Abstract
Following ischemia-reperfusion (I/R), platelet adhesion is thought to represent the initial event leading to remodeling and reocclusion of the vasculature. The mechanisms underlying platelet adhesion to the endothelium have not been completely established. Endothelial cells rendered ischemic acquire a procoagulant phenotype, characterized by fibrinogen accumulation. Therefore, we evaluated whether fibrinogen deposition during I/R mediates platelet adhesion. Using fluorescence microscopy, fibrinogen deposition and the accumulation of platelets were assessed in vivo in a model of intestinal I/R (1.5 hours/60 minutes). Fibrinogen accumulated in arterioles and venules early after the onset of reperfusion. The deposition of fibrinogen colocalized with large numbers of adherent platelets (520 ± 65 and 347 ± 81 platelets/mm2 in arterioles and venules). Pretreatment with an antifibrinogen antibody attenuated platelet adhesion. Intracellular adhesion molecule (ICAM)-1 served as a major receptor for fibrinogen, since fibrinogen deposition and platelet adhesion to the endothelial cell surface were markedly decreased in ICAM-1–deficient mice. The platelet IIb/β3 integrin plays a key role in fibrinogen-dependent platelet accumulation, because (1) platelet adhesion involved RGD-recognition sequences, and (2) platelets isolated from a patient with Glanzmann’s disease showed decreased interaction with the postischemic endothelium. Since platelets are demonstrated here to induce tyrosine phosphorylation in endothelial cells, platelet recruitment might contribute to the development of an inflammatory reaction during I/R.
IN INDUSTRIALIZED COUNTRIES, ischemia-reperfusion (I/R)-induced organ injury underlies many of the most important diseases, including angina pectoris, myocardial infarction, and stroke. Although many of the ischemic episodes can be reversed by medical interventions, the reintroduction of oxygenated blood initiates an inflammatory reaction (reperfusion injury), associated with leukocyte adhesion and emigration.1-4 In addition to leukocytes, platelets are recruited to the postischemic microvasculature very early after the onset of reperfusion.5,6 Upon activation, platelets generate oxygen radicals and release a variety of chemokines and growth factors.7-12 Therefore, platelet adhesion to the endothelium and intravascular platelet aggregation are thought to be the initial events leading to remodeling and reocclusion of the postischemic vessel segment. However, the mechanisms that underlie the interactions between platelets and endothelial cells during ischemia-reperfusion (I/R) have not been completely elucidated thus far.
Although originally envisioned as passive and inert, the endothelium has been shown to actively respond to stimuli. Following hypoxia/reoxygenation or I/R, vascular endothelial cells, which act as a nonthrombogenic surface under physiologic conditions, acquire a procoagulant phenotype. This procoagulant endothelial activity arises from changes in the synthesis and surface expression of endothelial proteins. Endothelial cell activation, eg, by hypoxia/reoxygenation, is known to induce tissue factor expression and to suppress thrombomodulin activity, leading to thrombin activation and promoting fibrin(ogen) deposition.13-16
While the accumulation of fibrin(ogen) driven by tissue factor has been reported during reperfusion of ischemic organs,17 the role of fibrin(ogen) in the pathogenesis of I/R injury has not been clearly defined thus far. Leukocytes, endothelial cells, and platelets are known to associate with fibrinogen. Fibrinogen binding to intercellular adhesion molecule (ICAM)-1 expressed on the endothelial cell surface has been demonstrated to mediate the adhesion of leukocytes to human umbilical vein endothelial cells (HUVECs) in vitro18 and to induce the attachment of monocytic HL-60 cells to the vascular wall of mesenteric venules in vivo.19Moreover, fibrinogen binding to αIIb/β3integrins on adjacent platelets is believed to represent the molecular substrate for platelet aggregation during primary hemostasis and to promote the adhesion of platelets to immobilized fibrinogen and to HUVEC monolayers in vitro.20 21 Fibrinogen accumulation might, therefore, directly contribute to platelet recruitment during postischemic reperfusion. However, the causative role of fibrinogen in mediating platelet adhesion during I/R has not been clearly established. The aim of the present in vivo study, therefore, was (1) to investigate the time course of fibrinogen accumulation during I/R, (2) to study the impact of fibrinogen deposition on platelet adhesion to the postischemic endothelium, and (3) to evaluate the role of ICAM-1 in fibrinogen accumulation during I/R.
MATERIALS AND METHODS
Animals.
Female Balb/c mice (Charles River, Sulzfeld, Germany) and C57BL/6J mice (wild-type, or ICAM-1–deficient), aged between 5 and 7 weeks (54 experimental animals and 54 platelet donors) were used. The ICAM-1 mutant strain was generated in the laboratory of Professor A.L. Beaudet (Houston, TX)22 and purchased from The Jackson Laboratory (Bar Harbor, ME). All experimental procedures were approved by the German legislation on protection of animals.
Surgical procedure.
The surgical procedure has been described in detail elsewhere.23 In brief, the mice were anesthetized by inhalation of isoflurane-N2O (FiO2 0.35, 0.015 l/l isoflurane; Forene, Abbott GmbH, Wiesbaden, Germany), and polyethylene catheters (PE 50, inner diameter [ID] 0.28 mm; Portex, Hythe, UK) were inserted into the left carotid artery and jugular vein. A segment of the jejunum was exteriorized and subjected to 1.5 hours of normothermic ischemia. Platelet-endothelial cell interactions in the postischemic microvasculature were investigated by intravital microscopy (IVM) 10 to 30 minutes after the onset of reperfusion.
Blood sampling and platelet preparation.
For IVM, murine platelets were isolated from whole blood and labeled with rhodamine-6G (50 μL 0.05% per mL whole blood; molecular weight, 479; Sigma-Aldrich, Deisenhofen, Germany), as previously described.23 For experiments with human platelets, whole blood was obtained from healthy, nonsmoking volunteers or from a patient with Glanzmann’s disease. The donors had not received acetylsalicylic acid or any other platelet inhibitor for at least 14 days before the experiments. Human platelets were isolated by centrifugation and labeled with rhodamine-6G as described for murine platelets.23
Before infusion, the platelet count and the purity of each platelet suspension (murine or human) were assessed by a Coulter ACT Counter (Coulter Corp, Miami, FL) and by flow cytometry. Platelet separation by differential centrifugation yielded a platelet suspension with negligible amounts of other cellular components. More than 99% of all platelets were labeled with rhodamine-6G. As reported earlier, platelet preparation did not increase platelet P-selectin expression, indicating the absence of platelet activation due to the separation procedure.23 A total of 100 × 106platelets stained with rhodamine-6G were transfused to achieve a labeled fraction in the recipient mouse of approximately 10% of all circulating platelets.23
Intravital fluorescence microscopy.
Using IVM, platelet-endothelial cell interactions were analyzed within submucosal arterioles and postcapillary venules of the exposed segment. The fluorescent platelets were infused via the jugular vein for 5 minutes starting 5 minutes after the onset of reperfusion. In each animal, the jejunal segment was scanned from the oral to the aboral section 10 to 30 minutes after the onset of reperfusion. Five nonoverlapping regions of interest were selected randomly, using a microscopic setup previously described.23 In each animal, 5 to 7 arterioles (mean diameter, 40 μm) and 5 to 7 postcapillary venules (mean diameter, 60 μm) were recorded on videotape.
Quantitative assessment of platelet-endothelial cell interactions within these vessels was performed off-line by frame-to-frame analysis of the videotaped images. Within both arterioles and postcapillary venules, platelet-endothelial cell interactions were classified as rolling (intermittent platelet adhesion) or firm adhesion of platelets. Rolling platelets were defined as platelets crossing an imaginary perpendicular line through the vessel at a velocity significantly lower than the centerline velocity in the microvessel; their numbers are given as cells per second per vessel diameter. Firm platelet adhesion was defined in each vessel segment as number of cells that did not move or detach from the endothelial lining within an observation period of 30 seconds. Platelet adhesion is quantified as number of cells per square millimeter endothelial surface, calculated from the diameter and length of the vessel segment observed.
Experimental groups.
Platelet-endothelial cell interactions were investigated under sham conditions without ischemia (n = 6) or in response to 1.5 hours ischemia (n = 6), respectively. IVM was performed 10 to 30 minutes after the onset of reperfusion. The role of fibrinogen for postischemic platelet-endothelial cell interaction was determined using a goat polyclonal antibody directed against mouse fibrinogen (4 mg/kg; Nordic Immunology, Tilburg, Netherlands; n = 6). The antibody was affinity-purified with fibrinogen-Sepharose (Sigma-Aldrich) before use and infused intravenously immediately before injection of fluorescent platelets. Control animals were subjected to I/R as described earlier. Goat anti-human IgG polyclonal control antibody (n = 3; 4 mg/kg; DAKO, Glostrup, Denmark) was infused intravenously after the onset of reperfusion. To assess the involvement of endothelial ICAM-1 in mediating postischemic platelet accumulation, wild-type platelets were transfused into ICAM-1–deficient animals in a separate group (n = 6). The possible role of the platelet fibrinogen receptor, the αIIb/β3 integrin (CD41/CD61), as a mediator of postischemic platelet-endothelial cell interactions was determined using the Arg-Gly-Asp (RGD) peptide Gly-Arg-Gly-Asp-Ser-Pro (GRGDSP, molecular weight, 587.7; BIOMOL, Hamburg, Germany), which is known to inhibit the interaction of fibrinogen with the platelet αIIb/β3integrin (n = 6). Purified GRGDSP peptide (10 mg/kg) was injected intravenously before the infusion of fluorescent platelets. As an independent approach to examine the role of the αIIb/β3 integrin in mediating postischemic platelet-endothelial cell interactions, human platelets derived from a patient with homozygous Glanzmann’s disease were infused into wild-type mice (n = 6). Platelets derived from healthy human volunteers served as controls (n = 6).
Fibrinogen binding to postischemic endothelial cells.
In a separate set of experiments, the binding of fibrinogen to endothelial cells (wild-type or ICAM-1–deficient; n = 3 per group) was investigated in vivo by video fluorescence microscopy according to Witte.24 Alexa 488-conjugated fibrinogen (17 mg/kg; Molecular Probes, Eugene, OR) was administered intravenously 30 minutes before the induction of ischemia. To assess the microvascular distribution of fibrinogen under physiologic conditions and in response to I/R, 5 arterioles and venules were recorded before ischemia, at the end of the ischemic period, as well as 5 and 30 minutes following reperfusion. To evaluate whether fibrinogen deposition is associated with platelet adhesion during reperfusion, rhodamine-6G–labeled platelets were prepared and infused, as described earlier. Using two different filter sets, both fibrinogen binding to the microvascular wall and platelet-endothelial cell interactions were visualized in identical vessel segments during initial reperfusion. Sham-operated animals without I/R followed the same protocol and served as controls (n = 2).
Immunohistology and electron microscopy.
Samples from the intestinal segment were taken 60 minutes after the onset of reperfusion and immediately placed in O.C.T compound (Tissue-Tek; Miles Inc, Elkhart, IN) and frozen in liquid nitrogen or fixed in paraformaldehyde, respectively. Endogenous peroxidase activity was blocked with methanol-H2O2 for 10 minutes at room temperature. For immunostaining of ICAM-1, acetone-fixed cryostat sections (6 μm) were incubated with biotin-conjugated hamster–anti-mouse ICAM-1 (3E2; Pharmingen, Hamburg, Germany) monoclonal antibodies. For staining of fibrinogen, rabbit anti-human fibrinogen polyclonal antibody (lot no. 097; DAKO) was added on paraffin sections (6 μm). This antibody cross-reacts with the corresponding mouse antigen. Tissue sections, prepared with isotype-matched primary antibodies, served as controls. Following incubation with the primary antibody, the sections were stained with commercially available peroxidase immunohistochemistry kits (Vectastain; Camon, Wiesbaden, Germany). An easily detectable reddish-brown-colored end product was obtained by development in H2O2/3-amino-9-ethylcarbazol. Counterstaining of the sections was performed using Mayer’s hemalaun.
For electron microscopy, tissue was excised from sham-operated control and I/R experiments (groups A and B). The samples were fixed and processed as described earlier.6 Ultrathin sections were cut and stained with uranyl acetate and lead citrate and examined under a Zeiss EM 900 transmission electron microscope (Zeiss, Oberkochen, Germany) operating at 80 kV.
Endothelial cell culture and exposure of cells to hypoxia/reoxygenation.
To investigate the effects of platelet adhesion on hypoxia/reoxygenation-induced endothelial cell activation, HUVECs were isolated from fresh umbilical cords and cultured in Endothelial Cell Growth Medium (Promo Cell, Heidelberg, Germany) with 2% fetal calf serum. For experimental use, HUVECs were plated (4.0 × 106 cells/flask) and grown to confluence (3 to 5 days) in Falcon Primaria culture flasks with 25 cm2 growth area (Becton Dickinson, Heidelberg, Germany). The cells were identified by their typical morphology. The endothelial cells were exposed to hypoxia (4 hours, 1 vol% O2) and reoxygenation (1 hour, respectively; 21 vol% O2, 5 vol% CO2) using an anaerobic jar (BBL Gas Pak System; Pierce, Rockford, IL).
Assessment of tyrosine phosphorylation.
Upon the onset of reoxygenation, 2 mL phosphate-buffered saline (PBS) (group 1), PBS containing isolated platelets (150 × 106 platelets/mL), or platelets plus fibrinogen (500 mg/dL) were added to the HUVECs. After 60 minutes of incubation, the plates were washed twice with PBS. The cells were lysed in 150 μL lysis buffer (pH 7.4, 10 μmol/l TRIS buffer, 10% sodium dodecyl sulfate [SDS], 1 mmol/L sodium vanadate, 10 mmol/L EDTA, and Complete protease inhibitor [Boehringer, Ingelheim, Germany] as recommended). It appears noteworthy that after washing of the plates (see above), only very few platelets remained attached to the endothelial cell culture. This implicates that the amount of platelet protein contained within the cell lysates is negligible. The whole-cell protein lysates (20 μg protein per lane) were separated on a 10% SDS-polyacrylamide gel electropheresis (PAGE) and transferred to a PVDF membrane (Boehringer). The membranes were blocked with PBS containing 0.3% gelatin and incubated for 1 hour with a mouse–anti-human phosphotyrosine antibody (1 μg/mL PBS, PY20; Transduction Laboratories, Lexington, UK). The membranes were washed 4 times and exposed to peroxidase-conjugated rabbit–anti-mouse IgG secondary antibody (diluted 1:2,000 in PBS; DAKO). Finally, the membranes were washed, incubated with Supersignal (Pierce), and exposed to x-ray film.
Statistics.
Data analysis was performed with a statistical software package (SigmaStat for Windows; Jandel Scientific, Erkrath, Germany). The Kruskal-Wallis test followed by Dunn’s method was used for the estimation of stochastic probability in intergroup comparisons. Mean values ± SEM are given. P values less than .05 were considered significant.
RESULTS
Platelet-endothelial cell interactions in response to I/R.
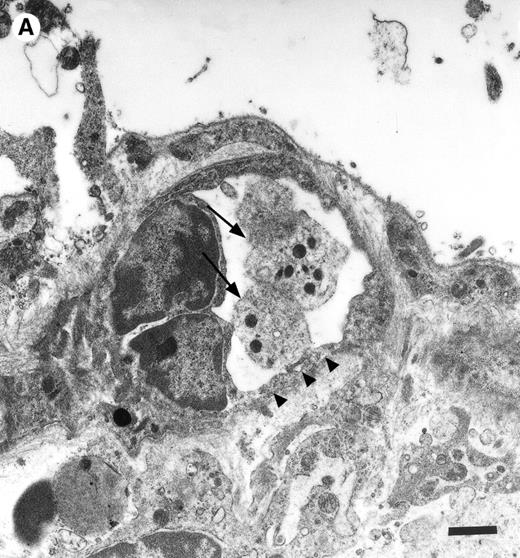
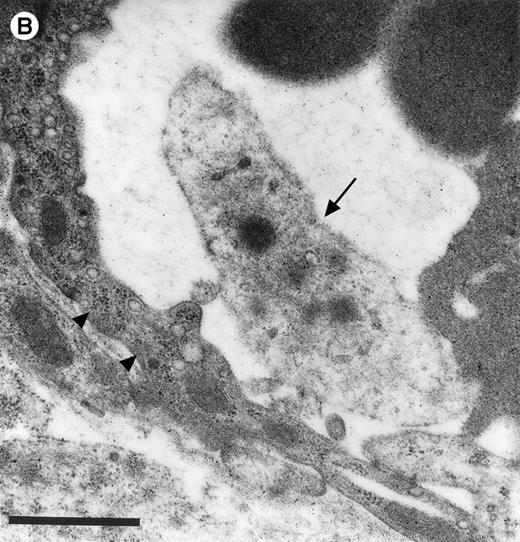
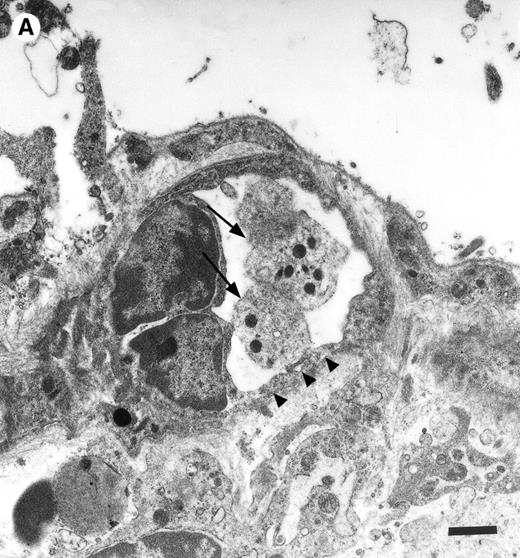
In the physiologic state without I/R, circulating platelets rarely interacted with the microvascular endothelium (Figs1 and 2). Few platelets were observed rolling along the endothelial cell lining of arterioles and postcapillary venules (0 ± 0 and 3 ± 1 platelets/s/mm, respectively). At the same time, only 26 ± 14 and 28 ± 11 platelets were found firmly attached per mm2endothelial cell surface of arterioles and venules, respectively. In contrast, 1.5 hours of ischemia dramatically enhanced platelet-endothelial cell interactions immediately after postischemic reperfusion (Figs 1 and 2). As reported earlier, postischemic platelet accumulation involved arterioles, as well as venules. More than 15 platelets/s/mm vessel diameter were seen rolling along the arteriolar and venular vessel wall, respectively. At the same time, the number of firmly adherent platelets had increased 20- and 12-fold in arterioles and venules, compared with sham-operated animals (Fig 1). Platelet aggregation was a prominent phenomenon. Electron microscopy demonstrated that single or aggregated platelets adhered directly to endothelial cells; obvious defects in the endothelial cell layer were not detected (Fig 3).
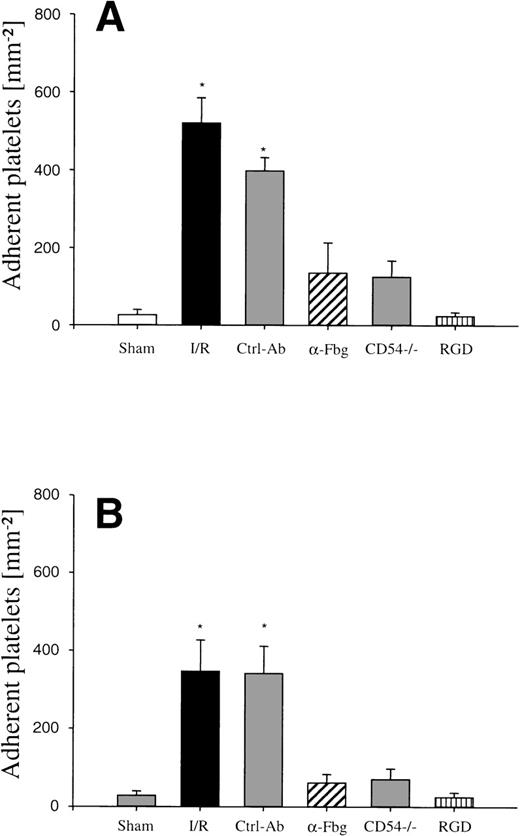
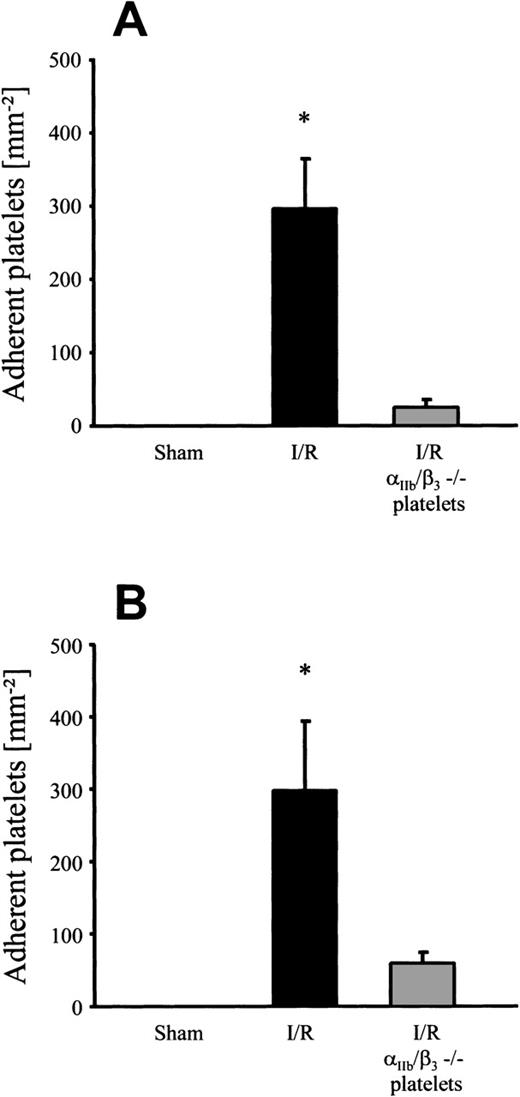
Platelet adhesion in response to I/R of the small intestine in vivo (ischemia time, 90 minutes). Platelet-endothelial cell interactions were investigated in arterioles (A) and venules (B) using IVM. Platelet adhesion was assessed following I/R, after pretreatment with an antibody directed to fibrinogen (-Fbg), or with control antibody (Ctrl-Ab), as well as in ICAM-1–deficient mice (CD54−/−), or after infusion of GRGDSP peptide (RGD), respectively. Sham-operated animals (Sham) served as controls. The number of adherent platelets is given per mm2 vessel surface. Mean ± SEM, n = 6 experimental animals per group. *P < .01 vsham, Dunn’s method.
Platelet adhesion in response to I/R of the small intestine in vivo (ischemia time, 90 minutes). Platelet-endothelial cell interactions were investigated in arterioles (A) and venules (B) using IVM. Platelet adhesion was assessed following I/R, after pretreatment with an antibody directed to fibrinogen (-Fbg), or with control antibody (Ctrl-Ab), as well as in ICAM-1–deficient mice (CD54−/−), or after infusion of GRGDSP peptide (RGD), respectively. Sham-operated animals (Sham) served as controls. The number of adherent platelets is given per mm2 vessel surface. Mean ± SEM, n = 6 experimental animals per group. *P < .01 vsham, Dunn’s method.
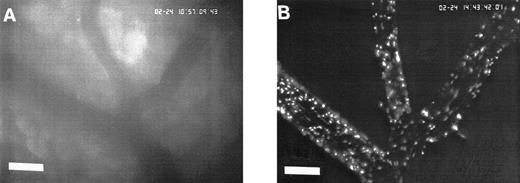
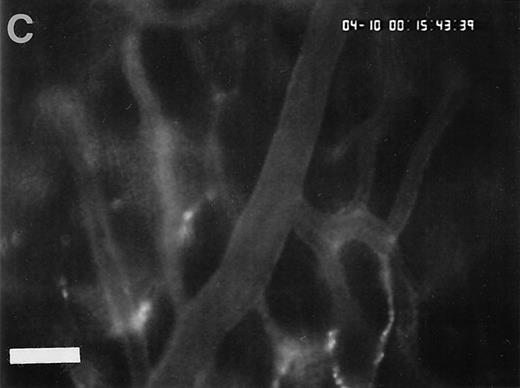
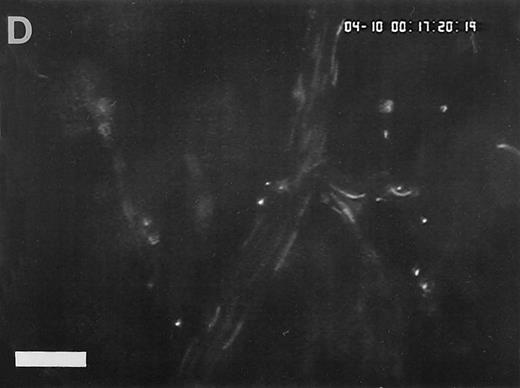
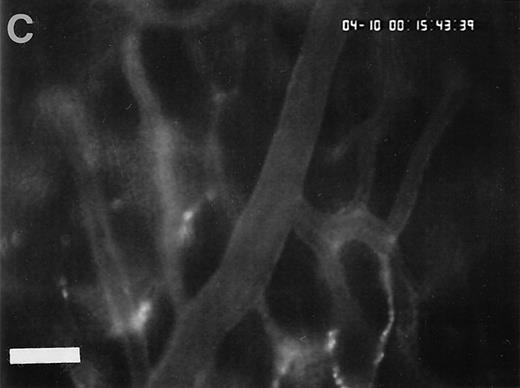
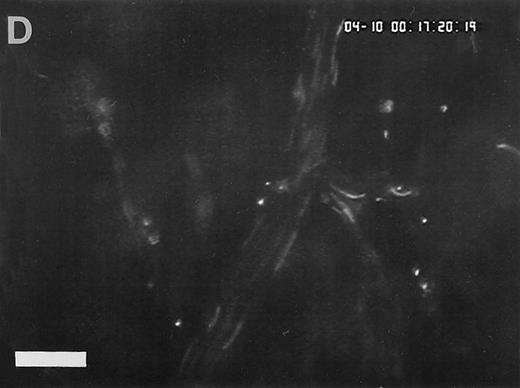
Sequence of photographs documenting I/R-induced platelet-endothelial cell interactions in vivo. Using IVM, rhodamine-6G–labeled platelets are visualized within 3 postcapillary venules before ischemia (A), as well as following 90 minutes of ischemia and 30 minutes of reperfusion (B). Few platelets adhere to the venular endothelium before I/R (A); the majority passes the vessel segment without interacting with the endothelial surface. In contrast, a large number of platelets are seen interacting with the endothelium 30 minutes after reperfusion (B). Monitor magnification 450×; bars represent 50 μm.
Sequence of photographs documenting I/R-induced platelet-endothelial cell interactions in vivo. Using IVM, rhodamine-6G–labeled platelets are visualized within 3 postcapillary venules before ischemia (A), as well as following 90 minutes of ischemia and 30 minutes of reperfusion (B). Few platelets adhere to the venular endothelium before I/R (A); the majority passes the vessel segment without interacting with the endothelial surface. In contrast, a large number of platelets are seen interacting with the endothelium 30 minutes after reperfusion (B). Monitor magnification 450×; bars represent 50 μm.
Platelets in postischemic microvasculature visualized by electron microscopy. There are no defects in the endothelial cell layer (arrowheads) Platelets (arrows) attach directly to endothelial cells (arrowheads). Bars represent 1 μm. Original magnifications: 7,000× (A) and 12,000× (B). NCL, endothelial cell nucleus.
Platelets in postischemic microvasculature visualized by electron microscopy. There are no defects in the endothelial cell layer (arrowheads) Platelets (arrows) attach directly to endothelial cells (arrowheads). Bars represent 1 μm. Original magnifications: 7,000× (A) and 12,000× (B). NCL, endothelial cell nucleus.
Role of fibrinogen for platelet-endothelial cell interactions during I/R.
To assess fibrinogen deposition during I/R, the vascular distribution of fibrinogen was determined using immunohistology. In sham-operated animals small amounts of fibrinogen were detectable within the vessel lumen. Fibrinogen accumulation on the endothelial surface was not observed. In contrast, fibrinogen sequestration on the luminal surface of arterioles and venules was a prominent phenomenon after I/R. To investigate the time course of fibrinogen binding to the endothelial cell surface during I/R in vivo, fluorescent fibrinogen was administered intravenously before the induction of 1.5 hours of ischemia. In the physiologic state (baseline conditions), Alexa 488-conjugated fibrinogen was found homogeneously distributed in the plasma. No fibrinogen deposition was detectable in arterioles or venules (Fig 4). Similarly, during the ischemic period, no significant accumulation of fibrinogen on the endothelial surface was observed. In contrast, reperfusion dramatically enhanced fibrinogen binding to the endothelium in the postischemic microvasculature. Within 1 to 5 minutes after the onset of reperfusion, streaks of fluorescent fibrinogen were observed along the endothelial lining. After 10 minutes of reperfusion, the gaps between the streaks were partially filled with fibrinogen, resulting in a heterogenous fibrinogen coat on the endothelial cell surface of the majority of all vessels studied. In most instances, significant fibrinogen deposition at the arteriolar and venular endothelial surface coincided with the adhesion of platelets in these areas (Fig 4).
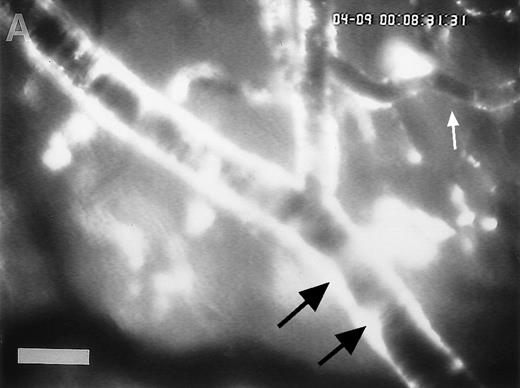
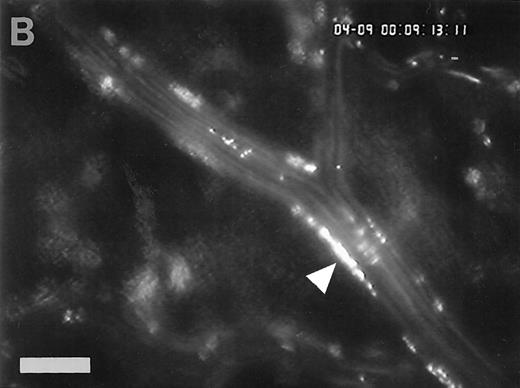
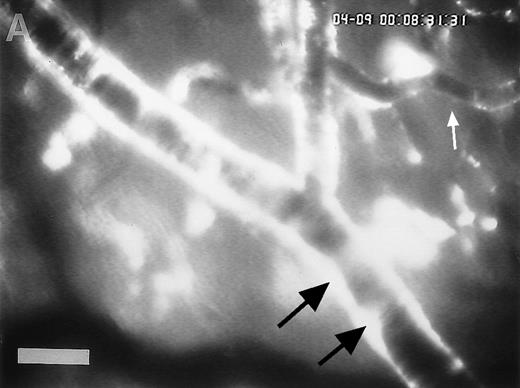
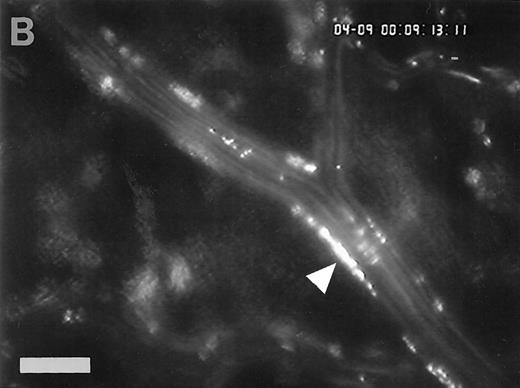
Accumulation of fibrinogen and platelets during I/R in vivo. Alexa 488-conjugated human fibrinogen (17 mg/kg) was administered intravenously 30 minutes before the induction of ischemia (left column). Rhodamin-labeled platelets were visualized in identical arterioles and venules using a different filter set (right column; see Materials and Methods). In wild-type animals (A,B), fibrinogen is bound unevenly to the vascular wall of arterioles and venules in the postischemic microvasculature. Areas with large amounts of fibrinogen (A, large arrow) can be seen besides regions without detectable fibrinogen deposition (A, small arrow). The accumulation of large amounts of fibrinogen colocalizes with platelet adhesion (B, arrowhead). In mice lacking ICAM-1 (C,D), the I/R-induced accumulation of fibrinogen and platelets is attenuated. Monitor magnification, 450×.
Accumulation of fibrinogen and platelets during I/R in vivo. Alexa 488-conjugated human fibrinogen (17 mg/kg) was administered intravenously 30 minutes before the induction of ischemia (left column). Rhodamin-labeled platelets were visualized in identical arterioles and venules using a different filter set (right column; see Materials and Methods). In wild-type animals (A,B), fibrinogen is bound unevenly to the vascular wall of arterioles and venules in the postischemic microvasculature. Areas with large amounts of fibrinogen (A, large arrow) can be seen besides regions without detectable fibrinogen deposition (A, small arrow). The accumulation of large amounts of fibrinogen colocalizes with platelet adhesion (B, arrowhead). In mice lacking ICAM-1 (C,D), the I/R-induced accumulation of fibrinogen and platelets is attenuated. Monitor magnification, 450×.
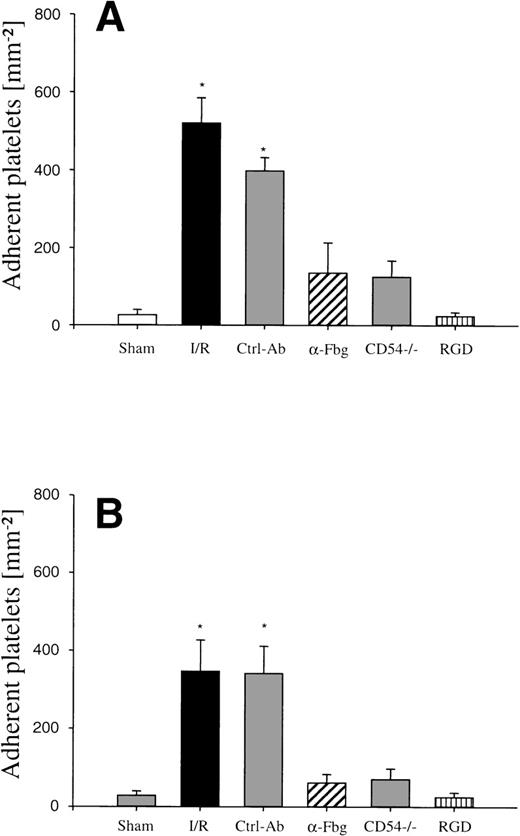
To evaluate the causative role of endothelial fibrinogen deposition for postischemic platelet adhesion in vivo, an affinity-purified polyclonal antibody directed against mouse fibrinogen was administered immediately before the onset of reperfusion. Whereas the antifibrinogen antibody had no effects on platelet rolling in arterioles and venules, the number of adherent platelets was significantly reduced. A mean of 135 ± 79 and 61 ± 22 platelets/mm2 was seen firmly attached to the endothelial cell surface of arterioles and venules, respectively, indicating that fibrinogen promotes platelet adhesion during I/R (Fig 1). In contrast, the goat anti-human IgG polyclonal control antibody had no effects on postischemic platelet accumulation in both arterioles and venules, respectively (Fig 1).
Role of ICAM-1 (CD54) for postischemic platelet-endothelial cell interactions.
To determine the role of ICAM-1 as an endothelial fibrinogen receptor, the accumulation of Alexa 488-conjugated fibrinogen during I/R was investigated in mice deficient in ICAM-1. No fibrinogen binding was seen under control conditions or during ischemia. During reperfusion, a moderate increase in endothelial fibrinogen binding occurred in few arterioles and venules (Fig 4), while the majority of the vessels studied showed no significant fibrinogen accumulation, indicating that ICAM-1 is in fact involved in mediating the deposition of fibrinogen at the postischemic endothelium. To evaluate whether ICAM-1–dependent fibrinogen sequestration on the endothelial surface might mediate platelet-endothelial cell interactions during postischemic reperfusion, fluorescent wild-type platelets were infused into ICAM-1–deficient animals (Fig 1). Whereas the number of rolling platelets did not differ from wild-type mice (23 ± 5 and 36 ± 4 platelets/s/mm in arterioles and venules, respectively), platelet adhesion was significantly reduced in the absence of endothelial ICAM-1: 125 ± 42 and 71 ± 27 platelets were seen firmly attached per mm2 endothelial cell surface of arterioles and venules, respectively, indicating that ICAM-1 is in fact involved in platelet recruitment during postischemic reperfusion.
To assess whether alterations in ICAM-1 expression might account for the changes in fibrinogen sequestration observed during I/R, immunohistochemistry was performed on cryostat sections of wild-type mice using anti–ICAM-1 monoclonal antibody. In sham-operated animals, ICAM-1 was constitutively expressed by the vascular endothelium of both venules and arterioles. However, no alteration in the expression of ICAM-1 was observed in response to I/R (not shown).
Involvement of the platelet αIIb/β3integrin in postischemic platelet-endothelial cell interactions.
To study the role of the platelet fibrinogen receptor (αIIb/β3 integrin) for postischemic platelet adhesion, GRGDSP-peptide, an antagonist to the αIIb/β3 integrin, was administered intravenously. While GRGDSP had no significant effects on the number of rolling platelets in postischemic arterioles and venules (18 ± 6 and 24 ± 5 platelets/s/mm), platelet adhesion in response to I/R was significantly attenuated (23 ± 10 and 24 ± 12 platelets/mm2 in arterioles and venules, Fig 1).
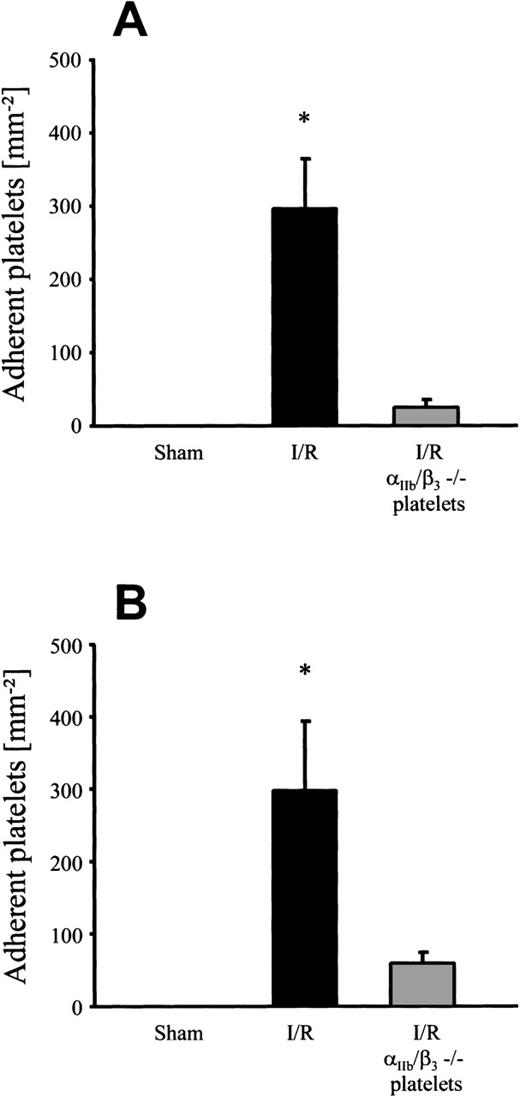
To examine the contribution of the αIIb/β3integrin to postischemic platelet adhesion, human platelets derived from a patient with homozygous Glanzmann’s disease were infused into wild-type mice during I/R. Whereas CD31, CD36, CD42, and CD63 were present on the surface of these platelets, they completely lacked the αIIb/β3 integrin complex (CD41/CD61), as confirmed by flow cytometry (data not shown). As a control, platelets derived from healthy human volunteers were transfused into mice before and after I/R. Under baseline conditions without I/R, no adhesion of normal human platelets to murine endothelium was observed (Fig5). However, I/R significantly increased the number of adherent platelets in both arterioles and venules (296 ± 68 and 298 ± 96 platelets/mm2, respectively). In contrast, when platelets lacking the αIIb/β3 integrin complex were transfused, only 24 ± 11 and 59 ± 16 adherent platelets/mm2were seen in postischemic arterioles and venules, respectively. Taken together, these results suggest that platelet adhesion to postischemic endothelial cells in vivo is αIIb/β3integrin-dependent and involves fibrinogen and endothelial ICAM-1.
Adhesion of human platelets in response to 90 minutes of ischemia and subsequent reperfusion. Human platelets derived from healthy human subjects or from a patient suffering from Glanzmann’s disease ( IIb/β3−/−) were labeled with rhodamine-6G and transfused into wild-type mice. Platelet-endothelial cell interactions were investigated in arterioles (A) and venules (B) using IVM. Sham-operated animals served as controls. The number of adherent platelets is given per mm2 vessel surface. Mean ± SEM, n = 6 experimental animals per group. *P < .01v sham, Dunn’s method.
Adhesion of human platelets in response to 90 minutes of ischemia and subsequent reperfusion. Human platelets derived from healthy human subjects or from a patient suffering from Glanzmann’s disease ( IIb/β3−/−) were labeled with rhodamine-6G and transfused into wild-type mice. Platelet-endothelial cell interactions were investigated in arterioles (A) and venules (B) using IVM. Sham-operated animals served as controls. The number of adherent platelets is given per mm2 vessel surface. Mean ± SEM, n = 6 experimental animals per group. *P < .01v sham, Dunn’s method.
Platelet adhesion enhances tyrosine phosphorylation in reoxygenated HUVECs.
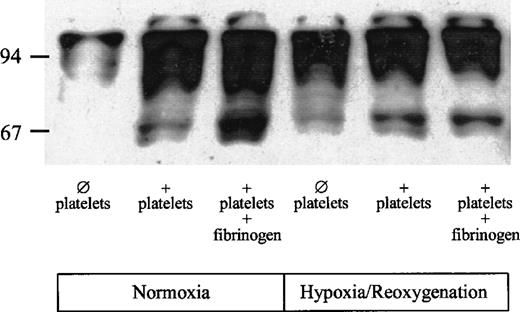
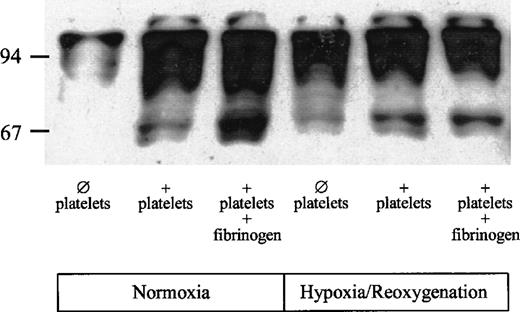
To determine whether platelet adhesion affects signaling pathways in endothelial cells, tyrosine phosphorylation in HUVECs was assessed in the absence or presence of platelets and exogenous fibrinogen. Under normoxic conditions in the absence of platelets, small amounts of phosphotyrosine were detectable (Fig 6). In the presence of platelets, phosphorylation of tyrosine residues was markedly enhanced. The antiphosphotyrosine antibody recognized several proteins, including a 65-kD, a 95-kD, and a 97-kD protein. Tyrosine phosphorylation of the 65-kD protein was enhanced further when exogenous fibrinogen was added to the HUVECs together with the platelets. Hypoxia/reoxygenation increased tyrosine phosphorylation in HUVECs. Despite the absence of platelets, a 65-kD protein, as well as a 95- and a 97-kD protein, was detectable. However, tyrosine phosphorylation of the 65-kD protein was markedly enhanced, in particular in the presence of both platelets and exogenous fibrinogen.
Effect of platelet adhesion on tyrosine phosphorylation in HUVECs. HUVECs were exposed to hypoxia (4 hours, 1 vol% O2) and reoxygenation (1 hour; 21 vol% O2, 5 vol% CO2). Upon onset of reoxygenation, 2 mL PBS (group 1), PBS containing isolated platelets (150 × 106platelets/mL), or platelets plus fibrinogen (500 mg/dL) were added to the HUVECs and incubated for 60 minutes (for details see Materials and Methods). HUVECs without hypoxia/reoxygenation served as controls.
Effect of platelet adhesion on tyrosine phosphorylation in HUVECs. HUVECs were exposed to hypoxia (4 hours, 1 vol% O2) and reoxygenation (1 hour; 21 vol% O2, 5 vol% CO2). Upon onset of reoxygenation, 2 mL PBS (group 1), PBS containing isolated platelets (150 × 106platelets/mL), or platelets plus fibrinogen (500 mg/dL) were added to the HUVECs and incubated for 60 minutes (for details see Materials and Methods). HUVECs without hypoxia/reoxygenation served as controls.
DISCUSSION
Atherosclerosis, a major cause of morbidity in industrialized countries, causes luminal narrowing of the affected blood vessel and can lead to a compromised perfusion of the supplied organ. Following ischemia, restoration of nutritive blood flow induces the adhesion of platelets to the injured endothelium.6 Platelet recruitment to the postischemic vasculature might represent the initial event, contributing to recurrence of luminal narrowing or even complete reocclusion.25 26 However, the molecular mechanisms underlying platelet-endothelial cell interactions in response to I/R have not been completely elucidated thus far.
The accumulation of fibrin(ogen) has been demonstrated following myocardial, hepatic, renal, and cerebral ischemia.17,27,28While in many instances, microvascular fibrinogen accumulation is associated with the recruitment of platelets,29 the causative role of fibrinogen in promoting platelet adhesion during postischemic reperfusion has not been established thus far. The present study provides several lines of evidence indicating that the deposition of fibrinogen onto the postischemic vessel wall significantly contributes to platelet adhesion in both arterioles and venules: (1) the microvascular accumulation of fibrinogen was a prominent phenomenon following I/R; (2) fibrinogen deposition colocalized with platelet adherence in vivo; and (3) treatment with an antifibrinogen antibody attenuated platelet adhesion in both arterioles and venules. Immunoinhibition of fibrinogen-receptor interactions did not affect platelet rolling or transient attachment. Therefore, in agreement with previous findings,20 fibrinogen appears to support firm and irreversible platelet attachment.
It appears noteworthy that the absolute number of adherent platelets was higher in arterioles as compared with venules. In vitro platelet adhesion onto fibrinogen is less efficient at the high wall shear rates, which occur in arterioles, but also in small postcapillary venules.20 Therefore, an initial capturing process is required to allow irreversible binding of platelets to fibrinogen. Platelet capturing or intermittent platelet adhesion can be mediated via P-selectin6,30 and by binding of the leucine-rich glycoprotein Ib (GPIb) to von Willebrand factor.20 The latter interaction, unlike the fibrinogen-GPIIb/IIIa interaction, is particularly efficient at high shear rates.20 Once captured, activation of platelets can occur, leading to an increase in the fibrinogen-binding affinity of the GPIIb/IIIa receptor complex and initiating firm and irreversible platelet adhesion. Similar to platelet capturing per se, the activation of platelets under flow is shear rate-dependent in that the shear rate must be above a threshold limit.31-33 Both, shear-dependent platelet capturing and activation may explain why platelet recruitment is more efficient in arterioles than in venules, despite a low resistance of the fibrinogen-GPIIb/IIIa interaction to tensile stress. Interestingly, after transfusion of human platelets, there was no difference in the number of adherent platelets in arterioles as compared with venules (Fig 5). This suggests that the molecular mechanisms that underlie shear-dependent platelet capturing function only within one species, but might be less effective in the xenogeneic setting.
Endothelial denudation was not observed in the present study, as demonstrated by electron microscopy. Instead, the deposition of fibrinogen occurred directly on the endothelial cell surface, indicating that the attachment of fibrinogen to endothelial cells might be mediated by a fibrinogen receptor present on the endothelium. Recently, the functional role of ICAM-1 as a novel fibrinogen receptor has been emphasized. ICAM-1 has been shown to contain a fibrinogen recognition site that is distinct from previously recognized ICAM-1 ligand binding regions.34 Through its ICAM-1 recognition, fibrinogen enhances the adhesion of leukocytes to endothelial cells in vitro and in vivo,18,19 and supports transendothelial monocyte migration.35 In addition, fibrinogen–ICAM-1 bridging has recently been demonstrated to mediate platelet adhesion to HUVECs in vitro.21 In the present study, the lack of endothelial ICAM-1 expression attenuated fibrinogen deposition to the postischemic vessel wall. In addition, in the absence of ICAM-1 platelet adhesion was significantly reduced, indicating that through its fibrinogen recognition, ICAM-1 promotes platelet adhesion to the postischemic endothelium in vivo. This mechanism might be of particular importance in mediating platelet adhesion to atherosclerotic lesions, which are characterized by the large deposition of fibrin(ogen) and the increased expression of ICAM-1.36-38 The established role of both fibrinogen and soluble ICAM-1 as a major risk factor for myocardial infarction further highlights the potential pathophysiologic relevance of platelet adhesion to endothelial cells via fibrinogen bridging to ICAM-1.39-41
Although both fibrinogen and ICAM-1 are constitutively expressed, fibrinogen deposition was absent under physiologic conditions and during ischemia. In contrast, within minutes after the onset of reperfusion, fibrinogen accumulation was drastically enhanced, which suggests that reoxygenation/reperfusion is required to promote endothelial recognition of the clotting factor. The mechanisms that initiate the interaction between soluble fibrinogen and endothelial cells remain unclear. An upregulation of the endothelial fibrinogen receptor might explain the increase in fibrinogen binding following ischemia. However, since an enhancement of ICAM-1 or fibrinogen expression requires de novo mRNA and protein synthesis, this mechanism is unlikely to contribute to fibrinogen deposition observed after 1.5 hours of ischemia. Accordingly, we were not able to detect any differences in the ICAM-1 expression before and after I/R. This suggests that changes in the affinity of fibrinogen to its receptor (or vice versa), rather than alterations in ICAM-1 surface expression, are involved in the regulation of fibrinogen-endothelial cell interaction. An increase in the adhesive properties of ICAM-1 might explain fibrinogen binding to the endothelium in response to I/R. However, in contrast to integrins that are known to undergo conformational changes upon activation,42 similar mechanisms leading to an increased adhesiveness of ICAM-1 have not been documented so far. Hence, fibrinogen is most likely to be modified in a way that increases its binding affinity to the endothelial surface. Endothelial cell activation, eg, by hypoxia/reoxygenation or by oxygen free radicals, is known to induce rapid upregulation of tissue factor expression13,14,43 and to suppress thrombomodulin activity in endothelial cells.15,16 Both tissue factor activation and suppression of thrombomodulin activity lead to thrombin release/activation. Thrombin generated by the postischemic endothelium and released by recruited platelets initiates proteolytic fibrinogen degradation, which results in fibrin attachment to the endothelium.13,14,44 Since Alexa-fibrinogen retains its fluorescence after incubation with thrombin, we cannot exclude that fibrin instead of fibrinogen deposition was visualized by in vivo fluorescence microscopy. In addition, the polyclonal antifibrinogen antibody used in the present study recognizes both fibrinogen and its degradation product fibrin. However, we were not able to identify the exact molecular characteristics of fibrinogen present after reperfusion, since an antibody that recognizes exclusively murine fibrin but not fibrinogen is currently not available. Nevertheless, in a baboon model of cerebral I/R, the presence of microvascular fibrin has been clearly demonstrated by Okada et al using the murine anti-human fibrin monoclonal antibody MH-1.17 Therefore, I/R-induced fibrinogen degradation, leading to fibrin deposition to the vessel wall, might in fact be the mechanism that initiates platelet adhesion during postischemic reperfusion. It is unclear thus far how proteolytic processing enhances fibrin(ogen) deposition to the endothelium. Gardiner et al45 proposed that proteolysis of soluble fibrinogen might increase the binding affinity of the clotting factor to ICAM-1 by complete exposure of the γ(117-133) sequence, which mediates the association between fibrin(ogen) and endothelial ICAM-1.46 Alternatively, small amounts of fibrinogen might be constitutively bound to ICAM-1. The degradation of soluble fibrinogen during reperfusion could lead to a polymerization of the resultant fibrin to the fibrinogen bound to ICAM-1. This polymerization would then enhance the deposition of fibrin- (ogen) to the endothelial cell layer, promoting platelet adhesion during I/R.
The αIIb/β3 integrin appears to be the platelet receptor that binds to fibrinogen and initiates platelet adhesion to the postischemic vessel wall. Both treatment with the RGD-containing peptide GRGDSP, which inhibits ligand binding to αIIb/β3 integrin, as well as the absence of functional αIIb/β3 integrin on the surface of platelets derived from patients with Glanzmann’s disease, attenuated platelet adhesion during I/R. This strongly suggests that platelets bind to postischemic endothelial cells in a process that involves αIIb/β3 integrin bridging to endothelium-bound fibrinogen. The relevance of this mechanism is stressed by the protective effects of αIIb/β3 antagonists in patients with unstable angina pectoris or myocardial infarction.47
Rupture of an atherosclerotic plaque initiates platelet adhesion and subsequent thrombus formation and ischemia of the supplied organ. As reported here, reperfusion after an ischemic episode also induces platelet adhesion, a process that may lead to recurrence of luminal narrowing and eventually reocclusion. It is now clear that the resultant reperfusion injury is one form of acute inflammation1-4 in which platelets might play an important role. As demonstrated earlier,6 platelets are among the first cells recruited to the postischemic microvasculature. Activated platelets release oxygen radicals and generate a variety of proinflammatory mediators, such as platelet-activating factor (PAF), interleukin-1 (IL-1), epithelial-derived neutrophil-activating factor-78 (ENA-78), neutrophil-activating peptide-2 (NAP-2), and RANTES.9-12,48-50 In the present study, we have demonstrated that platelet adhesion enhances tyrosine phosphorylation in endothelial cells. Supporting a role of platelets in inflammatory reactions, Kaplanski et al have reported that activated platelets induce IL-8 secretion from human umbilical vein endothelial cells.51 Although this indicates that platelet-endothelial cell interactions may modulate endothelial cell function and initiate endothelial cell activation, the mechanisms that underlie platelet-dependent signaling in endothelial cells have not been established thus far. Consistent with a role of ICAM-1 in vascular cell signal transduction, binding of fibrinogen to ICAM-1 expressed on the endothelial surface of the saphenous vein modulates vascular tone in an NO-independent mechanism.52 In addition, platelet adhesion to the endothelial cell layer allows close cell-to-cell contact. This might facilitate juxtacrine activation of the endothelial cells by soluble platelet mediators or by direct ligand-receptor interactions.53
In conclusion, we have demonstrated in vivo that fibrin(ogen) accumulates in the postischemic microvasculature early after the onset of reperfusion. This deposition of fibrin(ogen) onto the endothelial cell surface promotes platelet adhesion, involving fibrin(ogen)-αIIb/β3 integrin interactions. Since adherent platelets induce tyrosine phosphorylation, platelet recruitment is likely to significantly contribute to the manifestation of microvascular I/R injury.
ACKNOWLEDGMENT
The authors thank Elke Schütze, Katrin Baltzer-Quoast, and Sylvia Münzing for their excellent and skillful technical assistance. We are grateful to Dr Karin Auberger (Dr von Haunersches Kinderspital der Universität München, Munich, Germany) for providing the CD41/CD61-deficient platelets.
S.M. and G.E. contributed equally to this work.
Supported by research grant Biomed 2 Contract No. BMH4-CT95-0875 (DG12-SSMA).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Steffen Massberg, MD, Deutsches Herzzentrum München, Technical University of Munich, Lazarettstr 36, 80636 Munich, Germany; e-mail: massberg@icf.med.uni-muenchen.de.