Abstract
Wiskott-Aldrich syndrome (WAS) is an X-linked recessive disorder characterized by thrombocytopenia, eczema, and a progressive deterioration of immune function. WAS is caused by mutations in an intracellular protein, WASP, that is involved in signal transduction and regulation of actin cytoskeleton rearrangement. Because immune dysfunction in WAS may be due to an accelerated destruction of lymphocytes, we examined the susceptibility to apoptosis of resting primary lymphocytes isolated from WAS patients in the absence of exogenous apoptogenic stimulation. We found that unstimulated WAS lymphocytes underwent spontaneous apoptosis at a greater frequency than unstimulated normal lymphocytes. Coincident with increased apoptotic susceptibility, WAS lymphocytes had markedly attenuated Bcl-2 expression, whereas Bax expression did not differ. A negative correlation between the frequency of spontaneous apoptosis and the level of Bcl-2 expression was demonstrated. These data indicate that accelerated lymphocyte destruction by spontaneous induction of apoptosis may be one pathogenic mechanism by which the progressive immunodeficiency in WAS patients develops.
WISKOTT-ALDRICH SYNDROME (WAS) is a rare X-linked recessive disorder.1,2 Affected males are clinically characterized by eczema, thrombocytopenia with platelets of reduced size, immunodeficiency, and an increased susceptibility to hematopoietic malignancy and autoimmunity (Remold-O’Donnell et al3 and references therein). WAS patients suffer from chronic and recurrent opportunistic and viral infections as a consequence of immunologic abnormalities. A progressive deterioration of T-lymphocyte function in affected children accompanies the development of T lymphopenia by 6 years of age.4Cell-mediated immunity is further compromised by poor macrophage and neutrophil motility and chemotactic responses.4-7 WAS patients commonly have low isohemagglutinin titers and a depressed response to polysaccharide antigens3; but, their impaired humoral immunity has been at least partially attributed to T-lymphocyte dysfunction.8 WAS T lymphocytes have diminished proliferative responses to mitogens, some specific antigens, and allogeneic stimulation.3 Moreover, WAS T lymphocytes fail to proliferate or upregulate interleukin-2 gene expression in response to treatment with immobilized anti-CD3 antibody.9,10Deformation and deregulation of the actin cytoskeleton is likely to be the basis for many of the clinical defects associated with WAS, particularly immune dysfunction.3,10,11 WAS lymphocytes are frequently morphologically abnormal, with irregular and bulbous cellular projections,10,12 a paucity of microvilli,13 and a poorly delineated actin cortex.10 CD3-mediated stimulation of WAS T-lymphoblastoid cell lines results in abnormal actin polymerization, marked by the absence of specific cytoskeleton rearrangements that occur in normal T-lymphoblastoid cell lines.10 Epstein-Barr virus–transformed B-cell lines from WAS patients also have marked abnormalities in actin distribution and polymerization.11
The WAS gene was mapped to the short arm of the X chromosome at Xp11.2214 and identified in 1994.15 The gene encodes a 66-kD intracellular protein, WASP, that is expressed exclusively in blood cells15,16 throughout hematopoiesis.17 More than 138 unique mutations to WASP have been described,18 which include frameshifts and substitutions that generally nullify expression by causing mRNA instability and protein truncation.11,19-21 There is considerable variation in WAS severity; even members of the same kindred may have markedly different phenotypes,22 possibly indicating polygenic or environmental contributions. WAS severity may be partially determined by the level at which WASP is stably expressed.11 19-21
The function of WASP is unknown; however, it has been surmised that WASP is involved in the regulation of actin polymerization and actin cytoskeleton organization from observations that WAS lymphocytes, platelets, granulocytes, and monocytes are defective in regulating the cortical actin cytoskeleton (Remold-O’Donnell et al3 and references therein). In support of this presumption, N-WASP, a ubiquitous mammalian homolog of WASP, has been demonstrated to directly induce actin depolymerizaton and actin cytoskeleton reorganization in nerve growth factor-stimulated cells.23 Although WASP apparently lacks catalytic activity, the protein colocalizes with actin and interacts with WIP24 and PSTPIP,25 2 proteins that regulate actin polymerization. The Rho family small guanosine triphosphatases (GTPases), Cdc42 and Rac, which have been shown to specifically control actin cytoskeleton reorganization,26 interact with WASP.27-30 WASP also associates with numerous other proteins, including various tyrosine kinases (Btk, Itk, Tec, Fyn, and c-Src), adaptor proteins (Nck and Grb2), and phospholipase Cγ1, all of which are involved in lymphoid signal transduction.31-36 Therefore, it has been proposed that WASP functions as a molecular scaffold28 that docks and aligns other proteins for more specific interaction.37 Thus, WASP may link components of the cytoskeleton with key signal transduction elements to integrate signaling in response to various intrinsic or extrinsic stimuli to regulate actin cytoskeleton reorganization.
A WASP deficiency or the expression of dysfunctional WASP is probably detrimental to the development and survival of hematopoietic cell lineages.3 A uniformly nonrandom pattern of X-chromosome inactivation in all blood cells from WAS obligate heterozygous carriers and platelet loss in WAS patients suggests that mutations to WASP probably confer a growth and/or survival disadvantage to affected cells.3 Consequently, we reasoned that the development of lymphopenia and other progressive immune defects associated with WAS may be caused by an inherently decreased potential for survival and the accelerated destruction of peripheral lymphocytes.
In this study, we compared the levels of spontaneous apoptosis in unstimulated resting lymphocytes isolated from WAS patients and from normal, healthy controls. We found that WAS lymphocytes underwent spontaneous apoptosis at a greater frequency than did normal lymphocytes. Bcl-2 family members regulate the onset of apoptosis, acting as either agonists or inhibitors of programmed cell death.38 Because the deregulation of apoptosis in WAS lymphocytes might be caused by aberrant expression of one or more of the Bcl-2 family members, we determined that the level of Bcl-2 expression in WAS lymphocytes was attenuated. We suggest that the accelerated destruction of lymphocytes by spontaneous apoptosis could account for the progressive deterioration of immune function in WAS patients.
MATERIALS AND METHODS
Patient samples.
Samples from 5 male patients previously diagnosed with WAS were used in this study. At the time of analysis, patients no. 1 and 2 were 2.5 and 4 years of age, respectively. Patients no. 3 and 4 are siblings and were 4.5 and 14 years of age, respectively. Patient no. 5 was 14 months of age. Diagnosis of WAS was initially based on family history and presentation of immunodeficiency with recurrent infection, thrombocytopenia, platelets with reduced volume, and eczema. To define the WAS phenotype of the patients, clinical scores were assigned to each according to published recommendations.18 21 Patients no. 1, 2, 3, 4, and 5 were assigned clinical scores of 4, 4, 3, 3, and 3, respectively. The genotypes of 4 of 5 patients have been determined. Patient no. 1 has a point mutation, C290T, resulting in an amino acid substitution of R86C in exon 2. The genotype of patient no. 2 was not determined. Patients no. 3 and 4, the siblings, have a deletion mutation in exon 8 (John Bastian, personal communication, July 1999). Patient no. 5 has a splice site mutation (4-bp deletion in intron 8), resulting in the deletion of exon 8; patient no. 5 does not express WASP. Patients no. 1, 2, and 5 were treated at Childrens Hospital (CHLA) in Los Angeles, CA; each has since received bone marrow transplantations. Patients no. 3 and 4 were treated at Children’s Hospital in San Diego, CA. These studies were performed in accord with protocols approved by the Committee on Clinical Investigations of the Institutional Review Board at CHLA. None of the patients had been splenectomized. Normal controls (8) were obtained from either healthy volunteer adult donors (5) or healthy pediatric patients (3) at CHLA. No significant difference between the normal pediatric and normal adult means was noted (P = .978). Whenever possible, patient and normal blood was collected and processed under paired conditions. For the comparison of normal pediatric and normal adult blood, specimens were collected and processed under paired conditions.
Isolation and in vitro treatment of peripheral blood lymphocytes (PBL).
Peripheral blood was collected from WAS patients or normal, healthy individuals in heparinized Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ). Within 8 hours, mononuclear cells were isolated from whole blood by Ficoll-Hypaque (Pharmacia Biotech, Piscataway, NJ) density gradient centrifugation. Mononuclear cells were washed in Hanks’ balanced salt solution (HBSS; BioWhittaker, Walkersville, MD) and resuspended in R-10 medium (RPMI-1640 supplemented with 10% heat-inactivated human A+ serum [CHLA Blood Bank, Los Angeles, CA], 2 mmol/L L-glutamine [Gemini BioProducts, Calabasas, CA], 1 × 10−6 mol/L 2-mercaptoethanol [Sigma, St Louis, MO], and 1× penicillin-streptomycin [Gemini BioProducts]). Cells were plated at a density of 5 × 106 cells/mL in 100-mm tissue culture dishes (Corning, Corning, NY) and placed in a 37°C humidified incubator conditioned with 5% CO2 for 1 hour. Nonadherent PBL were collected and resuspended in R-10. PBL were plated at a density of 0.5 × 106 cells/mL in 24-well flat-bottomed dishes (Costar, Cambridge, MA) and maintained in a humidified atmosphere with 5% CO2 at 37°C for up to 10 days. Some experiments were performed after maintaining PBL in R-10 containing 10% heat-inactivated fetal calf serum (Summit Biotechnology, Fort Collins, CO) instead of human serum, but no differences in the apoptotic frequencies of PBL were apparent. PBL were not stimulated in vitro.
Detection of apoptosis.
Either of 2 methods, terminal deoxynucleotidyl transferase-mediated dUTP nicked end labeling (TUNEL) or annexin V-labeling, was used to mark apoptotic cells. For TUNEL,39 cells were harvested and washed in 3 mL phosphate-buffered saline (PBS; Sigma). At least 105 cells were stained on ice and in the dark with fluorochrome-conjugated antihuman antibodies recognizing specific cell surface markers to be used in immunophenotyping. Incubations with the antibodies were performed in PBS containing 0.1% human intravenous Ig (Sandoz Pharmaceuticals, East Hanover, NJ) for 15 minutes. All allophycocyanin- and phycoerythrin-conjugated antibodies were used according to the supplier’s recommendations (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA). Stained cells were washed in 3 mL PBS and fixed in 0.5 mL 2% paraformaldehyde (Sigma) on ice for 15 minutes. Cells were washed in 3 mL PBS and permeabilized in 0.5 mL PBS containing 0.5% Tween-20 (Sigma) and 0.2% bovine serum albumin (BSA; fraction V; Sigma). Samples were mixed gently and placed on ice for 15 minutes. Cells were washed in 3 mL PBS and resuspended in 50 μL TUNEL reaction mix containing 5 U terminal deoxynucleotidyl transferase (TdT; Promega, Madison, WI), 1× TdT reaction buffer (Promega; containing 100 mmol/L cacodylate buffer [pH 6.8], 1 mmol/L cobalt chloride, and 0.1 mmol/L dithiothreitol), and 10 μmol/L biotin-16-dUTP (Boehringer Mannheim Biochemicals, Indianapolis, IN). Cells were incubated in the reaction mix at 37°C for 30 minutes. Cells were washed in 3 mL PBS and resuspended in 100 μL labeling solution containing 4× SSC, 0.1% Triton X-100 (Sigma), 10% nonfat dry milk (Carnation, Glendale, CA), and 0.1% sodium azide (Sigma). Ten microliters of avidin-fluorescein (BDIS) was mixed with the cells. Samples were kept at room temperature for 30 minutes in the dark. Cells were washed in 3 mL PBS containing 0.1% Triton X-100. Cells were resuspended in 0.5 mL PBS containing 5 μg/mL propidium iodide (PI; Sigma) and 0.1% DNase heat-inactivated RNase A (Sigma). Negative control samples were treated in an identical manner except that TdT was omitted from the TUNEL reaction mix. Labeled cells were analyzed promptly by flow cytometry.
Annexin V-labeling was used to confirm the results of TUNEL.40 Briefly, cells were harvested and washed in 3 mL PBS. Cells were stained with immunophenotyping antibodies as described above. Cells were then washed in 3 mL PBS and resuspended at a concentration of 1 × 106 cells/mL in 1 × annexin V binding buffer (all reagents are supplied with the Apoptosis Detection Kit; R&D Systems, Minneapolis, MN). One hundred microliters (105) of cells was transferred to another tube. Ten microliters of fluorescein-conjugated annexin V (10 μg/mL) and 10 μL PI (50 μg/mL) were mixed with the cells. Samples were kept at room temperature for 15 minutes in the dark. Four hundred microliters of 1× binding buffer was used to dilute the labeled cells and analysis was performed by flow cytometry within 1 hour. Controls were prepared according to the recommendations of the supplier.
Determination of relative Bcl-2 expression levels.
The relative level of Bcl-2 expression in PBL was determined by intracellular staining using a hamster antihuman monoclonal antibody, an assay similar to that used by von Freeden-Jeffry et al.41 Briefly, at least 105 cells were harvested and stained with immunophenotyping antibodies, as described above. Cells were washed in 3 mL PBS and fixed in 0.5 mL 2% paraformaldehyde for 15 minutes on ice. Cells were washed in 3 mL PBS and permeabilized in 200 μL PBS containing 0.3% saponin (Sigma) and 0.2% BSA. All subsequent steps were performed in this buffer. Samples were placed on ice for 15 minutes. Two micrograms of anti–Bcl-2 antibody (clone 6C8; Pharmingen, San Diego, CA) was mixed with the cells. The negative control for measuring background fluorescence was prepared in a similar manner, except that 2 μg hamster IgG isotype control polyclonal antibody (Pharmingen) was added to the cells. Samples were placed on ice for 20 minutes. Cells were washed in 3 mL PBS and resuspended in 100 μL of the buffer. One microgram of fluorescein-conjugated mouse antihamster IgG antibody (clone G70-204; Pharmingen) was mixed with the cells. Samples were placed on ice in the dark for 20 minutes. Cells were washed in 3 mL PBS and resuspended in 0.5 mL 2% paraformaldehyde. Samples were stored at 4°C until analysis was performed by flow cytometry.
Flow cytometry and data analysis.
Analysis of TUNEL samples was performed using a FACSVantage flow cytometer (BDIS) equipped with an argon laser tuned to 488 nm and a helium-neon (HeNe) laser tuned to 633 nm. A 610 nm short-pass splitter was used to divert PI fluorescence to FL-3. After electronic compensation, FL-3 fluorescence was measured using both linear and logarithmic amplification; FL-1 (fluorescein), FL-2 (phycoerythrin), and FL-4 (allophycocyanin) fluorescences were measured using logarithmic amplification. The flow rate was not permitted to exceed 200 events per second. The data from at least 5 × 104events were collected for analysis of freshly isolated cells. For all other experiments, the data from at least 104 events were collected. The data were analyzed using CELLQuest software (BDIS). For analysis of TUNEL data, fragmented cells and debris were electronically excluded. Another region was set to electronically eliminate multiplet events from the analysis. A negative control sample (no TdT added) was used to establish TUNEL-negative and -positive regions, such that at least 99% of the events were in the lower TUNEL-negative region. Background events were then subtracted from the TUNEL-positive events.
All other flow cytometry was performed using a FACSCalibur instrument (BDIS) fitted with both argon and HeNe lasers. The data from at least 104 events were collected. Analysis of fluorescein-annexin V-labeled cells was performed according to the recommendations provided with the Apoptosis Detection Kit (R&D Systems). For analysis of annexin V binding data, fragmented cells and debris were electronically excluded. For analysis of Bcl-2 expression, a region was set to include the enriched lymphocyte population, excluding all other cells and debris. The same region was used for analysis of both normal and WAS lymphocytes so that differences in cell size and granularity, and possible differences in mitochondrial copy number on a per cell basis, were not reflected in the values reported. Background fluorescence was measured using a negative isotype control. The mean fluorescence intensity (MFI) reported for a given sample is the difference between the sample value and the background value.
Statistical analysis of results.
Number Crunching Statistical Systems software (Dr Gerry L. Hintze, Kayszille, UT) was used for the statistical analysis of data. Where applicable, an unbalanced repeated measures analysis of variance (ANOVA) was used to determine the significance of differences between normal and WAS sample values. This method eliminates bias possibly associated with analysis of unbalanced repeated measures. The TUNEL data were also analyzed as a set of randomly chosen duplicate repeated measures to test the validity of the first method of analysis. This analysis showed that the first method was indeed valid, confirming the statistical significance of the data. A probability level (P) of ≤.05 is termed significant. An analysis of correlation between Bcl-2 expression and level of apoptosis was performed using logarithmic transformation. A correlation coefficient (r2) was determined using the nonparametric Spearman method. Increases in the frequency of apoptosis over time were determined by linear regression.
RESULTS
WAS PBL undergo spontaneous apoptosis in vitro at a greater rate than normal PBL.
The levels of spontaneous apoptosis in unstimulated, resting PBL were determined in this study. To compare the frequency of apoptosis in WAS PBL and normal PBL, we determined the fraction of cells undergoing apoptosis by 2 quantitative methods. TUNEL39 and annexin V-labeling40 measure oligonucleosomal DNA degradation and externalization of phosphatidylserine, respectively, 2 hallmarks of apoptosis.42 43 Flow cytometry was used to quantify marked cells undergoing apoptosis immediately after PBL isolation or after a period of in vitro incubation. Importantly, PBL were not treated with cytokines or any other mitogenic or antigenic stimuli and neither was an extrinsic stimulus used to induce apoptosis.
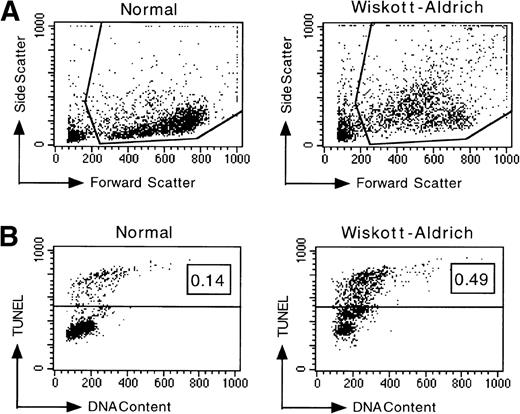
Representative TUNEL data shown in Fig 1show that a larger fraction of cells isolated from a WAS patient were undergoing spontaneous apoptosis compared with cells from a normal control at the time of analysis. Another prominent characteristic of apoptotic cells detectable by flow cytometry is cytoplasmic shrinkage, resulting in decreased forward light scatter and increased side light scatter, caused by increases in cellular density during volume reduction.44 A direct comparison of the light scattering properties of normal PBL with those isolated from a WAS patient showed that a relatively larger subpopulation of WAS PBL had apoptotic characteristics (Fig 1A). The results of an analysis of freshly isolated PBL performed immediately after isolation are shown in Fig 1C. The mean levels of apoptotic DNA fragmentation in freshly isolated PBL from the group of 5 WAS patients and the group of 8 normal individuals (5 adults and 3 children) differed significantly (P = .0123). WAS PBL underwent spontaneous apoptosis at a 10-fold greater frequency immediately after isolation. Considerable variation in the levels of spontaneous apoptosis in freshly isolated PBL from different WAS patients was evident, possibly reflecting genotypic differences. However, differences between the siblings are also noted and suggest that polygenic factors may determine the susceptibility to apoptosis in WAS PBL. Therefore, these data suggest that WAS PBL, as compared with normal PBL, may have a lower threshold of susceptibility to apoptogenic signals.
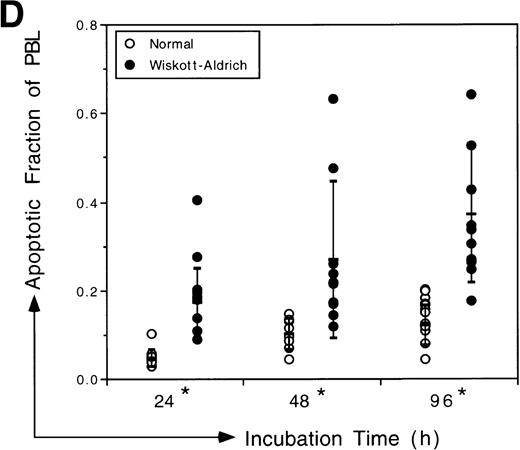
Analysis of apoptotic lymphocytes isolated from WAS patients. The levels of apoptosis were measured using TUNEL immediately after isolation or after incubation in vitro. PBL were not stimulated. (A) WAS lymphocytes had increased side scatter and decreased forward scatter, characteristics of apoptosis, after 4 days of incubation in vitro relative to normal lymphocytes. (B) Representative data acquired after TUNEL indicate that a higher fraction of WAS lymphocytes were undergoing apoptosis relative to normal, healthy donor lymphocytes after 4 days of incubation in vitro. The fraction of TUNEL-positive cells is indicated in the upper right corner. (C) WAS lymphocytes underwent apoptosis at a greater frequency than normal lymphocytes. Levels of apoptosis were measured immediately after isolation of PBL from WAS patients or from normal individuals using the TUNEL assay. N, the mean value for a group of 8 different normal controls (3 children and 5 adults). W, the mean value for a group of 5 different WAS patients. 1 through 5, the mean values for each individual WAS patient. Patient no. 5 was analyzed twice in 2 independent experiments (with 6 and 3 replicates); all other patients were analyzed once in single experiments (with 6 replicates). An unbalanced repeated measures analysis of variance (ANOVA) showed that the values for patients no. 2, 3, 4, and 5 differed significantly from the mean normal value (P ≤ .0001). The mean value for the group of 5 WAS patients (W) also differed significantly from the mean normal (P = .0123). n, the number of different samples; *, statistical significance. (D) WAS lymphocytes were more susceptible to apoptosis than normal lymphocytes after in vitro incubations of 24, 48, and 96 hours. Considerable variability in the apoptotic susceptibility of PBL from different WAS patients was apparent. Mean levels of apoptosis are reported in Table 1. Patient no. 1 was analyzed once in a single experiment, patient no. 2 was analyzed 3 times in 3 independent experiments, and patients no. 3, 4, and 5 were analyzed twice in 2 independent experiments. Results from 8 different normal controls (3 children and 5 adults) are shown. The number of repeated measures made in each experiment was varied, ranging from 2 to 6 (generally 4), depending on the number of PBL isolated from the blood samples. An unbalanced repeated measures ANOVA showed that differences between the groups, WAS and normal, are significant at 24, 48, and 96 hours (P = .000181, .00283, and .000190, respectively). *Statistical significance.
Analysis of apoptotic lymphocytes isolated from WAS patients. The levels of apoptosis were measured using TUNEL immediately after isolation or after incubation in vitro. PBL were not stimulated. (A) WAS lymphocytes had increased side scatter and decreased forward scatter, characteristics of apoptosis, after 4 days of incubation in vitro relative to normal lymphocytes. (B) Representative data acquired after TUNEL indicate that a higher fraction of WAS lymphocytes were undergoing apoptosis relative to normal, healthy donor lymphocytes after 4 days of incubation in vitro. The fraction of TUNEL-positive cells is indicated in the upper right corner. (C) WAS lymphocytes underwent apoptosis at a greater frequency than normal lymphocytes. Levels of apoptosis were measured immediately after isolation of PBL from WAS patients or from normal individuals using the TUNEL assay. N, the mean value for a group of 8 different normal controls (3 children and 5 adults). W, the mean value for a group of 5 different WAS patients. 1 through 5, the mean values for each individual WAS patient. Patient no. 5 was analyzed twice in 2 independent experiments (with 6 and 3 replicates); all other patients were analyzed once in single experiments (with 6 replicates). An unbalanced repeated measures analysis of variance (ANOVA) showed that the values for patients no. 2, 3, 4, and 5 differed significantly from the mean normal value (P ≤ .0001). The mean value for the group of 5 WAS patients (W) also differed significantly from the mean normal (P = .0123). n, the number of different samples; *, statistical significance. (D) WAS lymphocytes were more susceptible to apoptosis than normal lymphocytes after in vitro incubations of 24, 48, and 96 hours. Considerable variability in the apoptotic susceptibility of PBL from different WAS patients was apparent. Mean levels of apoptosis are reported in Table 1. Patient no. 1 was analyzed once in a single experiment, patient no. 2 was analyzed 3 times in 3 independent experiments, and patients no. 3, 4, and 5 were analyzed twice in 2 independent experiments. Results from 8 different normal controls (3 children and 5 adults) are shown. The number of repeated measures made in each experiment was varied, ranging from 2 to 6 (generally 4), depending on the number of PBL isolated from the blood samples. An unbalanced repeated measures ANOVA showed that differences between the groups, WAS and normal, are significant at 24, 48, and 96 hours (P = .000181, .00283, and .000190, respectively). *Statistical significance.
We examined the effects of maintaining isolated PBL in vitro for varying periods in medium containing 10% human A+ serum, which minimizes lymphocyte activation. The levels of spontaneous apoptosis in WAS PBL (5 patients) and normal PBL (5 adults and 3 children) differed significantly after incubations of 24, 48, and 96 hours (Fig 1D). We observed a steady increase in the fraction of WAS PBL undergoing spontaneous apoptosis over time. In contrast, the frequency of spontaneous apoptosis in normal PBL leveled off after 48 hours in vitro. Over the entire duration of the incubation, the difference between the increases in WAS and normal PBL tendency to undergo apoptosis was statistically significant (P < .0001). We observed that most of the cells undergoing apoptosis were resting in either G0 or G1 of the cell cycle (data not shown).
Annexin V-labeling confirmed and extended the results obtained using TUNEL to mark cells undergoing spontaneous apoptosis. Apoptotic cells are distinguished by flow cytometry from live and dead cells by fluorescein-conjugated annexin V binding and exclusion of the vital dye, PI, respectively (Fig 2A). As shown in Fig 2B, over the entire duration of incubation, WAS PBL from the 4 patients (no. 1 through 4) analyzed underwent spontaneous apoptosis at a significantly greater frequency than normal PBL (P = .025).
Annexin V staining confirmed that WAS lymphocytes underwent apoptosis at a greater frequency than normal lymphocytes. Representative data indicate that a higher percentage of WAS lymphocytes had externalized phosphatidylserine and bound annexin V compared with normal lymphocytes. Furthermore, a higher percentage of lymphocytes from WAS patients were inviable, no longer excluding PI, relative to lymphocytes from normal individuals. Apoptotic cells are represented by events in the lower right-hand quadrant, with viable and dead cells depicted by events in the lower left-hand and upper right-hand quadrants, respectively. The fraction of cells undergoing apoptosis and the fraction of dead cells are indicated at the right of each plot. (B) Both methods of analysis, annexin V staining and TUNEL, indicated that WAS lymphocytes underwent apoptosis at a greater frequency than normal lymphocytes. Samples from 4 different WAS patients (no. 1 through 4) and 6 different normal controls were analyzed. Statistical analysis by an unbalanced repeated measures ANOVA showed that the differences between WAS and normal levels of apoptosis as measured using annexin V were significant after in vitro incubations of 48 and 96 hours, as indicated by the asterisks (P = .0013 and .0181, respectively). TUNEL data (shown in Fig 1) is duplicated for easy comparison.
Annexin V staining confirmed that WAS lymphocytes underwent apoptosis at a greater frequency than normal lymphocytes. Representative data indicate that a higher percentage of WAS lymphocytes had externalized phosphatidylserine and bound annexin V compared with normal lymphocytes. Furthermore, a higher percentage of lymphocytes from WAS patients were inviable, no longer excluding PI, relative to lymphocytes from normal individuals. Apoptotic cells are represented by events in the lower right-hand quadrant, with viable and dead cells depicted by events in the lower left-hand and upper right-hand quadrants, respectively. The fraction of cells undergoing apoptosis and the fraction of dead cells are indicated at the right of each plot. (B) Both methods of analysis, annexin V staining and TUNEL, indicated that WAS lymphocytes underwent apoptosis at a greater frequency than normal lymphocytes. Samples from 4 different WAS patients (no. 1 through 4) and 6 different normal controls were analyzed. Statistical analysis by an unbalanced repeated measures ANOVA showed that the differences between WAS and normal levels of apoptosis as measured using annexin V were significant after in vitro incubations of 48 and 96 hours, as indicated by the asterisks (P = .0013 and .0181, respectively). TUNEL data (shown in Fig 1) is duplicated for easy comparison.
Both WAS B and T lymphocytes are more susceptible to apoptosis than normal B and T lymphocytes.
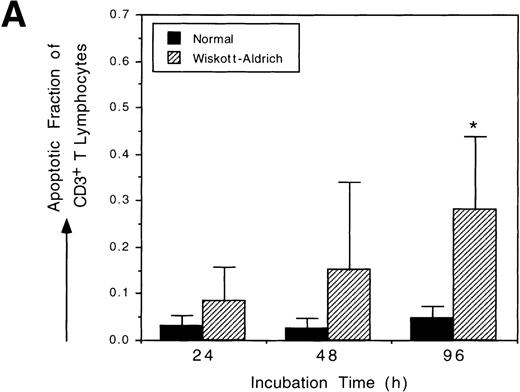
WAS patients have defects in both humoral and cell-mediated immunity; thus, we sought to determine if both T and B lymphocytes have increased susceptibility to spontaneous apoptosis. We measured the frequencies of spontaneous apoptosis in resting T and B lymphocytes isolated from WAS patients (no. 1 through 4) and normal controls after incubation in vitro. Figure 3A shows that unstimulated WAS CD3+ T lymphocytes undergo spontaneous apoptosis at a greater rate than do unstimulated normal CD3+ T lymphocytes. In vitro incubation augmented the level of spontaneous apoptosis in unstimulated T lymphocytes. Over time, the frequency of spontaneous apoptosis in WAS T lymphocytes increased more rapidly than occurred normally (P = .024). Unstimulated WAS CD19+ B lymphocytes also underwent spontaneous apoptosis at an increasing frequency during in vitro incubation (Fig 3B). The mean level of spontaneous apoptosis in WAS B lymphocytes was higher than in normal B lymphocytes at each time point. This trend was consistent, although statistical significance could not be demonstrated due to the presence of very low numbers of circulating B lymphocytes in our WAS patients. In vitro incubation also augmented spontaneous apoptosis in unstimulated WAS B lymphocytes by 2-fold over normal.
Both T and B lymphocytes from WAS patients are more susceptible to apoptosis than T and B lymphocytes from normal individuals. Analysis of apoptosis was performed using TUNEL after staining with immunophenotyping cell surface markers. Four different WAS patients (no. 1 through 4) and 4 different normal controls were analyzed. (A) CD3+ T lymphocytes from WAS patients underwent apoptosis at a greater frequency than did normal lymphocytes. Statistical significance was shown after 96 hours of incubation in vitro (P = .0239) by an unbalanced repeated measures ANOVA. (B) WAS B lymphocytes (CD19+) are more susceptible to apoptosis than were normal B lymphocytes. The mean levels of apoptosis were clearly different, although, because of small cell numbers, the standard errors were too high to show statistical significance. The scales used in (A) and (B) are identical.
Both T and B lymphocytes from WAS patients are more susceptible to apoptosis than T and B lymphocytes from normal individuals. Analysis of apoptosis was performed using TUNEL after staining with immunophenotyping cell surface markers. Four different WAS patients (no. 1 through 4) and 4 different normal controls were analyzed. (A) CD3+ T lymphocytes from WAS patients underwent apoptosis at a greater frequency than did normal lymphocytes. Statistical significance was shown after 96 hours of incubation in vitro (P = .0239) by an unbalanced repeated measures ANOVA. (B) WAS B lymphocytes (CD19+) are more susceptible to apoptosis than were normal B lymphocytes. The mean levels of apoptosis were clearly different, although, because of small cell numbers, the standard errors were too high to show statistical significance. The scales used in (A) and (B) are identical.
Bcl-2 expression is attenuated in WAS PBL.
Because Bcl-2 family members have been shown to regulate apoptosis in hematopoietic cells, the accelerated spontaneous apoptosis in WAS PBL could result from deregulated expression of regulatory factors, such as Bcl-2,45 that repress apoptosis. We examined the relative levels of Bcl-2 expression in WAS and normal PBL by measuring the MFI of cells stained indirectly with anti–Bcl-2 antibody in situ. Measurements were made either immediately after isolation of PBL (Table 1) or after in vitro incubation (Table 2). WAS PBL from 5 different patients had a significantly reduced level of Bcl-2 compared with normal PBL from 8 different individuals (Fig 4). The relative levels of Bcl-2 expressed in isolated PBL did not change significantly over time during in vitro incubation. Wide ranges in Bcl-2 expression are evident, both in PBL isolated from WAS patients and from normal controls. Analysis showed that PBL from patients no. 2 and 5 had Bcl-2 levels that differed significantly from normal (P = .00477 and .0254, respectively) at the initial time point. There was no significant difference between the levels of Bax46 expression in WAS and normal PBL (data not shown). Therefore, we have demonstrated that Bcl-2, an inhibitor of apoptosis, but not Bax, an activator of apoptosis, is aberrantly expressed in WAS PBL relative to normal PBL.
Bcl-2 expression in WAS lymphocytes is attenuated as compared with Bcl-2 expression in normal lymphocytes. MFI of the Bcl-2 signal was measured either immediately after isolation or after in vitro incubation for 24 or 48 hours. Samples from 5 different WAS patients and 7 different normal controls were analyzed. An unbalanced repeated measures ANOVA showed that the differences in Bcl-2 expression levels between the 2 groups, normal and WAS, at each time point (0, 24, and 48 hours) are significant (P = .0233, .00985, and .0192, respectively).
Bcl-2 expression in WAS lymphocytes is attenuated as compared with Bcl-2 expression in normal lymphocytes. MFI of the Bcl-2 signal was measured either immediately after isolation or after in vitro incubation for 24 or 48 hours. Samples from 5 different WAS patients and 7 different normal controls were analyzed. An unbalanced repeated measures ANOVA showed that the differences in Bcl-2 expression levels between the 2 groups, normal and WAS, at each time point (0, 24, and 48 hours) are significant (P = .0233, .00985, and .0192, respectively).
Correlation between the level of Bcl-2 expression and susceptibility to induction of spontaneous apoptosis in PBL.
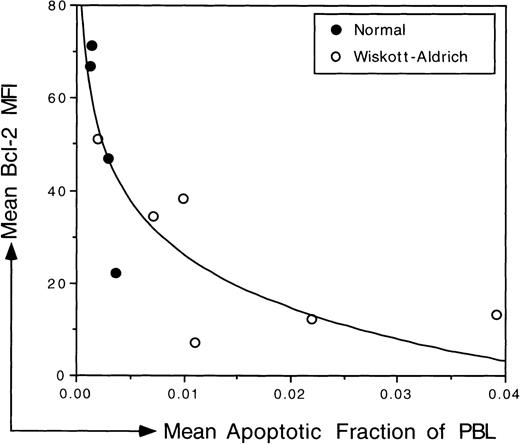
The relationship between Bcl-2 expression and susceptibility to induction of spontaneous apoptosis was studied in freshly isolated PBL from 5 different WAS patients and from 4 normal, healthy individuals. Relative Bcl-2 expression was measured immediately after isolation without an extended in vitro incubation. At the same time and using the same sample, we measured the level of apoptosis in the PBL population after TUNEL. As illustrated in Fig 5, there was an inverse relationship between Bcl-2 expression and the susceptibility to induction of spontaneous apoptosis (r2 = .744). Unstimulated normal PBL that expressed relatively high levels of Bcl-2 were less susceptible to induction of spontaneous apoptosis; the frequency of apoptosis in normal populations was reduced when compared with the frequency of apoptosis in WAS populations. In contrast, unstimulated WAS PBL that expressed relatively low levels of Bcl-2 tended to be more susceptible to induction of spontaneous apoptosis than were normal PBL.
Correlation between the relative level of Bcl-2 expression and the susceptibility to spontaneous apoptosis in PBL isolated from WAS patients and normal controls. The mean value for replicate measures (2 to 3) of the MFI of the Bcl-2 signal was plotted on the y-axis. The mean fraction of PBL undergoing spontaneous apoptosis was determined using TUNEL and was plotted on the x-axis. Both measurements were made immediately after isolation of PBL using the same sample. Samples from 5 different WAS patients were analyzed; patient no. 5 was analyzed twice in 2 independent experiments. The data were fitted logarithmically. There is an inverse correlation between Bcl-2 expression and the level of susceptibility to apoptosis (r2 = .744).
Correlation between the relative level of Bcl-2 expression and the susceptibility to spontaneous apoptosis in PBL isolated from WAS patients and normal controls. The mean value for replicate measures (2 to 3) of the MFI of the Bcl-2 signal was plotted on the y-axis. The mean fraction of PBL undergoing spontaneous apoptosis was determined using TUNEL and was plotted on the x-axis. Both measurements were made immediately after isolation of PBL using the same sample. Samples from 5 different WAS patients were analyzed; patient no. 5 was analyzed twice in 2 independent experiments. The data were fitted logarithmically. There is an inverse correlation between Bcl-2 expression and the level of susceptibility to apoptosis (r2 = .744).
DISCUSSION
We have shown that, in the absence of extrinsic apoptotic stimuli, resting WAS PBL undergo spontaneous apoptosis at a significantly greater frequency than resting normal PBL. We also have found that the expression of Bcl-2 is attenuated in WAS PBL. Because Bcl-2 inhibits the onset of apoptosis, insufficient levels of Bcl-2 expression could diminish the survival potential and shorten the life span of lymphocytes. We suggest then that peripheral T and B lymphocytes in WAS patients undergo accelerated spontaneous apoptosis as a consequence of a partial Bcl-2 deficiency. The accelerated destruction of peripheral T lymphocytes by spontaneous apoptosis could account for the progressive deterioration of T-lymphocyte function and lymphopenia in WAS patients. Moreover, a poor humoral response to certain specific antigens by WAS patients may not be strictly a consequence of T-lymphocyte abnormalities, as has been previously suggested.3,8 The accelerated destruction of memory B lymphocytes with diminished survival potential may preclude secondary immune responses.47 Therefore, chronic and recurrent infection in WAS patients may result from a failure to develop immunologic memory caused by accelerated spontaneous apoptosis of peripheral T and B lymphocytes.
Marked by DNA degradation, phosphatidylserine externalization, and cytoplasmic shrinkage, the abnormally high incidence of apoptosis in WAS lymphocytes suggests that inherent abnormalities of the actin cytoskeleton and cell surface glycoproteins3 may engender their self-destruction by programmed cell death (PCD). We found that the frequency of WAS PBL apoptosis was augmented to a greater extent than normal by in vitro incubation. This suggests that PCD was not triggered before isolation or in a manner dependent on the removal of lymphocytes from the periphery, where perhaps an extrinsic factor enabled survival. Moreover, because an external stimulus was not required to induce apoptosis in WAS PBL, we suggest that an internal mechanism induces unstimulated, resting WAS PBL to undergo spontaneous apoptosis at an accelerated frequency relative to normal PBL. The necessary impetus to undergo spontaneous apoptosis might be provided by the inherent abnormalities in WAS cells; these defects may conceivably trigger intrinsic apoptogenic signaling by surveillance proteins that monitor cellular damage.
Previous studies of WAS patients have demonstrated profound lymphopenia in peripheral lymphoid organs, corroborating our results and suggesting that our observations are clinically relevant. For example, the spleen and lymph nodes of WAS patients were largely devoid of T lymphocytes.48,49 Moreover, another case study of a WAS patient reported that the germinal centers and follicles of the lymph node and spleen were poorly developed or absent.49Hypocellularity and abnormal tissue architecture of these secondary lymphoid tissues in WAS patients is consistent with the our observation that WAS lymphocytes undergo accelerated spontaneous apoptosis and support the possible contention that WAS PBL have an inherently diminished potential for survival in vivo.
Studies of other affected blood cell lineages in WAS patients have also provided data that are consistent with our observations. It has been proposed that thrombocytopenia in WAS patients develops as a consequence of the accelerated destruction of platelets.3Studies of WAS heterozygotes have suggested that affected hematopoietic stem cells and their progeny that carry an active mutant X-chromosome are stringently selected against during development,3conceivably due to a loss of a capacity to either proliferate or survive. Affected blood cells in WAS heterozygotes may either fail to renew or to expand in a manner competitive with their normal, unaffected counterparts. Our study provides a unifying hypothesis that may explain the mechanism of the accelerated destruction of platelets and lymphocytes in WAS patients and the strict exclusion of affected blood cells in WAS carriers. We propose that mutations to WASP cause increased spontaneous apoptosis in all affected blood cells in both WAS patients and WAS carriers.
Mutations to WASP may obstruct hematopoiesis, but clearly the development of mature lymphocytes and platelets in WAS patients is not strictly precluded. Instead, it seems that WASP mutations are a detriment to the survival of mature blood cells in the periphery. In this study, we found abnormally low levels of Bcl-2 in resting, unstimulated WAS PBL. Because other studies have demonstrated the importance of Bcl-2 in regulating the survival of mature lymphocytes,50-52 our results suggest that the increased lability of WAS lymphocytes may be due to insufficient Bcl-2 expression. Interestingly, we note that the development and maintenance of lymphocytes in WAS patients is mirrored by lymphopoiesis in Bcl-2–deficient mice. Postnatally, it appears that lymphopoiesis in Bcl-2–deficient mice is normal; however, older mice eventually develop lymphopenia, as the thymus and spleen of these mice undergo massive apoptotic involution.53 Furthermore, whereas Bcl-2–deficient hematopoietic progenitor cells (HPC) in mouse chimeras differentiate into phenotypically mature lymphocytes, their progeny have markedly shortened life spans relative to the progeny of normal HPC.54 It seems, therefore, that murine Bcl-2 is dispensable for lymphocyte maturation but is required for the maintenance of viability afterward. We now describe a similar phenomena that occurs in WAS patients. Consistent with this observation, WASP-deficient mice, despite having apparently normal lymphopoiesis, have markedly decreased numbers of mature lymphocytes in the periphery relative to wild-type mice.55
Considerable variation in the levels of susceptibility to spontaneous apoptosis was found in PBL from different WAS patients. Despite genotypic identity and presumably similar environments, PBL isolated from the siblings, patients no. 3 and 4, had strikingly different levels of susceptibility to spontaneous apoptosis. PBL from patient no. 4 were about 3 times more sensitive to intrinsic apoptotic stimuli than were PBL from patient no. 3. Although both patients had grade 3 WAS, patient no. 3 is several years younger than patient no. 4. Because immunodeficiency associated with WAS is progressive, it is intriguing to speculate that such phenotypic differences may arise with the increasing age of the patient. Of course, there is the possibility that multiple genetic factors determine the clinical severity of WAS. Certainly, it is not uncommon to find that different members of the same kindred have different manifestations of the disease.18
We have observed considerable variation in Bcl-2 expression in freshly isolated PBL from different WAS patients (Table 1). The level of spontaneous apoptosis appears to be inversely related to the level of Bcl-2 expression. For example, PBL from WAS patient no. 1, which expressed relatively higher levels of Bcl-2 than PBL from the other patients, were less susceptible to the induction of spontaneous apoptosis. Because reduced Bcl-2 expression in WAS PBL could account for the increased susceptibility to intrinsic apoptotic stimuli, we speculate that WASP may be involved in signaling pathways that regulate Bcl-2 expression. Many mutations entirely nullify WASP expression, causing a clinically severe form of the disease.11 19-21Other mutations cause only trace amounts of WASP to be stably expressed and manifest a milder form of the disease. We suggest that WAS severity could be determined by the variable susceptibility of different patient’s lymphocytes to spontaneous apoptosis, which, in turn, could be determined by variable levels of Bcl-2 expression. If Bcl-2 expression is regulated by WASP-dependent signaling pathways, as we have proposed, then insufficient WASP activity could cause a reduction in the expression of Bcl-2 in WAS lymphocytes and thereby increase their sensitivity to apoptogenic signals. It will be of interest to examine the possible relationship between WASP insufficiency and the expression level of Bcl-2 using a larger number of WAS patients in future studies.
The function of WASP is not currently known; however, it has been suggested that, because WASP lacks catalytic activity, the protein may act as a molecular scaffold28 to coordinate the interactions of other signaling proteins that control the dynamic organization of the actin cytoskeleton. In support of this proposal, WASP interacts with Cdc42 and Rac,27-30 small GTPases of the Rho family that regulate the formation of filopodia and lamellipodia, respectively.26 Emerging now is the concept that, along with the actin cytoskeleton, the Rho family GTPases have the ability to coordinately regulate other cellular activities, such as cell cycle progression, activation of mitogen-activated protein (MAP) kinase signaling cascades, and cell survival.26,56 Several studies have demonstrated that Rho family GTPase activity is essential, not only to actin cytoskeleton dynamics, but also to cell survival.57-60 We suggest then that WASP might function as a scaffold for Cdc42- or Rac-dependent effector complexes that coordinately regulate actin cytoskeleton reorganization and cell survival in lymphocytes. This WASP-dependent complex may facilitate the activation of phosphoinositide 3-kinase (PI 3-kinase) by Cdc42.61 PI 3-kinase has been shown to be involved in both suppression of apoptosis62,63 and actin cytoskeleton dynamics.64,65 Alternatively, Btk, a protein kinase that regulates the expression of Bcl-XL in B lymphocytes,66 might become activated by a Cdc42- or Rac-dependent effector complex in a manner that requires its interaction with WASP.34,67,68 Perhaps Itk, another Tec kinase family member expressed in T lymphocytes and shown to interact with WASP,33 will be found to have a role analogous to Btk.69
If this is true, then the lack of WASP in WAS blood cells could result in irregular signaling through Cdc42 or Rac. A WASP deficiency may result in the random activation of other discrete pools of Rho family GTPase, possibly by the mechanism discussed by Reif and Cantrell.56 The nonspecific activation of different pools of Cdc42/Rac-effector complexes could result in sustained signaling through alternative pathways, such as the c-Jun N-terminal kinase or stress-activated protein kinase (JNK/SAPK) MAP kinase cascades,70,71 which may lead to the induction of apoptosis.72,73 In support of this idea, overexpression of activated Rho family GTPases markedly increases apoptosis.74-76 Consequently, in addition to the effect of decreased Bcl-2 expression by WAS lymphocytes, erratic signaling through Cdc42 or Rac, in the absence of sufficient WASP activity, could possibly promote the onset of apoptosis.
In conclusion, this study implicates a pathogenic mechanism in WAS that involves the accelerated apoptotic depletion of peripheral lymphocytes and that may thereby cause immunodeficiency in WAS patients. We suggest that the progressive immune dysfunction associated with WAS may develop as the result of an inability of the thymus to generate new T lymphocytes at a rate that is compensatory with their depletion in the periphery. The deregulation of apoptosis has now been associated with the pathogenesis of a variety of diseases.77,78 Moreover, in those diseases associated with increased apoptosis, it is apparent that the deficient expression of antiapoptotic regulatory factors, such as Bcl-2 and Bcl-XL, may be responsible for the decreased survival of affected cells. For example, in patients with X-linked agammaglobulinemia (XLA), in which B-lymphocyte development is arrested by mutations to the tyrosine kinase Btk,79 manifest immunodeficiency might develop, in part, due to the attrition of abnormally fragile pre-B lymphocytes. This was suggested by studies ofxid mice, an animal model for XLA, also carrying mutations in Btk,80 that demonstrated that the poor survival of peripheral B lymphocytes may be due to abnormal regulation of both Bcl-2 and Bcl-XL.50,66 In human immunodeficiency virus infection, CD4+ T lymphocytes undergo accelerated destruction,81-83 possibly due to diminished expression of Bcl-2,84,85 and immunodeficiency may then progress from insufficient compensatory lymphopoiesis.86 Thus, together with the results of this study, it would appear that deregulation of Bcl-2 family members may be a common mechanism involved in the pathogenesis of various immunodeficiencies. An analysis of larger numbers of WAS patients, in an effort to correlate susceptibility to spontaneous apoptosis and disease severity, will be important in understanding the clinical course of WAS. Future studies will also address the possible mechanisms by which WASP-dependent signaling pathways may regulate the survival of peripheral lymphocytes.
ACKNOWLEDGMENT
The authors express our gratitude to Dr Leo Mascarenhas and Dr John Bastian (Children’s Hospital, San Diego, CA) for their assistance in procuring blood samples from WAS patients, and Dr Hans Ochs for providing the genotypic information. We appreciate the efforts of Earl Leonard, our biostatistician. We thank Dr Donald Durden, Dr Jane Fountain, and Brile Chung for the critical reading of this manuscript. We are grateful to Flavia Thiemann and Matthieu DeClerck for their technical assistance. We gratefully acknowledge the CHLA Research Institute, and the Achievement Rewards for College Scientists Foundation (Los Angeles Chapter) for the support of S.L.R., the recipient of the John H. Richardson & Margaret Kersten Ponty Endowed Fellowship through the Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, CA.
Supported in part by Grants No. AI40581, HL54729, and HL54850 (Specialized Center of Research in Stem Cell Biology) from the National Institutes of Health. S.L.R. is a fellow of the Achievement Rewards for College Scientists Foundation and Childrens Hospital Los Angeles Research Institute.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kenneth I. Weinberg, MD, Division of Research Immunology and Bone Marrow Transplantation, Mail Stop #62, Childrens Hospital Los Angeles, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail: kweinberg@chla.usc.edu.