Abstract
Receptors used by natural killer (NK) cells to mediate natural cytotoxicity are poorly defined, although it is now clear that a number of adhesion molecules can serve this function. CD38 transduces signals on T- and B-cell lines, and we asked whether it could trigger lytic and secretory responses in human NK cells. By using an anti-CD38 monoclonal antibody in reverse antibody-dependent cellular cytotoxicity experiments, it is shown that CD38 engagement triggers cytotoxic responses by activated NK cells, but not by cytotoxic T lymphocytes or fresh NK cells. Cross-linking with anti-CD38 F(ab′)2 caused activated NK cells to release granzymes and cytokines, but did not trigger an increase in intracellular Ca2+. Fresh NK cells acquired CD38-dependent lytic function during activation with interleukin-2 (IL-2), and inhibitor studies suggested that IL-2 stimulated the de novo expression of proteins that act between CD38 and the lytic machinery in NK cells. The induction of proteins that link commonly expressed adhesion molecules to effector mechanisms could provide a paradigm for pathogen recognition by the innate immune system.
NATURAL KILLER (NK) CELLS, the primary lymphoid mediators of natural cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC), are controlled by positive and negative cytolytic signals.1,2 Negative signals are transduced by 2 classes of major histocompatibility complex (MHC) I-binding molecules: by receptors containing Ig-like domains known as KIRs in humans and receptors, including the Ly-49 family of proteins in mice and CD94/NKG2A in humans that have extracellular C-lectin domains,3,4 and by molecules with unknown specificity.5 The best-characterized positive triggering molecule on NK cells is CD16 (FcγRIIIA), the receptor responsible for mediating ADCC.1,6 CD16 associates noncovalently with γ and ζ homodimers and heterodimers that contain tyrosine-based activation motifs (ITAMs) in their cytoplasmic domains. Cross-linking of CD16 leads to phosphorylation of these ITAMs and ofsyk, ZAP-70, and phospholipase C-γ (PLC-γ), followed by a number of downstream events, including the release of intracellular Ca2+, that ultimately result in the exocytosis of cytolytic granules at the effector-target interface. It has been recently shown that activating forms of KIRs and Ly-49 follow a similar positive signaling pathway but use DAP-12, a homologue of ζ, as a signal transducer.3,7 8
Receptors mediating natural cytotoxicity, the innate ability of NK cells to lyse tumor or virally infected target cells in the absence of antibody, have been more difficult to characterize because multiple different receptors can trigger cytolysis. Thus, a monoclonal antibody (MoAb) to any 1 triggering molecule would only marginally inhibit lysis. However, it has been possible to bypass the normal receptor-ligand interaction and to study the capacity of individual molecules to transduce cytotoxic signals by using receptor-specific IgG MoAbs to redirect lysis of FcγR+ target cells.9,10 By using this approach, a number of cytotoxic triggering molecules have been identified, including CD2,11-13 CD44,14-16 CD69,17NKRP-1,18 CD40,19 B7.2,19 and NK-TR,20 suggesting that these molecules might be involved in natural cytotoxicity. Another molecule expressed by NK cells is CD38, a type II integral membrane receptor that binds to CD31 (PECAM-1) and has adenosine diphosphate (ADP)-ribosyl cyclase ectoenzymatic activity.21-23 CD38 is expressed early in the differentiation of CD34+ stem cells to lymphocytes and remains in mature, CD56+, CD16+ NK cells24,25 but not in resting B or T cells, although it is re-expressed in activated T cells. Ligation of CD38 on Jurkat T cells,26,27 immature B cells,28 and differentiated HL-60 cells29 by anti-CD38 MoAbs induces protein tyrosine phosphorylation. In Jurkat, but not in immature B cells or MHC-nonrestricted T cells,30 CD38 cross-linking also induces increases in intracellular Ca2+. A number of important functional consequences of CD38 engagement include upregulation of receptors such as B7.231 and CD73,32 apoptosis,27proliferation,31 and cytokine release.30
The potent signaling properties of CD38 in T-cell, B-cell, and granulocytic cell lines prompted us to ask whether CD38 might also serve as a cytotoxic triggering molecule on NK cells. We show here that an agonistic anti-CD38 MoAb redirects cell-mediated cytolysis and induces cytokine and granzyme release in activated NK cells. We conclude that the CD38 adhesion molecule is indeed a cytotoxic trigger on NK cells that could contribute significantly to the positive triggering events leading to natural cytotoxicity.
MATERIALS AND METHODS
Antibodies and reagents.
Phycoerythrin (PE)-anti-CD38 (H1T2), PE-anti-CD16 (3G8), and unconjugated anti-CD11a (G43-25B, IgG2b and HI111, IgG1) and anti-CD56 (B159, IgG1) were from Pharmingen (San Diego, CA). 3G8 (IgG1, anti-CD1633), OKT3 (IgG2a anti-CD334), W6/32 (IgG2a, anti-HLA35), and IB4 [IgG2a and F(ab′)2, anti-CD3836] have been described. OKT10 (IgG1 anti-CD38) and M300 (IgG1 myeloma protein, MOPC 300) were from the American Type Culture Collection (Manassas, VA). Actinomycin D was from Sigma Chemical Co (St Louis, MO).
Effector and target cells.
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats from normal National Institutes of Health Blood Bank (Bethesda, MD) donors by Ficoll-Hypaque density gradient separation. Peripheral blood lymphocytes (PBLs) were prepared from PBMCs by monocyte depletion using plastic adherence, followed in some experiments by nylon wool adsorption.15 PBLs were activated for 6 days (unless stated otherwise) in culture medium (RPMI 1640 plus 10% fetal calf serum [FCS], glutamine, penicillin, and streptomycin) containing 200 to 400 Cetus Units/mL recombinant human interleukin-2 (rhIL-2; Cetus-Chiron, Emeryville, CA) and, in some experiments, 5% Lymphocult-T (conditioned medium from phytohemagglutinin [PHA]-activated PBMCs; Biotest Diagnostics, Denville, NJ). This procedure induces cytotoxic activity in NK and CD56+ T cells but does not activate the great majority of T cells, which lack CD56.37 NK cells were purified from unactivated or activated PBLs by negative selection using a Vario MACS system with an NK isolation kit (Miltenyi Biotec, Auburn, CA) and were always greater than 95% CD16+ and less than 1% CD3+. Cytotoxic T lymphocytes (CTLs) were obtained from high buoyant density PBLs38 that had been activated for 4 days with culture medium containing 200 to 400 Cetus Units/mL rhIL-2 and 5% Lymphocult-T and then negatively selected for CD56 using the Vario MACS system. P815 (mouse FcγR+ mastocytoma) target cells (American Type Culture Collection) were labeled with 51Cr as described.39
Cytotoxicity assay.
Effector cells were incubated for 20 to 30 minutes at room temperature with or without MoAb (2.5 to 10 μg/mL) and diluted serially. Ten thousand 51Cr-labeled P815 target cells in 100 μL culture medium were then added to 100 μL of diluted effector cells, and cytotoxicity was measured in triplicate samples using a 4-hour51Cr-release assay.39 The percentage of specific lysis was calculated as follows: 100 × (CPMexperimental − CPMspontaneous)/(CPMmaximum − CPMspontaneous). Spontaneous release was 10% or less of the maximum in all experiments reported.
Surface expression.
Cells were incubated on ice for 30 minutes with either a fluorescein isothiocyanate (FITC)- or PE-conjugated MoAb, followed by a second incubation with an MoAb conjugated with the other fluorophore. They were washed twice and analyzed with a FACScan flow cytometer (Becton Dickinson Immunocytometry, San Jose, CA). Propidium iodide staining and forward and side light scatter were used to gate on viable cells, and a total of 5,000 to 10,000 cells were analyzed per measurement.
Effector-target conjugates.
Effector-target conjugates were detected by flow cytometry using a modification of a previously published procedure.40Activated NK cells were suspended in 5 mL phosphate-buffered saline (PBS) at a concentration of 2 × 106 cells/mL and 50 μL of 1 mmol/L FITC (Sigma) in ethanol was added. The cells were incubated for 5 minutes at 37°C, washed, centrifuged, and incubated on ice for 30 minutes with or without 2.5 μg of MoAb in a volume of 0.1 mL. After washing, Ficoll-Hypaque–fractionated P815 cells in a total volume of 0.1 mL RPMI 1640 were added at a target:effector ratio of 1.5:1. The cells were pelletted, incubated at room temperature for 1 hour, and gently resuspended in 0.5 mL PBS. NK cells could be distinguished from the P815 cells by flow cytometry on the basis of their lower side light scatter. Conjugates were calculated as the percentage of FITC+ cells having the same or higher side light scatter as the P815 cells. At least 10,000 FITC+ (NK) cells were analyzed, yielding 1,500 as the minimum number of conjugates detected in any experiment.
N-α-benzyloxycarbonyl-L-lysine thiobenzyl ester (BLT) thioesterase assay.
Serine esterase release from activated, purified NK cells was measured using a modification of a previously described method.41Briefly, 96-well Immulon microtiter immunoassay plates (Dynatech Laboratories, Chantilly, VA) were incubated overnight with 50 μL/well of 15 μg/mL MoAb in borate-buffered saline, pH 8.5. After 3 washes with PBS, 1 to 4 × 105 NK cells in 200 μL RPMI 1640 without phenol red and without serum but containing 400 U/mL IL-2 were added to each well. Plates were centrifuged for 5 minutes at 300g and incubated for 3 hours at 37°C in 5% CO2. After incubation, 50-μL aliquots of supernatants were transferred to a flat-bottom microtiter plate and 50 μL of 1 mmol/L 5′,5′-Dithio-bis-(2-nitrobenzoic acid) (DTNB; Sigma) in Hanks’ balanced salt solution (HBSS) was added to each well. Immediately after the addition of DTNB, plates were read at 414 nm using a Titertech (Huntsville, AL) Multiskan enzyme-linked immunosorbent assay (ELISA) reader to obtain blank values. One hundred microliters of 1 mmol/L BLT (Calbiochem, San Diego, CA) in HBSS was added to each well, and the plates were incubated at room temperature until a visible yellow color developed. The plates were again read in the ELISA reader, and blank values were subtracted.
Cytokine release.
Immulon 96-well microtiter immunoassay plates were incubated overnight with 50 μL/well of 25 μg/mL MoAb in borate-buffered saline, pH 8.5. After 3 washes with PBS, 3 × 105 IL-2–activated, purified NK cells in 200 μL complete medium were added to each well. After 20 hours of incubation at 37°C, cell-free supernatants were harvested and either tested immediately or stored at −20°C. Tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) levels were quantitated with Biosource International (Camarillo, CA) ELISA kits, following the manufacturer’s instructions. Limits of detection were 1 pg/mL for TNF-α and 4 pg/mL for IFN-γ.
Cytoplasmic Ca2+.
Cytoplasmic Ca2+ was measured by using the Fluo-3/AM fluorescent calcium marker, as described.42 Briefly, purified, IL-2–activated NK cells were washed with HBSS and loaded for 30 minutes at room temperature with 4 μmol/L Fluo-3/AM (Molecular Probes, Eugene, OR) in the presence of 0.06% Pluronic F-127 (Molecular Probes). Cells were diluted 5-fold in HBSS containing 1% FCS, incubated for 30 minutes at room temperature, washed 3 times, and kept at 4°C in HEPES-buffered saline (137 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L Na2HPO4, 5 mmol/L glucose, 1 mmol/L CaCl2, 0.5 mmol/L MgCl2, 1 mg/mL bovine serum albumin [BSA], and 10 mmol/L HEPES, pH 7.4). Five minutes before fluorescence-activated cell sorting (FACS) analysis, the Fluo-3–labeled cells were warmed to 37°C. They were then stimulated with 10 μg/mL of IB4 F(ab′)2, W6/32, or 3G8 MoAb, followed by the addition of 10 μg/mL of rabbit F(ab′)2 antimouse IgG (Southern Biotechnology, Birmingham, AL). Proper loading was checked by treating cells with 2 μg/mL of ionomycin.
RESULTS
CD38 is a cytotoxic triggering molecule in activated NK cells.
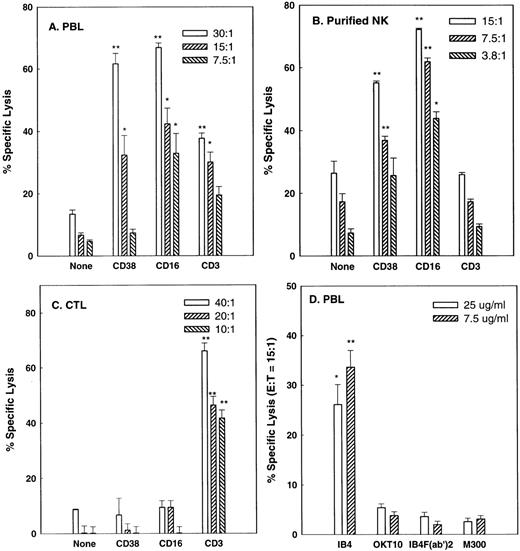
Although CD38 transduces a variety of signals in B-cell, T-cell, and granulocytic cell lines26-29 and induces a cytotoxic response in an MHC-unrestricted T-cell line,30 it is not known whether it has the capacity to trigger cytolysis in isolated subsets of PBLs. Therefore, PBLs from normal donors were incubated in reverse ADCC10 experiments with FcγR+ P815 target cells in the presence of either anti-CD38 MoAb or MoAbs against CD3 and CD16, known cytotoxic triggers in CTLs and NK cells, respectively. In these experiments, IgG MoAbs redirect lysis by simultaneously binding to FcγR on the target cells and to triggering molecules on the effector cells. P815 cells are of mouse origin and are not specifically recognized by antihuman CD38 or other mouse antihuman MoAbs used in this study. Figure 1A shows that IL-2–activated PBLs do in fact contain cells that mediate CD38-directed cytolysis. Purified NK cells and CTLs were then used to determine which types of cells mediate this activity. The purified NK cell preparation (Fig 1B) mediated moderate amounts of lysis of P815 cells, a relatively NK-resistant target, in the absence of MoAb. Lysis was not affected by anti-CD3 MoAb, indicating that levels of CTL contamination were negligible, but was markedly enhanced by the anti-CD38 and anti-CD16 MoAbs. By contrast, conventional CTL, depleted of NK cells and a small subset of T cells by CD56 negative selection, exhibited potent CTL activity, but failed to mediate CD38- or CD16-directed lysis. Removal of the Fc portion of IB4, the anti-CD38 MoAb used above, abrogated its capacity to mediated reverse ADCC, as expected, and OKT10, a less agonistic anti-CD38 MoAb than IB4,43 consistently mediated lysis at a lower level than IB4 (Fig 1D). FACS analysis (Fig 2) showed that all NK (CD16+) cells and the great majority of CTL expressed CD38. Thus, the inability of the anti-CD38 MoAb to trigger lysis by CTLs was not due to the lack of expression of CD38 on these cells. We conclude that CD38 is a cytotoxic trigger on activated NK cells but not on conventional CTLs.
CD38 is a cytotoxic triggering molecule for activated NK cells but not CTL. IL-2–activated PBLs (A), purified IL-2–activated NK cells (B), or CD56-depleted CTL (C) from separate donors were tested in reverse ADCC assays using anti-CD38 (IB4), anti-CD3 (OKT3), or anti-CD16 (3G8) MoAbs to redirect the lysis of P815 target cells. Effector:target (E:T) ratios are indicated in the legends. In (D), the indicated concentrations of intact IB4, IB4 F(ab′)2, intact OKT10 (a less agonistic anti-CD38 MoAb), and M300 (nonspecific IgG control) were used with PBLs at an E:T ratio of 15:1 to redirect lysis. All effector cells were preactivated as described in Materials and Methods. *P < .05, **P < .01 when compared with the no antibody control (A, B, and C) or the M300 control (D) at the same E:T ratio.
CD38 is a cytotoxic triggering molecule for activated NK cells but not CTL. IL-2–activated PBLs (A), purified IL-2–activated NK cells (B), or CD56-depleted CTL (C) from separate donors were tested in reverse ADCC assays using anti-CD38 (IB4), anti-CD3 (OKT3), or anti-CD16 (3G8) MoAbs to redirect the lysis of P815 target cells. Effector:target (E:T) ratios are indicated in the legends. In (D), the indicated concentrations of intact IB4, IB4 F(ab′)2, intact OKT10 (a less agonistic anti-CD38 MoAb), and M300 (nonspecific IgG control) were used with PBLs at an E:T ratio of 15:1 to redirect lysis. All effector cells were preactivated as described in Materials and Methods. *P < .05, **P < .01 when compared with the no antibody control (A, B, and C) or the M300 control (D) at the same E:T ratio.
Expression of CD38 on NK cells and CTLs. Contour diagrams of PBL incubated for 3 days in (A) medium alone, (B) medium containing IL-2, and (C) medium containing IL-2 plus 10 ng/mL actinomycin D. (D) Single-parameter histogram showing CD38 expression on CTLs. In (A) through (C), the NK (CD16+) cells were 95%, 93%, and 99% CD38+, respectively, and the mean CD38 fluorescence intensities on the CD16+CD38+ cells were 147, 134, and 232. In (D), cells were 90% positive for CD3 (not shown) and 80% positive for CD38.
Expression of CD38 on NK cells and CTLs. Contour diagrams of PBL incubated for 3 days in (A) medium alone, (B) medium containing IL-2, and (C) medium containing IL-2 plus 10 ng/mL actinomycin D. (D) Single-parameter histogram showing CD38 expression on CTLs. In (A) through (C), the NK (CD16+) cells were 95%, 93%, and 99% CD38+, respectively, and the mean CD38 fluorescence intensities on the CD16+CD38+ cells were 147, 134, and 232. In (D), cells were 90% positive for CD3 (not shown) and 80% positive for CD38.
Comparison of triggering functions of NK surface molecules.
Not all MoAbs against NK surface molecules consistently induced lytic responses. Table 1 shows that activated PBLs from most donors mediate CD38- and CD16-dependent lysis of P815 target cells at levels significantly above the antibody-independent background. By contrast, anti-MHC I and anti-CD11a MoAbs induced positive responses in only 18% and 0% of donors, respectively, although these same MoAbs bound to essentially all NK cells by FACS analysis (data not shown). Moreover, both triggering and nontriggering MoAbs were fully capable of inducing NK cell-target cell conjugate formation (Fig 3), indicating that conjugate formation, per se, is not a lytic signal in NK cells.
Conjugates between P815 and NK cells. Purified FITC-labeled activated NK cells were coated with MoAb of the indicated specificity, washed, and mixed with P815 cells at a ratio of 1 NK cell to 1.5 P815. The cells were pelletted, incubated for 1 hour at room temperature, resuspended, and analyzed for light scatter and green fluorescence. Conjugates were detected as FITC+ particles with side scatter greater or equal to that of P815 cells.
Conjugates between P815 and NK cells. Purified FITC-labeled activated NK cells were coated with MoAb of the indicated specificity, washed, and mixed with P815 cells at a ratio of 1 NK cell to 1.5 P815. The cells were pelletted, incubated for 1 hour at room temperature, resuspended, and analyzed for light scatter and green fluorescence. Conjugates were detected as FITC+ particles with side scatter greater or equal to that of P815 cells.
Induction of CD38-dependent lysis.
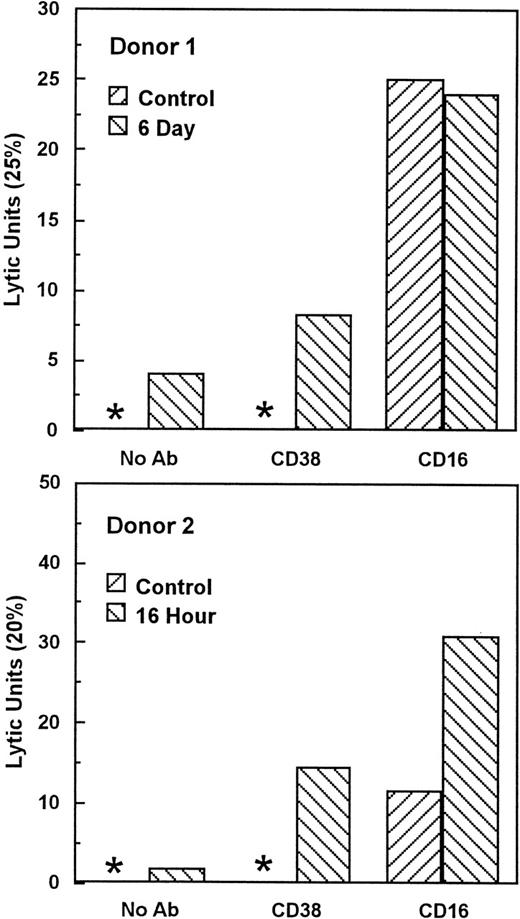
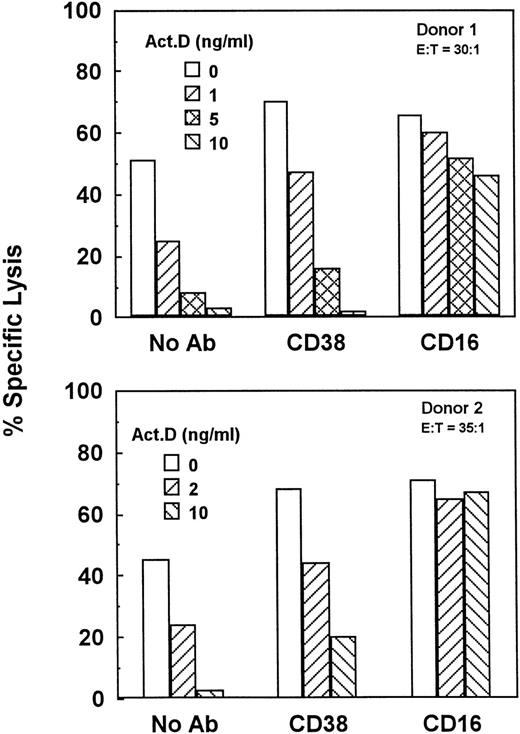
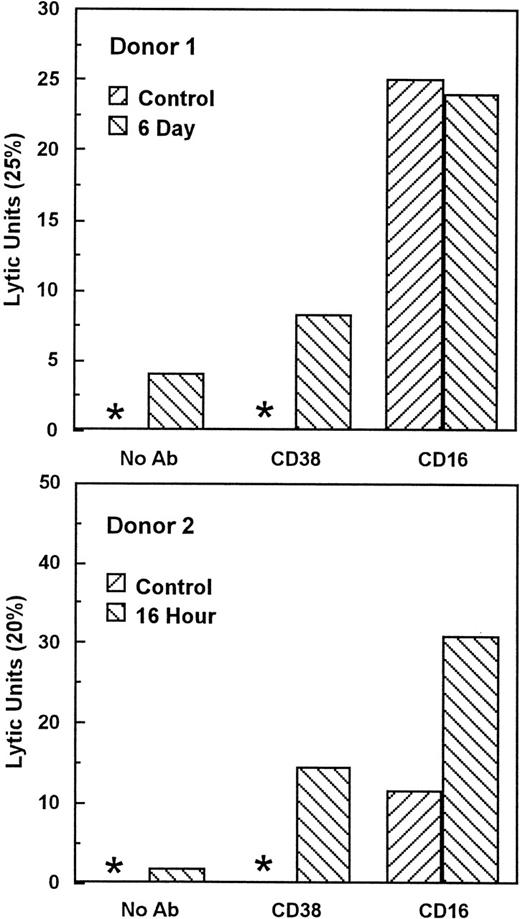
Unstimulated NK cells mediated little if any CD38-dependent lysis, but culture with IL-2 induced both CD38-dependent lysis and Ab-independent cytotoxicity (Fig 4). By contrast, unstimulated NK cells did mediate CD16-directed lysis, indicating that they were competent killer cells before activation (Fig 4). CD38 expression was similar on both activated and unstimulated cells (Fig 2B and A), indicating that the acquisition of CD38 triggering function was not due to enhanced CD38 surface expression. To determine whether de novo gene expression was required for the induction of CD38-directed lysis, PBLs were activated in the presence of varying concentrations of the transcription inhibitor, actinomycin D. As shown in Fig 5, actinomycin D, even at 10 ng/mL, had little effect on the acquisition or maintenance of lysis mediated by CD16, indicating that it did not impair the cytolytic function of NK cells in general. By contrast, actinomycin D strongly inhibited the induction of CD38-dependent lysis and Ab-independent cytotoxicity (Fig5), but had a negligible effect on CD38 expression (Fig 2C). These data suggest that IL-2 induced the expression of at least 1 protein, distinct from CD38 itself, that was required for CD38 lytic function.
IL-2 activation induces CD38 triggering function. Cells from 2 normal donors were cultured with 200 U/mL IL-2 for the indicated times and tested for the ability to lyse P815 target cells in the presence of medium alone (no Ab), anti-CD38, or anti-CD16 MoAbs. Lytic units were calculated from the E:T ratio giving 25% (donor 1) or 20% (donor 2) lysis (lytic units = 100/E:T). Asterisks indicate that lysis was less than 25% and 20% for donors 1 and 2, respectively, at all E:T. This experiment has been repeated 4 times with similar results. CD38-directed lysis appeared in different donors as early as 16 hours and as late as 4 days.
IL-2 activation induces CD38 triggering function. Cells from 2 normal donors were cultured with 200 U/mL IL-2 for the indicated times and tested for the ability to lyse P815 target cells in the presence of medium alone (no Ab), anti-CD38, or anti-CD16 MoAbs. Lytic units were calculated from the E:T ratio giving 25% (donor 1) or 20% (donor 2) lysis (lytic units = 100/E:T). Asterisks indicate that lysis was less than 25% and 20% for donors 1 and 2, respectively, at all E:T. This experiment has been repeated 4 times with similar results. CD38-directed lysis appeared in different donors as early as 16 hours and as late as 4 days.
Gene transcription is required for the induction of natural cytotoxicity and CD38-dependent lysis. PBLs from 2 separate donors were activated for 3.5 days in medium containing 200 U/mL IL-2 and the indicated concentrations of actinomycin D. Cells were then tested for the ability to mediate natural cytotoxicity (No Ab) or cytotoxicity directed by anti-CD38 or anti-CD16 MoAbs.
Gene transcription is required for the induction of natural cytotoxicity and CD38-dependent lysis. PBLs from 2 separate donors were activated for 3.5 days in medium containing 200 U/mL IL-2 and the indicated concentrations of actinomycin D. Cells were then tested for the ability to mediate natural cytotoxicity (No Ab) or cytotoxicity directed by anti-CD38 or anti-CD16 MoAbs.
CD38 cross-linking triggers degranulation and cytokine release.
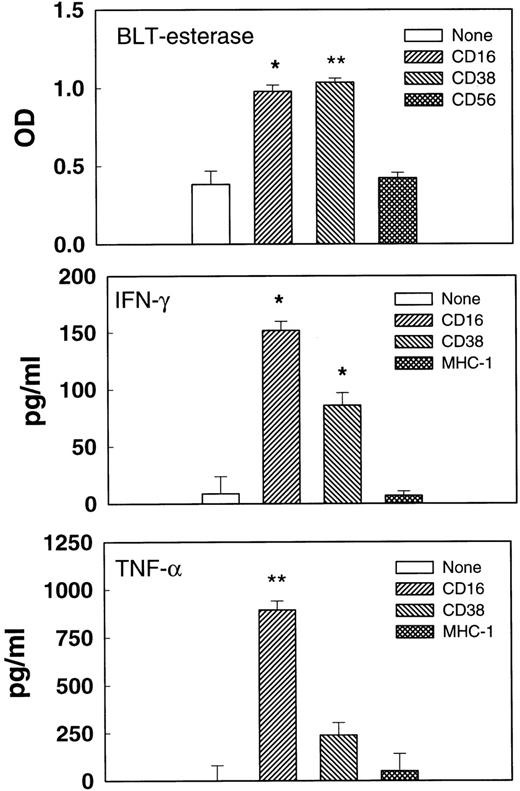
Cell-mediated cytolysis requires the formation of multicellular conjugates between effector and target cells and could involve ligation of several different receptors on the killer cells. To determine whether the cross-linking of CD38 alone could trigger functional responses, activated, purified NK cells were incubated with immobilized MoAbs, and the supernatants were tested for esterase or cytokine release (Fig 6). Immobilized anti-CD38 F(ab′)2 promoted esterase, IFN-γ, and TNF-α release at levels that were clearly above the medium control, but that were usually lower than those induced by anti-CD16 MoAb. By contrast, MoAbs against CD56 and MHC I, 2 molecules expressed on NK cell surfaces, failed to trigger cytokine or esterase release. Esterase release results from the exocytosis of stored cytolytic substances such as perforin and granzymes, whereas cytokine release requires de novo protein synthesis. Thus, the cross-linking of CD38 with immobilized F(ab′)2 triggered 2 fundamentally different processes used in NK effector responses.
Triggered degranulation and cytokine release. Purified IL-2–activated NK cells were incubated in microtiter wells coated with no Ab, anti-CD16 MoAb, anti-CD38 F(ab′)2, anti-CD56 MoAb, or anti–MHC-1 MoAb for 3 (esterase release) or 20 (cytokine release) hours. Supernatants were removed and tested for BLT-esterase (upper panel), TNF- (middle panel), or IFN-γ (lower panel). Cells from different donors were used for measuring esterase and cytokine release. *P < .05. **P < .01 as compared with the no antibody control.
Triggered degranulation and cytokine release. Purified IL-2–activated NK cells were incubated in microtiter wells coated with no Ab, anti-CD16 MoAb, anti-CD38 F(ab′)2, anti-CD56 MoAb, or anti–MHC-1 MoAb for 3 (esterase release) or 20 (cytokine release) hours. Supernatants were removed and tested for BLT-esterase (upper panel), TNF- (middle panel), or IFN-γ (lower panel). Cells from different donors were used for measuring esterase and cytokine release. *P < .05. **P < .01 as compared with the no antibody control.
Lack of CD38-induced Ca2+ mobilization in NK cells.
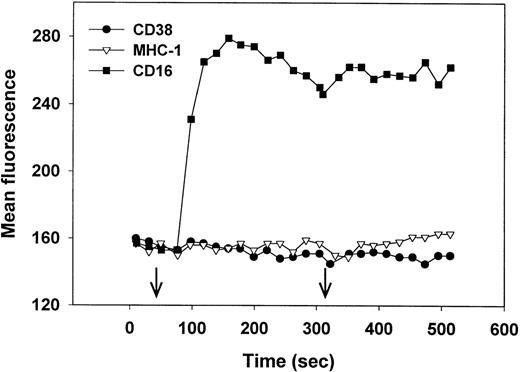
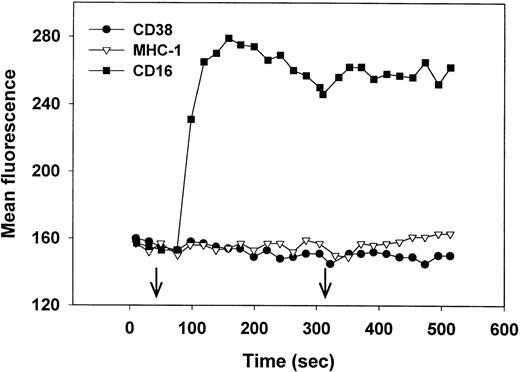
Cross-linking of CD38 induces an increase in intracellular Ca2+ in Jurkat,27 but not in immature B cells28 or MHC-nonrestricted CTLs.30 We therefore asked whether CD38 cross-linking would trigger Ca2+ mobilization in NK cells. Fluo-3–loaded NK cells, isolated from PBLs that mediated high levels of CD38-dependent lysis (Fig 1A) were treated with either anti-CD38 F(ab′)2, anti-CD16 MoAb (positive control), or anti-MHC I MoAb (negative control), followed by further cross-linking (right arrow) with antimouse Ig. As seen in Fig 7, only the anti-CD16 MoAb induced an increase in intracellular Ca2+. Similar results were obtained using biotin-MoAbs and streptavidin as a cross-linker (data not shown). We conclude that CD38 cross-linking in NK cells does not trigger Ca2+ mobilization and that the CD38 and CD16 signaling pathways differ in this regard.
Cross-linking of CD16 but not CD38 induces an increase in intracellular Ca2+. Purified, IL-2–activated NK cells were loaded with Fluo-3, warmed to 37°C, and, at the time indicated by the left arrow, treated with anti-CD38 (IB4) F(ab′)2 (•) anti–MHC-1 (W6/32) MoAb (▿), or anti-CD16 (3G8) MoAb (▪). At the time indicated by the right arrow, antibodies were further cross-linked by the addition of rabbit F(ab′)2 antimouse IgG. Cytolysis mediated by PBLs from this donor is shown in Fig 1A. This experiment has been repeated 4 times, with similar results.
Cross-linking of CD16 but not CD38 induces an increase in intracellular Ca2+. Purified, IL-2–activated NK cells were loaded with Fluo-3, warmed to 37°C, and, at the time indicated by the left arrow, treated with anti-CD38 (IB4) F(ab′)2 (•) anti–MHC-1 (W6/32) MoAb (▿), or anti-CD16 (3G8) MoAb (▪). At the time indicated by the right arrow, antibodies were further cross-linked by the addition of rabbit F(ab′)2 antimouse IgG. Cytolysis mediated by PBLs from this donor is shown in Fig 1A. This experiment has been repeated 4 times, with similar results.
DISCUSSION
It is well known that NK cells lyse tumor and virally infected cells in the absence of Ab, but the receptors used in target cell recognition are poorly defined. In this report, we show that CD38 is a receptor that could potentially contribute to natural cytotoxicity mediated by IL-2–activated NK cells. The signaling capacity of CD38 was demonstrated by using an anti-CD38 MoAb to mimic its interaction with CD31, a ligand for CD38 that is present in especially high amounts on endothelial cells. The choice of target cell is particularly important in these studies. P815 was chosen not only because it expresses FcγR and is of mouse origin (and therefore does not interact with the mouse antihuman receptor antibodies used here), but also because it is relatively resistant to natural cytotoxicity. Natural cytotoxicity is the result of several positive signaling interactions being balanced by negative signals. An NK target such as K562 that expresses low levels of MHC I molecules is lysed so efficiently that no further increases can be seen on the addition of antibody. On the other hand, very high engagement of inhibitory molecules could totally block redirected lysis (eg, see Meyaard et al5). P815 falls between these 2 extremes. In fact, we did attempt to use an endothelial cell line that expresses high levels of CD31 as a target cell (data not shown). Natural cytotoxicity of this target was quite high, and anti-CD38 or anti-CD31 were unable to significantly inhibit lysis, presumably because other triggering molecules contributed to cytotoxic signaling.
The killing capacity of CD38 appeared to be mediated primarily by activated NK cells. Conventional CTLs and unactivated NK cells expressed CD38 on their surfaces and mediated lysis through CD3 and CD16, respectively, but failed to kill through CD38. Because of our separation procedures, we could not establish whether the CD56+ T cells were also triggered by CD38 ligation. This NK-like subset of T cells comprises 2% to 3% of total PBLs and is the primary mediator of CD3 directed lysis in IL-2–activated PBLs. By contrast, the more abundant conventional CTL are CD3+, CD56− and require T-cell receptor (TCR) cross-linking in addition to cytokine for activation.9 Recent studies by Cesano et al30 provide evidence on the role of CD38 in the activation of CD56+, CD3+ T cells. In their experiments, the IB4 anti-CD38 MoAb triggered low levels of cytolysis by the CD56+, CD3+ TALL line and no lysis by unfractionated LAK cells that were 90% T cells. In addition, anti-CD38 failed to induce an increase in intracellular Ca2+ levels in both cell types. However, IB4 did induce low, but significant levels of esterase release by both cell types, and gave robust cytokine responses. Thus, to the extent that the TALL cells and unfractionated LAK represent CD56+ T cells, it appears that this subset of cells behaves similar to NK cells in response to anti-CD38, except that they are less cytotoxic.
The acquisition of CD38 triggering capacity by NK cells during activation with IL-2 was blocked by actinomycin D, an inhibitor of transcription, suggesting that CD38 lytic function depends on the de novo synthesis of 1 or more proteins that are essential components of the lytic pathway. Because actinomycin D had no significant effect on CD38 expression, the newly synthesized proteins must have acted downstream of CD38. There are 2 known pathways by which lymphocytes lyse target cells in 4 hours (the time used in the current study): granule exocytosis and cross-linking of death receptors such as Fas on target cells.44 We demonstrate here that both anti-CD38 and anti-CD16 MoAbs induce degranulation (BLT-esterase release) of NK cells; in addition, the target cells used in this study did not express Fas (CD95) either by functional or FACS analysis (data not shown). Thus, CD16 and CD38 most likely killed through the granule exocytosis pathway. Because actinomycin D had little effect on CD16-mediated lysis on either activated or fresh NK cells, it is unlikely that the putative, IL-2–induced proteins were involved in the degranulation process itself. Thus, it is likely that IL-2 induced the synthesis of proteins that act between CD38 and degranulation. Previously, we demonstrated that CD44, like CD38, acquired cytotoxic triggering capacity during NK cell activation and that this gain in function was actinomycin inhibitable but not dependent on receptor expression.45 Thus, the de novo synthesis of proteins that link adhesion molecules to the killing machinery may be an important component of NK cell-mediated lysis in general.
In the Jurkat T-cell line, CD38 ligation induces the tyrosine phosphorylation of several proteins used in TCR signaling, including ζ, ZAP-70, and PLC-γ1, as well as proteins in the Raf-1/MAP kinase pathway.26 Cross-linking of CD38 on Jurkat cells also induces an increase in intracellular Ca2+ and apoptosis, both of which require an intact TCR.27 CD16 ligation on NK cells initiates a signaling pathway that mirrors that of the TCR in that it induces phosphorylation of ζ and homologous γ subunits, phosphorylation of ZAP-70 and syk,1 and an increase in intracellular Ca2+.46 In addition, it has been reported that CD16 associates with CD38 on NK cell surfaces.36 Thus, it seemed possible that the CD38 and CD16 triggering pathways on NK cells might merge either at the level of CD16 itself or downstream of CD16. However, the fact that CD38 cross-linking does not induce an increase in intracellular Ca2+, whereas CD16 ligation does, suggests that the 2 triggering molecules use different signaling pathways.
The number of adhesion molecules that can serve as cytotoxic triggers on NK cells, which now include CD2, CD38, CD44, and CD69, continues to increase, suggesting that the process of natural cytotoxicity may not be triggered by specialized NK receptors, but may instead reflect the level of expression of downstream proteins that confer triggering capacity on commonly expressed receptors. This mechanism of triggering might be fundamental to the cellular arm of innate immunity, but would be avoided by cellular mediators of acquired immunity, in which it would override a highly specific interaction with one that was less specific. Thus, it is not surprising that CD38 and CD44 trigger lysis on NK cells, but fail to do so when expressed on bulk CTLs generated from PBLs15 (although some CTL clones mediate CD44-directed lysis47,48). It has been shown that CD44 is a cytotoxic trigger in neutrophils,49 but it remains to be established how extensively cellular mediators of innate immunity use adhesion molecules to initiate cytolytic responses.
F.M. was supported by AIRC (Milan, Italy), TELETHON (Rome, Italy), and by the AIDS and TB Projects (Higher Institute of Medicine, Rome, Italy).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to David M. Segal, PhD, Bldg 10, Room 4B36, NIH, Bethesda, MD 20892-1360.