The glycosaminoglycan hyaluronate (HA) is part of the extracellular environment in bone marrow. We show here that HA activates signal transduction cascades important for hemopoiesis. In myeloid and lymphoid long-term bone marrow cultures (LTBMC), treatment with hyaluronidase (HA’ase) results in reduced production of both progenitor and mature cells. Exogeneous HA added to LTBMC had the opposite effect: it enhanced hematopoiesis. The effect of HA is mediated through two different HA receptors on bone marrow macrophage-like cells, one of which is CD44 while the other is unknown. HA induces bone marrow macrophages to secrete IL-1β (CD44-dependent) and IL-6 (CD44-independent). The two receptors address different signal transduction pathways: CD44 links to a pathway activating p38 protein kinase while the other yet unknown receptor induces Erk activity. There was no difference of the effect of HA and HA’ase on hematopoiesis in LTBMC and on cytokine production by macrophages in CD44-deficient mice compared with wild-type mice, indicating that the CD44 hyaluronate receptor and its signal transduction can be compensated for. Our data suggest a regulatory role for the extracellular matrix component HA in hematopoiesis and show the induction of signal transduction by HA receptors.
THE BEHAVIOR OF CELLS, reflected by their program of gene expression, is guided and controlled by microenvironmental cues. The bone marrow represents an instructive example of such microenvironmental influences. Hematopoiesis in bone marrow depends on the mutual interaction of hematopoietic stem cells and progenitor cells with stromal cells, and on the interaction of these cells with components of the extracellular matrix (ECM).1-3 The ECM in bone marrow consists mainly of fibronectin, hemonectin, thrombospondin, collagens, laminin, and the glycosaminoglycans (GAGs) heparan sulfate, dermatan, chondroitin sulfate (CS), and hyaluronic acid (HA).4 5
The development of hematopoietic cells in the bone marrow can be mimicked in vitro, at least in part, in so-called long-term bone marrow culture (LTBMC).6 LTBMC contains the cellular constituents of bone marrow and supports the generation of progenitors and their differentiation into certain lineages. During culture, ECM is produced mainly by stromal cells, and components of the ECM such as heparan sulfate, CS, and fibronectin are required for the development of hematopoietic progenitors.7-10 HA has also been detected in LTBMC but it is not known whether it plays a role in hematopoiesis.11
This study has been motivated by our interest in the function of CD44 proteins which are the major known HA receptors on the surface of cells.12 Hematopoietic progenitor cells in the bone marrow and progenitor cell lines carry one or several different CD44 proteins on their surface,13,14 all of which carry an HA-binding motif in the N-terminal domain.15,16 As CD44 is required for myelopoiesis in LTBMC,17 18 it is conceivable that HA binding is an essential step in hematopoiesis. In this study we examine whether HA addition or its depletion by treatment with hyaluronidase (HA’ase) influences hematopoiesis in LTBMC. We report that HA enhances and HA’ase inhibits both myelopoiesis and lymphopoiesis. HA exerts this effect by binding to a subpopulation of adherent stromal cells and by inducing the production of interleukin-1β (IL-1β) and IL-6. Enriched bone marrow–derived macrophages (BMDM) respond to HA by the release of both cytokines. Antibodies directed against the HA-binding motif of CD44 inhibit IL-1β secretion but not IL-6 release by macrophages, indicating that BMDM carry two HA receptors, both of which mediate signal transfer in response to HA. The signal transduction pathway from CD44 to IL-1β involves p38 MAP kinase phosphorylation, clearly distinguished from the other HA induced pathway leading to Erk activation and IL-6 release. Our data support the idea that, in addition to other extracellular matrix components, HA controls hematopoiesis at an early progenitor stage before commitment toward the myeloid and lymphoid lineages.
MATERIALS AND METHODS
Cell lines.
Rat fibrosarcoma (RFS) cells19 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS). Their supernatant was used as a source of HA.19 Wehi-3B cells20 were cultured in RPMI supplemented with 10% FCS. The conditioned medium (CM) was used as a source of IL-3.20 L929 cells21 were cultured in DMEM supplemented with 10% FCS. Their CM was used as a source of macrophage colony-stimulating factor (M-CSF).21 An IL-7 producer cell line was kindly provided by Dr H. Karasuyama (Basel Institute for Immunology, Basel, Switzerland)22 and cultured in RPMI with 10% FCS. The murine T-cell lymphoma cell line HA923 was provided by Dr D. Naor (Jerusalem). It was used as a positive control for CD44-dependent HA binding.
Mice.
C57Bl/6, DBA, (C57Bl/6 × DBA)F1, BALB/c, and 129 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were used for the experiments at the age of 6 to 12 weeks. CD44−/− mice were obtained from Dr T.W. Mak (University of Toronto, Toronto, Canada)24and back-crossed into a C57Bl/6 background. All animals were kept under specific pathogen-free conditions.
LTBMC.
Myeloid LTBMC were established as described.6 In brief, mice were killed by cervical dislocation, and femurs and tibias were removed. Bone marrow cells were flushed out of the bones with phosphate-buffered saline (PBS) containing 2% FCS. Bone marrow cells (1 × 106 cells/mL) were cultured in DMEM supplemented with 20% horse serum (Linaris, Bettingen, Germany) and hydrocortisone (10−6 mol/L; Sigma, St Louis, MO) in 6-well plates at 33°C in a humid atmosphere containing 5% CO2. Cultures were fed weekly by changing half of the culture medium. Nonadherent cells were collected from the culture medium, counted, and used for flow cytometry or assayed for colony-forming units (CFU). HA or HA’ase, respectively, was added to LTBMC medium before culture set-up and before medium changes.
Lymphoid LTBMCs were performed as described.25 Briefly, bone marrow cells were cultured in RPMI supplemented with 5% FCS, 5 × 10−5 2-mercaptoethanol (2-ME) and 2 mmol/L L-glutamine, at 37°C and 5% CO2 in a humid atmosphere. Cultures were fed weekly by changing half of the medium.
BMDM cultures.
BMDM cultures were obtained using a protocol previously described.27 Bone marrow cells were flushed from femurs and tibias with PBS containing 2% FCS. The bone marrow cells were dispersed at 1 × 106 cells per mL in DMEM containing 2 mmol/L glutamine, 0.37% (wt/vol) NaHCO3, 10% (vol/vol) heat-inactivated FCS, and 10% (vol/vol) L929 cell-conditioned medium. Cultures were maintained at 37°C in a 5% CO2atmosphere for 5 days. The medium was changed and cells were used between days 6 and 8 thereafter. Ninety-nine percent of cells in the monolayer were esterase-positive and were phagocytic for latex particles. The cells were starved for 12 hours before stimulation with HA.
CFU assay.
Bone marrow cells or nonadherent cells from LTBMC were plated at a concentration of 1 × 104 cells/mL in semisolid methylcellulose supplemented with 30% FCS, 1% bovine serum albumin (BSA), 10−4 mol/L 2-ME, 2 mmol/L L-glutamine (StemCell Technologies, Vancouver, Canada), and 15% Wehi-3B CM as a source of IL-3.28 Plates were cultured at 37°C in 5% CO2 atmosphere. Colonies were counted under the microscope after 7 days of culture. Other cytokines used for determination of lineage-specific progenitors were granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF, and erythropoietin (Boehringer Mannheim, Mannheim, Germany), M-CSF (supernatant of L929 cells) and IL-7 (supernatant of a producer cell line, see Cell lines).
Flow cytometry.
The monoclonal antibody (MoAb) KM 81 was obtained from American Type Culture Collection (ATCC; Rockville, MD; no. TIB241), phycoerythrin (PE)-labeled anti-mouse CD11b/CD18 was obtained from Cedarlane (Ontario, Canada). Rooster comb HA (Sigma) and chondroitin sulphate A (CS-A; Fluka, Buchs, Switzerland) were fluorescein isothiocyanate (FITC)-labeled as previously described.29 For flow cytometry, 5 × 105cells were incubated with PE- or FITC-labeled antibodies at a concentration of 10 μg/mL at 4°C for 30 minutes and washed twice with FACS-buffer (PBS, 3% FCS, 0.01% NaN3). Fluorescence was analyzed on a FACStar Plus flow cytometer (Becton Dickinson, Heidelberg, Germany).
Immunohistochemistry.
For examination of the HA binding ability of adherent cells from LTBMC, cells were fixed with 3% paraformaldehyde, washed with PBS, and incubated with FITC-labeled HA in PBS at 4°C for 30 minutes. Cultures were washed and inspected by fluorescence microscopy. The cytoskeleton was stained as follows: Cells of LTBMC were washed twice with PHEM-buffer (60 mmol/L PIPES, 25 mmol/L HEPES, 10 mmol/L EGTA, 2 mmol/L MgCl2, pH 6.9), fixed with 2.5% glutaraldehyde (Serva, Heidelberg, Germany) in stabilizing buffer (20 mmol/L HEPES, 5 mmol/L EGTA, 2 mmol/L phenylmethylsulfone fluorid (PMSF), 1 mmol/L MgSO4, 1 g/L Na-tosyl-L-argininemethylester, 20% glycerol, pH 6.8) at 37°C for 15 minutes. Thereafter, cells were treated with 50 to 100 μL staining solution (3.3 μmol/L phalloidine-rhodamine in methanol 1:10 in PHEM-buffer) at 37°C for 2 hours. The stained probes were evaluated by fluorescence microscopy.
Enzyme-linked immunosorbent assay (ELISA).
LTBMC or BMDMC were stimulated by HA (100 μg/mL), and CM was collected and kept frozen in aliquots at −80°C until use. CM samples were checked by ELISA (Endogen, Woburn, MA) for IL-1α, IL-1β, IL-3, IL-4, IL-6, IL-10, tumor necrosis factor-α (TNF-α), and GM-CSF according to the instructions of the manufacturer.
Protein analysis.
Western blot analysis of signal transduction kinases was performed according to standard procedures. Cells were lysed in sample buffer (20% glycerol, 4% sodium dodecyl sulfate [SDS], 0.16 mol/L Tris pH 6.8, 4% 2-ME, 0.05% bromphenol blue). Lysates were sonicated and heated at 100°C for 5 minutes. Proteins were resolved by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immunobilon-PVDF membrane (Millipore, Bedford, UK). Immunostaining was performed using anti–phospho-p38 and anti–phospho-Erk antibodies (New England Biolabs, Beverly, MA; cat. nos. 9210 and 9101, respectively). Horseradish peroxidase–linked anti-rabbit antibodies (DAKO, Hamburg, Germany) were used as secondary antibodies. The staining was visualized using the enhanced chemiluminescence (ECL) reaction (Amersham, Freiburg, Germany). To control for protein loading, the membranes were stripped and incubated with anti-p38 and anti-Erk antibodies (Santa Cruz Biotech, Santa Cruz, CA; SC535 and SC1647).
RESULTS
HA’ase treatment inhibits hematopoiesis in LTBMC.
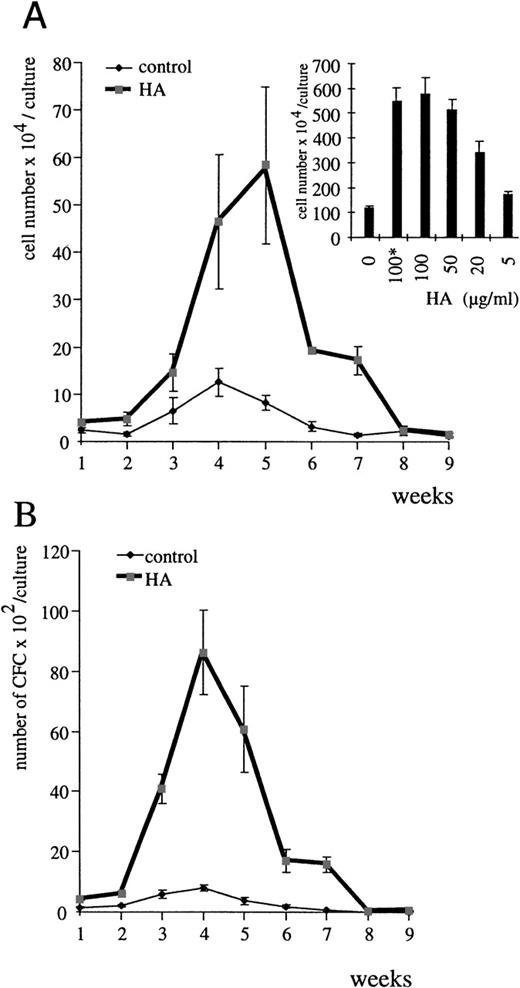
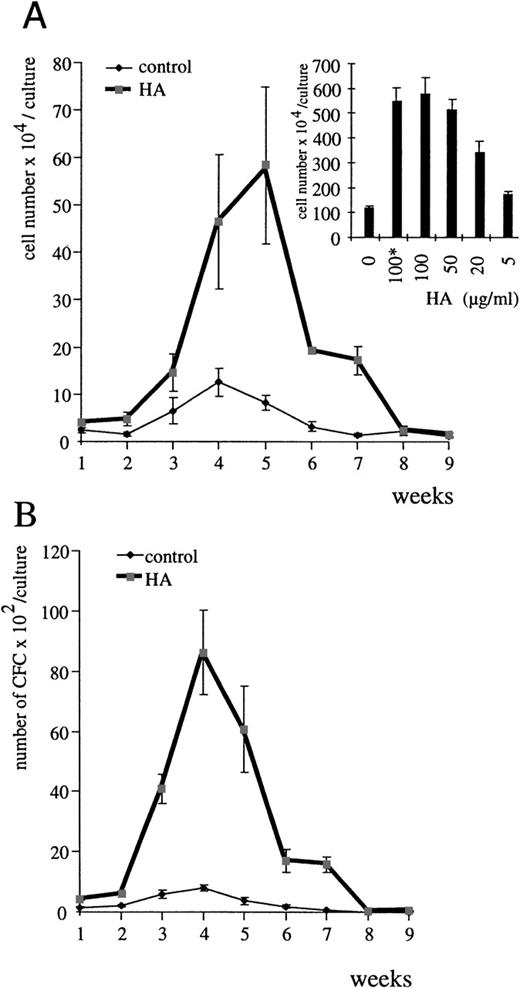
To assess a role for HA in hematopoiesis, we examined the effect of HA deprivation on the production of nonadherent cells in myeloid and lymphoid LTBMC. To achieve HA deprivation, LTBMC were cultured in the presence of HA’ase. The production of nonadherent cells in both lymphoid (Table 1) and myeloid cultures (Fig 1A) was severely inhibited by HA’ase treatment throughout the course of the culture. The inhibition was dose-dependent (see inset in Fig 1A).
Influence of HA’ase on myelopoiesis in LTBMC. (A) Nonadherent cells from myeloid LTBMC in the absence of HA’ase (control) or treated with HA’ase (Streptomyces HA’ase, Calbiochem; 0.1 U/mL) were harvested once per week, and their number was counted. Standard error (SE) was calculated from results obtained with 3 independent cultures. The insert shows the dose-dependent effect of HA’ase on the production of nonadherent cells in LTBMC after 4 weeks in culture. The bars indicate SE calculated from 3 independent wells each. (B) The production of clonogenic cells in either control LTBMC or in LTBMC treated with HA’ase (0.1 U/mL) was measured by the CFU assay (Materials and Methods). Nonadherent cells were harvested from LTBMC and plated in semisolid culture in the presence of IL-3 (Materials and Methods). SE was calculated from results obtained from 3 independent wells.
Influence of HA’ase on myelopoiesis in LTBMC. (A) Nonadherent cells from myeloid LTBMC in the absence of HA’ase (control) or treated with HA’ase (Streptomyces HA’ase, Calbiochem; 0.1 U/mL) were harvested once per week, and their number was counted. Standard error (SE) was calculated from results obtained with 3 independent cultures. The insert shows the dose-dependent effect of HA’ase on the production of nonadherent cells in LTBMC after 4 weeks in culture. The bars indicate SE calculated from 3 independent wells each. (B) The production of clonogenic cells in either control LTBMC or in LTBMC treated with HA’ase (0.1 U/mL) was measured by the CFU assay (Materials and Methods). Nonadherent cells were harvested from LTBMC and plated in semisolid culture in the presence of IL-3 (Materials and Methods). SE was calculated from results obtained from 3 independent wells.
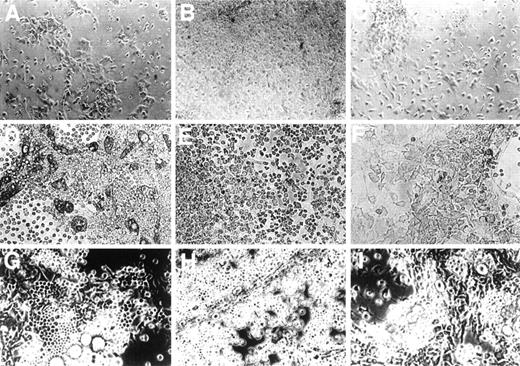
HA’ase treatment did not alter the morphology of adherent stromal cells, but the ratio of progenitors to stromal cells rapidly changed after the first week of culture. Areas of hematopoiesis, “cobblestone areas,” appeared much later and were significantly smaller in HA’ase-treated cultures (Fig 2, see different time points in C, F, and I) than in control cultures (Fig2, A, D, and G). To quantitate this observation, we compared the proliferative capacity of adherent cells in control and HA’ase-treated LTBMC by measuring 3H-thymidine incorporation. HA’ase treatment reduced thymidine incorporation to less than 10% (not shown), a reduction that matched the reduction of proliferating cells within the cobblestone areas of the adherent cell layer (compare Fig 2D and F). Consistant with the decreased number of hematopoietic areas, the number of clonogenic cells in the LTBMC as measured by CFU assay was dramatically reduced by HA’ase treatment (Fig 1B and Table 1).
Influence of HA and of HA’ase on formation of the adherent layer. Primary bone marrow cells were cultured in 6-well plates without any addition (A, D, and G) or with the addition of 100 μg/mL of HA (B, E, and H) or with 0.1 U/mL HA’ase (C, F, and I). Photographs were taken after 1 (top panel), 4 (middle panel), and 8 (bottom panel) weeks of culture. Only adherent cells are shown. In (D), (E), (G), and (H), typical cobblestone-like cells are visible, which are increased in number in (E) and (H), whereas in (F) and (I) such cells are hardly detectable. The magnification was 400-fold.
Influence of HA and of HA’ase on formation of the adherent layer. Primary bone marrow cells were cultured in 6-well plates without any addition (A, D, and G) or with the addition of 100 μg/mL of HA (B, E, and H) or with 0.1 U/mL HA’ase (C, F, and I). Photographs were taken after 1 (top panel), 4 (middle panel), and 8 (bottom panel) weeks of culture. Only adherent cells are shown. In (D), (E), (G), and (H), typical cobblestone-like cells are visible, which are increased in number in (E) and (H), whereas in (F) and (I) such cells are hardly detectable. The magnification was 400-fold.
To define whether HA’ase acted directly on proliferation of the progenitor cells, we examined the effect of HA’ase on colony formation of primary bone marrow progenitor cells in methylcellulose. Depending on the type of progenitor to be determined, different cytokines were added. For polypotent and bipotent myeloid progenitors, IL-3 and GM-CSF, respectively, were added. For erythroid burst-forming colonies, IL-3 together with erythropoietin was added. For monopotent macrophage and granulocyte progenitors, M-CSF and G-CSF, respectively, were added, and for lymphoid progenitors, IL-7 was added. Colony formation by any of these treatments was not influenced by the presence of HA’ase (not shown), suggesting that HA’ase does not act directly on progenitor cells. Furthermore, this result shows that HA’ase exerted no toxic effects on progenitors, but rather specifically inhibited hematopoiesis at the level of stromal cells.
Exogeneously added HA increases cell production in LTBMC.
The HA’ase data speak for a regulatory role of HA in hematopoiesis. This conclusion is further supported by the effects we observed when HA was added exogenously to LTBMC. Both commercially available HA (Fig 3) and HA from CM of HA-producing RFS cells (not shown)19 stimulated the yield of myeloid (Fig3A) and lymphoid (Table 1) nonadherent cells in LTBMC in a dose-dependent manner. The optimal HA concentration required to increase the number of nonadherent cells in LTBMC was 100 μg/mL (inset in Fig 3A). Measurement of the endogenous concentration of HA in the supernatant of LTBMC by staining of HA with stain-all26was less than 10 μg/mL (below detection level). After Proteinase K treatment of LTBMC and vigorous shaking, HA could be measured in the supernatant (15 to 20 μg/mL). Exogenously added HA was easily detected and within the 7-day period between medium change no change in HA concentration was measurable. The total number of clonogenic cells in LTBMC was also increased (Fig 3B and Table 1). Both the number of cells attached to plastic and the number of cells forming areas of hematopoiesis were significantly higher in the HA-treated cultures than in control cultures (see Fig 2B, E, and H in comparison with A, D, and G). The HA-dependent increase was detectable as early as 7 days after the start of the cultures. To rule out any contamination of the HA preparations with cytokines, we heat-treated the HA preparations before addition to LTBMC. The heat treatment had no influence on the results (Fig 3A inset, and not shown).
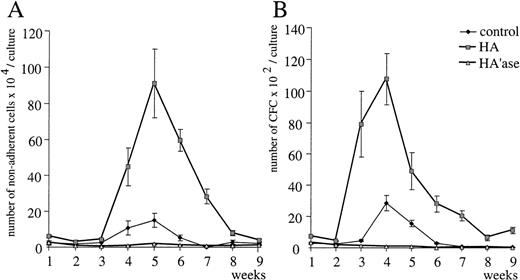
HA stimulates myelopoiesis in LTBMC. (A) Nonadherent cells from myeloid LTBMC in the absence of HA (control) or treated with HA (100 μg/mL; rooster comb HA from Sigma) were determined each week. SE was calculated from results obtained from 3 independent wells. The inset shows the dose-dependent effect of HA on the production of nonadherent cells in myeloid LTBMC after 4 weeks in culture. The asterisk indicates the use of heat-treated HA (1 hour at 95°C). The bars indicate the SE determined from results obtained from 3 independent wells. (B) Clonogenic cells in myeloid LTBMC grown in the absence of HA (control) or presence of HA (100 μg/mL) were determined at the time indicated by plating of nonadherent cells in semisolid methylcellulose culture medium in the presence of IL-3 (Materials and Methods). SE was calculated from results obtained from 3 different wells.
HA stimulates myelopoiesis in LTBMC. (A) Nonadherent cells from myeloid LTBMC in the absence of HA (control) or treated with HA (100 μg/mL; rooster comb HA from Sigma) were determined each week. SE was calculated from results obtained from 3 independent wells. The inset shows the dose-dependent effect of HA on the production of nonadherent cells in myeloid LTBMC after 4 weeks in culture. The asterisk indicates the use of heat-treated HA (1 hour at 95°C). The bars indicate the SE determined from results obtained from 3 independent wells. (B) Clonogenic cells in myeloid LTBMC grown in the absence of HA (control) or presence of HA (100 μg/mL) were determined at the time indicated by plating of nonadherent cells in semisolid methylcellulose culture medium in the presence of IL-3 (Materials and Methods). SE was calculated from results obtained from 3 different wells.
Consistent with the data obtained by HA depletion, HA appeared to act on stromal cells in that HA did not influence colony outgrowth from committed progenitors in methylcellulose supplied with any of the cytokines specified above (not shown). Addition of CS-A, another GAG found in the ECM of bone marrow, did not further stimulate hematopoiesis in vitro over a wide range of concentrations (data not shown), demonstrating the specificity of HA action. Thus, complementing the data obtained by HA’ase treatment, addition of exogenous HA promotes both myelopoiesis and lymphopoiesis.
HA upregulates IL-1β and IL-6 production in LTBMC.
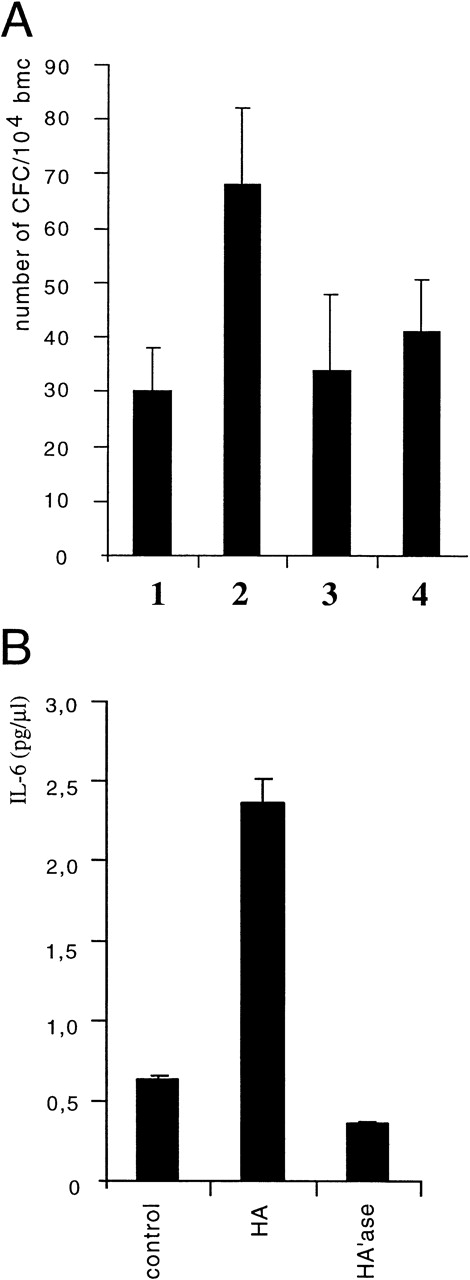
Because HA did not directly act on progenitors during growth in methylcellulose but rather appeared to influence hematopoiesis via stromal cells, its mode of action could be either to stimulate stromal cells to act directly on progenitor cells, or to produce and/or release an activity that promotes progenitor proliferation. The first assumption seemed unlikely, because in transwell assays where we separated nonadherent cells (including progenitors) from stromal cells by a membrane, HA treatment of stromal cells still resulted in an increased number of nonadherent cells in the transwell (not shown). To test for the latter possibility, CM from control cultures and from HA-treated cultures were examined for the ability to stimulate myeloid colony formation in methylcellulose of primary bone marrow cells in the presence of suboptimal concentrations of IL-3. CM from HA-treated cultures indeed enhanced both the colony size (not shown) and the number of colonies (Fig 4A) compared with control CM. We conclude that CM from HA-treated cultures contained a soluble colony-promoting activity produced by stromal cells.
HA stimulates the production of a colony promoting activity in LTBMC. (A) LTBMC was treated with HA (100 μg/mL). CM was harvested at day 28 and tested for colony-promoting activity. Freshly isolated bone marrow cells were added to methylcellulose cultures and the colony formation by myelopoietic progenitors was determined in the presence of suboptimal doses of IL-3 (5% CM of Wehi-3B cells) and the addition of 20% of CM from either control LTBMC (lanes 1 and 3) or of CM from LTBMC treated with HA (lanes 2 and 4). After 7 days of culture, the numbers of colonies were counted. In lanes 3 and lane 4, hematopoietic progenitors were determined similarly to lanes 1 and 2, but antibodies directed against IL-6 (100 μg/mL) were added in addition. SE was calculated from 3 independent assays. (B) CM of control LTBMC or LTBMC cultured for 4 weeks in the presence of HA (100 μg/mL) or in the presence of HA’ase (0.1 U/mL) was assayed for IL-6 by ELISA.
HA stimulates the production of a colony promoting activity in LTBMC. (A) LTBMC was treated with HA (100 μg/mL). CM was harvested at day 28 and tested for colony-promoting activity. Freshly isolated bone marrow cells were added to methylcellulose cultures and the colony formation by myelopoietic progenitors was determined in the presence of suboptimal doses of IL-3 (5% CM of Wehi-3B cells) and the addition of 20% of CM from either control LTBMC (lanes 1 and 3) or of CM from LTBMC treated with HA (lanes 2 and 4). After 7 days of culture, the numbers of colonies were counted. In lanes 3 and lane 4, hematopoietic progenitors were determined similarly to lanes 1 and 2, but antibodies directed against IL-6 (100 μg/mL) were added in addition. SE was calculated from 3 independent assays. (B) CM of control LTBMC or LTBMC cultured for 4 weeks in the presence of HA (100 μg/mL) or in the presence of HA’ase (0.1 U/mL) was assayed for IL-6 by ELISA.
This activity could represent one or several of the cytokines known to act on bone marrow stem cells and early hematopoietic progenitors.28 Therefore, the HA-CM was examined by ELISA for the presence of GM-CSF and IL-1α, -1β, -3, -4, -6, and -10. The level of IL-6 was upregulated 4- to 5-fold in LTBMC derived fom one of several strains of mice (C57Bl/6xDBA)F1, DBA, BALB/c, or 129) upon treatment with HA (Fig 4B). Among the other cytokines, only IL-1β levels were slightly but consistantly enhanced upon HA treatment (not shown). To prove that the colony-promoting activity of CM from HA-treated cultures was indeed caused by the production of IL-6, we added cytokine-neutralizing antibodies. The effect of the HA-CM on CFU formation was reduced to the level of control CM by IL-6–specific antibodies (Fig 4A). Hence, we can conclude that HA induces in LTBMC the release of IL-6 (and weakly of IL-1β) from adherent stromal cells, which promotes the proliferation of hematopoietic progenitors.
HA binds to the surface of a subpopulation of stromal cells in LTBMC.
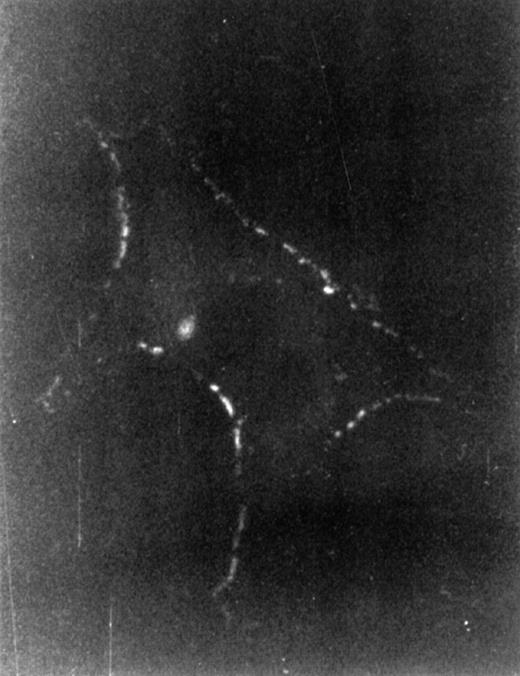
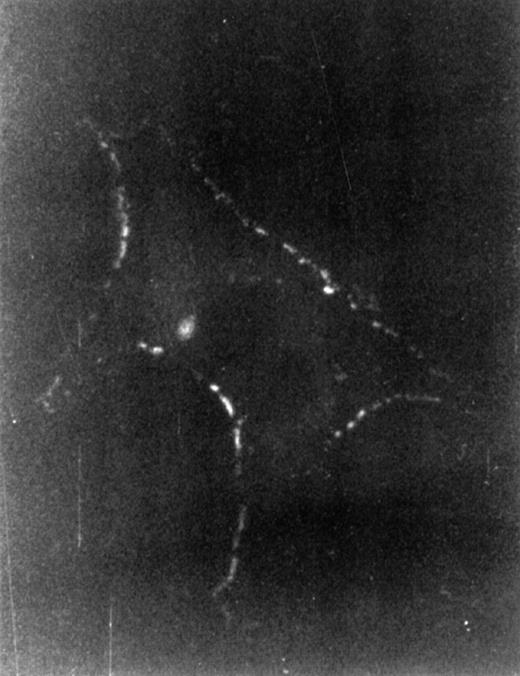
To investigate which cell type responded to HA by cytokine production, we first examined LTBMC cells for binding FITC-labeled HA. To this end, the cells to be examined were incubated with FITC-HA either directly or after treatment with HA’ase to remove endogenously produced HA that might preoccupy putative receptors, and thus to increase the binding sensitivity. Incubation with FITC-HA was done at room temperature or at 4°C for 30 minutes. Nonadherent cells from LTBMC in steady state (after 4 weeks in culture) showed no staining as determined by flow cytometry (not shown). The adherent cells were paraformaldehyde-fixed before incubation with FITC-HA at 4°C. In contrast, patchy staining was detected with stromal cells with a typical macrophage morphology (Fig 5). Cobblestone areas, however, showed no staining (data not shown). Based on this observation, we focused our attention on the stromal population, because we considered these cells candidates for HA-induced signaling. The macrophage nature of the HA-stained cells was confirmed by the presence of CD11b antigen (data not shown).
Binding of HA to macrophage cells. After 4 weeks, LTBMC nonadherent cells were washed out, adherent cells were treated for 30 minutes at 37°C with HA’ase (5 U/mL), and then fixed with 3% paraformaldehyde and incubated with FITC-labeled HA at 4°C for 30 minutes. Immunofluorescence-staining of a representative cell is shown. Magnification was 1,000-fold. Similar staining was observed without HA’ase treatment.
Binding of HA to macrophage cells. After 4 weeks, LTBMC nonadherent cells were washed out, adherent cells were treated for 30 minutes at 37°C with HA’ase (5 U/mL), and then fixed with 3% paraformaldehyde and incubated with FITC-labeled HA at 4°C for 30 minutes. Immunofluorescence-staining of a representative cell is shown. Magnification was 1,000-fold. Similar staining was observed without HA’ase treatment.
HA induces cytokine release from BMDM.
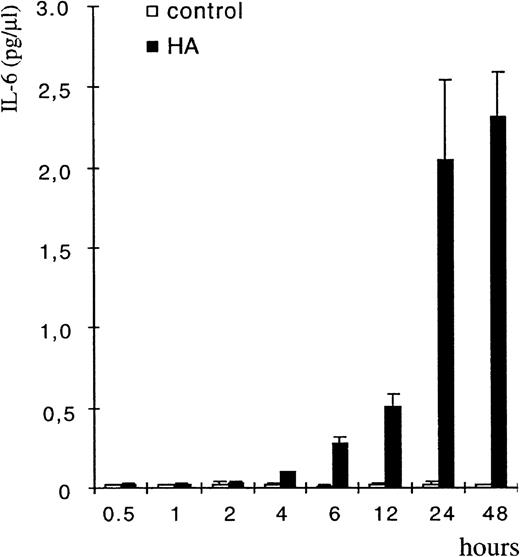
To prove that BMDM were the targets of HA and the source of HA-induced cytokine release, bone marrow cells were cultured in the presence of M-CSF to enrich for macrophages. Enriched macrophages were stained by FITC-labeled HA similarly to the monocytic cells in LTBMC (not shown). HA caused upregulation of cytokine release, both of IL-6 and of IL-1β, with a lag period of 2 to 4 hours as measured by ELISA (Fig 6 and Fig 9). BMDM from all mice strains tested [(C57Bl/6xDBA)F1, DBA, BALB/c, or 129] were inducible for both cytokines, and the induction of IL-1β was considerably stronger in the enriched macrophage preparation than in LTBMC.
HA treatment of BMDM upregulates IL-6 production. BMDM were isolated as described in Materials and Methods and were then treated with HA (100 μg/mL). CM was collected at the indicated times and tested for the presence of IL-6 by ELISA. The error bars indicated SE calculated from results obtained from 3 independent wells.
HA treatment of BMDM upregulates IL-6 production. BMDM were isolated as described in Materials and Methods and were then treated with HA (100 μg/mL). CM was collected at the indicated times and tested for the presence of IL-6 by ELISA. The error bars indicated SE calculated from results obtained from 3 independent wells.
HA-induced cytoskeletal rearrangement in BMDM.
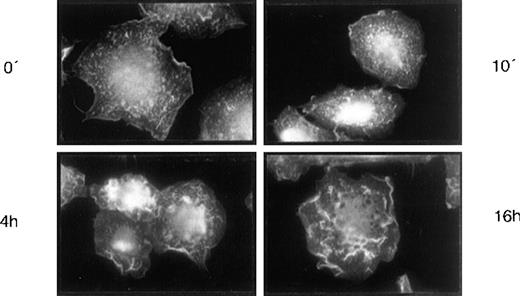
The brief response kinetic for cytokine release (Fig 6) is compatible with a direct action of HA on signal transduction triggering cytokine synthesis and release. Further to this, we detected a second type of HA-induced response in BMDM which may also be the result of a signal transduction process. At 4 hours after HA addition, the BMDM were stained with phalloidine-rhodamine, which showed that the actin cytoskeleton was rearranged (Fig 7). Such changes in the cytoskeleton are indicative of signal transduction.30 The changes in the cytoskeleton are comparable to those that occur during macrophage activation (not shown).
HA-induced actin rearrangement in LTBMC adherent cells. Adherent cells of LTBMCs after 4 weeks in culture were incubated with HA (100 mg/μL) for 10 minutes, 4 hours, and 16 hours, respectively. Staining of the actin cytoskeleton by phalloidine-rhodamine (Materials and Methods) is shown. Magnification was 1,000-fold.
HA-induced actin rearrangement in LTBMC adherent cells. Adherent cells of LTBMCs after 4 weeks in culture were incubated with HA (100 mg/μL) for 10 minutes, 4 hours, and 16 hours, respectively. Staining of the actin cytoskeleton by phalloidine-rhodamine (Materials and Methods) is shown. Magnification was 1,000-fold.
Two hyaluronate receptors mediate signal transduction.
The action of HA on enriched BMDM raises two questions: Which is the relevant surface receptor for HA, and which signal transduction pathway is this receptor connected to? Because CD44 is a plausible receptor candidate, we investigated the effect of an antibody that blocks the HA-binding motif of CD44 (KM81), on cytokine production of BMDM. Because antibodies may bind to the Fc-receptor on macrophages and thereby activate the cells, we used F(ab′)2 fragments of KM81 antibodies for the blocking studies. Interestingly, KM81 F(ab′)2 fragments reduced HA-induced IL-1β release, but not that of IL-6 (Fig 8). The KM81 F(ab′)2 fragments were functional in that they blocked the binding of HA to the cell line HA9 (a derivative of the murine lymphoma LB17 selected for HA binding to CD4423), and they were functional throughout the experiment (24 hours) because the medium from the macrophages treated with HA and KM81 F(ab′)2 for 24 hours still blocked the binding of HA to the HA9 cell line (data not shown). These results indicate the presence of at least two relevant HA receptors on BMDM: CD44 and an yet unknown receptor.
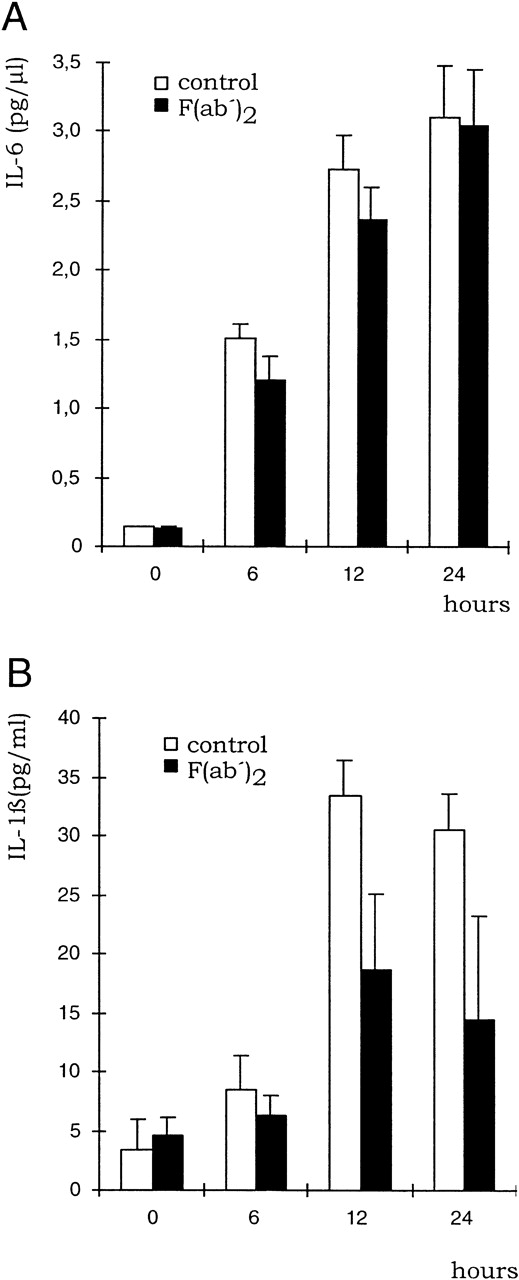
Influence of KM81 MoAb on cytokine production by macrophages upon HA treatment. BMDM were treated with HA (100 μg/mL) for the times indicated either in the presence or absence of F(ab′)2 fragments of the pan CD44 antibody KM81 (20 μg/mL). The concentration of IL-6 (A) and IL-1β (B) was determined in the supernatant by ELISA. SE was calculated from results obtained from 3 independent wells each.
Influence of KM81 MoAb on cytokine production by macrophages upon HA treatment. BMDM were treated with HA (100 μg/mL) for the times indicated either in the presence or absence of F(ab′)2 fragments of the pan CD44 antibody KM81 (20 μg/mL). The concentration of IL-6 (A) and IL-1β (B) was determined in the supernatant by ELISA. SE was calculated from results obtained from 3 independent wells each.
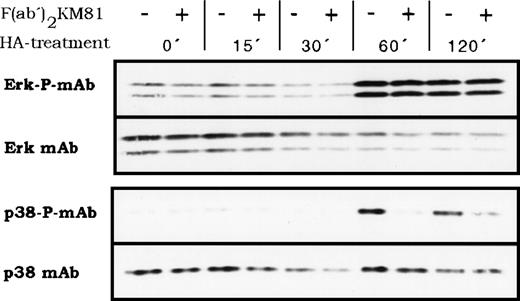
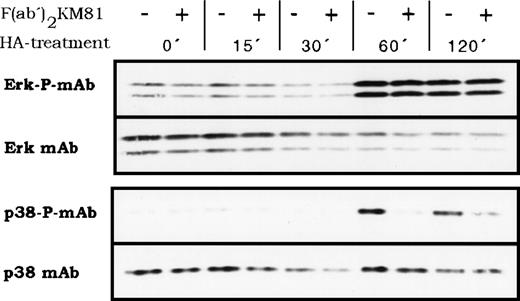
The two different HA receptors likely feed into different signal transduction pathways that activate protein kinases and, finally, different transcription factors. We explored whether HA could activate members of the proline-directed protein kinases that phosphorylate transcription factors. Indeed, BMDM responded to HA treatment with the activation of proline-directed protein kinases as detected by antibodies recognizing the phosphorylated forms of Erk and p38 (Fig 9). The lag period of activation for both kinases was between 30 and 60 minutes and activation persisted beyond 120 minutes. The question of whether the two postulated HA receptors link into different pathways was addressed by blocking CD44 with antibody KM81 F(ab′)2 fragments. KM81 F(ab′)2 fragments blocked specifically the HA-induced activation of p38 but not that of Erk (Fig 9), indicating that indeed at least 2 different HA receptors mediate HA signaling and that they stimulate 2 different signal transduction pathways. The antibody KM81 thus defines a pathway from CD44 through p38 (or through a coactivated protein kinase not yet detected) to IL-1β. However, IL-6 secretion depends on stimulation of another HA receptor and parallels the activation of Erk.
HA-induced activation of proline-directed protein kinases in BMDM. BMDM were stimulated with HA (100 μg/mL) for the indicated times, then lysed, and protein samples were analyzed by Western blotting for the presence of phosphorylated p38 kinase and phosphorylated Erk. The same membranes were stripped and stained with anti p38 or Erk antibodies, respectively. Where indicated, cells were preincubated for 15 minutes at 4°C with KM81 F(ab′)2 fragments (20 μg/mL) before HA stimulation.
HA-induced activation of proline-directed protein kinases in BMDM. BMDM were stimulated with HA (100 μg/mL) for the indicated times, then lysed, and protein samples were analyzed by Western blotting for the presence of phosphorylated p38 kinase and phosphorylated Erk. The same membranes were stripped and stained with anti p38 or Erk antibodies, respectively. Where indicated, cells were preincubated for 15 minutes at 4°C with KM81 F(ab′)2 fragments (20 μg/mL) before HA stimulation.
Function of CD44 in bone marrow is compensated for in CD44-deficient mice.
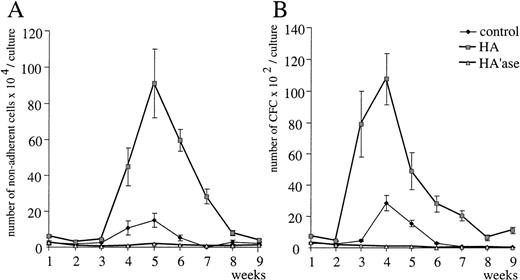
The dependence of IL-1β induction by HA on CD44 suggests that this function might be missing in CD44 knock-out mice. Therefore, we investigated whether LTBMC originating from bone marrow of CD44−/− mice showed any deficiency in hematopoiesis and whether bone marrow macrophages could respond to HA. CD44−/− mice were obtained by gene disruption.24 Bone marrow cells from wild-type and from CD44−/− mice were cultured in the presence or absence of HA. In both wild-type and CD44−/−LTBMC, exogeneous HA stimulated the generation of nonadherent cells as well as of clonogenic cells (Fig 10). Stromal cells from CD44−/− LTBMC also bound FITC-HA to an extent similar to that of stromal cells from wild-type bone marrow (not shown). Conversely, treatment of LTBMC from both sources with HA’ase led to dramatic inhibition of hematopoiesis (Fig10). The number of both nonadherent cells and hematopoietic precursors was reduced. HA also induced the release of IL-6 and IL-1β in cultures of macrophages derived from CD44−/−bone marrow (not shown). As expected, IL-1β induction was not inhibited by KM81 F(ab′)2 fragments in macrophages derived from CD44−/− mice (not shown).
Influence of HA and HA’ase on myeloid LTBMC from CD44 knock-out mice. Myeloid LTBMC from CD44-deficient mice were untreated or treated with HA or HA’ase as described in the legends to Figs 1 and3. The number of nonadherent cells (A) and hematopoietic progenitors (B) were measured once per week. SE was calculated from results obtained from 3 independent wells.
Influence of HA and HA’ase on myeloid LTBMC from CD44 knock-out mice. Myeloid LTBMC from CD44-deficient mice were untreated or treated with HA or HA’ase as described in the legends to Figs 1 and3. The number of nonadherent cells (A) and hematopoietic progenitors (B) were measured once per week. SE was calculated from results obtained from 3 independent wells.
DISCUSSION
This report addresses the role of the extracellular matrix component HA of the bone marrow microenvironment in hematopoiesis. We show that HA is not only a passive structural component, but a necessary and a specific signal-inducing molecule for hematopoiesis in the bone marrow. Specifically, HA’ase treatment of murine LTBMC abrogates lymphopoiesis and myelopoiesis, whereas addition of HA to LTBMC enhances lymphopoiesis and myelopoiesis. HA regulates a decisive step before the commitment of polypotent progenitor cells.
The mode of action of HA is to induce the release of cytokines from BMDM, particularly of IL-6 and IL-1β, but not of GM-CSF, IL-3, IL-4, IL-10, and TNF-α. IL-1β has been reported to be released in response to HA from both peripheral and bone marrow macrophages.31,32 To our knowledge, this manuscript reports for the first time an activation of IL-6 release by a GAG. HA binding to macrophages led to changes in the cytoskeleton and to the activation of p38 kinase and Erk kinase, both of which are key enzymes in different signal transduction pathways.33F(ab′)2 fragments of antibodies directed against the HA-binding domain of the HA receptor CD44 reduce the IL-1β induction, indicating a role for CD44-mediated signaling to IL-1β. This observation is in agreement with a previous report.32 The IL-6 induction by HA on the same bone marrow macrophages was not inhibited by the KM81 F(ab′)2 fragments, suggesting that a different receptor for HA is involved. Antibody blocking of the protein kinase activation confirms the existence of two different receptors, both of which interact with HA as p38 kinase was activated in a CD44-dependent manner while Erk kinase activation was independent of CD44. Thus, we conclude that on BMDM, HA binding to CD44 leads to activation of p38 kinase and to induction of IL-1β. On the other hand, the binding of HA to a still unknown receptor leads to the activation of Erk kinase and the stimulation of IL-6 release.
The induction kinetics of Erk and p38 after HA ligation by both CD44 and the unknown HA receptor are quite slow compared with signaling activation by receptor tyrosine kinases. Recently, however, the activation of two receptor tyrosine kinases, DDR1 and DDR2, has also been described to be slow, similar to the kinetics we observed here. Strikingly, DDR1 and DDR2 are also receptors for extracellular matrix components, namely for collagens.34 35
The induction of cytokines may explain the stimulatory effect of HA on hematopoiesis. IL-6, together with CSFs, is known to increase proliferation and to maintain primitive hematopoietic progenitor cells.36-39 Furthermore, IL-6 also stimulates megakaryocytopoiesis and the formation of monocytic colonies from hematopoietic progenitor cells.40-42 In agreement with these findings, IL-6–deficient mice have reduced the number of early progenitors in the bone marrow and in blood, suggesting that proliferation and differentiation of hematopoietic progenitors are impaired in these mice.43 IL-1β, in contrast, seems to have only minor effects on hematopoiesis44,45 and no profound effect on cell number, morphology, and colony-forming cells in LTBMC.46 In line with these results, IL-1β–deficient mice have no hematopoietic phenotype.47 Thus, we conclude that the decisive step promoting hematopoiesis by HA in LTBMC, and by analogy in bone marrow, is the induction of IL-6 in BMDM.
It has been shown that CD44 is involved in the regulation of hematopoiesis, although the mechanism of its action is not yet well understood. The application of panCD44 MoAbs led to an inhibition of hematopoiesis in LTBMC from different species.17,18,48 CD44 antibodies capable of stimulating hematopoiesis in LTBMC have also been described.49,50a These data suggest that CD44 proteins are engaged in steps and mechanisms other than mediating an HA effect (see also the discussion in ref 18).
The best-characterized HA receptor on the surface of cells is CD44. ICAM-1 on rat liver endothelial cells has been described as capable of binding HA,51 but recent studies showed that this observation was based on an artefact.52 Recently, an HA receptor termed RHAMM on Ras-transformed fibroblasts was defined as part of the HARC complex.53,54 An MoAb directed against a portion of the published cDNA sequence of RHAMM recognized an intracellular HA binding protein but not a cell-surface protein.55,56 Therefore, RHAMM was not considered as a relevant receptor in our experimental context. Interestingly, the presence of two HA binding receptors on the same cell has also been described on a brain endothelial cell line,57 one of which is CD44, while the other is yet unknown.
From the fact that HA-dependent IL-1β induction in BMDM is mediated by CD44, one could expect that this induction does not occur in CD44 knock-out mice. This is not the case, suggesting that the CD44 HA binding function is compensated for in the knock-out mice. The compensating molecule is yet unknown. The assumption of existence of a compensatory mechanism is further corroborated by the finding that hematopoiesis is by and large normal in CD44 knock-out mice,24 whereas a functional relevance of CD44 proteins has been deduced from biochemical studies.17,18 An explanation for this apparent contradiction between the results obtained with knock-out animals and molecular biological analysis might be that CD44 substitutes can only be established during early embryogenesis. Consistent with this assumption is the observation that a conditional antisense-mediated elimination of CD44 from keratinocytes showed a striking skin phenotype,58 whereas in knock-out animals the skin develops perfectly normally.24Similarly, a defined limb phenotype in embryogenesis was created by antibody interference with CD44 function in the apical ectodermal ridge, which resulted in repression of mesenchyme proliferation59 while CD44-deficient mice develop normal limbs.24
Our experiments have identified a new function for the ECM component HA in the activation of cytokine release through two different signaling pathways and have added evidence to the increasing number of examples that the components of the ECM are not only space-filling and structural molecules, but also have specific regulatory functions in gene expression.
ACKNOWLEDGMENT
We thank Martin Hegen, Andreas Herrlich, Axel Knebel, Harald König, and Carsten Weiβ for fruitful discussion and technical advice. The CD44 knock-out mice were kindly provided by R. Schmits (Homburg, Germany) and T.W. Mak (Toronto, Canada).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
NOTE ADDED IN PROOF
During the preparation of this manuscript, a paper was published60 which reported that HA stimulates IL-6 expression in dendritic cells.
Author notes
Address reprint requests to Helmut Ponta, PhD, Forschungszentrum Karlsruhe, Institute of Genetics, PO Box 3640, D-76021 Karlsruhe, Germany; e-mail: genetik@igen.fzk.de.