Abstract
Although adenosine diphosphate (ADP), per se, is a weak platelet agonist, its role as a crucial cofactor in human blood platelet functions has now been clearly demonstrated in vitro and in vivo. The molecular basis of the ADP-induced platelet activation is starting to be understood since the discovery that 2 separate P2 purinergic receptors may be involved simultaneously in the activation process. However, little is known about how ADP plays its role as a cofactor in platelet activation and which signaling pathway initiated by a specific agonist can be modulated by the released ADP. To investigate these points, we took advantage of a model of platelet activation through the thrombin receptor PAR1 in which both ADP scavengers and phosphoinositide 3-kinase (PI 3-kinase) inhibitors have been shown to transform the classical irreversible aggregation into a reversible one. We have observed that, among the different PI 3-kinase products, the accumulation of phosphatidylinositol 3,4-bisphosphate [PtdIns(3,4)P2] was dramatically and specifically attenuated when ADP was removed by apyrase treatment. A comparison between the effects of PI 3-kinase inhibitors and apyrase strongly suggest that the late, ADP-dependent, PtdIns(3,4)P2accumulation is necessary for PAR1-induced irreversible aggregation. Using selective antagonists, we found that the effect of ADP was due to the ADP receptor coupled to inhibition of adenylyl cyclase. Finally, we found that both ADP and PI 3-kinase play an important role in PAR1-dependent reorganization of the cytoskeleton through a control of myosin heavy chain translocation and the stable association of signaling complexes with the actin cytoskeleton.
THROMBIN, A POTENT agonist for platelets, is known to induce the synthesis of both phosphatidylinositol 3,4-bisphosphate [PtdIns(3,4)P2] and phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3], 2 phosphoinositides phosphorylated at the D3 position of the inositol ring by phosphoinositide 3-kinases (PI 3-kinases). The D3-phosphoinositides generated by the various PI 3-kinases are now considered as second messengers capable of binding protein modules, including src homology region 2 (SH2) or pleckstrin homology (PH) domains, present in their targets,1 thus regulating specific signaling pathways. In platelets stimulated by thrombin, the synthesis of PtdIns(3,4,5)P3 is rapid and transient,2,3 whereas PtdIns(3,4)P2 accumulates upon increasing stimulation times.4-6 Using platelets from thrombasthenic patients or Arg-Gly-Asp-Ser (RGDS)-treated platelets, we have demonstrated that the synthesis of a major part of PtdIns(3,4)P2 is dependent on the engagement of αIIbβ3 integrin.7 It is important to note that fibrinogen binding to its receptor αIIbβ3 is not sufficient per se to induce a full activation of this pathway, because aggregation is also required, as demonstrated by using thrombin-treated platelets in the absence of stirring.3 Furthermore, platelets adhering to a fibrinogen matrix require adenosine diphosphate (ADP) for spreading, phosphorylation of focal adhesion kinase (p125FAK), and synthesis of PtdIns(3,4)P2.8,9 These results suggest that the level of PtdIns(3,4)P2 is regulated by complex mechanisms involving cross-talk between different signaling pathways. Although the accumulation of PtdIns(3,4)P2 in stimulated platelets is thought to play an important role,3,7,9 its function and target remain unknown. It has been suggested that PI 3-kinase and its products may play a role in irreversible aggregation, possibly by maintaining the αIIbβ3 integrin in its activated state.10 These lipids may also be involved in mediating actin filament uncapping and specific filopodial actin assembly.11
We have recently shown that washed human platelets stimulated by ADP in the presence of fibrinogen were unable to accumulate significant amounts of PtdIns(3,4)P2.12 Indeed, as compared with thrombin, ADP is a weak platelet agonist inducing only reversible aggregation with partial and reversible cytoskeleton reorganization.12-14 Again, this observation indicates that fibrinogen binding to αIIbβ3 is not sufficient per se to induce the accumulation of PtdIns(3,4)P2 and that the absence of accumulation of this lipid correlates with a reversible aggregation. However, although ADP is a weak agonist per se, the use of ADP receptor antagonists or of enzymes capable of degrading ADP leads to clear attenuation of platelet responses to low thrombin concentrations or other agonist-like collagen.13-15 Present at very high concentrations in the platelet dense granules,13,14 ADP is secreted when platelets are stimulated by other aggregating agents. Among all platelet-released substances, ADP has been shown to be selectively responsible for the stabilization of thrombin-induced platelet aggregates.16 17
The molecular basis of ADP-induced platelet activation is only beginning to be understood with the finding that 2 separate P2 receptors could be involved simultaneously in the activation process.18-22 The P2 family of receptors is composed of 2 classes of receptors, namely the P2X receptors, which are ligand-gated ion channels, and the P2Y receptors, which belong to the serpentine G protein-coupled receptor family.23 In the case of platelets, the P2Y1 receptor is coupled to calcium mobilization and has been shown to be responsible for ADP-induced shape change. In addition, a not yet identified P2 receptor negatively coupled to adenylyl cyclase seems to be necessary for the completion of the aggregation response.18-20 Selective antagonists and inhibitors have recently been developed that allow specific discrimination between P2Y1 and P2/adenylyl cyclase–dependent responses. Adenosine 2′-phosphate 5′-phosphate (A2P5P) is a selective P2Y1antagonist,18-20,24 whereas AR-C66096 has been found to selectively block the inhibitory effect of ADP on adenylyl cyclase.18 The pharmacology of AR-C66096 is strikingly similar to that of the well-known antiplatelet drug clopidogrel, which inhibits selectively ADP-induced platelet aggregation by blocking the effect of ADP on adenylyl cyclase.25
We have investigated here how ADP plays its role as a cofactor in platelet activation and whether a specific signaling pathway, PI 3-kinase activation, is modulated by released ADP. We took advantage of 2 models of reversible aggregation previously described10,26 in which the PAR1 thrombin receptor is stimulated by the peptide SFLLRNP (TRAP) in the presence of an ADP-scavenger26 or in the presence of PI 3-kinase inhibitors.10 We demonstrate that ADP plays a key and specific role in the late accumulation of PtdIns(3,4)P2induced by TRAP through its receptor coupled to inhibition of adenylyl cyclase. The late and sustained activation of a PI 3-kinase, which is responsible for the ADP-dependent production of PtdIns(3,4)P2, was found to be necessary for the irreversible aggregation of platelets stimulated by TRAP. Finally, we show that the cytoskeletons from TRAP-stimulated platelets display major differences in their myosin heavy chain and RhoA content, depending on the presence of ADP and synthesis of PtdIns(3,4)P2.
MATERIALS AND METHODS
Reagents.
Apyrase was purified from potatoes as previously described.27 Its specific ADPase activity was 12.5 U/mg. TRAP was purchased from Bachem (Budendorf, Switzerland), rabbit anti-p85α antibody from Upstate Biotechnology Inc (Lake Placid, NY), rabbit anti-p125FAK, and anti-RhoA antibodies from Santa Cruz Biotechnology Inc (Santa Cruz, CA). LY294002 was purchased from Biomol Research Laboratories, Inc (Plymouth, PA). Enhanced chemiluminescence (ECL) Western blotting reagents and [32P]-orthophosphate were obtained from Amersham International (Buckinghamshire, UK). AR-C66096, formerly known as FLP66096 or ARL66096, was a generous gift from ASTRA Charnwood (Loughborough, UK). All other reagents were from Sigma (St Louis, MO) unless otherwise indicated.
Preparation of washed human platelets.
Human blood was collected from a forearm vein, and platelet suspensions were prepared as previously described.27 In some experiments, platelets were labeled with sodium [32P]-orthophosphate (400 μCi/mL) for 1 hour at 37°C during a first washing step in Tyrode's buffer lacking phosphate. The final resuspending medium (pH 7.35) was a Tyrode's buffer containing 2 mmol/L Ca2+, 1 mmol/L Mg2+, 0.35% human serum albumin (Etablissement de Transfusion Sanguine, Strasbourg, France), and apyrase (0.02 U/mL) to prevent desensitization of platelet responses to ADP. Platelets were stored at 37°C throughout the experiments, and the cell count was adjusted in the final suspension to 7.5 × 105/μL using a Sysmex 100 particle counter (Merck Clevenot, Nogent-sur-Marne, France).
Platelet aggregation studies.
Aggregation was measured at 37°C by a turbidimetric method using a dual-channel Payton aggregometer (Payton Associates, Scarborough, Ontario, Canada). A 1.45-mL aliquot of nonlabeled or32P-labeled platelet suspension was stirred at 1,100 rpm and activated by the addition of TRAP in the absence or presence of 1 U/mL apyrase or one of the following selective ADP receptor antagonists: 100 μmol/L ATPαS, an antagonist of both P2Y1 receptor and the unidentified P2 receptor coupled to adenylyl cyclase; 100 μmol/L A2P5P, a selective P2Y1antagonist20,24; or 1 μmol/L of the recently described ATP analogue AR-C66096, a strong and selective antagonist of the P2 receptor coupled to inhibition of adenylyl cyclase.18 28
Measurement of adenylyl cyclase activity.
A 450-μL aliquot of washed platelets was stirred at 1,100 rpm in an aggregometer cuvette and the following reagents were added at 30-second intervals: prostaglandin E1 (PGE1); A2P5P, AR-C66096, or ATPαS; and TRAP at indicated concentrations. One minute later, the reaction was stopped by addition of 50 μL of ice-cold 6.6 mol/L perchloric acid. Perchloric acid extracts were centrifuged at 11,000g for 5 minutes to eliminate protein precipitate, and cyclic adenosine monophosphate (cAMP) was isolated from the supernatants as described.20 The upper aqueous phase was freeze-dried, and the dry residue was dissolved in the buffer provided with the commercial radioimmunoassay kit for cAMP measurement (Amersham).
Cytoskeleton extraction.
Reactions were stopped and the cytoskeleton was immediately isolated as described previously.3 Cytoskeletal material was collected by centrifugation (12,000g for 10 minutes at 4°C), washed once with Csk buffer (50 mmol/L Tris-HCl, pH 7.4, 10 mmol/L EGTA, 1 mmol/L Na3VO4, 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 1 mmol/L phenylmethylsulfonylfluoride) containing 0.5% (vol/vol) Triton X-100 and then twice with Csk buffer without Triton X-100.
Gel electrophoresis and immunoblotting.
Cytoskeletal proteins were solubilized in the electrophoresis sample buffer, boiled for 5 minutes, separated on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and visualized by Coomassie Blue staining or transferred onto a nitrocellulose membrane (Gelman Sciences, Ann Arbor, MI) for immunoblotting as reported previously.3 Immunodetections were performed with relevant antibodies and reactions were visualized using the ECL chemiluminescent system.
Lipid extraction and analysis.
Reactions were stopped by the addition of 2 vol of chloroform/methanol and lipids were extracted, separated, deacylated, and finally analyzed by high-performance liquid chromatography (HPLC) on a partisphere SAX column (Whatman International Ltd, Maidstone, UK) as reported previously.29
RESULTS
The apyrase-induced reversible aggregation of TRAP-stimulated human platelets is associated with a lack of PtdIns(3,4)P2accumulation.
As previously shown by Lau et al,26 when platelets were stimulated with 40 μmol/L TRAP, the addition of apyrase caused a reversion of aggregation, starting at 60 to 80 seconds (Fig 1A). Figure 1B indicates that treatment of platelets with the ADP scavenger led to a dramatic change in [32P]PtdIns(3,4)P2 accumulation induced by TRAP. Interestingly, the responses were quite similar until 40 seconds, and then a large decrease in the intensity of labeling was observed in the presence of apyrase, followed by a rapid disappearance of this lipid, which was complete at 3 minutes. This strongly suggests a lack of sustained PI 3-kinase activation and points out a degradation pathway occurring under these conditions. These results clearly indicate a major role of ADP in the late increase in PtdIns(3,4)P2 occurring after 1 minute in TRAP-stimulated platelets. In contrast, the production of [32P]PtdIns(3,4,5)P3 evoked by TRAP was rapid (maximal at 20 seconds), transient, and not significantly affected by apyrase (Fig 1C). The level of [32P]PtdIns(3)P was low under resting conditions and did not change significantly upon TRAP stimulation either in the presence or in the absence of apyrase (not shown).
ADP is involved in the accumulation of [32P]PtdIns(3,4)P2 induced by TRAP. [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP in the absence or the presence of 1 U/mL apyrase (− and + in [A], • and ○ in [B] and [C]) for various periods of time. (A) Aggregation was measured as described in Materials and Methods. (B) Time course of PtdIns(3,4)P2 accumulation. (C) Time course of PtdIns(3,4,5)P3 accumulation. Lipids were analyzed using an HPLC technique as indicated in Materials and Methods. Data are from 1 representative experiment of 3 independent experiments that gave very similar results.
ADP is involved in the accumulation of [32P]PtdIns(3,4)P2 induced by TRAP. [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP in the absence or the presence of 1 U/mL apyrase (− and + in [A], • and ○ in [B] and [C]) for various periods of time. (A) Aggregation was measured as described in Materials and Methods. (B) Time course of PtdIns(3,4)P2 accumulation. (C) Time course of PtdIns(3,4,5)P3 accumulation. Lipids were analyzed using an HPLC technique as indicated in Materials and Methods. Data are from 1 representative experiment of 3 independent experiments that gave very similar results.
Effect of apyrase on PtdIns, PtdIns(4)P, PtdIns(4,5)P2, and phosphatidic acid metabolism induced by TRAP.
The specificity of ADP action as a cofactor in the accumulation of PtdIns(3,4)P2 induced by TRAP was further assessed by measuring the level of the other classes of phosphoinositides and of phosphatidic acid (PtdOH). As shown in Fig2, when TRAP was used as an agonist, PtdIns, PtdIns 4P, and PtdIns(4,5)P2 displayed a characteristic pattern of labeling not significantly modified by suppression of ADP. As previously observed by Hartwig et al,30 we could measure a net and steady increase in PtdIns(4)P labeling in TRAP-treated platelets. This increase was much more obvious than upon thrombin addition5 and probably subsequent, beside an increased synthesis, to an absence of persistent phospholipase C activation. The activation of PtdIns 4-kinase was not dependent on the presence of ADP. The increase in PtdIns(4,5)P2 labeling (Fig 2) presented some fluctuations from one experiment to another, as is often the case in platelet phosphoinositide metabolic studies, yet apyrase effects were always negligible. Apyrase did not modify the overall kinetics of PtdOH formation (Fig 2), although removal of ADP did lead to a more rapid decrease in PtdOH levels. It is noteworthy that, in contrast to the sustained increase of [32P]PtdOH synthesis observed upon thrombin addition, even at low thrombin concentrations (unpublished results), 40 μmol/L TRAP induced a transient increase in the labeling of PtdOH. This time course of PtdOH production might be correlated with the more transient cytosolic free Ca2+ increase measured in the presence of TRAP in comparison with thrombin.31 Together, the results from Figs1B and 2 indicate that ADP has a specific effect as a cofactor of TRAP on PtdIns(3,4)P2 metabolism, because TRAP alone (Fig 1B) or ADP alone12 were unable to induce the late accumulation of this phosphoinositide.
Time courses of [32P]PtdIns, [32P]PtdIns(4)P, [32P]PtdIns(4,5)P2, and [32P]PtdOH production and effects of released ADP in TRAP-stimulated platelets. Time courses of [32P]-labeling of PtdIns, PtdIns(4)P, PtdIns(4,5)P2, and PtdOH in [32P]-labeled platelets activated by 40 μmol/L TRAP in the absence (•) or the presence (○) of 1 U/mL apyrase. Radioactivity incorporated into various phospholipids was quantified using HPLC as in Fig 1. Results are representative of 3 experiments with similar results.
Time courses of [32P]PtdIns, [32P]PtdIns(4)P, [32P]PtdIns(4,5)P2, and [32P]PtdOH production and effects of released ADP in TRAP-stimulated platelets. Time courses of [32P]-labeling of PtdIns, PtdIns(4)P, PtdIns(4,5)P2, and PtdOH in [32P]-labeled platelets activated by 40 μmol/L TRAP in the absence (•) or the presence (○) of 1 U/mL apyrase. Radioactivity incorporated into various phospholipids was quantified using HPLC as in Fig 1. Results are representative of 3 experiments with similar results.
The late and sustained activation of PI 3-kinase is required for PtdIns(3,4)P2 accumulation and irreversible aggregation.
As already reported,10 when platelets were pretreated with PI 3-kinase inhibitors, the aggregation induced by TRAP became reversible (not shown), as in the absence of ADP (Fig 1). However, under these conditions, the synthesis of all PI 3-kinase products was fully inhibited (not shown). Interestingly, addition of the 2 unrelated PI 3-kinase inhibitors after 2 minutes of stimulation, when aggregation and PtdIns(3,4)P2 production were at their maximum, induced the reversion of aggregation in a dose-dependent manner (Fig 3A and B). At this stage of stimulation, the rapid and transient PtdIns(3,4,5)P3synthesis (Fig 1) has already occurred and was not affected (not shown). In contrast, Fig 3C shows that the level of PtdIns(3,4)P2 dramatically decreased immediately upon addition of the PI 3-kinase inhibitors, demonstrating a very active turnover of this phosphoinositide and a sustained activation of PI 3-kinase between 2 and 4 minutes. Interestingly, the decrease in PtdIns(3,4)P2 was rapidly followed by the disaggregation mechanism. Moreover, the addition of PI 3-kinase inhibitors at any time up to 2 minutes of stimulation was without effect on the aggregation response until 2 minutes, but, once at its maximum, the aggregation became reversible (not shown). These results demonstrate a key role of a PI 3-kinase active after 2 minutes of stimulation in the accumulation of PtdIns(3,4)P2 and the stabilization of aggregation induced by TRAP. Results from Fig 3 also strongly suggest that the late formation of PtdIns(3,4)P2 was actually the cause of irreversible aggregation.
The late and sustained activation of PI 3-kinase is required for PtdIns(3,4)P2 accumulation and irreversible aggregation induced by TRAP in the presence of ADP. Platelet aggregation was measured as described in Materials and Methods. Various concentrations of the 2 unrelated PI 3-kinase inhibitors, wortmannin (A) or LY294002 (B), were added 2 minutes after stimulation with 40 μmol/L TRAP, as indicated by the arrow. Data are representative of 3 independent experiments with very similar results. (C) [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP. After 2 minutes, 50 nmol/L wortmannin (•), 25 μmol/L LY 294002 (○), or 0.1% Me2SO (▪) was added. The reactions were stopped at different times by addition of chloroform/methanol and the level of [32P]PtdIns(3,4)P2 was measured by HPLC as indicated in Materials and Methods.
The late and sustained activation of PI 3-kinase is required for PtdIns(3,4)P2 accumulation and irreversible aggregation induced by TRAP in the presence of ADP. Platelet aggregation was measured as described in Materials and Methods. Various concentrations of the 2 unrelated PI 3-kinase inhibitors, wortmannin (A) or LY294002 (B), were added 2 minutes after stimulation with 40 μmol/L TRAP, as indicated by the arrow. Data are representative of 3 independent experiments with very similar results. (C) [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP. After 2 minutes, 50 nmol/L wortmannin (•), 25 μmol/L LY 294002 (○), or 0.1% Me2SO (▪) was added. The reactions were stopped at different times by addition of chloroform/methanol and the level of [32P]PtdIns(3,4)P2 was measured by HPLC as indicated in Materials and Methods.
Effect of selective ADP receptor antagonists on TRAP-induced platelet aggregation and PtdIns(3,4)P2 accumulation.
To assess which ADP receptor may be involved in the contribution to PtdIns(3,4)P2 formation by TRAP, we used selective antagonists that are known to discriminate between them. Figure 4A shows that 100 μmol/L ATPαS and 1 μmol/L AR-C66096 could reverse TRAP-induced platelet aggregation in a manner similar to that of apyrase, whereas 100 μmol/L of the selective P2Y1 antagonist A2P5P did not. In relation to this, ATPαS or AR-C66096 inhibited TRAP-induced accumulation of PtdIns(3,4)P2, whereas A2P5P was ineffective (Fig 4B). Again, this effect was selective for this lipid, because the other phosphoinositides were not affected by these compounds (data not shown). These results strongly suggest that the cofactor role of ADP in PtdIns(3,4)P2 accumulation and irreversible aggregation may be due to its P2 receptor coupled to inhibition of adenylyl cyclase.
Effect of selective ADP receptor antagonists on TRAP-induced platelet aggregation and PtIns(3,4)P2accumulation. [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP in the absence or in the presence of the selective P2Y1 antagonist A2P5P (100 μmol/L), the selective antagonist of the P2 receptor coupled to adenylyl cyclase AR-C66096 (1 μmol/L), or an antagonist of both ADP receptors ATPS (100 μmol/L). (A) Aggregation was measured as described in Materials and Methods. (B) Time course of PtdIns(3,4)P2 accumulation. Lipids were immediately extracted and [32P]PtdIns(3,4)P2 was separated and quantified as in Fig 1. Results are expressed as the percentage of PtdIns(3,4)P2 produced, with 100% being the maximal production observed upon TRAP stimulation, and are the means ± SD from 2 independent experiments.
Effect of selective ADP receptor antagonists on TRAP-induced platelet aggregation and PtIns(3,4)P2accumulation. [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP in the absence or in the presence of the selective P2Y1 antagonist A2P5P (100 μmol/L), the selective antagonist of the P2 receptor coupled to adenylyl cyclase AR-C66096 (1 μmol/L), or an antagonist of both ADP receptors ATPS (100 μmol/L). (A) Aggregation was measured as described in Materials and Methods. (B) Time course of PtdIns(3,4)P2 accumulation. Lipids were immediately extracted and [32P]PtdIns(3,4)P2 was separated and quantified as in Fig 1. Results are expressed as the percentage of PtdIns(3,4)P2 produced, with 100% being the maximal production observed upon TRAP stimulation, and are the means ± SD from 2 independent experiments.
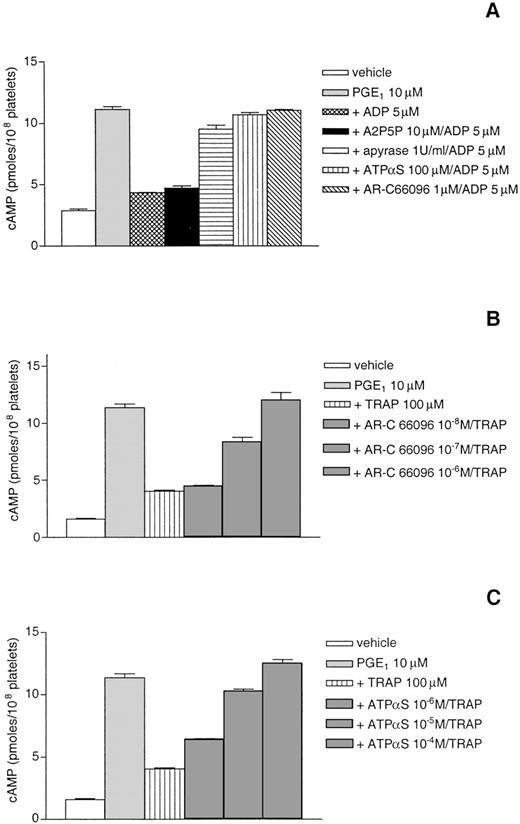
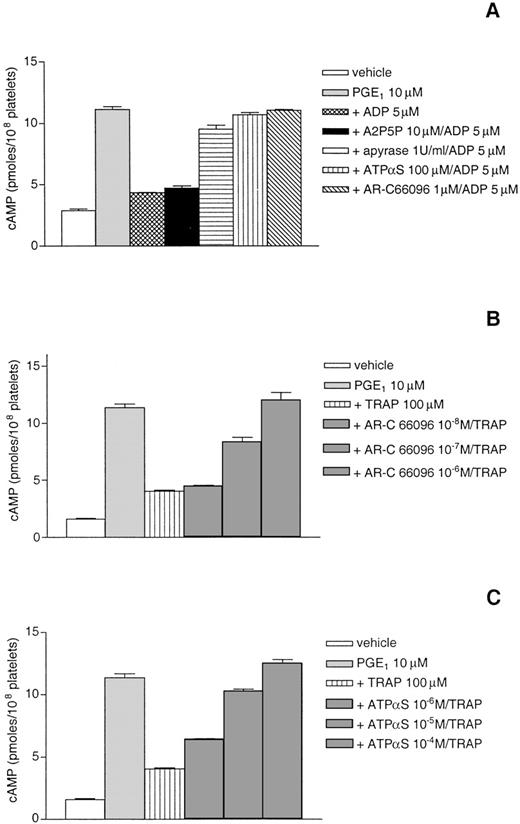
TRAP-induced reversion of cAMP formation by PGE1 involves released ADP.
To assess further the role of this signaling pathway, apyrase and the various ADP receptor antagonists were tested for their ability to inhibit the effect of TRAP on cAMP accumulation. For this purpose, adenylyl cyclase was stimulated by incubation of platelets with 1 μmol/L PGE1 in the presence of ADP receptor antagonists and, 1 minute later, the addition of ADP or TRAP. As shown in Fig 5A, apyrase, ATPαS, and AR-C66096 were able to block the inhibitory effect of ADP on adenylyl cyclase, whereas A2P5P did not, which confirmed previous work.18 20Under these experimental conditions, reversion of cAMP formation by a high concentration of TRAP (100 μmol/L) was due to released ADP, because this effect was inhibited in a dose-dependent manner by ATPαS and AR-C66096, with a complete inhibition at 10−4mol/L and 10−6 mol/L, respectively (Fig 5B and C).
TRAP-induced reversion of cAMP formation by PGE1 involves released ADP. The effects of apyrase and various ADP receptor antagonists on cAMP accumulation were measured in intact platelets stimulated by 5 μmol/L ADP (A). The effects of various concentrations of AR-C 66096 and ATPS on cAMP accumulation were measured in intact platelets stimulated by a high concentration of TRAP (100 μmol/L) (B) as described in Materials and Methods. Results are the means of triplicates ± SEM from 1 representative experiment of 3 independent experiments that gave very similar results.
TRAP-induced reversion of cAMP formation by PGE1 involves released ADP. The effects of apyrase and various ADP receptor antagonists on cAMP accumulation were measured in intact platelets stimulated by 5 μmol/L ADP (A). The effects of various concentrations of AR-C 66096 and ATPS on cAMP accumulation were measured in intact platelets stimulated by a high concentration of TRAP (100 μmol/L) (B) as described in Materials and Methods. Results are the means of triplicates ± SEM from 1 representative experiment of 3 independent experiments that gave very similar results.
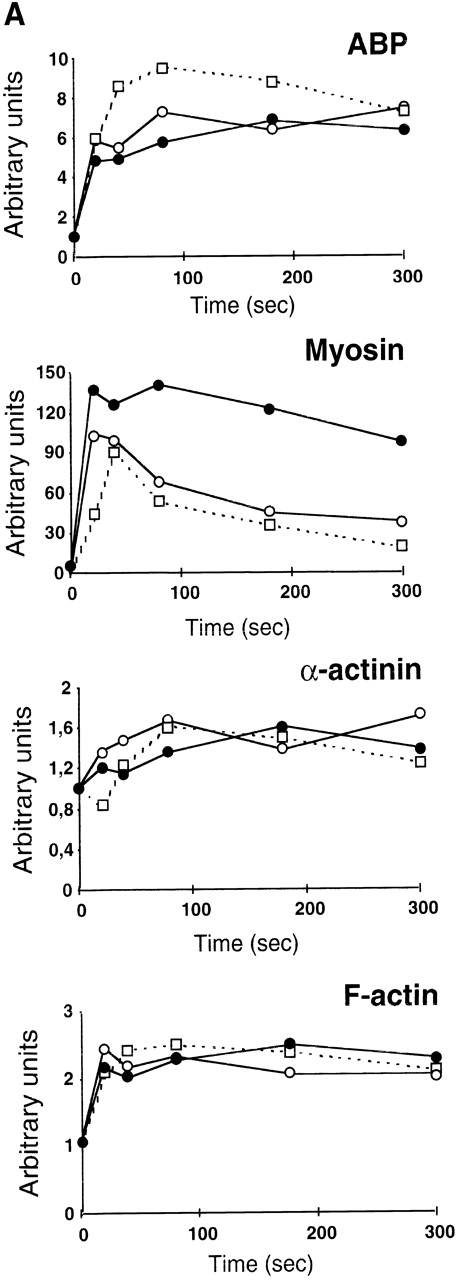
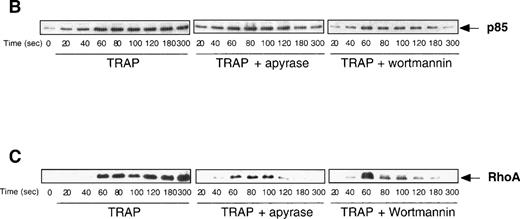
ADP scavengers and PI 3-kinase inhibitors alter similarly the cytoskeleton reorganization induced by TRAP.
The dramatic reorganization of the actin cytoskeleton observed during platelet aggregation is thought to play an important role in the stabilization of aggregation and in the formation of functional signaling complexes. Based on the results described above, we decided to investigate the potential changes that may occur in TRAP-induced actin cytoskeleton reorganization in the absence of ADP or when PI 3-kinase is inhibited. Interestingly, Fig 6A clearly indicates that, among the major proteins found in the actin cytoskeleton from TRAP-aggregated platelets, the content of 200-kD myosin heavy chain was strongly decreased when ADP was absent or when PI 3-kinase was inhibited. In contrast, these inhibitors did not induce significant changes in the content of F-actin, α actinin, and actin-binding protein (ABP). To evaluate the formation of signaling complexes linked to the actin cytoskeleton, we compared the time courses of relocation to the cytoskeleton of the p85α subunit of PI 3-kinase and of its potential regulator, RhoA. Figure 6B indicates that TRAP induced a significant association of p85α with the cytoskeleton, reaching a plateau at approximately 2 minutes. In contrast, in the presence of apyrase or PI 3-kinase inhibitors, after 2 minutes, a reversion of the association of p85α with the cytoskeleton was observed. An even more dramatic difference was found in the translocation of RhoA upon addition of apyrase or PI 3-kinase inhibitors (Fig 6B) that paralleled effects of these agents on p85α.
Both apyrase and the PI 3-kinase inhibitor wortmannin specifically affect the association of myosin heavy chain with the cytoskeleton and the organization of signaling complexes. (A) Cytoskeletons were isolated from 40 μmol/L TRAP-treated platelets at various time points in the absence (•) or in the presence of 1 U/mL apyrase (○) or 100 nmol/L wortmannin (□). Cytoskeletal proteins were separated by a 7.5% SDS-PAGE and visualized by Coomassie Blue staining. Actin and the major actin-binding proteins were quantified by densitometric analysis (ScanMaker IIHR, Microtek, Germany). The relocation of p85 (B) and RhoA (C) to the cytoskeleton was analyzed by Western blotting with specific antibodies. Data shown are representative of 3 independent experiments with similar results.
Both apyrase and the PI 3-kinase inhibitor wortmannin specifically affect the association of myosin heavy chain with the cytoskeleton and the organization of signaling complexes. (A) Cytoskeletons were isolated from 40 μmol/L TRAP-treated platelets at various time points in the absence (•) or in the presence of 1 U/mL apyrase (○) or 100 nmol/L wortmannin (□). Cytoskeletal proteins were separated by a 7.5% SDS-PAGE and visualized by Coomassie Blue staining. Actin and the major actin-binding proteins were quantified by densitometric analysis (ScanMaker IIHR, Microtek, Germany). The relocation of p85 (B) and RhoA (C) to the cytoskeleton was analyzed by Western blotting with specific antibodies. Data shown are representative of 3 independent experiments with similar results.
DISCUSSION
In a recent study dealing with ADP stimulation of washed platelets, in the presence of exogenous fibrinogen to allow αIIbβ3 engagement, we have observed a relationship between reversible aggregation, absence of PtdIns(3,4)P2 accumulation, and a large reduction of the amount of myosin heavy chain and RhoA in the cytoskeleton.12 These data, together with our previous report of a parallelism between aggregation extent and PtdIns(3,4)P2 labeling in thrombin-stimulated platelets,3 suggest that irreversible aggregation may be linked to the late accumulation of PtdIns(3,4)P2 in human platelets. The present study shows that, in another model of reversible aggregation, ie, activation of human platelets by TRAP in the presence of the ADP scavenger apyrase,26 PtdIns(3,4)P2is synthesized in the very early stages of activation but then quickly disappears. In contrast, in the presence of released ADP, TRAP leads to an irreversible aggregation and to a large increase in the production of PtdIns(3,4)P2 up to 3 minutes of stimulation. The metabolism of the other phosphoinositides, including PtdIns(3,4,5)P3 and PtdIns(3)P, is not significantly modified by apyrase, indicating the specificity of this mechanism.
However, based on these results alone, it is difficult to know whether the accumulation of this lipid is a cause or consequence of the irreversible aggregation. We show here that PI 3-kinase inhibitors, added when aggregation is at its maximum after 2 minutes of stimulation, are able to induce a very rapid and dramatic decrease in the level of PtdIns(3,4)P2 followed by a disaggregation of platelets. Although a potential role of PI 3-kinase as a protein kinase cannot be totally excluded, these results strongly suggest a role for the late accumulation of PtdIns(3,4)P2 in strengthening aggregation. The particularly active turnover of this phosphoinositide indicates that its accumulation results from a sustained PI 3-kinase activation rather than the inhibition of PtdIns(3,4)P2hydrolysis. Moreover, the fact that PtdIns(3,4,5)P3 does not accumulate in the presence of ADP scavengers suggests that a 5-phosphatase32 does not play a critical role in the late and large increase of PtdIns(3,4)P2. Therefore, we propose that ADP is playing its essential role as cofactor of TRAP via, notably, the late activation of a PI 3-kinase and the production of PtdIns(3,4)P2. A C2 domain-containing PI 3-kinase, activated by αIIbβ3 engagement, has recently been described in platelets.33 This enzyme produces PtdIns(3)P, which is then phosphorylated to PtdIns(3,4)P2 by a PtdIns(3)P 4-kinase. This new route could be compatible with our results; however, in our model, the binding of fibrinogen to its receptor αIIbβ3 is not sufficient per se to lead to PtdIns(3,4)P2 production, because ADP-dependent signaling is clearly necessary.
Because we have shown that TRAP (Fig 1) or ADP alone12 are not sufficient per se to induce the accumulation of PtdIns(3,4)P2, an important question is how a combination of these agents can induce a full activation. One explanation might be that αIIbβ3 exposure to its ligand must reach a certain level, obtained upon addition of 2 weak agonists (ie, TRAP and ADP),24 so that the formation of strong focal complexes might occur. The outside/in signaling of αIIbβ3 is thought to be linked to the recruitment, around the β3 cytoplasmic tail, of signaling complexes and cytoskeletal proteins.34 These complexes may be different according to the degree of αIIbβ3 activation and the mechanical strengths acting through this integrin.
Another possibility, based on the role of ADP in enhancing the secretion response induced by other agonists,35 could be that other adhesive receptors have to cooperate with αIIbβ3 for full signaling through the integrin. Consistent with this idea, a recent study suggests a modulation of αIIbβ3 function by thrombospondin,36 which is released upon platelet activation.
The results obtained using selective ADP receptor antagonists strongly suggest that the P2 receptor negatively coupled to adenylyl cyclase is the ADP receptor required for the reinforcing role of ADP. This observation is of consequence in terms of antithrombotic pharmacology,37-39 because the antiplatelet drug clopidogrel, acting through this ADP receptor, inhibits thrombosis in humans.25 The intracellular machinery involved in these processes is currently under investigation. One can speculate that, beside the inhibition of cAMP formation, the release of β/γ subunits from heterotrimeric G-proteins may be critical.
Surprisingly, the purinergic antagonists were able to totally reverse the inhibition of cAMP formation caused by TRAP (Fig 5). At first glance, this is in apparent contradiction with previously published work showing that PAR1 stimulation leads to ADP-independent inhibition of adenylyl cyclase by a Gi coupling mechanism in isolated platelet membranes40,41 or intact cells.42 This discrepancy may come from the fact that inhibition of adenylyl cyclase is not measurable without stimulation of cAMP production by PGE1 or PGI2 and that, in contrast to Giesberts et al,42 who first stimulated platelets by TRAP or thrombin and then, 5 minutes later, added PGI2, we increased the cAMP levels by PGE1 1 minute before stimulation by TRAP. This increase in cAMP level may result in a decrease of efficiency of TRAP to directly activate Gi, probably via a partial desensitization of the receptor,43 and thus may exacerbate the effect of secreted ADP. This is consistent with the fact that, in similar experiments, we observed that thrombin at high concentration (>0.05 U/mL) did inhibit cAMP production by PGE1 in an ADP-independent manner, whereas, at lower concentrations, the role of ADP was clear (not shown). Moreover, we show here that PAR1 does not require ADP to induce the reversible phase of platelet aggregation. In contrast, ADP plays a pivotal role to get an irreversible aggregation, indicating its involvement in a rather late phase of platelet activation induced by TRAP. Based on recent results demonstrating the necessity of both Gi and Gq pathways to obtain ADP-induced reversible aggregation,21 22 it is conceivable that PAR1-mediated reversible aggregation by itself also requires these 2 pathways.
The impact of released ADP and the importance of newly synthesized PtdIns(3,4)P2 for reorganization of the cytoskeleton and translocation of signaling enzymes were also evaluated in this study. The cytoskeletal content of actin binding protein, α-actinin, and F-actin increases upon TRAP stimulation, with no effect of apyrase or wortmannin. In contrast, these treatments markedly reduce the amount of myosin associated with the cytoskeletal fraction and affect the stability of the signaling complexes associated with the actin cytoskeleton. It is likely that the translocation of myosin to the cytoskeleton results from its binding to actin filaments,44a process regulated by phosphorylation of myosin light chain (MLC). Beside the role of the Ca2+/calmodulin-dependent MLC kinase in the initial responses induced by thrombin, the RhoA-dependent regulation of MLC phosphorylation might also occur.45 This is supported by the striking parallelism observed here between the low cytoskeletal content of myosin and RhoA and the reversible aggregation. Because PI 3-kinase inhibitors and apyrase had similar effects, it is tempting to suggest that ADP may play its role in the reorganization of the cytoskeleton via potentiating PtdIns(3,4)P2 synthesis. We are currently investigating whether this lipid could contribute to the stabilization of focal contacts and, in turn, to the irreversible ligand binding to αIIbβ346 in TRAP-stimulated platelets.
Together, our results emphasize the role of ADP as a cofactor and may explain its involvement in stabilizing platelet aggregates induced by other agonists.16,17 It is also noteworthy that ADP is involved in platelet spreading,8,9 a mechanism that requires PI 3-kinase activity.47 A better understanding of the molecular mechanisms involved in ADP-dependent regulation of the accumulation of PtdIns(3,4)P2 in TRAP-stimulated platelets, as well as the identification of the targets of this phosphoinositide, may lead to new pharmacological strategies to modulate platelet aggregation or spreading in vivo.
ACKNOWLEDGMENT
The authors thank Dr G. Mauco for helpful discussions.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Bernard Payrastre, PhD, INSERM U 326, Hôpital Purpan, 31059 Toulouse, France.

![Fig. 1. ADP is involved in the accumulation of [32P]PtdIns(3,4)P2 induced by TRAP. [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP in the absence or the presence of 1 U/mL apyrase (− and + in [A], • and ○ in [B] and [C]) for various periods of time. (A) Aggregation was measured as described in Materials and Methods. (B) Time course of PtdIns(3,4)P2 accumulation. (C) Time course of PtdIns(3,4,5)P3 accumulation. Lipids were analyzed using an HPLC technique as indicated in Materials and Methods. Data are from 1 representative experiment of 3 independent experiments that gave very similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4156/5/m_blod42429001x.jpeg?Expires=1766622752&Signature=SGjbpk8cgYdxAMEltey8F8s2AeOOhP3RoJVzJFaawDL2wWoEmxd4FRsXsr2hStDrnXHZq3B7s3eo~F~diuJw9xD34NySQ4McjU7b2gxWmj~ha4fjeqUbJQwOUfIRmAIp5x18uNlFuk9r0AkwhgkEtvE~5e5f3TVMknDh3J~jjY6yokKRxPuQt3LDb7CicRxZl1mnE4wbvR7ySi51T-LpULFsobk5o-00JADOT3JR~QulJcrdaQ3VxkdZGrUp46L8Rl0REJOc9JZBD39C45hMtYl4Rv7t3SC7av2plgq8Y5ec9F0yDy~NK54brAtLOz8-T7gyslmiT2dVEA-g1mINKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Time courses of [32P]PtdIns, [32P]PtdIns(4)P, [32P]PtdIns(4,5)P2, and [32P]PtdOH production and effects of released ADP in TRAP-stimulated platelets. Time courses of [32P]-labeling of PtdIns, PtdIns(4)P, PtdIns(4,5)P2, and PtdOH in [32P]-labeled platelets activated by 40 μmol/L TRAP in the absence (•) or the presence (○) of 1 U/mL apyrase. Radioactivity incorporated into various phospholipids was quantified using HPLC as in Fig 1. Results are representative of 3 experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4156/5/m_blod42429002x.jpeg?Expires=1766622752&Signature=JEbE2~xKYcMsVg1oVBqKFN5YSNrdxkOClXmhbAma4OJe3nEYQaEzIwrEhFsQnEwkytTXHhObG60F28htMOO3-WnQu-Wv8zDKTgsalfhpd589LMh2cG6PHEQWq2FDUgict2surtnp4VGbcdwhGe~3F~-gZbHRYKLggm0tuzP3w3huIua5aitJGuG5leI2G4ZX1MX134Wf4uPeMw2d1dlV4s0trmaTBFkYoU9bOi06iRN89tw5DGGEn~ifrEdbzatB-XtEwhKa~VRrnxGCjZg~c9ClORMspErke7SxqOzz4L3hR-begUID-Ee3ocTC4BxEspSFptbZZdjvwC3qJ9mEBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. The late and sustained activation of PI 3-kinase is required for PtdIns(3,4)P2 accumulation and irreversible aggregation induced by TRAP in the presence of ADP. Platelet aggregation was measured as described in Materials and Methods. Various concentrations of the 2 unrelated PI 3-kinase inhibitors, wortmannin (A) or LY294002 (B), were added 2 minutes after stimulation with 40 μmol/L TRAP, as indicated by the arrow. Data are representative of 3 independent experiments with very similar results. (C) [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP. After 2 minutes, 50 nmol/L wortmannin (•), 25 μmol/L LY 294002 (○), or 0.1% Me2SO (▪) was added. The reactions were stopped at different times by addition of chloroform/methanol and the level of [32P]PtdIns(3,4)P2 was measured by HPLC as indicated in Materials and Methods.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4156/5/m_blod42429003x.jpeg?Expires=1766622752&Signature=b7dp7pbBQeFQtebPCAuhCt9JjTOVrKh1WQQx-Lk9yht~V9hTboi9OcBrSlxP-qghWDPgv~T31lptfRtF8SjkSptx3C1m9hkinRgvk8~PZ~V2Idst7mG9glbAkORLkEKF9L2A~MmRv4qE5gX~FcPUU3D1FSz1w8DHlQXlEjL9otbl1p6KL-TF0j8NpGFYAnnKWZIyPm6wDTWUcIaFSCg366ww7uNyt9ENmo6EYy-fpVt4ZGrhpgTD-yTN0uCdj7FI6ciuvpKMkThi2kW7S-4HOcfXU3HCuyLt1Yhq5CJ1YcLZPikvvYE9Arvqba63npw0LGvn3YRX6-OXobNWkw8AlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of selective ADP receptor antagonists on TRAP-induced platelet aggregation and PtIns(3,4)P2accumulation. [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP in the absence or in the presence of the selective P2Y1 antagonist A2P5P (100 μmol/L), the selective antagonist of the P2 receptor coupled to adenylyl cyclase AR-C66096 (1 μmol/L), or an antagonist of both ADP receptors ATPS (100 μmol/L). (A) Aggregation was measured as described in Materials and Methods. (B) Time course of PtdIns(3,4)P2 accumulation. Lipids were immediately extracted and [32P]PtdIns(3,4)P2 was separated and quantified as in Fig 1. Results are expressed as the percentage of PtdIns(3,4)P2 produced, with 100% being the maximal production observed upon TRAP stimulation, and are the means ± SD from 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4156/5/m_blod42429004x.jpeg?Expires=1766622752&Signature=NAfgzIism7Exj-RWAluCEjM~oKWABxYRRjfFAxqyzGndsNs5RSqrPaUH035Fg~Z7rmA4alksUx~gNed~WnevCHbM2vHtnhP19a59D4CZwpbvZfXmh1E9zfSSyvr~YeBkd8AxnWIgkwFOVnpay05rxV4ZAskGH6uQTUDOWWSk~jn6mPXT2QlcZypc0lHG8cwwtvmNWN2vMXY7cVUPnSW-VrshqYfGFF3pwsTLO1hlhptUBbyigHtphfi7Al~C~h7A6fORQoo-BrW-s7GcJDDNg4x9aGFfXmn4hYCUhJqRU8Jp4Ly3OTik9~xZLdcwHsGfD96eHYFW0goiFPat3zNA3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. ADP is involved in the accumulation of [32P]PtdIns(3,4)P2 induced by TRAP. [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP in the absence or the presence of 1 U/mL apyrase (− and + in [A], • and ○ in [B] and [C]) for various periods of time. (A) Aggregation was measured as described in Materials and Methods. (B) Time course of PtdIns(3,4)P2 accumulation. (C) Time course of PtdIns(3,4,5)P3 accumulation. Lipids were analyzed using an HPLC technique as indicated in Materials and Methods. Data are from 1 representative experiment of 3 independent experiments that gave very similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4156/5/m_blod42429001x.jpeg?Expires=1766622753&Signature=ngf10o~QuEQqELM~VlYoyf0DdFx9iPAoylXD2TnhCJUrC1Z7w46Wn2-h8RvDNrm8yz7XGXhLKxYl7Zobiq3AAiwY5gKd8HimlpCN9LV9~AkNOkQmY3PpHFUPKWmzUCrPBl1jZZLXufd3oerhgz6kHfoGGiSUUU~d2jd0om1VAemcV9KCrahTgTxCkWsgQX13gDxOGslaRgv-NddJVShaABa0n6uDHSOrLDdIV1PXqRTvU4xWu8oe-OALThmaEWFnnNScNen0F9I2E7MasEPwBwpzVTT1IOlApDSIahUEZvPJpXTyr~IFS7IOR3QDgnfY4aEPjGggebTx7EBQ1cfTRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Time courses of [32P]PtdIns, [32P]PtdIns(4)P, [32P]PtdIns(4,5)P2, and [32P]PtdOH production and effects of released ADP in TRAP-stimulated platelets. Time courses of [32P]-labeling of PtdIns, PtdIns(4)P, PtdIns(4,5)P2, and PtdOH in [32P]-labeled platelets activated by 40 μmol/L TRAP in the absence (•) or the presence (○) of 1 U/mL apyrase. Radioactivity incorporated into various phospholipids was quantified using HPLC as in Fig 1. Results are representative of 3 experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4156/5/m_blod42429002x.jpeg?Expires=1766622753&Signature=dRYMiZBUWPj4yk5jlM6eV4uGhelKPN6q4i1Z5MfYVo6eD5Wzbx-RzDNuIzUQMPTANDpVOdo9E8uorQ0a19Bn3UjK429Aer~hqK~tbt4-WpnCdFvlAs1VILc8ZMO7A1tAOykGvOSRhpOB2mp6JXSRnCa7cfSRQvmMrRWUO7uT6VF6pzWdefVjsIlSQ7Ld1Z2x5Bp27TRbXreyd7Ao~2EjtBW2S0laonmoya-y6bVWAlT4llO7wu4Ohy~dLLQnzLr44Z2XcXiK6uScy6QVsrbj60BOwtgdk3GEjtcwSnGeXRy3Vh8wsiN2TT4BMSKEgNn6T00fNugWmY9jfIHl8QuBTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. The late and sustained activation of PI 3-kinase is required for PtdIns(3,4)P2 accumulation and irreversible aggregation induced by TRAP in the presence of ADP. Platelet aggregation was measured as described in Materials and Methods. Various concentrations of the 2 unrelated PI 3-kinase inhibitors, wortmannin (A) or LY294002 (B), were added 2 minutes after stimulation with 40 μmol/L TRAP, as indicated by the arrow. Data are representative of 3 independent experiments with very similar results. (C) [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP. After 2 minutes, 50 nmol/L wortmannin (•), 25 μmol/L LY 294002 (○), or 0.1% Me2SO (▪) was added. The reactions were stopped at different times by addition of chloroform/methanol and the level of [32P]PtdIns(3,4)P2 was measured by HPLC as indicated in Materials and Methods.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4156/5/m_blod42429003x.jpeg?Expires=1766622753&Signature=tFaijOR~1ywsZZ4Llwg4Ai7x15K8mXqP7UW26RxYyB4EkOgoFGdD7JUCMnMP8e1RlZqEnn5Diazi1kJ~YputXP0e7uZaY2ZgqAnbzmhus-cmA-KdSEyNQwBhlLgnON0tKcIPtseiJ2ndEbQRnveT~QohwbXtbwEfmd4JbVOXhrh~MIiWHaIWlDo2iA-vCYMgRkdTfgy0kmSk8hhQcWLRQXOYJp83lOXc6psR0PHjyXhDPIh2g~3vt8LtSVWCIP29TgHkxOVazrHWmjW91IutsbVlUoZiRnXMSdPY88GroFGna8mDi8tBKZgQm0NjwPXkU5245oe8AOtOgjw0XVOa4Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of selective ADP receptor antagonists on TRAP-induced platelet aggregation and PtIns(3,4)P2accumulation. [32P]-labeled human platelets (7.5 × 108/mL) were stimulated with 40 μmol/L TRAP in the absence or in the presence of the selective P2Y1 antagonist A2P5P (100 μmol/L), the selective antagonist of the P2 receptor coupled to adenylyl cyclase AR-C66096 (1 μmol/L), or an antagonist of both ADP receptors ATPS (100 μmol/L). (A) Aggregation was measured as described in Materials and Methods. (B) Time course of PtdIns(3,4)P2 accumulation. Lipids were immediately extracted and [32P]PtdIns(3,4)P2 was separated and quantified as in Fig 1. Results are expressed as the percentage of PtdIns(3,4)P2 produced, with 100% being the maximal production observed upon TRAP stimulation, and are the means ± SD from 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4156/5/m_blod42429004x.jpeg?Expires=1766622753&Signature=TufjdsV8vWmSgTjsflyK1qgh0e5Wh85xELQ9qc~AmQ7H4Z0yxH1-MjP0eEbrgL~83~xkdJlTYQswwHhZAPO65flWgv9PJ5KFMd51H-hAmBrtM-QeAEXeg9EOyY4GvKYsOxtv9BuiL~UIkMPPX1ZxkndKHC5VV~CmWaTFRlxWSHM6uyby2iSYV3-jztZNgJDWAgUFgjLl8VWq~UR18M8sqU9~fHNmDh4tT0Bzr0M3zupiJYcMhrmQrk5xjKSe-BZICbPwP8BPfwMB7gxVtN18EwQroLvo7S2k-rF4jS49LwU~soMzK34iQaNMFD303N~7KKKcUuIE968L73KBmsIIyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)