Abstract
Platelets are an interesting model for studying the relationship betwen adhesion and mitogen-activated protein (MAP) kinase activation. We have recently shown that in platelets, ERK2 was activated by thrombin and downregulated by IIbβ3integrin engagement. Here we focused our attention on the c-Jun NH2-terminal kinases (JNKs) and their activation in conditions of platelet aggregation. We found that JNK1 was present in human platelets and was activated after thrombin induction. JNK1 phosphorylation was detected with low concentrations of thrombin (0.02 U/mL) and after 1 minute of thrombin-induced platelet aggregation. JNK1 activation was increased (fivefold) when fibrinogen binding to IIbβ3 integrin was inhibited by the Arg-Gly-Asp-Ser (RGDS) peptide or (Fab′)2 fragments of a monoclonal antibody specific for IIbβ3, demonstrating that, like ERK2, IIbβ3 integrin engagement negatively regulates JNK1 activation. Comparison of JNK1 activation by thrombin in stirred and unstirred platelets in the presence of RGDS peptide showed a positive regulation by stirring itself, independently of IIbβ3 integrin engagement, which was confirmed in a thrombasthenic patient lacking platelet IIbβ3. The same positive regulation by stirring was found for ERK2. These results suggest that MAP kinases (JNK1 and ERK2) are activated positively by thrombin and stirring. In conclusion, we found that JNK1 is present in platelets and can be activated after thrombin induction. Moreover, this is the first report showing that two different MAP kinases (ERK2 and JNK1) are regulated negatively by IIbβ3 engagement and positively by mechanical forces in platelets.
MITOGEN-ACTIVATED protein kinases (MAP kinases) are a family of serine-threonine kinases1activated by many stimuli, including growth factors and hormones, in proliferative cells.2 This family consists of three subgroups. The extracellular signal-regulated kinases (ERKs) are involved in proliferation, adhesion, and cell progression.3,4 The p38MAP kinase and the c-Jun amino-terminal kinases (JNKs) or stress-activated protein kinases (SAPKs), which include the 46-kD JNK1 and 55-kD JNK2 isoforms, seem to be involved in apoptosis.5,6 Activation of MAP kinases requires dual phosphorylation at threonine and tyrosine residues.7 Moreover, different kinases activate ERKs, JNKs, and p38MAP kinase. ERKs, JNKs, and p38MAP kinase are phosphorylated by MAP kinase/ERK kinase (MEK)1/2, MEK4, and MEK3/6, respectively.8-10 The JNKs cascade is activated by various stresses11 (UV irradiation, heat shock, x-ray irradiation, osmotic shock), inflammatory cytokines12 (tumor necrosis factor [TNF]-α and interleukin-1), and weakly by growth factors. Components of the JNK pathways are dependent on MAP kinase kinase (MKKs), a serine/threonine kinase, p21-activated kinase (PAK), and Ras-like guanosine triphosphate (GTP)–binding proteins cdc42 and Rac.13-15
Platelets are nonproliferative cells and are a good model for studying the signal transduction of MAP kinases and new functions. In platelets, two MAP kinases, ERK and p38MAP kinase, have been identified.16,17 The ERK cascade is activated by thrombin, or collagen, and seems to involve MEK1/2 and protein kinase C (PKC).18,19 Recently, we found that αIIbβ3 downregulates the ERK2 activation induced by thrombin if engaged in fibrinogen-mediated platelet aggregation.20 This was the first report of an integrin downregulating ERK2 activation during cell adhesion. p38MAP kinase is activated by thrombin and seems to be involved in the phosphorylation of PLA2.21 The last subgroup of MAP kinases, JNKs, has not been identified in platelets.
We determined the conditions of JNK activation during thrombin-induced platelet aggregation. We also assessed the involvement of αIIbβ3 integrin engagement in JNK activation and compared it with that in ERK activation. We found that JNK1 was (1) present and activated after thrombin induction; (2) regulated negatively by αIIbβ3 engagement; and (3) regulated positively by stirring, like ERK2 activation. This is the first report showing that JNK1 is present and active in platelets and has a similar regulation to ERK2. To our knowledge, this is also the first report suggesting a direct mechanical induction of MAP kinases in platelets.
MATERIALS AND METHODS
Reagents.
Bovine thrombin, synthetic peptides Arg-Gly-Asp-Ser (RGDS), Arg-Gly-Glu-Ser (RGES), leupeptin, and aprotinin were purchased from Sigma (St Louis, MO). Protein-G PLUS-Agarose and the rabbit polyclonal antibody directed against JNK1 (C-17) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and rabbit polyclonal antibody directed against the phosphorylated forms of JNKs and ERKs was obtained from Promega (Madison, WI). Donkey antirabbit horseradish peroxidase–conjugated IgG was obtained from Jackson Immuno Research (West Grove, PA) and [γ-32P]adenosine triphosphate (ATP; 167 tera becquerel [Tbq]/mmol) was obtained from ICN (Irvine, CA). Electrophoresis reagents were obtained from Bio-Rad (Richmond, CA) and Euromedex (Souffelweyersheim, France), and chemical products were obtained from Merck (Darmstadt, Germany) and Prolabo (Paris, France). Nitrocellulose sheets were purchased from Schleicher & Schuell (Dassel, Germany). (Fab′)2 fragments of the mouse monoclonal IgG AP-2 specific for αIIbβ3 were an extremely generous gift from Dr T.J. Kunicki (Scripps Clinic, La Jolla, CA).22
Normal donors and patient.
Normal donors were not treated with any drug during the 2 weeks before blood donation given with informed consent. A patient with Glanzmann’s thrombasthenia is characterized by absence of platelet aggregation and fibrinogen binding, as previously described.23
Platelet preparation and aggregation.
Venous blood was obtained from healthy donors with their informed consent. Platelets were isolated and washed by differential centrifugation in citrate buffer, pH 6, containing 10−4 mmol/L PGE1, 140 mmol/L NaCl, 5 mmol/L KCl, 12 mmol/L trisodium citrate, 10 mmol/L glucose, and 12.5 mmol/L sucrose, and then in the same buffer but without PGE1. The platelet pellet was resuspended in 10 mmol/L HEPES pH 7.4, 140 mmol/L NaCl, 3 mmol/L KCl, 0.5 mmol/L MgCl2, 5 mmol/L NaHCO3, and 10 mmol/L glucose. Cell concentration was adjusted to 5 × 108/mL. Platelets (0.4 mL) were preincubated, without stirring, at 37°C for various times, with various agonists. Platelet aggregation was then initiated by adding bovine thrombin with or without constant stirring (1,200 rpm) in an aggregometer cuvette (Chronolog dual beam aggregometer). Aggregation was measured and is expressed as a percentage change in the transmission of light, with the blank sample (buffer without platelets) defined as 100%.
Immunoblotting.
Samples were subjected to immunoblotting as described previously.24 Briefly, platelet aggregation was stopped by addition of 40 μL of denaturing buffer (10% [wt/vol] sodium dodecyl sulfate [SDS], 100 mmol/L NaCl, 50 mmol/L Tris, 50 mmol/L NaF, 5 mmol/L EDTA, 40 mmol/L β-glycerophosphate, 200 mmol/L sodium orthovanadate, 5 μg/mL leupeptin, 10 μg/mL aprotinin, pH 7.4), and the samples were heated at 95°C for 5 minutes. Proteins were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE), in a 12% polyacrylamide gel. Proteins were transferred to nitrocellulose filters by semidry transfer (Enprotech, Natick, MA). Filters were then incubated for 1 hour at room temperature with the polyclonal primary antibody anti-JNK1-P (1:5,000) or anti-JNKs (1:2,000) or anti-ERK2-P (1:20,000) or anti-ERKs (1:20,000). The membranes were washed five times with Tris saline buffer without milk and were then incubated with horseradish peroxidase–conjugated goat antirabbit IgG (1:20,000) for 45 minutes at room temperature. Immunoreactive bands were detected by chemiluminescence using the Amersham ECL enhanced chemiluminescence system (Amersham, Arlington Heights, IL).
Immunoprecipitation.
Platelet lysates were diluted in 50 mmol/L HEPES pH 7.4, 150 mmol/L NaCl, 10% (vol/vol) glycerol, 1% (vol/vol) Triton X-100 (Sigma), 1.5 mmol/L MgCl2, 10 μg/mL of leupeptin, 0.5 mmol/L sodium vanadate, and 2 mmol/L EGTA, and were incubated overnight at 4°C with anti-JNK1 antibody (2 μg/sample). They were then incubated with protein-G PLUS agarose (40 μL, vol/vol) for 1 hour ar 4°C. Immune complexes were collected by centrifugation. The sample was then resuspended in 20 μL of kinase buffer (20 mmol/L HEPES, pH 7.4, 10 mmol/L MgCl2, 10 mmol/L p-nitrophenyl phosphate, 1 mmol/L dithiothreitol) containing GST-Jun (2 μg), 50 μmol/L unlabeled ATP, and 5 μCi of [γ-32P]ATP per sample.24After 10 minutes at 37°C, samples were subjected to SDS-PAGE, and the gel was stained with Coomassie Blue (Sigma), dried, and autoradiographed.
RESULTS AND DISCUSSION
Thrombin induces JNK1 activation during platelet aggregation.
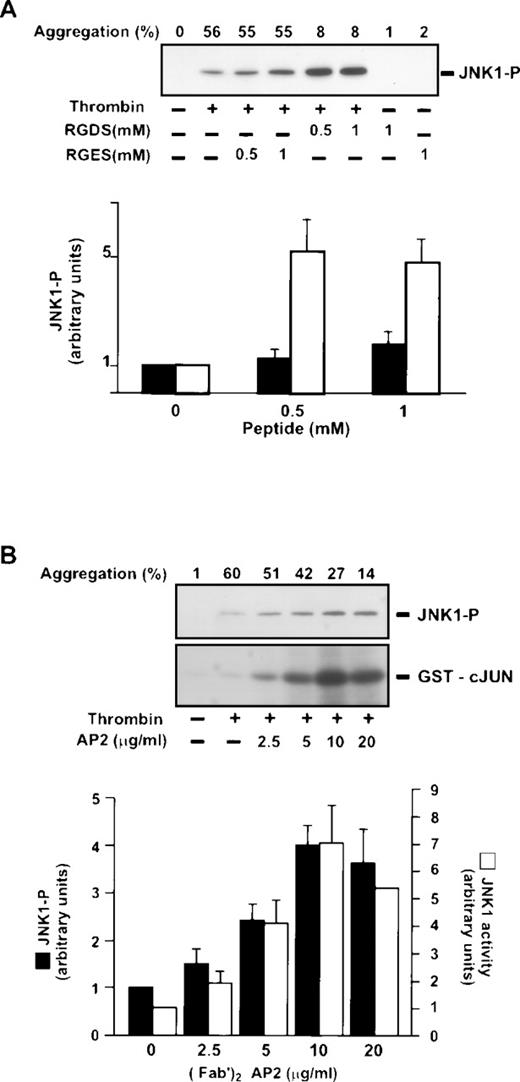
We first determined the conditions for thrombin-induced JNK1 activation by incubating platelets with various concentrations of thrombin (0 to 1 U/mL) for 2 minutes under stirring conditions (Fig 1A). Phosphorylation was investigated by Western blotting using an antibody that recognized phosphorylated JNK1 and JNK2 (JNK1-P and JNK2-P). In resting platelets and after induction with thrombin (0.01 U/mL), no phosphorylated JNK1 and/or JNK2 were found. JNK1-P was detected only after treatment with 0.02 U/mL thrombin, reaching a maximum intensity at 0.2 U/mL. Moreover, we used an antibody that recognized phosphorylated and nonphosphorylated JNKs as internal control to ensure equal JNKs loading. We assessed JNK1 kinase activity in the same thrombin conditions, after JNK1 immunoprecipitation, by in vitro phosphorylation of GST-cJUN (see Materials and Methods). No GST-cJUN phosphorylation was observed in resting platelets. In contrast, a significant increase in JNK1 activity was detectable with 0.01 U/mL thrombin and reached a maximum at 0.05 U/mL thrombin. The fact that JNK1 activity appears at lower concentrations of thrombin (0.01 U/mL) relative to its state of phosphorylation is probably a result of the difference in the sensitivity of the methods used, and/or in the antibodies used for phosphorylation and kinase activity. Thus, JNK1 activation occurred during thrombin-induced platelet aggregation. No JNK2 activation was detected in the same conditions.
Effect of thrombin on JNK1 activation. Platelets were incubated at 37°C with various concentrations of thrombin for 2 minutes (A) or in the presence or absence of thrombin (1 U/mL) for various times (B) as described in Materials and Methods. The reaction was stopped by addition of lysis buffer containing SDS for Western blotting and Triton X-100 for immunoprecipitation. JNK phosphorylation and total JNKs were analyzed by Western blotting using a polyclonal antibody recognizing JNK1-P and JNK2-P and a polyclonal antibody recognizing total JNKs, respectively. For JNK activity, phosphorylated GST-cJun was followed after immunoprecipitation of JNK1, with [32P] ATP. These autoradiographs shown are typical of at least three experiments. (A) Dose-dependent effect of thrombin on JNK activation. Washed platelets were stimulated by incubation with various concentrations of thrombin (0 to 1 U/mL) for 2 minutes with stirring. (B) Time course of thrombin-mediated JNK activation. Washed platelets were stimulated by incubation with 1 U/mL thrombin with stirring.
Effect of thrombin on JNK1 activation. Platelets were incubated at 37°C with various concentrations of thrombin for 2 minutes (A) or in the presence or absence of thrombin (1 U/mL) for various times (B) as described in Materials and Methods. The reaction was stopped by addition of lysis buffer containing SDS for Western blotting and Triton X-100 for immunoprecipitation. JNK phosphorylation and total JNKs were analyzed by Western blotting using a polyclonal antibody recognizing JNK1-P and JNK2-P and a polyclonal antibody recognizing total JNKs, respectively. For JNK activity, phosphorylated GST-cJun was followed after immunoprecipitation of JNK1, with [32P] ATP. These autoradiographs shown are typical of at least three experiments. (A) Dose-dependent effect of thrombin on JNK activation. Washed platelets were stimulated by incubation with various concentrations of thrombin (0 to 1 U/mL) for 2 minutes with stirring. (B) Time course of thrombin-mediated JNK activation. Washed platelets were stimulated by incubation with 1 U/mL thrombin with stirring.
We used phosphorylation and kinase assays to investigate the time course of JNK1 activation upon treatment with 1 U/mL thrombin under stirring conditions (Fig 1B). In resting platelets, no JNK1 phosphorylation or activity was detected. In contrast, JNK1 phosphorylation and activity that was detected after 30 seconds or 1 minute, depending on the experiment, reached a maximum between 120 and 180 seconds. In these conditions of JNK1 phosphorylation, immunoblots were probed with an antibody recognizing phosphorylated and nonphosphorylated JNKs and confirmed equal levels of total JNKs in the different platelet lysates. JNK1 was activated during platelet aggregation, raising questions about the respective involvement of αIIbβ3 integrin engagement or thrombin activation.
JNK1 activation is dependent on thrombin stimulation and is upregulated by inhibition of αIIbβ3engagement.
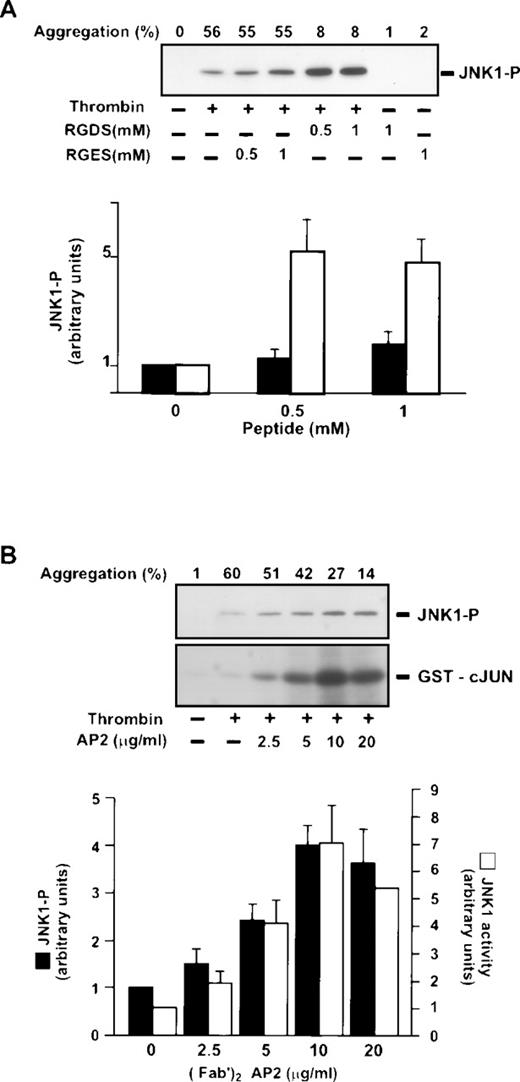
We investigated thrombin-induced JNK1 activation in conditions of inhibition of fibrinogen binding to integrin αIIbβ3, and consequently, inhibition of aggregation. The involvement of αIIbβ3 in JNK1 activation was assessed using the RGDS peptide, a competitive inhibitor of fibrinogen binding to αIIbβ3. In resting platelets, preincubation with RGDS alone, in the absence of thrombin, did not activate JNK1 (Fig 2A and B). In thrombin-activated platelets, however, preincubation with RGDS for 30 seconds (0.5 and 1 mmol/L), which completely inhibited platelet aggregation, did not prevent, but rather enhanced JNK1 phosphorylation and activity after 2 minutes of thrombin activation (Fig 2A). Quantification by densitometry demonstrated 471.2% ± 91.0% more JNK1 phosphorylation in the presence of 1 mmol/L RGDS. A control RGES peptide, which did not block platelet aggregation, did not significantly affect (179.8% ± 37.0%) the thrombin-induced phosphorylation of JNK1. We confirmed these results, by blocking fibrinogen binding using (Fab′)2 fragments of the anti-αIIbβ3 monoclonal antibody AP-2 preincubated for 5 minutes before thrombin addition. Thrombin-induced JNK1 phosphorylation and activity were increased dose-dependently by addition of AP-2 (Fab′)2, reaching a plateau at 10 μg/mL (Fig 2B). At this concentration (10 μg/mL), the values obtained were similar to those for inhibition by RGDS: 409.6% ± 53.3% JNK1 phosphorylation and 644.2% ± 111.0% JNK1 activity. Thus, the results of both experiments (RGDS and [Fab′]2 fragments) suggest that activation of the JNK1 pathway (1) occurs during thrombin aggregation independently of αIIbβ3 engagement, and moreover, (2) is downregulated by fibrinogen binding to αIIbβ3. This negative control of JNK1 activation is possibly because of the presence and/or activation of phosphatases in the same compartment of JNK1 in conditions of platelet aggregation. Different phosphatases are associated with cytoskeleton upon thrombin stimulation. PP2A, a serine/threonine phosphatase, which can dephosphorylate MAP kinases in proliferative cells, is present in the platelet cytoskeleton after thrombin induction.25Moreover, tyrosine phosphatases like SHP1 are also present in the cytoskeleton after αIIbβ3engagement.26 The other possibility is that the kinases involved in the activation of JNK1 are partitioned in different compartments after thrombin induction in the presence or absence of αIIbβ3engagement.
Effect of inhibition of IIbβ3 engagement on thrombin-induced JNK1 activation. Washed platelets were preincubated at 37°C for 30 seconds in the presence or absence of peptides RGDS and RGES (0.5 mmol/L and 1 mmol/L) (A) or with various concentrations of the (Fab′)2 fragment of the anti-IIbβ3 monoclonal antibody AP-2 (0 to 20 μg/mL) for 5 minutes (B). They were then incubated with 0.2 U/mL with stirring for 2 minutes. JNK1 phosphorylation was studied by Western blotting and JNK1 activity by phosphorylation of GST-cJun, as described in Fig 1. Autoradiographs were scanned with a laser densitometer. For each experiment, the ratio of JNK1-P or GST-cJun was normalized to that of platelets treated with thrombin alone and is expressed as a relative intensity. Results are the means ± SEM for four experiments.
Effect of inhibition of IIbβ3 engagement on thrombin-induced JNK1 activation. Washed platelets were preincubated at 37°C for 30 seconds in the presence or absence of peptides RGDS and RGES (0.5 mmol/L and 1 mmol/L) (A) or with various concentrations of the (Fab′)2 fragment of the anti-IIbβ3 monoclonal antibody AP-2 (0 to 20 μg/mL) for 5 minutes (B). They were then incubated with 0.2 U/mL with stirring for 2 minutes. JNK1 phosphorylation was studied by Western blotting and JNK1 activity by phosphorylation of GST-cJun, as described in Fig 1. Autoradiographs were scanned with a laser densitometer. For each experiment, the ratio of JNK1-P or GST-cJun was normalized to that of platelets treated with thrombin alone and is expressed as a relative intensity. Results are the means ± SEM for four experiments.
Positive and negative regulation of thrombin-induced JNK1 activation: Comparison with ERK2 activation.
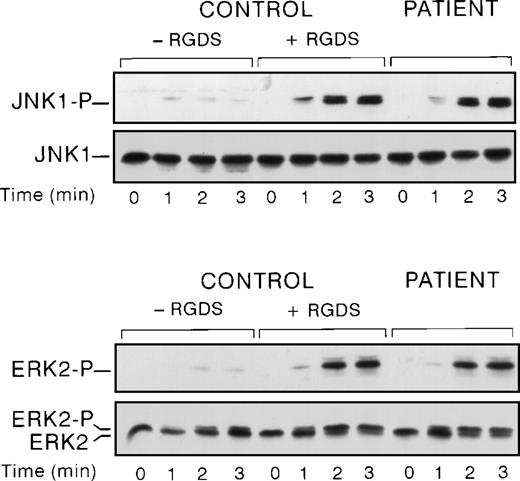
We have previously shown that αIIbβ3engagement negatively regulates ERK2 activation.20 So, we compared thrombin-induced JNK and ERK activation in a patient with Glanzmann’s thrombasthenia, a disease that is characterized by a quantitative defect in platelet αIIbβ3. In these conditions, JNK1 activation in stirred platelets was higher in the patient than in the control (Fig 3). The level of upregulation in the patient and control with RGDS was similar, strongly suggesting that this upregulation was caused by the absence of αIIbβ3 engagement rather than by any effect of RGDS itself. To ensure that the differences in the levels of JNK-P between control and a patient with Glanzmann’s thrombasthenia are not caused by differences in JNK protein levels, immunoblots were probed with an antibody directed against phosphorylated and nonphosphorylated JNKs. In these conditions, no difference in JNK protein levels was observed. Finally, we also demonstrated ERK2 overactivation, as previously described.20
Comparison of JNK1 and ERK2 activation in a Glanzmann’s thrombasthenia patient and control. Washed platelets from control in the presence or absence of RGDS peptide and patient were stimulated with 0.2 U/mL of thrombin under stirring conditions at the indicated times. Then platelet lysates were solubilized and analyzed for ERK2 and JNK1 activation.
Comparison of JNK1 and ERK2 activation in a Glanzmann’s thrombasthenia patient and control. Washed platelets from control in the presence or absence of RGDS peptide and patient were stimulated with 0.2 U/mL of thrombin under stirring conditions at the indicated times. Then platelet lysates were solubilized and analyzed for ERK2 and JNK1 activation.
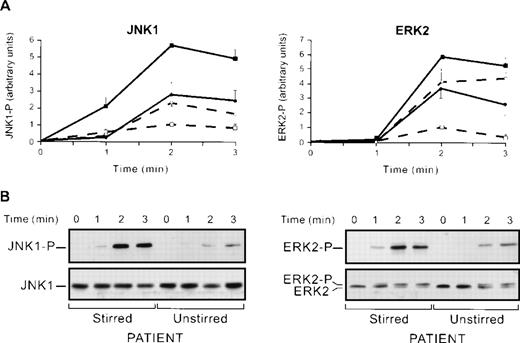
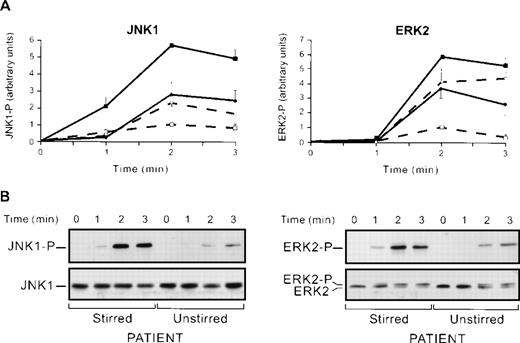
Thus, JNK1 maximal activation is obtained during thrombin activation of platelets in conditions in which αIIbβ3, and thus, aggregation are blocked (RGDS and monoclonal anti-αIIbβ3) or absent (thrombasthenia). However, all conditions implied that platelets were stirred. To test whether stirring per se might affect JNK1 activation, we compared thrombin-induced JNK1 activation in the absence or presence of stirring with or without the RGDS peptide. In stirred platelets, in the presence of the RGDS peptide, JNK1 was overactivated (566% ± 29% v100% in the absence of RGDS) 2 minutes after the addition of thrombin (Fig 4A). Interestingly, the absence of stirring, whether in the presence or absence of RGDS, led only to a partial overactivation (278% ± 69%) of JNK1. This is a strong indication that stirring per se, or a factor depending on it, may represent up to 50% of the JNK1 upregulation. The fact that stirring yields maximal JNK1 activation in the presence versus the absence of RGDS suggests that the remaining 50% upregulation is caused by the blocking of αIIbβ3 engagement, per se. To test whether positive regulation of JNK1 by stirring was specific of this MAP kinase, we next studied ERK2 activation by Western blotting using an anti-ERK-P antibody, in the same conditions of JNK1 activation (Fig 4A). We showed, as previously described,20 that ERK2 was overactivated in stirred platelets with the RGDS peptide. This ERK2 overactivation that reached 584% ± 21% after 2 minutes of thrombin induction (0.2 U/mL) was greater than that reported in a previous study,20 probably because of the high sensitivity of the anti-ERK-P antibody used in our system. Stirred (584% ± 21%) and unstirred (365% ± 70%) platelets compared in the presence of RGDS after 2 minutes of thrombin induction suggested that ERK2 activation was also partly regulated by a factor dependent on stirring, but this positive regulation may be weaker for ERK2 (219%) than for JNK1 (288%). Thus, (1) ERK2 and JNK1 activation induced by thrombin were both negatively regulated by αIIbβ3 engagement in aggregation itself, and (2) stirring, per se, or a factor dependent on stirring is involved, as a positive regulator in JNK1 activation and to a lesser extent in ERK2 activation.
Regulation of JNK1 and ERK2 activation. (A) JNK1 and ERK2 activation in stirred and unstirred platelets in the presence or absence of RGDS peptide. Washed platelets were preincubated with or without RGDS peptide (1 mmol/L) for 30 seconds and stimulated with thrombin (0.2 U/mL) for various times, in the presence or absence of stirring (+ stirring and + RGDS, ▪; + stirring and − RGDS, □; − stirring and + RGDS, •; − stirring and − RGDS, ○). The JNK1 phosphorylation (JNK1) and ERK2 phosphorylation (ERK2) of the lysates were then assessed. Autoluminographs were scanned with a laser densitometer. For each experiment, the ratio of phosphorylated JNK1 and ERK2 was normalized to that of stirred platelets treated with thrombin alone (2 minutes) expressed as a relative intensity. Results are the means ± SEM for four experiments. (B) Effect of stirring on thrombin-induced JNK1 and ERK2 activation in a thrombasthenic patient. Stirred and unstirred platelets from Glanzmann’s thrombasthenia patient were stimulated with thrombin (0.2 U/mL) for various times. The JNK1 phosphorylation and ERK2 phosphorylation and total JNKs and ERKs of the lysates were investigated.
Regulation of JNK1 and ERK2 activation. (A) JNK1 and ERK2 activation in stirred and unstirred platelets in the presence or absence of RGDS peptide. Washed platelets were preincubated with or without RGDS peptide (1 mmol/L) for 30 seconds and stimulated with thrombin (0.2 U/mL) for various times, in the presence or absence of stirring (+ stirring and + RGDS, ▪; + stirring and − RGDS, □; − stirring and + RGDS, •; − stirring and − RGDS, ○). The JNK1 phosphorylation (JNK1) and ERK2 phosphorylation (ERK2) of the lysates were then assessed. Autoluminographs were scanned with a laser densitometer. For each experiment, the ratio of phosphorylated JNK1 and ERK2 was normalized to that of stirred platelets treated with thrombin alone (2 minutes) expressed as a relative intensity. Results are the means ± SEM for four experiments. (B) Effect of stirring on thrombin-induced JNK1 and ERK2 activation in a thrombasthenic patient. Stirred and unstirred platelets from Glanzmann’s thrombasthenia patient were stimulated with thrombin (0.2 U/mL) for various times. The JNK1 phosphorylation and ERK2 phosphorylation and total JNKs and ERKs of the lysates were investigated.
To confirm the positive regulatory effect of stirring on MAP kinases, we compared ERK2 and JNK1 activation in stirred and unstirred platelets from a thrombasthenic patient lacking platelet αIIbβ3 (Fig 4B). In the conditions of identical levels of total JNKs proteins loaded, the activation of JNK1 and ERK2 in stirred platelets was higher than that in unstirred platelets, confirming that stirring was a positive regulator of JNK1 and ERK2 activation. We also confirmed that positive regulation by stirring was stronger for JNK1 than for ERK2 as judged by a lower stirred and unstirred ratio (x3) for ERK2 and x5 for JNK1 (Fig4B). Altogether, our data suggest that JNK1 and ERK2, though to a lesser extent, are activated by thrombin activation and stirring, and downregulated by αIIbβ3 integrin engagement. It is tempting to correlate these results with data showing that the fluid shearing of endothelial cells increases the activation of JNK much more than that of ERK.27 We investigated the involvement of stirring itself or of a factor released from platelets dependent on stirring in the positive regulation by quantifying serotonin release in condition of stirred and unstirred platelets in the presence or absence of the RGDS peptide. No differences in serotonin release depending on the conditions used were found (results not shown). The thromboxane analogue, U46619, activated ERK2 and JNK1 (results not shown). We therefore tested the effect of thrombin-induced thromboxane A2 formation as a secondary activator of MAP kinase. In our conditions, flurbiprofen, which blocks cyclooxygenase 1, had no effect on thrombin-induced JNK1 and ERK2 activation, suggesting that thromboxane A2 formation was not involved (results not shown). Thus, though our data cannot totally exclude the role of secreted factors, they are consistent with stirring itself being involved, in the upregulation of JNK1 and ERK2 activation.
The role of MAP kinases in platelet physiology is unclear. However, recent work28 has suggested that the Ras-initiated MAP kinase pathway suppresses integrin activation. Although these conclusions stem from work in Chinese hamster ovary cell line, a model system may be very different from platelets. It is tempting to speculate that in the physiopathologic conditions of shear stress and of platelet activation, MAP kinases (JNK1 and ERK2) may serve as a suppressor of αIIbβ3 activation and may act as a negative regulator of platelet activation. The partial downregulation of JNK1 and ERK2 would thus be part of a feedback loop of integrin engagement on MAP kinases activation.
We found that JNK1 and ERK2 were similarly regulated, but it is not clear whether both activation pathways involved a common determinant in platelets, and at which level of the signal transduction pathway the MAP kinases were affected by the engagement of αIIbβ3 integrin. It is well-known that Ras cascade activation is involved in the activation of ERKs, but the role of Ras in JNKs activation has only been recently shown.27,29 30 Thus, thrombin-induced JNK and ERK activation may involve a common pathway, and their negative regulation may occur upstream of the JNKs and ERKs. In conclusion, this is the first report showing (1) that JNK1 is present and can be activated in platelets, and (2) that mechanical forces induce ERK1 and JNK2 activation in platelets.
ACKNOWLEDGMENT
The authors thank Dr T.J. Kunicki for generously donating (Fab′)2 fragments from the mouse monoclonal IgG AP-2 antibody specific for αIIbβ3. The authors also thank E. Savariau and R. Nancel for graphic work.
Supported by Institut National de la Recherche Médicale and by grants from Ligue Nationale contre le Cancer (Comité de Paris), Fondation de France, and Association Pour la Recherche Contre le Cancer (A.R.C.) (No 9697).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Marijke Bryckaert, PhD, U348 INSERM, IFR Circulation Lariboisière, Hôpital Lariboisière, 41 Bvd de la Chapelle, 75475 Paris Cedex 10, France; e-mail:marijke.bryckaert@inserm.lrb.ap-hop-paris.fr.

![Fig. 1. Effect of thrombin on JNK1 activation. Platelets were incubated at 37°C with various concentrations of thrombin for 2 minutes (A) or in the presence or absence of thrombin (1 U/mL) for various times (B) as described in Materials and Methods. The reaction was stopped by addition of lysis buffer containing SDS for Western blotting and Triton X-100 for immunoprecipitation. JNK phosphorylation and total JNKs were analyzed by Western blotting using a polyclonal antibody recognizing JNK1-P and JNK2-P and a polyclonal antibody recognizing total JNKs, respectively. For JNK activity, phosphorylated GST-cJun was followed after immunoprecipitation of JNK1, with [32P] ATP. These autoradiographs shown are typical of at least three experiments. (A) Dose-dependent effect of thrombin on JNK activation. Washed platelets were stimulated by incubation with various concentrations of thrombin (0 to 1 U/mL) for 2 minutes with stirring. (B) Time course of thrombin-mediated JNK activation. Washed platelets were stimulated by incubation with 1 U/mL thrombin with stirring.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/11/10.1182_blood.v94.11.3800/4/m_blod42325001w.jpeg?Expires=1768017940&Signature=DAQdbfwtW5Qu17jC987BVe~Ij5gCr5ZURyO8twpSDE0~Az7GwNfnGtDQk-2HzV5mY8KCw8PsZ6xnGLTtykJgZJ3VXfxTbGBrcbDucxmELlHZ67WcJ~Mom73RcLQ1QFXCyIoidT5R~6hL8JZbux1B4gJMyYVteOZ1WJzpGCezoqPQMeKoVCcC4ev~eeKWp8v5sGG-IBZZ2g5rEAJYUYnss88jfuKCUhPTUtQ~lgZc54INiAJESU5i8Og97I5nLQX-xKRVwyvy5jQtMgkWfiHM0kh4VoR45RbR572g5YPKIokTl~Pw~hJBLBnloX8PWdGA5QRTkWFiJ9AaRRgWQGFiRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 1. Effect of thrombin on JNK1 activation. Platelets were incubated at 37°C with various concentrations of thrombin for 2 minutes (A) or in the presence or absence of thrombin (1 U/mL) for various times (B) as described in Materials and Methods. The reaction was stopped by addition of lysis buffer containing SDS for Western blotting and Triton X-100 for immunoprecipitation. JNK phosphorylation and total JNKs were analyzed by Western blotting using a polyclonal antibody recognizing JNK1-P and JNK2-P and a polyclonal antibody recognizing total JNKs, respectively. For JNK activity, phosphorylated GST-cJun was followed after immunoprecipitation of JNK1, with [32P] ATP. These autoradiographs shown are typical of at least three experiments. (A) Dose-dependent effect of thrombin on JNK activation. Washed platelets were stimulated by incubation with various concentrations of thrombin (0 to 1 U/mL) for 2 minutes with stirring. (B) Time course of thrombin-mediated JNK activation. Washed platelets were stimulated by incubation with 1 U/mL thrombin with stirring.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/11/10.1182_blood.v94.11.3800/4/m_blod42325001w.jpeg?Expires=1768017941&Signature=p3JtKoVbEJppBgyJhH5-pW~-H7V8N9d5aJ-8kO~x64~EKoddHToav05wDu-fMiTfcJJMIqK3XrJl1qVnjRGFk6JRICyEG9KJfGZojzGceaOGcvJHioLCDZRznmHi-kiDua2JYI~kbqZp4EjmLTx46BwblXcPfvGZBsNjBo9OzU6S3jD4awtnmfhMeIklBfZ-EnQ01ildzk5qvUUEMxHs0n-6YBiClM5Lo5bwrSjRddGROAdKe80Chgakm3GWZzKGkptbPDfIp1koQ9YEKsblD7tFBBEqEigFjnKWy60GkgbhnmSVDkNC3T~DjiaXYtsGS05OkY7X7ijvtf5bN9sZeg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)