiac disease, ulcerative jejunitis, enteropathic nonlymphomatous mucosa in EATL, and EATL itself. In keeping with this, EATL is a well-recognized complication of the two former conditions. The immunophenotypic features of nonlymphomatous mucosa in type B EATL in which the neoplastic cells are CD3ε+, CD8+, and CD56+7 illustrates this point more graphically. In the three cases studied, the IEL in the enteropathic, nonlymphomatous mucosa expressed the same CD3ε+, CD8+, CD56+ phenotype as the lymphoma. In one of these cases these CD56+ IEL were present distant from the ileal lymphoma mass in both duodenal and gastric mucosa. This finding is similar to that reported by Cellier et al,15 who detected immunophenotypically aberrant IEL in rectal biopsy specimens from their cases of nonresponsive celiac disease.
The interpretation of the results reported in this study should take into account other studies which have shown that IEL isolated from intestinal mucosa are oligoclonal and that the same dominant clones may be found at different sites along the small intestine.17 18Had this been a factor one would expect a dominant clone to be present in many, if not all, biopsy specimens from uncomplicated celiac disease, which was not the case (Fig 1A). The PCR finding of a dominant T-cell clone within DNA extracted from full-thickness intestinal mucosa, which includes the T-cell rich lamina propria, is, thus, strong evidence of a neoplastic population. This interpretation is reinforced by the finding of the same neoplastic clone in EATL and nonlymphomatous mucosa and in the mucosa from cases of ulcerative jejunitis and subsequent lymphoma.
On the basis of previous molecular analyses5 13 and the immunophenotypic findings in this study, it would seem safe to conclude that the monoclonal IEL in patients with complications of coeliac disease are neoplastic, although they are not cytologically abnormal and they do not form tumor masses. The accumulation of phenotypically aberrant, monoclonal IEL appears to be the first step in the genesis of EATL. With the recognition that patients with nonresponsive celiac disease and/or ulcerative jejunitis are in fact suffering from a neoplastic T-cell disorder, possibly involving most of the gastrointestinal tract, gastroenterologists will increasingly turn to hematological oncologists for help in treating these difficult patients. It remains to be seen whether current chemotherapeutic regimes have anything to offer in this respect or whether new strategies will need to be devised. Further cell and molecular biological investigations are indicated particularly to establish the precise relationship between the neoplastic IEL and the cells of fully developed EATL.
ACKNOWLEDGMENT
The authors thank Dr A.D. Rogers, Dr L. Krenacs, and Prof P. Ciclitera for permission to use their cases.
Supported by the Leukaemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Peter G. Isaacson, MD, Department of Histopathology, Royal Free and University College Medical School, University St, London WC1E 6JJ, UK.