Abstract
Cell-mediated immunity, especially the cytotoxic T lymphocyte (CTL), provides resistance to Epstein-Barr virus (EBV), as is demonstrated by the occurrence of posttransplant lymphoproliferative disease in immunosuppressed patients. We set out to use dendritic cells (DCs) to elicit anti–EBV-specific CTLs in culture. In unselected, HLA-B8+ donors, monocyte-derived mature DCs were pulsed with the HLA-B8–restricted EBNA-3A peptide, FLRGRAYGL, and added to autologous T cells for 7 days at a DC:T ratio of 1:5 to 1:60. The cultured cells specifically lysed EBNA-3A peptide-pulsed, HLA-B8+, B-lymphoblastoid cell lines in a 5-hour51Cr-release assay. The generation of CTLs did not require the addition of interleukin-2. In comparison, monocytes were weak antigen-presenting cells. DCs were then infected with recombinant vaccinia-EBNA-3A. Vaccinia infection significantly decreased the viability of immature DCs after 3 days of culture (to 25% to 45%) but had a smaller effect on mature DC recovery (40% to 70%). To decrease these cytopathic effects and to expand the potential use of vaccinia vectors for DC therapy in immunocompromised patients, we successfully used psoralen and UV-inactivated virus. Mature DCs pulsed with either live or inactivated vaccinia EBNA-3A virus could elicit strong EBNA-3A–specific CTLs. Therefore, mature DCs are powerful stimulators of EBV-specific CTLs and their major histocompatibility complex class I products can even be charged with UV-inactivated recombinant vaccinia.
EPSTEIN-BARR VIRUS (EBV) is a ubiquitous human γ-herpes virus that has been associated with several malignant diseases. These diseases include nasopharyngeal carcinoma, Burkitt’s lymphoma, approximately 50% of Hodgkin’s disease, and lymphoproliferative disorders in the immunocompromised patient.1,2 Posttransplant lymphoproliferative disorders (PTLPD) of B cells can develop in the setting of organ transplants and in hematopoietic stem cell transplantation; for the latter, the incidence can be as high as 10%.3 Complete regression of PTLPD has been reported in 40% of patients after reduction or discontinuation of immunosuppressive therapy, but this is less feasible in marrow transplantation, because a likely result is a flare of graft-versus-host disease. These findings, as well as the observation of clinically apparent virus replicative lesions in T-cell immunocompromised patients,4 strongly suggest an important role for cell-mediated immune responses in the control of EBV. Further evidence comes from studies in which enriched populations of EBV-specific cytotoxic T lymphocytes (CTLs), generated from normal donors, are adoptively transferred to bone marrow transplant patients.5-8 The transferred cells provide efficient prophylaxis and demonstrable treatment of immunoblastic lymphoma.
We wanted to assess the feasibility of using dendritic cells (DCs) to elicit CTL responses to EBV. DCs are the most potent antigen-presenting cells (APCs),9,10 and their role in resistance against experimental malignancies11-18 and infections19 20 is well described. It is now possible to generate large numbers of DCs from bone marrow, cord blood, and peripheral blood. If DCs could elicit EBV-specific CTL responses, this would be advantagous for generating CTL lines, because DCs can be generated in a much shorter time frame than the EBV-transformed lymphoblastoid cell lines (LCL) that are now in use. Also, DCs could potentially be used to actively boost a patient’s EBV-specific immunity, in contrast to passive transfer of chronically stimulated T-cell lines.
EBV establishes a growth-transforming infection of B lymphocytes. Infection is associated with the expression of 6 virus-encoded nuclear antigens (EBNA-1, -2, -3A, -3B, -3C, and -LP) and 2 latent membrane proteins (LMP-1 and -2). The primary and memory CD8+ CTL response in healthy EBV carriers is markedly skewed toward HLA allele-specific epitopes drawn from the EBNA-3A, -3B, and -3C subset of latent proteins.21-23 Reactivities to other EBV latent antigens are less frequent. In lymphoproliferative disorders in the immunocompromised patient, the full array of latent EBV antigens is expressed.24,25 In this study, we wanted to investigate the use of DCs to generate CTL responses in HLA-B8+, healthy, EBV+ carriers to the immunodominant EBV antigen EBNA-3A in a relatively short culture assay of 7 days. To initially test the use of DCs, we used the well-described HLA-B8+ T-cell epitope FLRGRAYGL from the EBNA-3A antigen. Then, to expand this method for use with other HLA-types and EBV antigens, we tested recombinant vaccinia virus as a source of antigen.21 26 We will show that DCs can be strong stimulators of EBV-specific CTL responses in culture and, remarkably, that UV-inactivated recombinant vaccinia virus can serve as a source of EBV antigen.
MATERIALS AND METHODS
Culture Medium
The medium used for generation of DCs and for CTL induction was RPMI-1640 supplemented with 10 mmol/L HEPES, 5 mmol/L L-glutamine, 20 μg/mL of gentamicin, and either 1% plasma (heparinized) or 5% pooled or single donor human serum, heat-inactivated for 30 minutes at 56°C.
Cytokines
We purchased recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; Sargramostim Leukine; 1.4 × 106 U/250 μg) from Immunex (Seattle, WA), recombinant interleukin-4 (IL-4; 4.1 × 107 U/mg) from Genzyme (Cambridge, MA), and IL-2 from Schiapparelli Biosystems (Fairfield, NJ). Lymphocult was puchased from Biotest (Dreieich, Germany).
Cell Lines
EBV-transformed B-LCLs were established by culturing peripheral blood mononuclear cells (PBMCs) of HLA class I-typed donors with supernatant from the marmoset line B95.8 in the presence of 1 μg/mL cyclosporin A in medium supplemented with 20% fetal calf serum (FCS). The TAP−/−, HLA-A2.1+ T2 cell line from the American Type Culture Collection (ATCC; Manassas, VA) was used as targets for testing the cytotoxic activity of influenza-specific CTLs. The BSC40 monkey kidney line (ATCC) was grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% FCS and used in plaque assays to titer recombinant vaccinia virus stocks. The RK13 rabbit kidney line (ATCC) was grown in DMEM supplemented with 15% FCS and used for expansion of the vaccinia virus stock.
Mononuclear Cell Subsets
PBMCs.
PBMCs were isolated from leukocyte-enriched buffy coats by standard density gradient centrifugation on Ficoll-Paque (Pharmacia, Uppsala, Sweden). T-cell–enriched (ER+) and T-cell–depleted (ER−) populations were prepared by rosetting with neuraminidase-treated (Vibrio cholerae neuraminidase; Calbiochem, La Jolla, CA) sheep red blood cells (Colorado Serum Co, Denver, CO).
T cells.
E-rosetted T cells were further purified by removal of monocytes, natural killer (NK) cells, and major histocompatibility complex (MHC) class II-positive cells by panning with antibodies to CD11b, CD16, and HLA-DR, as described.27
DCs.
A total of 2.5 × 106 ER− cells were plated in 3 mL volumes in 6-well tissue culture dishes (Falcon, Lincoln Park, NJ) in complete medium containing 1% human plasma. GM-CSF and IL-4 were added at final concentrations of 500 U/mL of IL-4 and 1,000 U/mL of GM-CSF. Cytokines and medium were replenished on days 2, 4, and 6.28,29 On day 7, nonadherent cells were collected and transferred to new 6-well plates. The cultures were supplemented with monocyte conditioned medium (MCM; final concentration, 50% vol/vol) to induce final maturation of the DCs that were harvested 48 hours later.30
Vaccinia Virus
Virus stocks.
We used recombinant vaccinia virus (rVV), expressing the EBV latent gene EBNA-3A or LMP-121 or the influenza matrix gene, vJL3 (vJL3 was kindly provided to us by B. Moss, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD).31 The control was the parental vaccinia virus construct that is negative for thymidine kinase (rVV-TK−).26
Vaccinia virus expansion.
Vaccinia virus stocks were expanded using adherent rabbit kidney cells, RK13 cells. Contaminating vaccinia proteins were digested with trypsin (GIBCO BRL, Life Technologies, Grand Island, NY). The virus preparation also was centrifuged through a sucrose gradient to further remove proteins and peptides.
Virus inactivation.
A psoralen (Sigma, Steinheim, Germany) stock solution (1 mg/mL, 50% H2O/50% ethanol) was added to the viruses (2 × 106 to 1 × 107 plaque-forming units [PFU]) at a concentration of 10 μg/mL in a flat-bottom 96-well plate (Costar, Cambridge, MA) and incubated for 10 minutes at room temperature. Then, to inactivate the virus, the preparation was irradiated in a Stratalinker 1800 UV cross-linking unit (Stratagene, La Jolla, CA) equipped with five 365-nm UV bulbs for 6 minutes (PLWUV, psoralen long wave UV).32
Viral titers.
Plaque-forming activities of active and inactivated virus were determined by serial dilutions on a BSC40 cell layer. Plaques were counted after rVV infection of BSC40 cells at a titration starting from 109 PFU/mL to 103 PFU/mL, in duplicates in 6-well plates. A titer of 0 was recorded when no plaques formed on the BSC40 cell monolayer after 2 days.
Influenza Virus
Influenza A virus (PR8, Puerto Rico/8/34; source: allantoic fluid) was purchased from Spafas Inc (Storrs, CT).
Antigens
Synthetic peptides.
The EBNA-3A peptides, FLRGRAYGL and QAKWRLQTL, were purchased from Biosynthesis (Lewisville, TX). The EBNA-3A peptide FLRGRAYGI was purchased from Genemed Synthesis (San Francisco, CA). All peptides were greater than 95% pure by mass spectrometry and high-performance liquid chromatography (HPLC). Stock solutions in dimethyl sulfoxide (DMSO) were kept at −70°C. The influenza A virus matrix peptide GILGFVFTL was used to pulse T2 cells for 51Cr release assays.
Antigen-pulsing of DCs.
DCs were harvested out of the 6-well plates after 24 or 48 hours of MCM and resuspended in 1% plasma at 1 × 107/mL. rVV was added at a multiplicity of infection (MOI) of 1:1 or 2:1 and was incubated for 1 hour at 37°C. DCs were infected with influenza virus in serum-free RPMI for 1 hour at 37°C at an MOI of 0.5:1. Peptide pulsing of mature DCs was performed for 1 hour in RPMI at room temperature at a final concentration of 10 μmol/L. DCs were washed 3 times and used to stimulate bulk cultures of purified syngeneic T cells in 96- or 24-well plates (Costar) at DC to T-cell ratios of 1:5 to 1:60.
Fluorescence-Activated Cell Sorting (FACS) Analysis of Cell Populations and Vaccinia Infection
Serological HLA class I typing.
Buffy coats were typed with monoclonal antibodies (MoAbs) to HLA-B8 (One Lambda, Canoga Park, CA) and HLA-A2 (ATCC) and analyzed by FACScan. Target LCL lines were HLA-typed at Memorial Sloan Kettering Cancer Center (New York, NY).
T cells.
T cells were phenotyped by staining with Simultest CD4-fluorescein isothiocyanate (FITC)/CD8-phycoerythrin (PE) or Simultest control IgG1-FITC/IgG2a-PE from Becton Dickinson (BD; San Jose, CA). To document purity of the panned T cells, we verified the absence of cells that stained for CD56-PE (BD) and HLA-DR-PE (BD), prepanning and postpanning.
DCs.
MoAbs to the following surface antigens were used: HLA-DR-PE, CD14-PE, CD25-PE (all BD), CD86-PE (PharMingen, San Diego, CA), CD83-PE (Coulter Corp, Miami, FL), and the antivaccinia hemagglutinin antibody VV1-4G9. PE-conjugated F(ab′)2 goat antimouse IgG (γ and light chain; Tago, Burlingame, CA) was used as a secondary antibody. Cells were phenotyped with the panel of MoAbs and analyzed on a FACScan.
Annexin V/propidium iodide (PI) staining.
Annexin V/PI staining (Kamiya, Seattle, WA) was used to monitor cytopathic effects on uninfected DCs and DCs infected with live and PLWUV-inactivated rVV. The DCs were plated at 1 × 105/200 μL of 5% pooled human serum per well in flat-bottom 96-well plates. Each day after infection, the cells were stained with 5 μL of Annexin V and 5 μL of PI and immediately analyzed on a FACScan.
Intracellular staining.
DCs and B-LCLs were fixed with 4% paraformaldehyde, washed, and permeabilized with 1% saponin for 30 minutes at 4°C. Anti-LMP-133 (Dako, Glostrup, Denmark) and antivaccinia antibodies (VV1-2F10, VV3-5B8, VV4-2F6, and VV1-6B6) were added for 30 minutes. Cells were washed in phosphate-buffered saline (PBS) containing 0.1% saponin, 0.1% azide, 1% FCS, and 1% human serum and then incubated with 1:250 PE- or 1:100 FITC-conjugated goat antimouse IgG (Biosource, Camarillo, CA). Cells were washed twice and analyzed by FACScan.
T-Cell Responses
Allogeneic MLR.
Uninfected and infected DCs were added in graded doses as stimulators for 2 × 105 purified, allogeneic T cells in 96-well flat-bottom plates. Proliferation was determined on day 5 with the addition of 4 μCi/mL of 3H-TdR for 8 to 12 hours to triplicate wells.
Induction of CTL responses.
A total of 2 × 105 or 1 × 106purified T cells were cultured in 96- or 24-well plates with graded doses of peptide-pulsed DCs or rVV-infected or uninfected DCs, in a total volume of 200 μL or 1 mL of 5% single donor serum. In 4 of 6 experiments using FLRGRAYGL-pulsed DCs as stimulators, cultures were set up with and without the addition of IL-2 (50 IU/mL) on day 3 postsetup. When using the QAKWRLQTL peptide, longer culture times were required to detect strong CTLs. T cells were restimulated with peptide-pulsed DCs on day 7, Lymphocult was added on days 1 and 7, and the cultures were harvested on day 14. To assay CTLs, cells from the 24-well plates were transferred to 96-well plates.
51Cr release assay for effector CTLs.
LCLs and T2 cells were incubated with peptide (10 μmol/L) for 1 hour at room temperature and then labeled for 1 hour with 400 μCi of Na51CrO4 (1 mCi/mL, sterile stock; New England Nuclear, Boston, MA) at 37°C. The cells were washed 4 times and resuspended at 2 × 105/mL, and 1 × 104 target cells were added to each well of a 96-well plate to give effector:target ratios of 10:1 to 30:1. Spontaneous and total release samples were prepared by adding the targets to wells containing RPMI alone or a final concentration of 0.33% sodium dodecyl sulfate (SDS), respectively. The plates were centrifuged for 2 minutes at 15g and incubated for 5 hours at 37°C. At the termination of the assay, the supernatants were collected with absorption cartridges using a harvesting press (Skatron Instruments Inc, Sterling, VA) and counted in a γ-counter. All tests were conducted in triplicate, and the percentage of specific lysis was calculated from the following formula: 100 × ([release by CTL − spontaneous release]/[total release − spontaneous release]). Spontaneous release was 15% to 25% of the total release.
RESULTS
CTL Induction With EBNA-3A (FLRGRAYGL) Peptide-Pulsed DCs
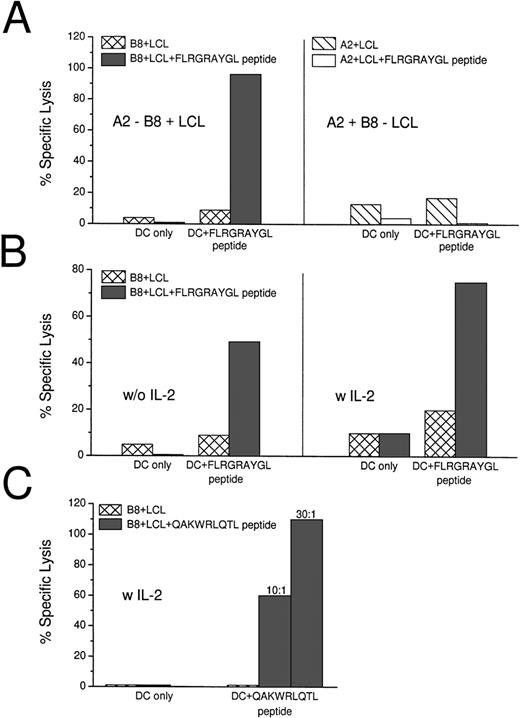
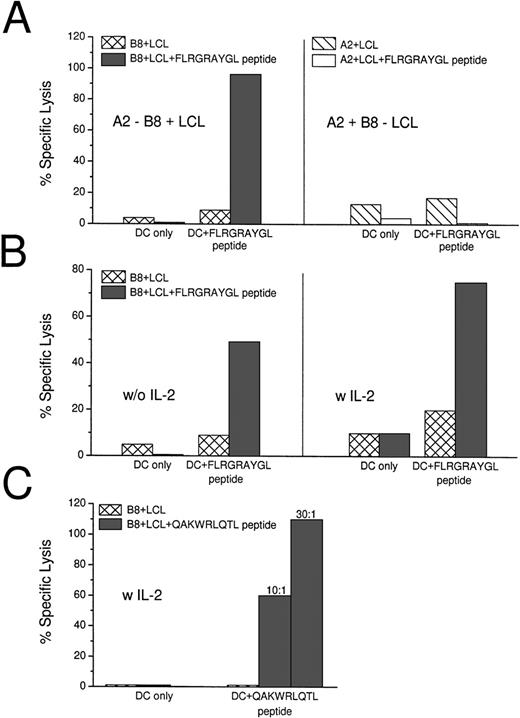
Peptide-pulsed and unpulsed DCs were added to autologous T cells and, after 7 days, the cultured cells were tested for killing activity on HLA-matched (B8+) and mismatched (A2+) LCL lines in a standard 5-hour 51Chromium release assay. In 6 of 6 HLA-B8+ donors, mature DCs that were pulsed with the HLA-B8+ dominant peptide FLRGRAYGL induced strong CTL responses in culture (Table 1). Effector:target ratios of 10:1 to 20:1 were sufficient to detect specific lysis of HLA-matched LCLs, and the killing was restricted to B8+ targets (Fig 1A). Different ratios of DCs to T cells were tested. A DC:T-cell ratio of 1:60 was sufficient to induce CTLs (Table 1). In 4 of 6 experiments, IL-2 (50 IU/mL) was added on day 3 of cultures, but this was not required for DCs to elicit CTL responses. High CTL responses were not further increased through the addition of IL-2, but weaker responses could be enhanced (Table 1 and Fig 1B). As has been described before,34,35 B-LCL could only serve as a CTL target if pulsed with exogenous peptide. The B-LCLs used throughout our experiments were transformed with the viral strain B95.8, which carries sequence variations in the EBNA-3A gene compared with the EBV strain A found in the western hemisphere, eg, the Leucine in the FLRGRAYGL peptide is mutated to Isoleucine in B95.8.36 Therefore, we used another peptide (QAKWRLQTL) that is the same in the different viral strains to stimulate syngeneic T cells (Fig 1C). In repeated experiments using different peptide concentrations for pulsing our DCs (10 μmol/L to 100 nmol/L, data not shown), we still could not detect lysis of the B-LCLs alone. The B-LCLs had to be pulsed with exogenous peptide to serve as targets.
Induction of EBV-specific CTLs with peptide-pulsed DCs. (A) Peptide (FLRGRAYGL)-pulsed DCs induce HLA class I-restricted lysis of peptide-pulsed B8+ LCLs after 7 days of coculture. The CTLs did not lyse unpulsed HLA-matched LCL or peptide-pulsed B8− LCL. The effector:target ratio was 20:1. (B) When the induction of CTL was relatively low, the response could be enhanced through the addition of IL-2 (with 50 IU/mL) on day 3 of the 7-day culture. (C) QAKWRLQTL-pulsed DCs induce HLA class I-restricted lysis of peptide-pulsed B8+ LCLs after 14 days of coculture. The culture was restimulated with peptide pulsed DCs on day 7 and IL-2 was added on days 1 and 7. Two effector:target ratios are shown (10:1 and 30:1).
Induction of EBV-specific CTLs with peptide-pulsed DCs. (A) Peptide (FLRGRAYGL)-pulsed DCs induce HLA class I-restricted lysis of peptide-pulsed B8+ LCLs after 7 days of coculture. The CTLs did not lyse unpulsed HLA-matched LCL or peptide-pulsed B8− LCL. The effector:target ratio was 20:1. (B) When the induction of CTL was relatively low, the response could be enhanced through the addition of IL-2 (with 50 IU/mL) on day 3 of the 7-day culture. (C) QAKWRLQTL-pulsed DCs induce HLA class I-restricted lysis of peptide-pulsed B8+ LCLs after 14 days of coculture. The culture was restimulated with peptide pulsed DCs on day 7 and IL-2 was added on days 1 and 7. Two effector:target ratios are shown (10:1 and 30:1).
Live and UV-Inactivated Vaccinia Infection of DCs
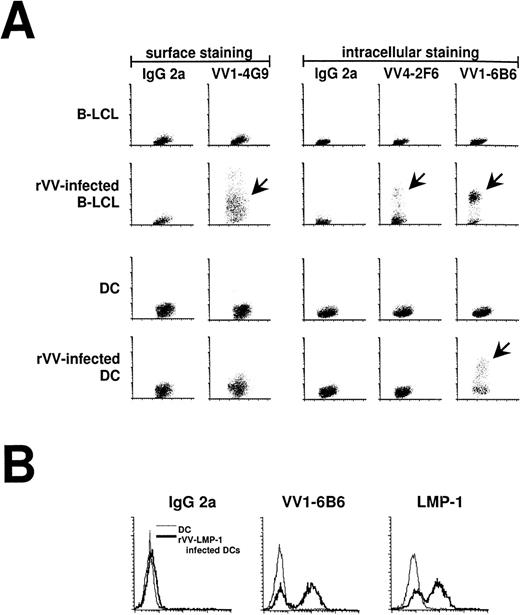
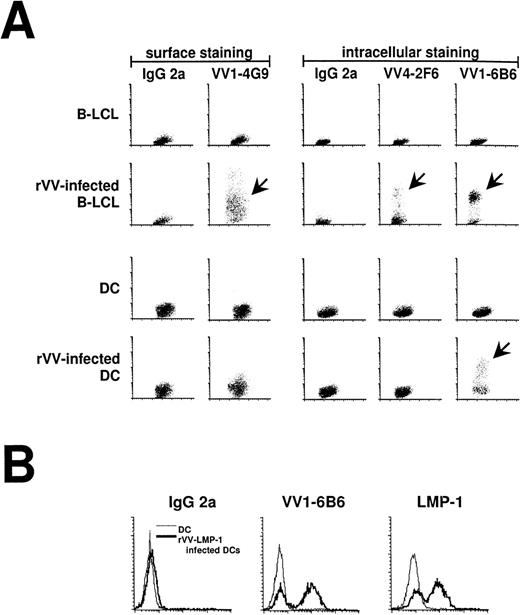
Immature (no addition of MCM) and mature DCs were infected with rVV at an MOI of 2:1. Expression of viral protein was monitored by FACS with a panel of MoAbs reacting with 1 early and 2 late proteins in the vaccinia replication cycle. These IgG2a antibodies stain an early vaccinia virus protein (VV1-6B6), the late vaccinia hemagglutinin (VV1-4G9), and the late D8L virion/surface protein (VV4-4G9; Table 2). When all of the antibodies were tested on rVV infected B-LCL and DCs, the B-LCLs expressed early and late vaccinia antigens, whereas DCs only expressed early antigens (Fig 2A). We also stained rVV-LMP-1–infected DCs with LMP-1 antibody and the VV1-6B6 antibody. Both proteins were expressed under the control of the early promoters and resulted in the same staining pattern (Fig 2B). The VV1-6B6 early antigen was used in all of our experiments to monitor the percentage of infected DCs.
Antibody staining 16 hours after infection of mature DCs with rVV. (A) Infection of DCs and LCL was compared using FACS staining (y-axis) and MoAbs against early and late vaccinia virus proteins (Table 2). VV1-4G9 was a surface stain, whereas all other antibodies required cell permeabilization. IgG2a was used as an isotype control. Infected DCs (arrows) only stained for the early vaccinia virus protein recognized by VV1-6B6, whereas infected LCL (arrows) stained for early and late vaccinia virus proteins. (B) The VV1-6B6 antibody was compared with the LMP-1 antibody, after infection of mature DCs with rVV-LMP-1. The VV1-6B6 and LMP-1 are early proteins, with the latter being an EBV protein. Both antibodies stained the same percentage of DCs, thereby confirming the accuracy of the VV1-6B6 antibody.
Antibody staining 16 hours after infection of mature DCs with rVV. (A) Infection of DCs and LCL was compared using FACS staining (y-axis) and MoAbs against early and late vaccinia virus proteins (Table 2). VV1-4G9 was a surface stain, whereas all other antibodies required cell permeabilization. IgG2a was used as an isotype control. Infected DCs (arrows) only stained for the early vaccinia virus protein recognized by VV1-6B6, whereas infected LCL (arrows) stained for early and late vaccinia virus proteins. (B) The VV1-6B6 antibody was compared with the LMP-1 antibody, after infection of mature DCs with rVV-LMP-1. The VV1-6B6 and LMP-1 are early proteins, with the latter being an EBV protein. Both antibodies stained the same percentage of DCs, thereby confirming the accuracy of the VV1-6B6 antibody.
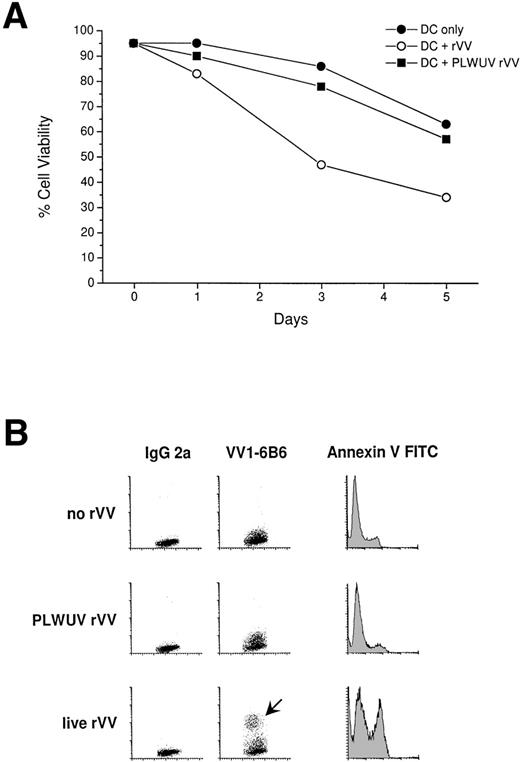
Immature DCs were infected at a higher frequency (40% to 80%) than mature DCs (15% to 65%). DC viability was tested 1 to 5 days postinfection with trypan blue exclusion (Fig 3A) and Annexin V/PI FACS staining (Fig 3B). Viability was decreased relative to uninfected DCs. For immature DCs, viable cell recovery was 25% to 45%, whereas for mature DCs, recovery was 40% to 70% on day 3 after infection (Fig 3A). Vaccinia virus is a known cytopathic virus, and, as the results from Annexin V/PI staining indicated, infection led to an increase in the number of DCs undergoing apoptosis (Fig 3B). In parallel studies, we have noted that, at early time points, vaccinia virus infection blocks the maturation of immature DCs.37
Cytotoxicity of rVV towards mature DCs. (A) DCs were recultured at 1 × 105/200 μL of 5% human serum in 1 well of a 96-well flat-bottom well plate, and cell viability was tested with trypan blue staining for 5 days. Live and PLWUV-inactivated, rVV-infected, and uninfected DCs were compared. Cell viability of rVV-infected mature DCs decreased significantly by day 3 compared with PLWUV rVV-infected and uninfected DCs. (B) Viability of uninfected and infected mature DCs was monitored with Annexin V and PI staining (PI stain not shown). The VV1-6B6 antibody indicated the rate of infection. Mature DCs were infected with live and PLWUV-inactivated rVV-LMP-1 at an MOI of 2:1. Twenty-four to 30 hours later, Annexin V FITC staining was performed and immediately analyzed by FACScan. DCs infected with live virus showed greater than 30% Annexin V+ cells, whereas uninfected and PLWUV rVV-infected DCs showed less than 10% positive cells.
Cytotoxicity of rVV towards mature DCs. (A) DCs were recultured at 1 × 105/200 μL of 5% human serum in 1 well of a 96-well flat-bottom well plate, and cell viability was tested with trypan blue staining for 5 days. Live and PLWUV-inactivated, rVV-infected, and uninfected DCs were compared. Cell viability of rVV-infected mature DCs decreased significantly by day 3 compared with PLWUV rVV-infected and uninfected DCs. (B) Viability of uninfected and infected mature DCs was monitored with Annexin V and PI staining (PI stain not shown). The VV1-6B6 antibody indicated the rate of infection. Mature DCs were infected with live and PLWUV-inactivated rVV-LMP-1 at an MOI of 2:1. Twenty-four to 30 hours later, Annexin V FITC staining was performed and immediately analyzed by FACScan. DCs infected with live virus showed greater than 30% Annexin V+ cells, whereas uninfected and PLWUV rVV-infected DCs showed less than 10% positive cells.
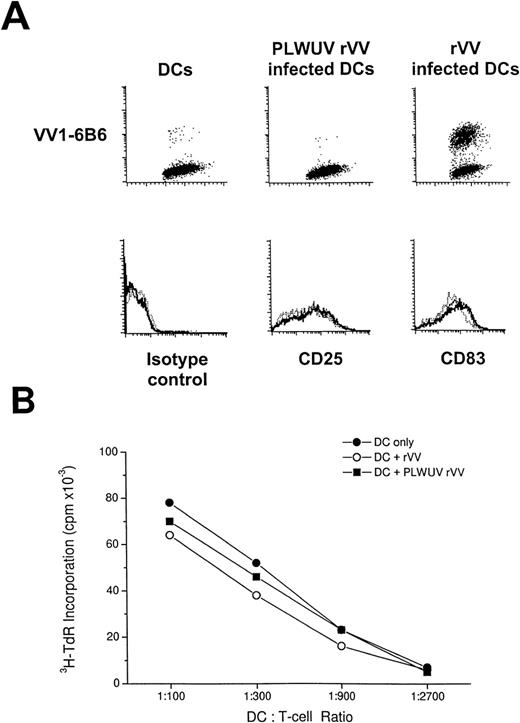
To decrease the cytopathic effect of vaccinia virus on DCs and to expand the potential use of vaccinia vectors in immunocompromised patients, we used psoralen and UVA-inactivated, nonreplicating vaccinia virus to infect mature DCs. Inactivation of rVV was monitored by a plaque-forming assay with BSC40 cells, as well as with FACS staining to follow synthesis of vaccinia virus proteins. Plaques were counted after infection with live and UV-inactivated rVV at a titration starting at 109 PFU/mL to 103 PFU/mL, in duplicates in 6-well plates. There were no plaques detectable with PLWUV-inactivated rVV in 3 separate experiments. To prevent protein or peptide contamination, viral preparations were digested with trypsin and centrifuged through a sucrose gradient. After infection of DCs with PLWUV-inactivated virus, an MoAb against an early vaccinia protein (VV1-6B6) gave a decreased staining (0% to 15%) compared with live rVV infected DCs (Fig 3B).
Mature DCs that were infected with live or PLWUV-inactivated rVV had the same phenotype and function as uninfected DCs. Specifically, infected and uninfected DCs expressed high levels of CD83, CD86, HLA-DR, and CD25, as is typical of mature DCs (Fig 4A), and these DCs were potent stimulators of allogeneic T-cell responses (Fig 4B). The efficacy of rVV-infected DCs in stimulating the MLR might reflect DC function before their death from infection or the capacity of the responding T cell to rescue the DC.
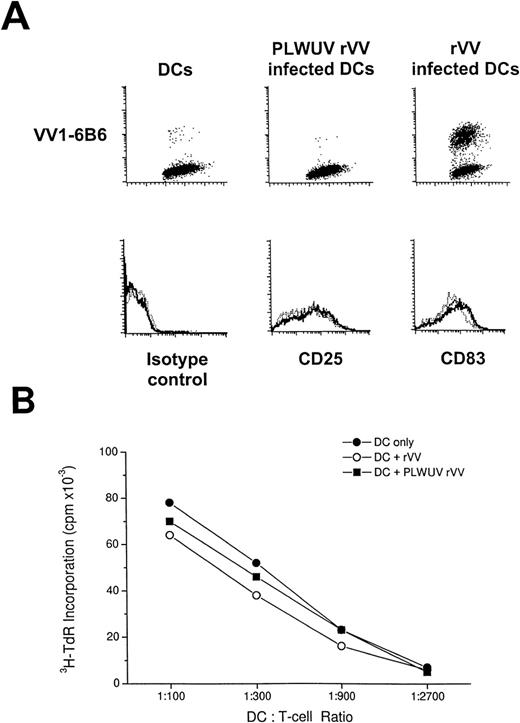
FACS analysis of rVV-infected mature DCs. (A) The top row shows staining for VV1-6B6 early vaccinia protein in uninfected, PLWUV rVV-TK− and live rVV-TK−-infected DCs at an MOI of 2:1. The lower row shows comparable staining for CD25 and CD83, 2 markers for DC maturation, on uninfected DCs (···), PLWUV rVV-infected DCs (—-), and rVV-infected DCs (—). (B) rVV-infected DCs stimulate T-cell proliferative responses in the MLR. Bulk T cells were used as responders towards graded doses of allogeneic DCs that were uninfected or infected with rVV-TK− and PLWUV rVV-TK−. Cultures were pulsed on day 5 for 8 hours with 4 μCi/mL of 3H-TdR. Results are the means of triplicates (mean cpm).
FACS analysis of rVV-infected mature DCs. (A) The top row shows staining for VV1-6B6 early vaccinia protein in uninfected, PLWUV rVV-TK− and live rVV-TK−-infected DCs at an MOI of 2:1. The lower row shows comparable staining for CD25 and CD83, 2 markers for DC maturation, on uninfected DCs (···), PLWUV rVV-infected DCs (—-), and rVV-infected DCs (—). (B) rVV-infected DCs stimulate T-cell proliferative responses in the MLR. Bulk T cells were used as responders towards graded doses of allogeneic DCs that were uninfected or infected with rVV-TK− and PLWUV rVV-TK−. Cultures were pulsed on day 5 for 8 hours with 4 μCi/mL of 3H-TdR. Results are the means of triplicates (mean cpm).
CTL Induction With DCs Infected With Live and PLWUV-Inactivated rVV
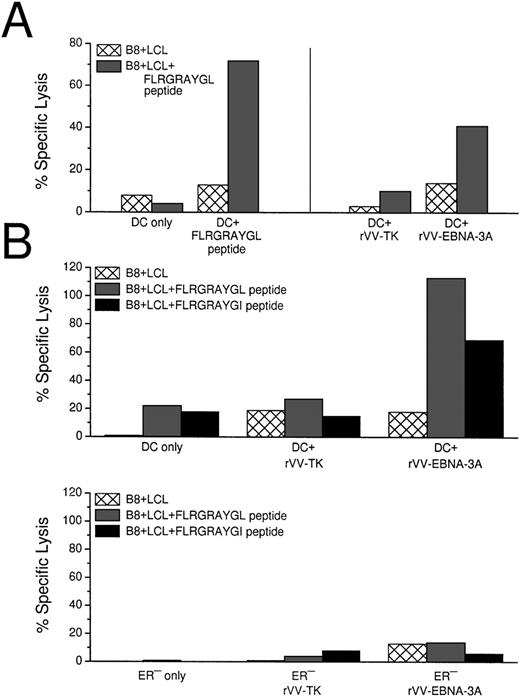
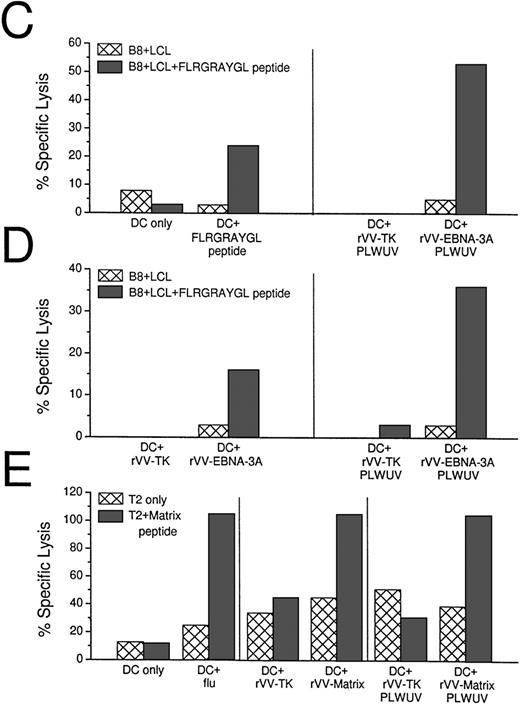
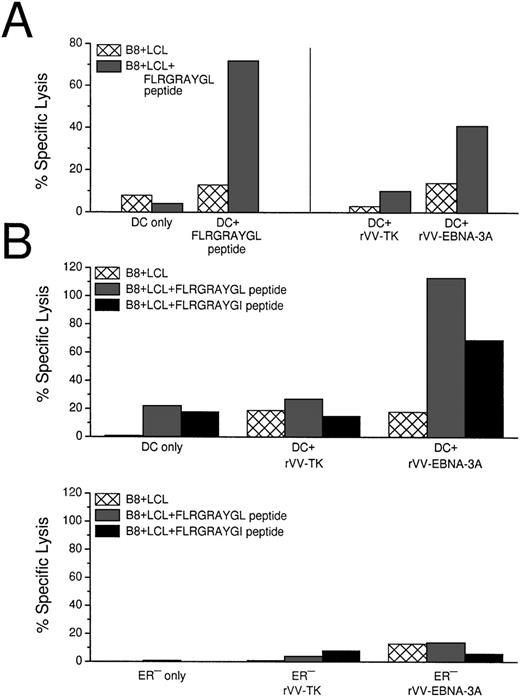
Because HLA-B8+ donors gave a reliable CTL response after stimulation with EBNA-3A peptide-pulsed DCs, we used live rVV-EBNA-3A to deliver antigens to the MHC class I products of mature DCs. In 3 HLA-B8+ positive random donors, we could elicit EBNA-3A–specific responses with vaccinia-infected DCs (Fig 5A). Because rVV-EBNA-3A is derived from the B95.8 viral strain, containing a mutated FLRGRAYGL peptide, we tested if rVV infected DCs could elicit CTLs against B-LCLs pulsed with either FLRGRAYGL or FLRGRAYGI (Fig 5B). rVV-EBNA-3A–infected DCs elicited good CTL responses in 7-day culture against both targets. Because FLRGRAYGI binds more weakly to the MHC class I molecule, the lysis was slightly decreased relative to FLRGRAYGL-pulsed B-LCLs.
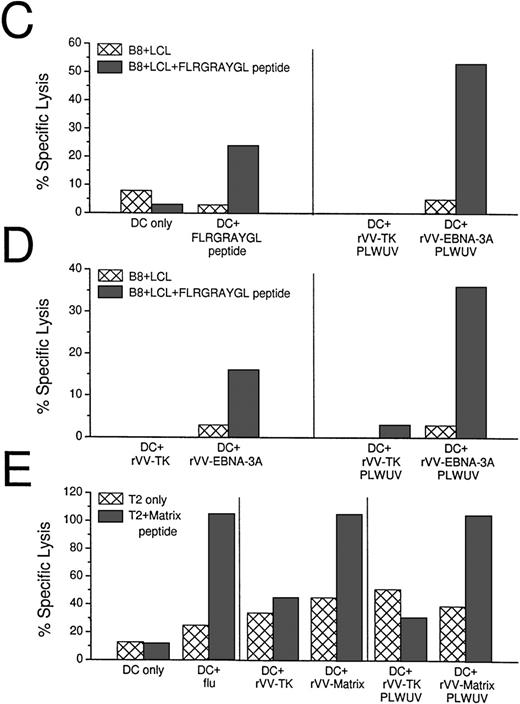
CTL induction with peptide-pulsed and live rVV-infected DCs. (A) Comparison of EBNA-3A peptide-pulsed DCs with rVV-infected DCs. Live rVV-TK− was used as negative control. (B) Comparion of monocytes and DCs as APCs in eliciting EBNA-3A–specific CTL responses in a 7-day culture assay. The top panel shows that rVV-EBNA-3A–infected DCs (DC:T cell ratio 1:30) stimulate T cells that recognize FLRGRAYGL- and FLRGRAYGI-pulsed HLA B8+ B-LCLs. The lower panel from the same experiment shows that rVV-EBNA-3A–infected monocytes (ER−:T-cell ratio 1:2) are unable to stimulate T cells that recognize FLRGRAYGL- or FLRGRAYGI-pulsed HLA B8+ B-LCLs. (C) Comparison of EBNA-3A peptide-pulsed DCs with PLWUV, rVV-EBNA-3A–infected DCs; PLWUV rVV-TK− was used as a negative control. (D) Comparison of live versus PLWUV rVV-EBNA-3A–infected DCs. (E) Comparison of DCs infected with live influenza A or with live or PLWUV rVV-influenza-matrix in eliciting responses to the immunodominant matrix peptide for HLA-A2.1. In all of the experiments, the DC:T-cell ratio was 1:30, the effector T-cell:target cell ratio was 1:20, and no IL-2 was used.
CTL induction with peptide-pulsed and live rVV-infected DCs. (A) Comparison of EBNA-3A peptide-pulsed DCs with rVV-infected DCs. Live rVV-TK− was used as negative control. (B) Comparion of monocytes and DCs as APCs in eliciting EBNA-3A–specific CTL responses in a 7-day culture assay. The top panel shows that rVV-EBNA-3A–infected DCs (DC:T cell ratio 1:30) stimulate T cells that recognize FLRGRAYGL- and FLRGRAYGI-pulsed HLA B8+ B-LCLs. The lower panel from the same experiment shows that rVV-EBNA-3A–infected monocytes (ER−:T-cell ratio 1:2) are unable to stimulate T cells that recognize FLRGRAYGL- or FLRGRAYGI-pulsed HLA B8+ B-LCLs. (C) Comparison of EBNA-3A peptide-pulsed DCs with PLWUV, rVV-EBNA-3A–infected DCs; PLWUV rVV-TK− was used as a negative control. (D) Comparison of live versus PLWUV rVV-EBNA-3A–infected DCs. (E) Comparison of DCs infected with live influenza A or with live or PLWUV rVV-influenza-matrix in eliciting responses to the immunodominant matrix peptide for HLA-A2.1. In all of the experiments, the DC:T-cell ratio was 1:30, the effector T-cell:target cell ratio was 1:20, and no IL-2 was used.
To evaluate the ability of other APCs to induce CTL responses, we used the ER− fraction of the sheep-rosetted PBMCs, which consisted mainly of monocytes. In contrast to DCs, monocytes were not able to elicit peptide specific CTLs in a 7-day culture system (Fig5B).
We then tested DCs infected with PLWUV-inactivated rVV. These DCs were as effective as DCs infected with live vaccinia in stimulating EBNA-3A–specific CTLs (Fig 5B and C). To confirm the use of PLWUV rVV as a vector for delivering antigen to DCs, we compared live and PLWUV-inactivated rVV carrying the influenza matrix gene. As shown in Fig 5D, DCs infected with live and inactivated rVV induced a comparable matrix peptide-specific CTL response to influenza-infected DCs.
DISCUSSION
Several laboratories have described a strong CD8+ T-cell response in healthy EBV+ carriers to the latent antigens of EBV. The dominant response is directed to EBNA-3A, -3B, and -3C gene products.1,2,22,26 These conclusions are based on CTL responses that are generated by repetitive stimulation of bulk mononuclear cells with B-LCLs and IL-2, followed by limiting dilution clonal analysis. However, relatively little information is available on the APCs that are responsible for eliciting this response. The EBV-infected B cell is likely to be important, but we also feel that DCs need to be studied for at least 2 reasons. First, even though DCs are not known to express the CD21 CR2 complement receptor responsible for EBV binding to host cells, it is possible that DCs capture EBV antigens from B cells that have undergone apoptosis.38Second, delivery of EBV antigens to DCs, using viral vectors as an example, could provide a new route whereby DCs would be used in therapy to induce stronger immunity in patients suffering from EBV-associated malignancies, eg, PTLPD, Hodgkin’s disease, and nasopharyngeal carcinoma.
In this report, we have begun to analyze the capacity of DCs to elicit CD8+ CTL immunity. We have found that mature DCs, after exposure to EBNA-3A peptide or infection with rVV-EBNA-3A, serve as efficient stimulators of CTL responses from healthy blood donors. A comparison of monocytes and DCs in eliciting EBV-specific CTL responses showed that DCs are more potent in generating memory responses to a dominant EBNA-3A–restricted peptide. At this time, we know that B-LCL can serve as APCs, but they are less efficient than DCs on a per cell basis and also require addition of exogenous IL-2 (data not shown). In our experience, DCs elicit CTL responses without the addition of exogenous cytokines, presumably because the mature type of DC that we used is known to be capable of eliciting strong IL-2 and cytokine production directly from CD4+ T-cell–depleted CD8+ cells.39 40
There remains a puzzling limitation to the CTLs that were generated with DCs, which is the same limitation that has also been described for T-cell clones generated with LCLs as APCs.34,35 The limitation is that EBNA-3A–specific CTLs do not lyse HLA-B8+ targets directly. We considered that our HLA-B8+ targets might express a different epitope than our FLRGRAYGL-pulsed DCs, because of known sequence variations between the donor’s EBV sequence and the laboratory B95.8 strain used to generate the B-LCL.36,41 However, the same findings were made in repeated experiments using the QAKWRLQTL peptide, which is conserved between the different viral strains. Again, the HLA-matched B-LCL targets had to be enhanced through peptide pulsing. Therefore, our results are similar to those of other reports34 35 that B-LCLs do not seem to present endogenous EBNA peptides in sufficient amounts.
There are several reports of the successful use of T-cell lines for the prophylaxis and treatment of PTLPD,6,8 as well as 1 report on the use of CTL lines in the treatment of patients with EBV+ Hodgkin’s disease.42 The successful reduction in lymphoma and EBV viral burden is likely due to the action of EBV-specific CTLs after this passive immunotherapy. In these studies, B-LCLs were used as APCs to stimulate the formation of CTLs, and this required 4 to 6 weeks. Instead, our data encourage the use of DCs for generating EBV-specific responses for passive immunotherapy. Monocyte-derived DCs can be reliably generated in 7 to 9 days, and these mature DCs induce strong, HLA-restricted CTLs in 7 days.
The other approach would be to use DCs for active rather than passive immunotherapy, ie, DCs would be charged with EBV antigens ex vivo and reinfused to directly immunize patients. Active immunization might provide longer lasting and stronger T-cell memory. The longevity of passively transferred immune cells varies in different reports. Walter et al43 followed cytomegalovirus (CMV)-specific T cells by analysis of rearranged T-cell receptor genes and detected the transferred cells for at least 12 weeks. Heslop et al7 showed persistence of EBV-specific T cells for 18 months after adoptive transfer and after restimulation of CTLs ex vivo. If DCs were used to actively immunize patients, the patient might develop long-term memory and be able to repeatedly generate CTLs after exposure to EBV antigens. In contrast, memory may not readily be established if one transfers chronically stimulated, effector type cells that are programmed to die quickly after withdrawal of cytokines or antigen encounter (antigen-induced cell death).
The use of EBV peptides to charge DCs with antigen has some severe limitations in the context of immunotherapy. First, the peptides need to be defined and tailored to the patient’s HLA haplotype. Second, a single peptide may elicit a form of immunity that is easy for tumors to escape, because most immunogenic peptides are not known to be sequences that are essential for the transforming ability of the virus. We considered here vaccinia vectors, because an excellent panel of recombinant vaccinia viruses exists, each encoding one of the 8 latent EBV genes.26 There are reports in which rVV has been used to deliver antigens to mouse DCs and human DCs.44,45Although vaccinia virus is a known cytopathic virus, none of the prior reports address the degree of toxicity that is encountered when using this virus. The toxicity of vaccinia virus is more prominent in immature DCs that are also infected to a greater extent than our current preparation of mature DCs. In addition, infection with rVV blocks the maturation of DCs before any detectable cytolysis.37
We have infected B-LCLs and immature and mature DCs with rVV and monitored the rate of infection with vaccinia antibodies that react with early and late vaccinia proteins. The VV1-6B6 antibody stains a protein that is inferred to be under the control of an early promotor that becomes active immediately after infection, because necessary transcription factors are brought into the cells within the infectious particles. Infected B-LCLs and DCs stain positively for VV1-6B6, as well as anti–LMP-1 when using rVV-LMP-1, which is under the control of an early promotor. In agreement with others,46,47 we find that macrophages and DCs ex vivo are nonpermissive for vaccinia virus because no late viral proteins are produced. This could be confirmed by staining with the VV1-4G9 antibody, which stains the vaccinia hemagglutinin protein A56R (controlled by a late promoter) on infected B-LCLs but not on DCs.48 The same findings are evident with VV4-2F6 that stains the late vaccinia protein D8L.49Consequently, we have used VV1-6B6 to monitor the percentage of infected DCs. We observe consistently that immature DCs are infected to a higher degree than mature DCs. Testing cell viability with trypan blue exclusion up to 5 days after infection shows a significant decrease in the viability of immaturely infected DCs. To exclude the possibility that the MCM we have used to induce maturation is responsible for this toxicity, we have performed comparative studies with prostaglandin E2 (PGE2; 10 μg/mL) and tumor necrosis factor-α (1,000 IU/mL) to induce maturation. No difference in cell viability as well as percentage of infection of mature DCs has been observed (data not shown). Staining with Annexin V FITC and PI shows that rVV-infected DCs undergo apoptosis faster and to a higher percentage compared with uninfected DCs (Fig 3B). However, before cell death, the uninfected and infected mature DCs have the same phenotype and function, as detected by the capacity to stimulate allogeneic T cells (Fig 4). The mechanism underlying the nonpermissive state for virus replication in macrophages as well as DCs is not known. There is evidence that the initiator of the apoptosis signal is ds RNA.50 Clearly, the maturation state of the DC somehow influences the degree of infection with rVV.
To increase the possibility of using rVV in immunocompromised patients and to possibly decrease the cytopathic effect of vaccinia, we have used vaccinia inactivated by psoralen and long-wave UV light.32 PLWUV is known to target nucleic acids preferentially and to introduce chemical cross-links in the viral genome, creating nonreplicating virus. We have confirmed this by titration on BSC40 cells and also by a decrease in protein synthesis in DCs infected with PLWUV-treated rVV. The extent of protein synthesis, as monitored with the VV1-6B6 antibody, is quite variable from one experiment to another, probably because of the random nature with which UV light cross-links the viral genome. The PLWUV-treated rVV retains some toxicity, albeit delayed compared with live rVV (Fig 3A). This has been described for other cells and is not surprising, because ds RNA is still produced.51 52 Also, some of the cytopathic effects are potentially caused by viral enzymes that are brought into the cell with the infectious particles.
However, remarkably, DCs that are infected with PLWUV-treated rVV retain the capacity to present the recombinant EBV antigens. This is also the case with rVV expressing the matrix gene from influenza virus. This finding is reminiscent of prior work in which heat-inactivated or UV-inactivated influenza virus can be efficiently presented by human DCs.53 The current interpretation of these findings is that DCs are such efficient APCs that they are able to elicit immunity with relatively small amounts of viral protein synthesis. The use of PLWUV-treated rVV should make it possible to charge DCs from any individual with EBV antigens without first having to define the peptide that is appropriate for his or her HLA type. Furthermore, PLWUV-treated rVV may have value for immunotherapy of immunosupressed patients, especially if the CD8 repertoire remains intact, or in posttreatment vaccination protocols.
ACKNOWLEDGMENT
The authors thank Judy Adams and Frank Isdell for assistance with graphics and flow cytometry respectively. We are grateful to Dr B. Moss for recombinant vaccinia matrix virus. We thank Dr C. Münz for critical discussion of the manuscript.
Supported by grants from the National Institutes of Health (AI 40874 to R.M.S. and AI 39516 to N.B.), the Cancer Research Institute (New York, NY), and the National Cancer Institute (to M.G.K.). M.S. was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Marion Subklewe, MD, Laboratory of Cellular Physiology and Immunology, Rockefeller University, New York, NY 10021-6399; e-mail: subklem@rockvax.rockefeller.edu.